Novel Functionalized Cellulose Microspheres for Efficient Separation of Lithium Ion and Its Isotopes: Synthesis and Adsorption Performance
Abstract
1. Introduction
2. Results and Discussion
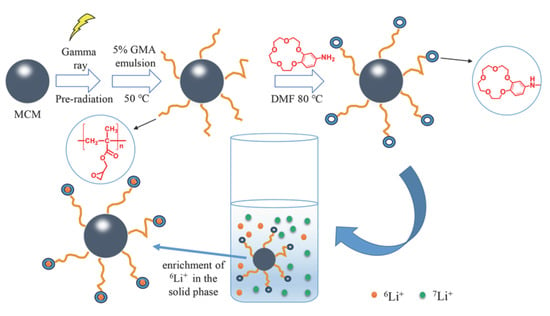
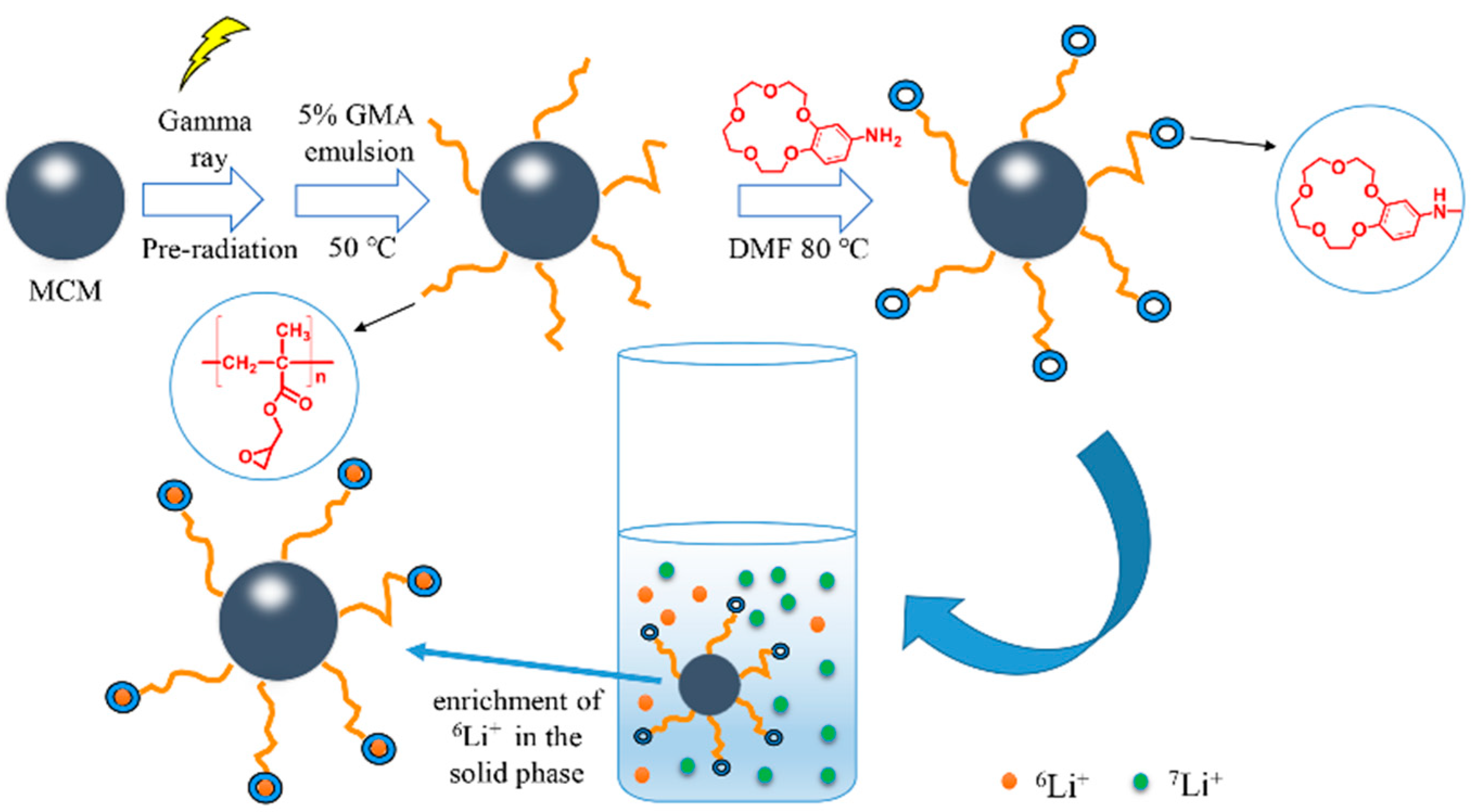
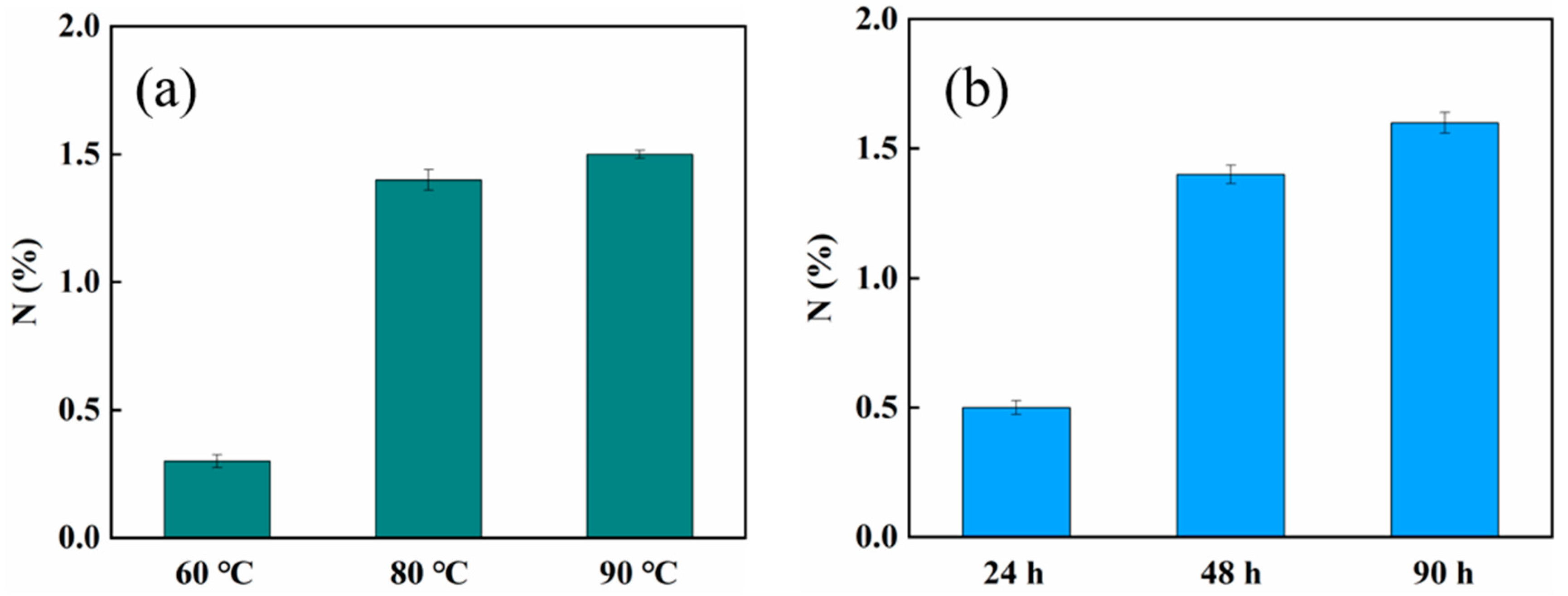
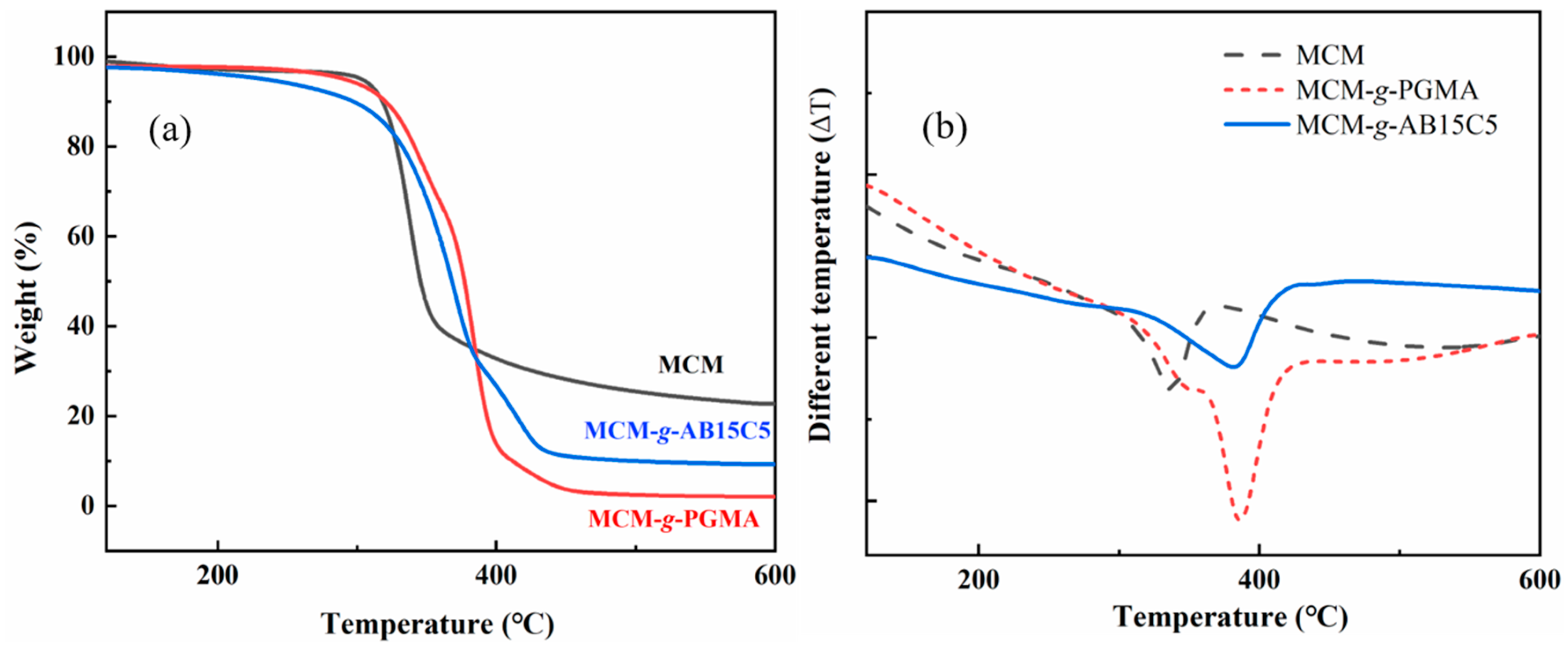
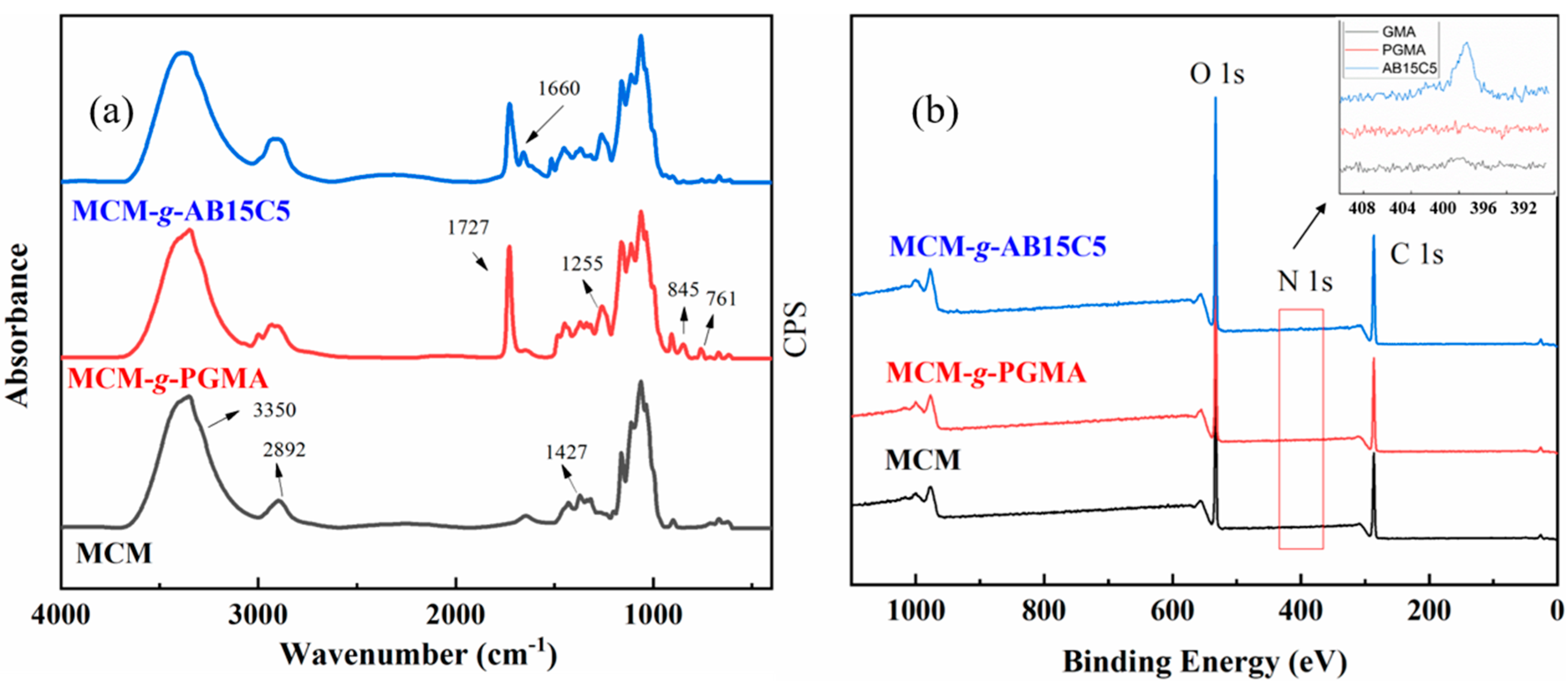
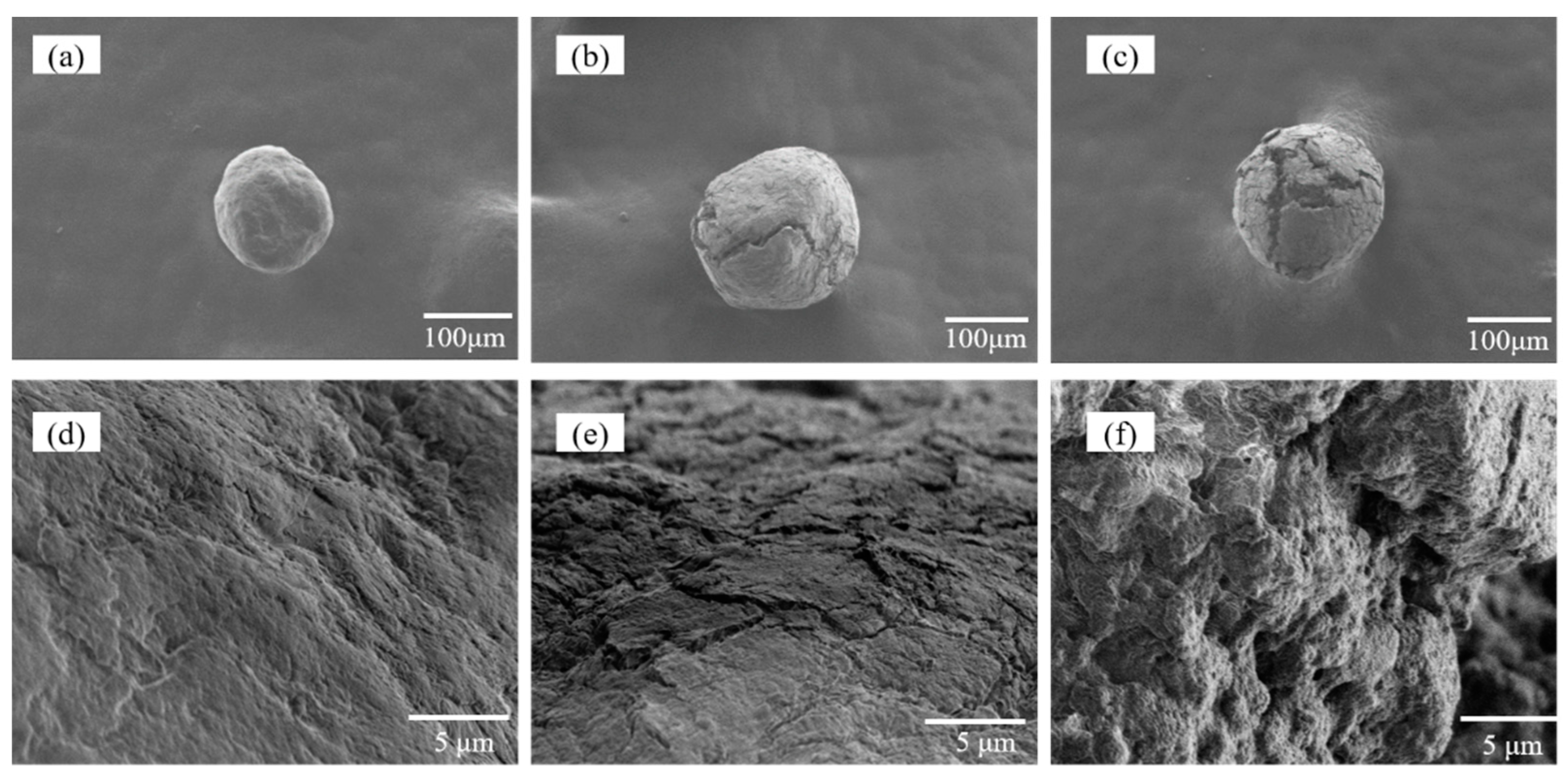
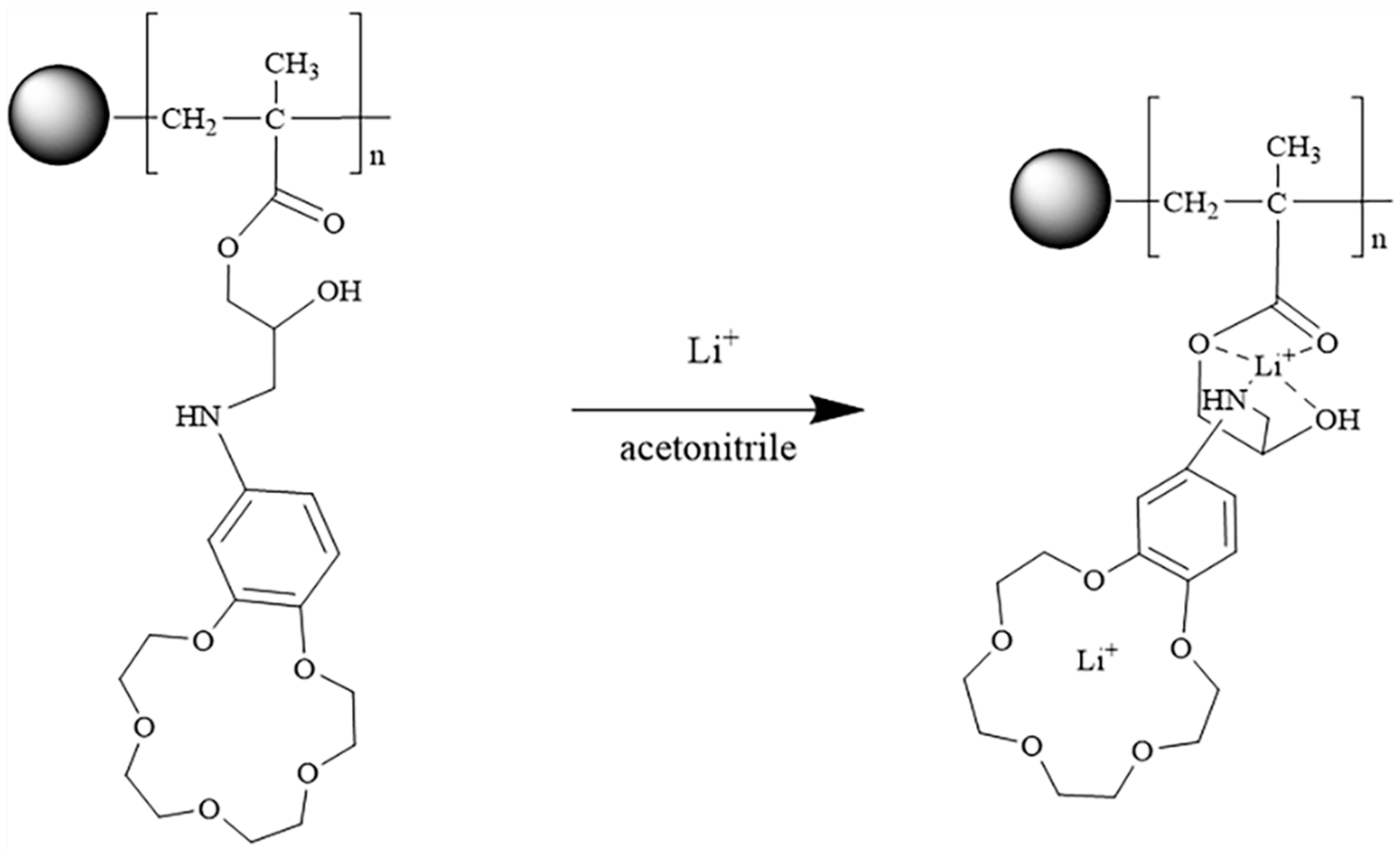
2.1. Synthesis and Characterization of the Adsorbent
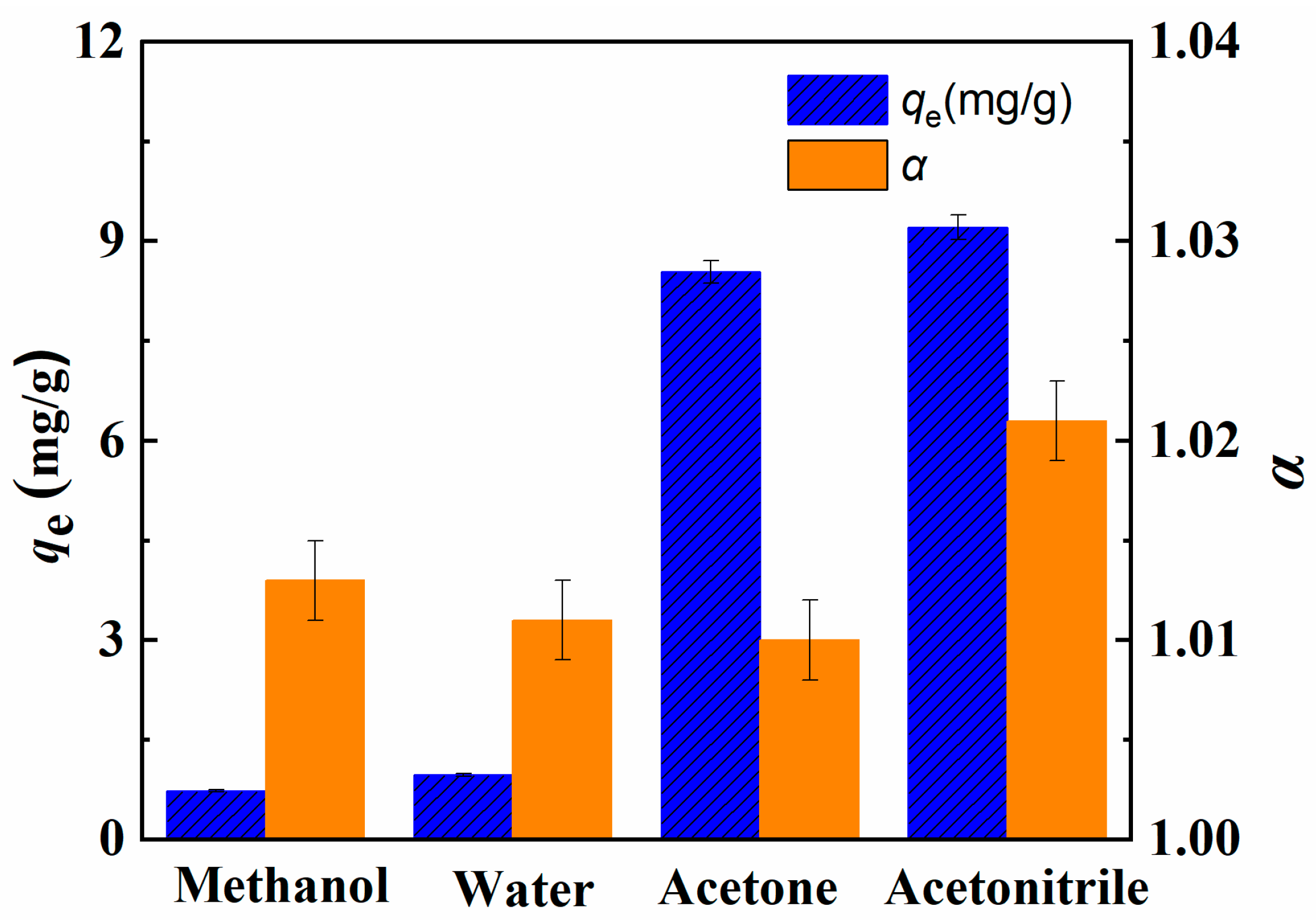
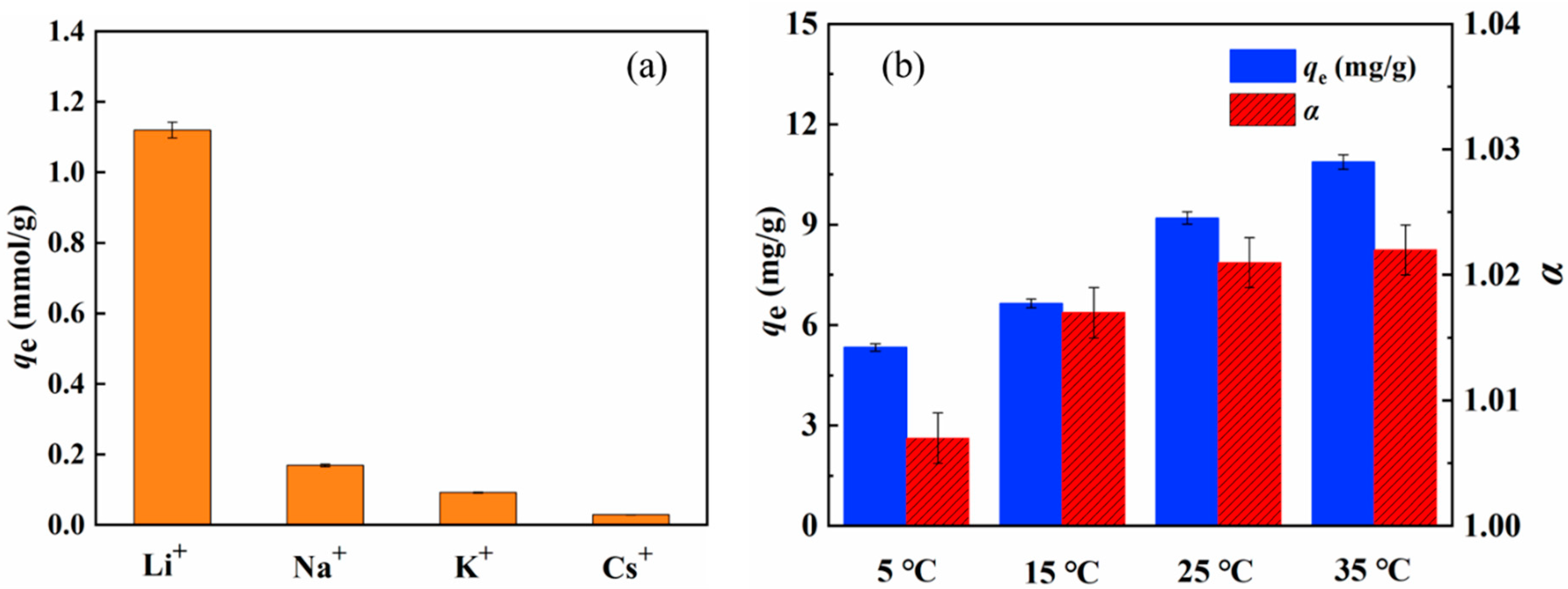
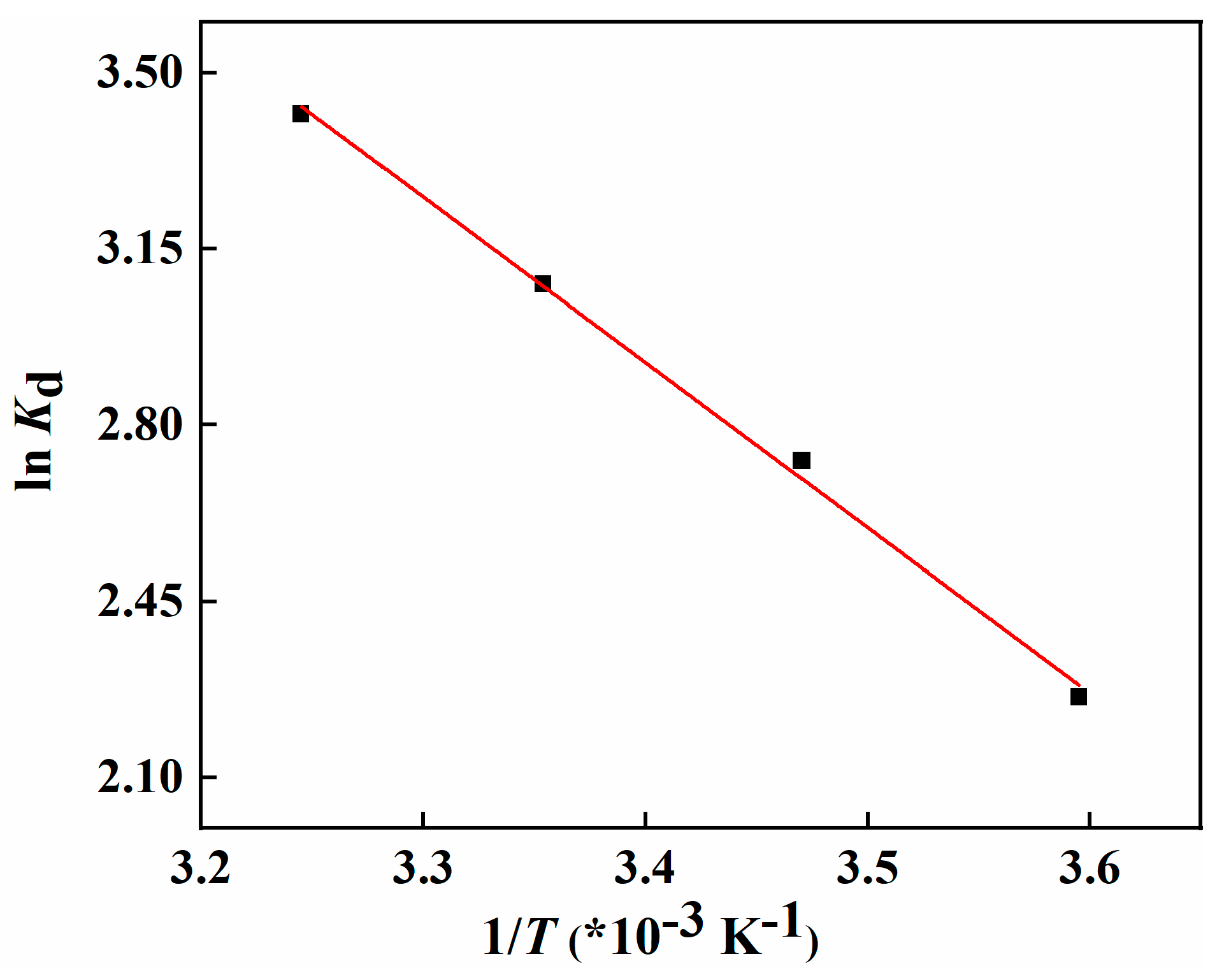
2.2. Adsorption Uptake of Li+ And Separation Factor of 6Li/7Li
3. Materials and Methods
3.1. Materials
3.2. Preparation of MCM-g-PGMA and MCM-g-AB15C5
3.3. Characterization
3.4. Adsorption of Li+ and Its Isotopes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef]
- Michiels, E.; De Bièvre, P. Absolute isotopic composition and the atomic weight of a natural sample of lithium. Int. J. Mass Spectrom. Ion Phys. 1983, 49, 265–274. [Google Scholar] [CrossRef]
- Nishizawa, K.; Takano, T.; Ikeda, I.; Okahara, M. Extractive Separation of Lithium Isotopes by Crown Ethers. Sep. Sci. Technol. 1988, 23, 333–345. [Google Scholar] [CrossRef]
- Liu, Y.; Inoue, Y.; Hakushi, T. Molecular Design of Crown Ethers. VII. Syntheses and Cation Selectivities of Unsubstituted 12-to 16-Crown-4. Bull. Chem. Soc. Jpn. 1990, 63, 3044–3046. [Google Scholar] [CrossRef]
