Physical–Chemical Composition and Quality Related Changes in “Ruaner” Pear (Pyrus ussuriensis) During Freezing–Thawing Period
Abstract
:1. Introduction
2. Results
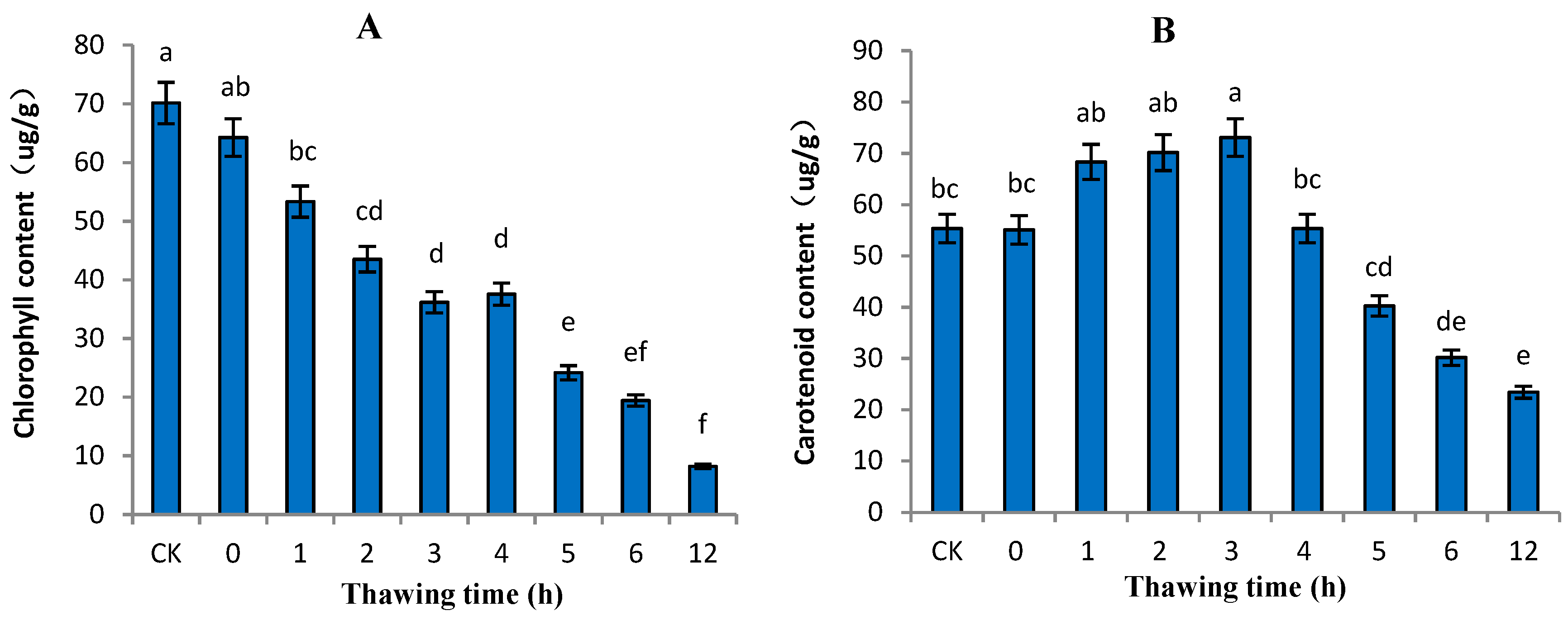
