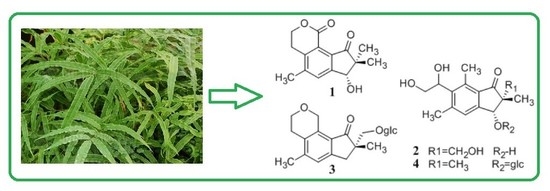
Four New Pterosins from Pteris cretica and Their Cytotoxic Activities
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Spectral Data
3.5. Cytotoxic Activity
3.6. Acid Hydrolysis of 3–4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Editorial Committee of Flora of China of Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1999; pp. 10–15. [Google Scholar]
- Yong, L.; Yao, Z.S. Resource investigation of medicine plants in Pteris genus in Jiangxi. Jiangxi For. Sci. Technol. 1996, 6, 29–30. [Google Scholar]
- Ouyang, D.W.; Ni, X.; Xu, H.Y.; Chen, J.; Yang, P.M.; Kong, D.Y. Pterosins from Pteris multifida. Planta Med. 2010, 76, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.J.; Huang, X.B.; Lv, Z.C. Isolation and identification of flavonoids components from Pteris vittata L. SpringerPlus 2016, 5, 1649. [Google Scholar] [CrossRef] [PubMed]
- Harinantenaina, L.; Matsunami, K.; Otsuka, H. Chemical and biologically active constituents of Pteris multifida. J. Nat. Med. 2008, 62, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Zhu, D.Y. Reviews on sesquiterpenoids from spices of Pteridaceae-Structures, physical and chemical properties and spectroscopic characteristics of 1H-inden-1-one sesquiterpenoids. Chem. Res. 2004, 15, 72–76. [Google Scholar]
- Lu, J.; Huang, Y.Z.; Chen, S.J.; Liu, J.Q.; Shu, J.C. Review on pterosins in ferns. Chin. Tradit. Pat. Med. 2019, 42, 160–171. [Google Scholar]
- Kim, J.W.; Kim, H.P.; Sung, S.H. Cytotoxic pterosins from Pteris multifida roots against HCT-116 human colon cancer cells. Bioorg. Med. Chem. Lett. 2017, 27, 3144–3147. [Google Scholar] [CrossRef]
- Yahara, Y.; Takemori, H.; Okada, M. Pterosin B prevents chondrocyte hypertrophy and osteoarthritis in mice by inhibiting Sik3. Nat. Commun. 2016, 27, 1–12. [Google Scholar] [CrossRef]
- Hsu, F.; Huang, C.; Chen, Y. Antidiabetic Effects of Pterosin A, a Small-Molecular-Weight Natural Product on Diabetic Mouse Models. Diabetes 2013, 62, 628–638. [Google Scholar] [CrossRef]
- Chen, J.J.; Wang, T.C.; Yang, C.K. New Pterosin sesquiterpenes and antitubercular constituents from Pteris ensiformis. Chem. Biodivers. 2014, 45, 1903–1909. [Google Scholar]
- Liu, J.; Shu, J.; Zhang, R. Two new pterosin dimers from Pteris mutifida Poir. Fitoterapia 2011, 82, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.C.; Pan, J.H.; Zhang, L.; Ren, X.J.; Liu, J.Q. Sesquiterpenes from Pteris multifida. Chin. Tradit. Pat. Med. 2011, 33, 2104–2107. [Google Scholar]
- Shu, J.; Liu, J.; Zhong, Y.; Liu, L.; Zhang, R. Two new pterosin sesquiterpenes from Pteris multifida Poir. Phytochem. Lett. 2012, 5, 276–279. [Google Scholar] [CrossRef]
- Kuraishi, T.; Murakami, T.; Taniguchi, T.; Kobuki, Y.; Maehashi, H.; Tanaka, N.; Saiki, Y.; Chen, C.M. Chemical and chemotaxonomical studies of Ferns. LIV. Pterosin derivatives of the genus Microlepia (Pteridaceae). Chem. Pharm. Bull. 1985, 33, 2305–2312. [Google Scholar] [CrossRef]
- Tanaka, N.; Satake, T.; Takahashi, A.; Mochizuki, M.; Murakami, T.; Saiki, Y.; Yang, J.Z.; Chen, C.M. Chemical and chmotaxonomical studies of Ferns. XXXIX. Chemical studies on the constituents of Pteris bella TAGAWA and Pteridium aquilinum subsp. wightianum (WALL) SHICH. Chem. Pharm. Bull. 1982, 30, 3646–3652. [Google Scholar] [CrossRef]
- Murakami, T.; Taguchi, S.; Nomura, Y.; Tanaka, N.; Satake, T.; Saiki, Y.; Chen, C.M. Weitere Indan-1-on-derivate der gattung Pteris. Chem. Pharm. Bull. 1976, 24, 1961–1964. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, F.R.; Lu, M.C.; Hsieh, P.W.; Wu, M.J. New benzoyl glucosides and cytotoxic pterosin sesquiterpenes from Pteris ensiformis Burm. Molecules 2008, 13, 255–266. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Fukuoka, M.; Yoshihira, K.; Natrori, S. Circular dichroism and confromations of pterosins, 1-indanone derivatives from Bracken. Chem. Pharm. Bull. 1979, 27, 731–741. [Google Scholar] [CrossRef]