- Nishizawa, K.; Ishino, S.I.; Watanabe, H.; Shinagawa, M. Lithium Isotope Separation by Liquid-Liquid Extraction Using Benzo-15-Crown-5. J. Nucl. Sci. Technol. 1984, 21, 694–701. [Google Scholar] [CrossRef]
- Otake, K.; Suzuki, T.; Kim, H.J.; Nomura, M.; Fujii, Y. Novel Syntheses Method of Phenol Type Benzo-15-Crown-5 Ether Resin and its Application for Lithium Isotope Separation. J. Nucl. Sci. Technol. 2006, 43, 419–422. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, X.L.; Gu, L.; Bao, F.F.; Xu, X.X.; Pang, C.Y.; Gu, Z.G.; Li, Z. A green strategy for lithium isotopes separation by using mesoporous silica materials doped with ionic liquids and benzo-15-crown-5. J. Radioanal. Nucl. Chem. 2014, 300, 843–852. [Google Scholar] [CrossRef]
- Pei, H.; Yan, F.; Ma, X.; Li, X.; Liu, C.; Li, J.; Cui, Z.; He, B. In situ one-pot formation of crown ether functionalized polysulfone membranes for highly efficient lithium isotope adsorptive separation. Eur. Polym. J. 2018, 109, 288–296. [Google Scholar] [CrossRef]
- Pei, H.; Yan, F.; Liu, H.; Li, T.; Wang, M.; Li, J.; Ma, X.; Cui, Z.; He, B. Formoxylbenzo-15-crown-5 ether functionalized PVA/NWF composite membrane for enhanced 7Li+ enrichment. J. Taiwan. Inst. Chem. E 2019, 97, 496–502. [Google Scholar] [CrossRef]