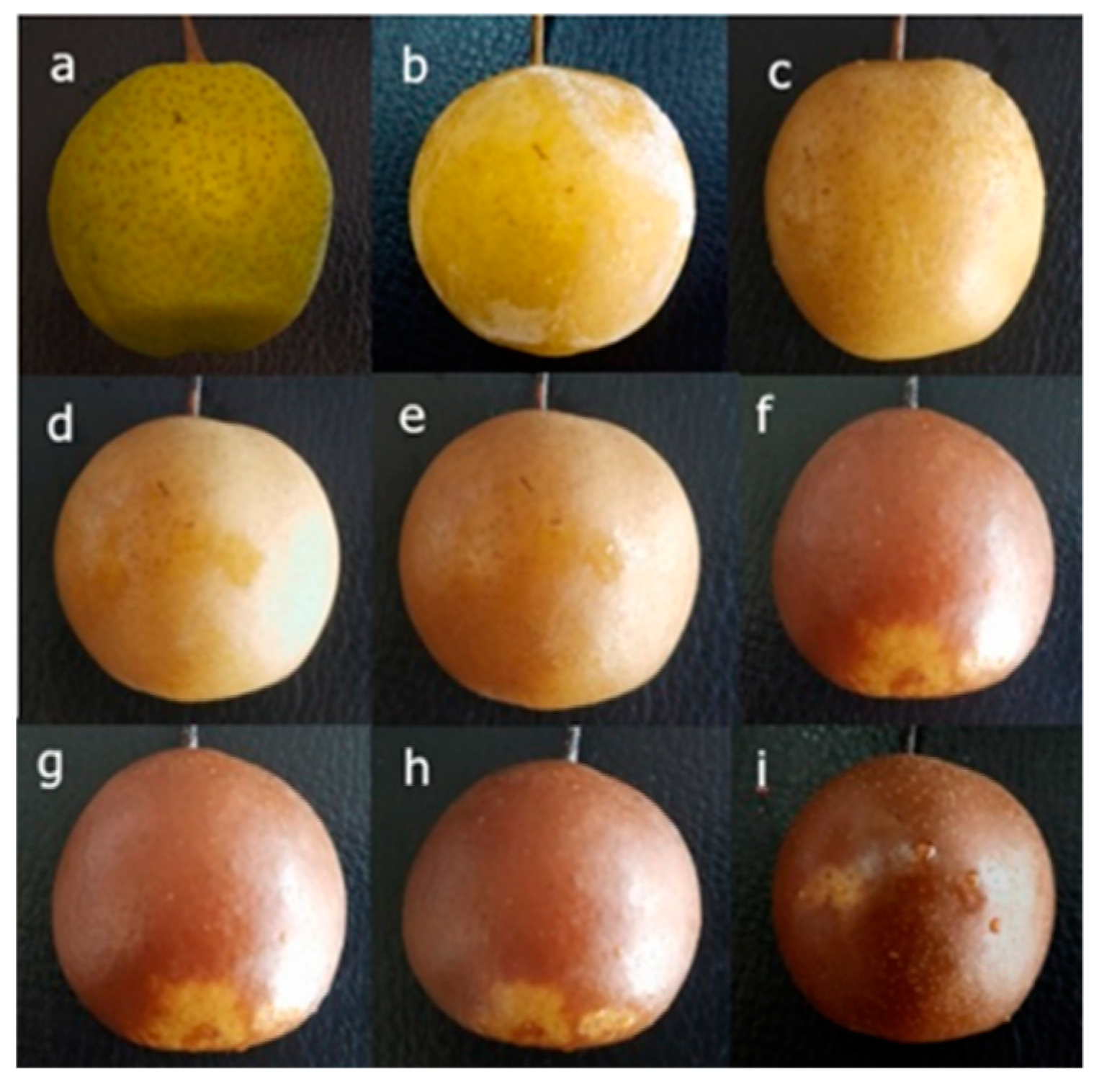
2.1. Variation in Pigments in “Ruaner” Pear
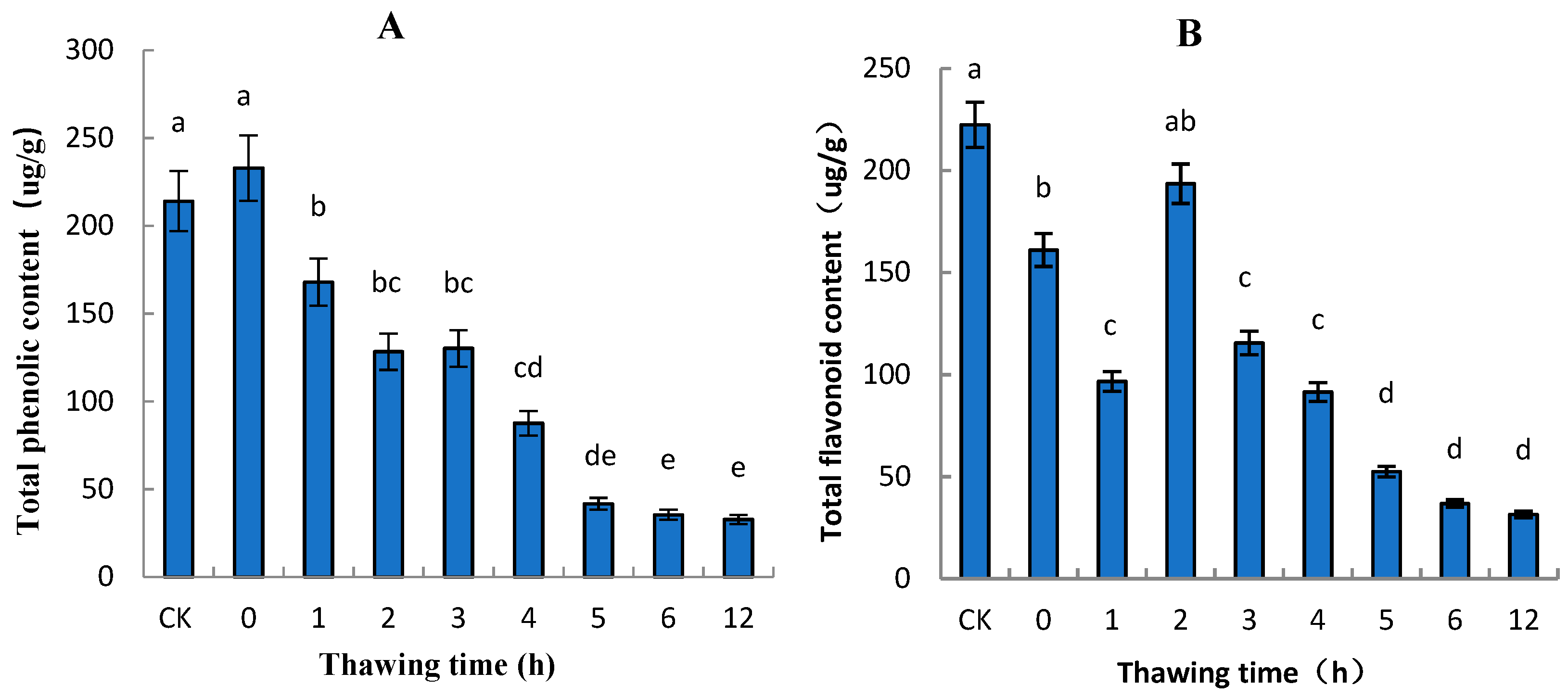
2.2. Total Phenolic and Total Flavonoid Contents of “Ruaner” Pear