- Mohamed, K.M. Phenylpropanoid glucosides from Chrozophora oblique. Phytochemistry 2001, 58, 615–618. [Google Scholar] [CrossRef]
- Xin, W.B.; Chou, G.X.; Wang, Z.T. Triterpenoids and saponins from leaves of Uncaria hirsute. Helv. Chim. Acta 2009, 92, 638–644. [Google Scholar] [CrossRef]
- Mohammad, R.H.; Nur-E-Alam, M.; Lahmann, M.; Parveen, I.; Tizzard, G.J.; Coles, S.J.; Fowler, M.; Drake, A.F.; Heyes, D.; Thoss, V. Isolation and characterisation of 13 pterosins and pterosides from bracken (Pteridium aquilinum (L.) Kuhn) rhizome. Phytochemistry 2016, 128, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, J.; Pan, L.L.; Chen, J.Y.; Liu, R.H.; Zhu, G.H.; Huang, H.L.; Shu, J.C.; Shao, F.; Liang, Y.H.; et al. Six new furostanol glycosides from Smilax glauco-china and their cytotoxic activity. J. Asian Nat. Prod. Res. 2017, 19, 754–765. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | - | 206.1 | - | 209.7 |
| 2 | - | 53.4 | - | 57.0 |
| 3 | 4.80 (s) | 77.1 | 4.84 (s) | 77.4 |
| 4 | 7.80 (s) | 132.4 | 7.35 (s) | 126.9 |
| 5 | - | 144.4 | - | 146.6 |
| 6 | - | 144.1 | - | 140.4 |
| 7 | - | 123.9 | - | 138.2 |
| 8 | - | 134.0 | - | 133.1 |
| 9 | - | 155.1 | - | 155.6 |
| 10 | 1.10 (s) | 20.0 | 3.73 (d, J = 11.0) | 67.1 |
| 3.68 (d, J = 11.0) | ||||
| 11 | 1.25 (s) | 22.6 | 1.15 (s) | 19.2 |
| 12 | 2.49 (s) | 20.3 | 2.58 (s) | 22.4 |
| 13 | 3.06 (m) | 27.0 | 5.32 (dd, J = 4.9, 8.3) | 72.7 |
| 14 | 4.49 (m) | 67.9 | 3.93 (dd, J = 8.3, 11.4) | 65.6 |
| 4.55 (m) | 3.64 (dd, J = 4.9, 11.4) | |||
| 15 | - | 163.8 | 2.76 (s) | 15.2 |
| Position | 3 | 4 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | - | 211.9 | - | 211.2 |
| 2 | - | 51.2 | - | 52.8 |
| 3 | 2.80 (d, J = 17.0) | 38.5 | 4.84 (s) | 86.0 |
| 3.50 (d, J = 17.0) | ||||
| 4 | 7.22 (s) | 126.9 | 7.52 (s) | 127.8 |
| 5 | - | 146.4 | - | 146.2 |
| 6 | - | 132.5 | - | 140.7 |
| 7 | - | 136.2 | - | 139.1 |
| 8 | - | 130.1 | - | 131.4 |
| 9 | - | 153.9 | - | 152.7 |
| 10 | 4.10 (d, J = 9.4) | 74.86 | 1.08 (s) | 22.3 |
| 3.48 (d, J = 9.4) | ||||
| 11 | 1.14 (s) | 21.8 | 1.28 (s) | 22.8 |
| 12 | 2.35 (s) | 19.9 | 2.57 (s) | 22.0 |
| 13 | 2.74 (t, J = 5.5) | 27.2 | 5.32 (dd, J = 4.9, 8.4) | 72.7 |
| 14 | 3.98 (m) | 65.7 | 3.92 (dd, J = 8.4, 11.5) | 65.5 |
| 3.63 (dd, J = 4.9,11.5) | ||||
| 15 | 5.08 (d, J = 4.8) | 67.5 | 2.74 (s) | 15.2 |
| glc-1 | 4.20 (d, J = 7.9) | 104.7 | 4.57 (d, J = 7.8) | 105.8 |
| glc-2 | 3.21 (m) | 71.5 | 3.36 (m) | 71.7 |
| glc-3 | 3.20 (m) | 77.9 | 3.42 (m) | 78.0 |
| glc-4 | 3.03 (m) | 74.9 | 3.28 (m) | 75.3 |
| glc-5 | 3.28 (m) | 78.1 | 3.27 (m) | 78.2 |
| glc-6 | 3.78 (dd, J = 1.6, 11.8) | 62.8 | 3.85 (dd, J = 2.0, 12.0) | 62.8 |
| 3.61 (dd, J = 5.7, 11.8) | 3.75 (dd, J = 5.4, 12.0) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Peng, C.; Cheng, S.; Liu, J.; Ma, Q.; Shu, J. Four New Pterosins from Pteris cretica and Their Cytotoxic Activities. Molecules 2019, 24, 2767. https://doi.org/10.3390/molecules24152767
Lu J, Peng C, Cheng S, Liu J, Ma Q, Shu J. Four New Pterosins from Pteris cretica and Their Cytotoxic Activities. Molecules. 2019; 24(15):2767. https://doi.org/10.3390/molecules24152767
Chicago/Turabian StyleLu, Jian, Caiying Peng, Shuang Cheng, Jianqun Liu, Qinge Ma, and Jicheng Shu. 2019. "Four New Pterosins from Pteris cretica and Their Cytotoxic Activities" Molecules 24, no. 15: 2767. https://doi.org/10.3390/molecules24152767
APA StyleLu, J., Peng, C., Cheng, S., Liu, J., Ma, Q., & Shu, J. (2019). Four New Pterosins from Pteris cretica and Their Cytotoxic Activities. Molecules, 24(15), 2767. https://doi.org/10.3390/molecules24152767