- Torrejos, R.E.C.; Nisola, G.M.; Park, M.J.; Shon, H.K.; Seo, J.G.; Koo, S.; Chung, W.-J. Synthesis and characterization of multi-walled carbon nanotubes-supported dibenzo-14-crown-4 ether with proton ionizable carboxyl sidearm as Li+ adsorbents. Chem. Eng. J. 2015, 264, 89–98. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Ye, G.; Pu, N.; Wu, F.; Wang, Z.; Huo, X.; Xu, J.; Chen, J. Macrocyclic ligand decorated ordered mesoporous silica with large-pore and short-channel characteristics for effective separation of lithium isotopes: Synthesis, adsorptive behavior study and DFT modeling. Dalton Trans. 2016, 45, 16492–16504. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Ye, G.; Song, Y.; Liu, F.; Huo, X.; Chen, J. Well-defined functional mesoporous silica/polymer hybrids prepared by an ICAR ATRP technique integrated with bio-inspired polydopamine chemistry for lithium isotope separation. Dalton Trans. 2017, 46, 6117–6127. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Pei, H.; Pei, Y.; Li, T.; Li, J.; He, B.; Cheng, Y.; Cui, Z.; Guo, D.; Cui, J. Preparation and Characterization of Polysulfone-graft-4′-aminobenzo-15-crown-5-ether for Lithium Isotope Separation. Ind. Eng. Chem. Res. 2015, 54, 3473–3479. [Google Scholar] [CrossRef]
- Zeng, Y.; Pei, H.; Wang, Z.; Yan, F.; Li, J.; Cui, Z.; He, B. Chitosan-graft-benzo-15-crown-5-ether/PVA Blend Membrane with Sponge-Like Pores for Lithium Isotope Adsorptive Separation. ACS Omega 2018, 3, 554–561. [Google Scholar] [CrossRef]
- Fakhre, N.A.; Ibrahim, B.M. The use of new chemically modified cellulose for heavy metal ion adsorption. J. Hazard. Mater. 2018, 343, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H.; Sohsah, M.A.; Ghoneim, M.M. The effects of hazardous ions adsorption on the morphological and chemical properties of reactive cloth filter. Radiat. Phys. Chem. 2009, 78, 976–985. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Földváry, C.M.; Takács, E. Radiation-induced grafting of cellulose for adsorption of hazardous water pollutants: A review. Radiat. Phys. Chem. 2010, 79, 848–862. [Google Scholar] [CrossRef]
- Li, W.; Ju, B.; Zhang, S. A green l-cysteine modified cellulose nanocrystals biosorbent for adsorption of mercury ions from aqueous solutions. RSC Adv. 2019, 9, 6986–6994. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, J.; Yuan, W.; Yi, Y.; Zhao, L. Recovery of Au(III) by radiation synthesized aminomethyl pyridine functionalized adsorbents based on cellulose. Chem. Eng. J. 2016, 283, 504–513. [Google Scholar] [CrossRef]
- O’sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Barsbay, M.; Kavaklı, P.A.; Tilki, S.; Kavaklı, C.; Güven, O. Porous cellulosic adsorbent for the removal of Cd (II), Pb(II) and Cu(II) ions from aqueous media. Radiat. Phys. Chem. 2018, 142, 70–76. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpaa, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Madrid, J.F.; Nuesca, G.M.; Abad, L.V. Gamma radiation-induced grafting of glycidyl methacrylate (GMA) onto water hyacinth fibers. Radiat. Phys. Chem. 2013, 85, 182–188. [Google Scholar] [CrossRef]
- Xu, M.; Ao, Y.; Wang, S.; Peng, J.; Li, J.; Zhai, M. Efficient adsorption of 1-alkyl-3-methylimidazolium chloride ionic liquids onto modified cellulose microspheres. Carbohydr. Polym. 2015, 128, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. The thermal performance of the polysaccharides extracted from hardwood: Cellulose and hemicellulose. Carbohydr. Polym. 2010, 82, 39–45. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Hu, X.; Wang, X.; Niu, J.; Ma, T. Adsorption of Cationic Dyes on a Cellulose-Based Multicarboxyl Adsorbent. J. Chem. Eng. Data 2013, 58, 413–421. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Senan, P. Adsorptive potential of sulfonated poly(glycidylmethacrylate)-grafted cellulose for separation of lysozyme from aqueous phase: Mass transfer analysis, kinetic and equilibrium profiles. Colloid. Surf. A 2011, 377, 156–166. [Google Scholar] [CrossRef]
- Kavner, A.; Shahar, A.; Black, J.; Young, E.D. Iron isotope electroplating: Diffusion-limited fractionation. Chem. Geol. 2009, 267, 131–138. [Google Scholar] [CrossRef]
- Davoudi, M.; Mallah, M.H. Enrichment of 6Li using dispersive liquid–liquid microextraction as a highly efficient technique. Ann. Nucl. Energy 2013, 62, 499–503. [Google Scholar] [CrossRef]
- Wang, W.; Julaiti, P.; Ye, G.; Huo, X.; Chen, J. Controlled architecture of macrocyclic ligand functionalized polymer brushes from glass fibers using surface-initiated ICAR ATRP technique for adsorptive separation of lithium isotopes. Chem. Eng. J. 2018, 336, 669–678. [Google Scholar] [CrossRef]
- Izatt, R.M.; Bradshaw, J.S.; Pawlak, K.; Bruening, R.L.; Tarbet, B.J. Thermodynamic and Kinetic Data for Macrocycie Interaction with Neutral Molecules. Chem. Rev. 1992, 92, 1261–1354. [Google Scholar] [CrossRef]
- Kim, D.W.; Jeon, Y.S.; Jeong, Y.K.; Suh, M.Y.; Joe, K.S. Lithium isotope separation by chemical exchange with polymer-bound azacrown compounds. J. Radioanal. Nucl. Chem. 1995, 189, 219–227. [Google Scholar] [CrossRef]
- Yan, F.; Liu, H.; Pei, H.; Li, J.; Cui, Z.; He, B. Polyvinyl alcohol-graft-benzo-15-crown-5 ether for lithium isotopes separation by liquid–solid extraction. J. Radioanal. Nucl. Chem. 2017, 311, 2061–2068. [Google Scholar] [CrossRef]
- Alizadeh, B.; Ghorbani, M.; Salehi, M.A. Application of polyrhodanine modified multi-walled carbon nanotubes for high efficiency removal of Pb(II) from aqueous solution. J. Mol. Liq. 2016, 220, 142–149. [Google Scholar] [CrossRef]
- Keypour, H.; Zebarjadian, M.H.; Rezaeivala, M.; Afkhami, A. Competitive 7Li NMR study of the stoichiometry, stability and thermodynamic data for the complexation of Li+, Mn2+, Zn2+ and Cd2+ ions with two asymmetrical branched pentadentate (N5) amines containing pyridine moiety in ionic liquid–acetonitrile mixtures. J. Mol. Struct. 2014, 1075, 525–533. [Google Scholar] [CrossRef]
- Huang, W.; Liu, S.; Liu, J.; Zhang, W.; Pan, J. 2-Methylol-12-crown-4 ether immobilized PolyHIPEs toward recovery of lithium(i). New J. Chem. 2018, 42, 16814–16822. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Yang, D.; Zheng, X.; Wang, Y.; Pan, J.; Zhang, T.; Xu, J.; Qiu, F.; Yan, Y.; et al. A facile strategy toward ion-imprinted hierarchical mesoporous material via dual-template method for simultaneous selective extraction of lithium and rubidium. J. Clean. Prod. 2018, 171, 264–274. [Google Scholar] [CrossRef]
- Ma, F.; Qu, R.; Sun, C.; Wang, C.; Ji, C.; Zhang, Y.; Yin, P. Adsorption behaviors of Hg(II) on chitosan functionalized by amino-terminated hyperbranched polyamidoamine polymers. J. Hazard. Mater. 2009, 172, 792–801. [Google Scholar] [CrossRef]
Sample Availability: Samples of MCM-g-PGMA and MCM-g-AB15C5 are available from the authors. |
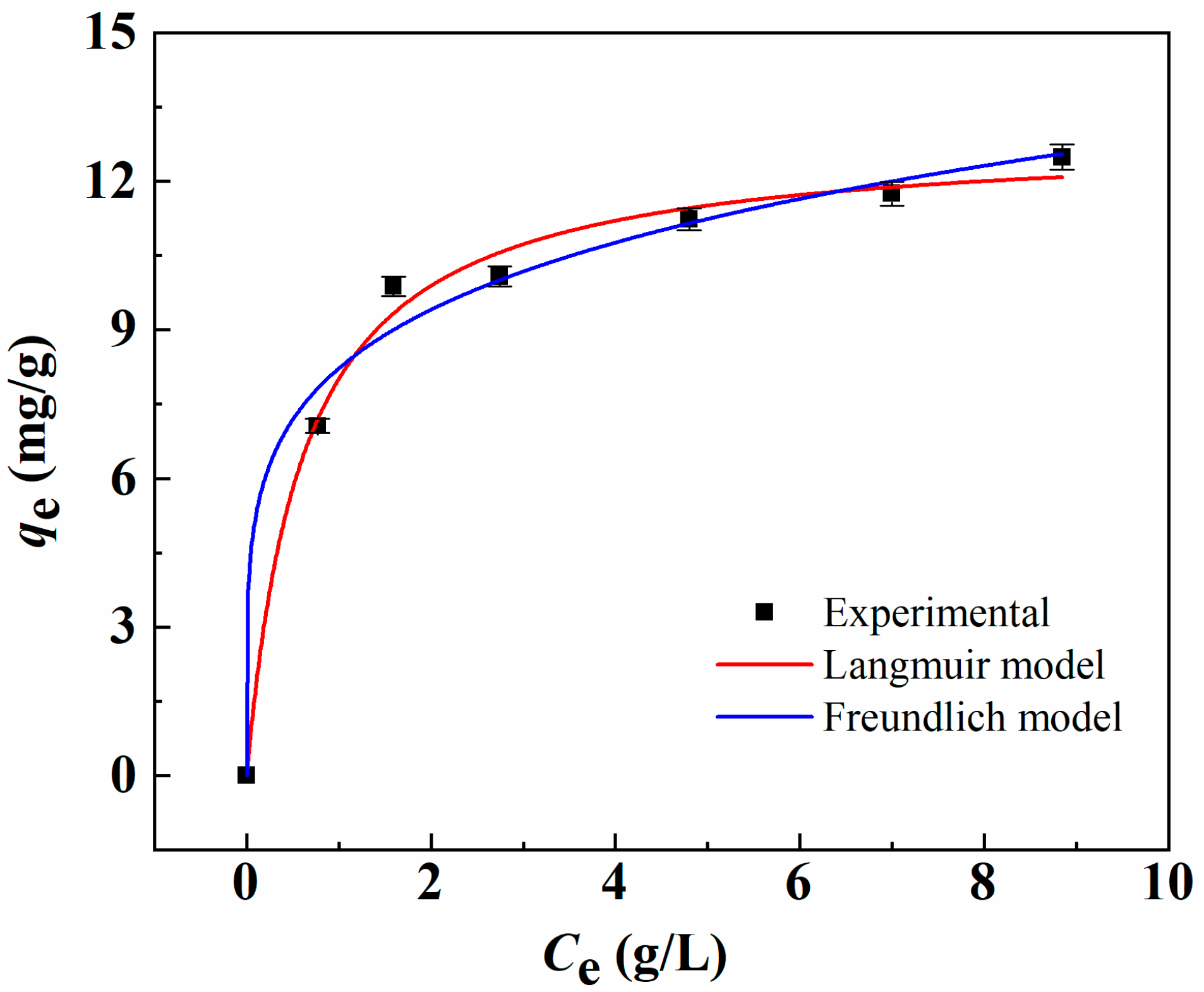
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| KL (L·mg−1) | qm (mg·g−1) | R2 | Kf ((mg·g−1) (L·mg)1/n) | n | R2 |
| 1.63 | 12.90 | 0.993 | 8.22 | 5.15 | 0.987 |
| Adsorbents | Adsorption Capacity (mg·g−1) | Reference |
|---|---|---|
| Macroporous polymer foam (2-methylol-12-crown-4) | 3.15 | Huang et al. (2018) [37] |
| Imprinted hierarchical porous silica (12-crwon-4) | 0.166 | Xu et al. (2018) [38] |
| Glass fiber mats (4′-aminobenzo-15-crown-5) | 6.46 | Wang et al. (2018) [31] |
| Mesoporous silica/polymer hybrids (4′-aminobenzo-15-crown-5) | 6.97 | Liu et al. (2017) [12] |
| Mesoporous silicas (4′-aminobenzo-15-crown-5) | 5.14 | Liu et al. (2016) [11] |
| Multi-walled carbon nanotubes (Hydroxy-dibenzo-14-crown-4 ether) | 2.11 | Torrejos et al. (2015) [10] |
| MCM-g-AB15C5 | 12.90 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, I.; Xu, C.; Peng, J.; Han, D.; Liu, S.; Zhai, M. Novel Functionalized Cellulose Microspheres for Efficient Separation of Lithium Ion and Its Isotopes: Synthesis and Adsorption Performance. Molecules 2019, 24, 2762. https://doi.org/10.3390/molecules24152762
Chen I, Xu C, Peng J, Han D, Liu S, Zhai M. Novel Functionalized Cellulose Microspheres for Efficient Separation of Lithium Ion and Its Isotopes: Synthesis and Adsorption Performance. Molecules. 2019; 24(15):2762. https://doi.org/10.3390/molecules24152762
Chicago/Turabian StyleChen, Ichen, Chenxi Xu, Jing Peng, Dong Han, Siqi Liu, and Maolin Zhai. 2019. "Novel Functionalized Cellulose Microspheres for Efficient Separation of Lithium Ion and Its Isotopes: Synthesis and Adsorption Performance" Molecules 24, no. 15: 2762. https://doi.org/10.3390/molecules24152762
APA StyleChen, I., Xu, C., Peng, J., Han, D., Liu, S., & Zhai, M. (2019). Novel Functionalized Cellulose Microspheres for Efficient Separation of Lithium Ion and Its Isotopes: Synthesis and Adsorption Performance. Molecules, 24(15), 2762. https://doi.org/10.3390/molecules24152762