2.3. Variation in Firmness, Water Content, Soluble Solids Content (SSC), and Titratable Acidity (TA) of “Ruaner” Pear
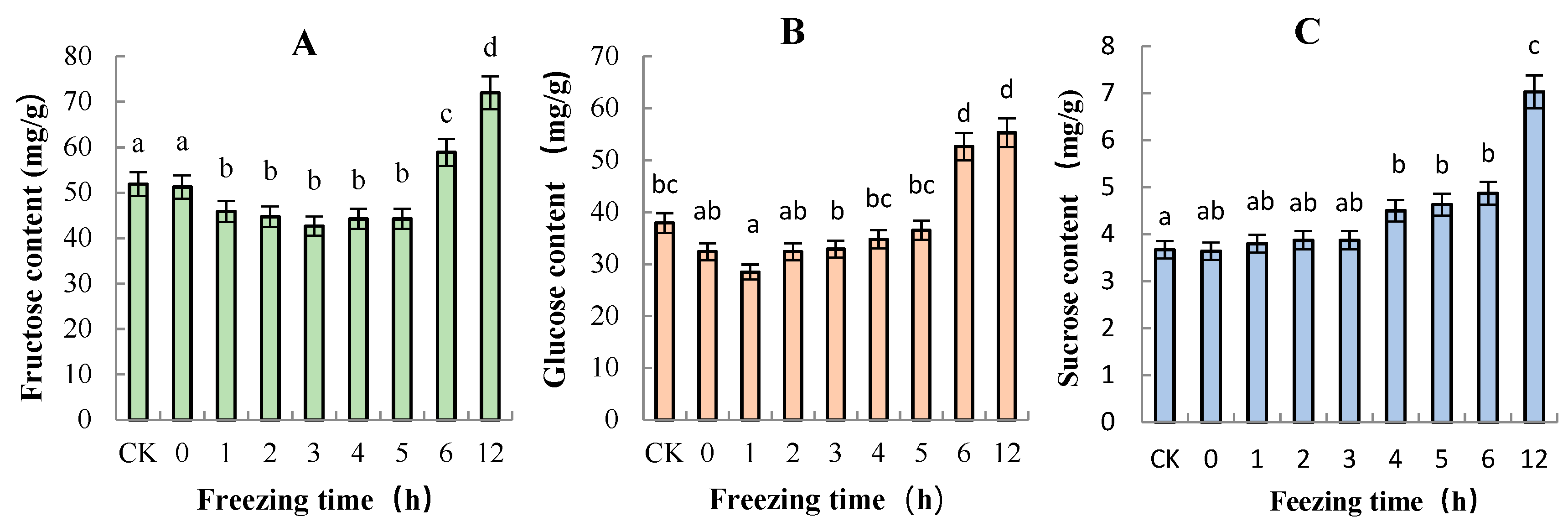
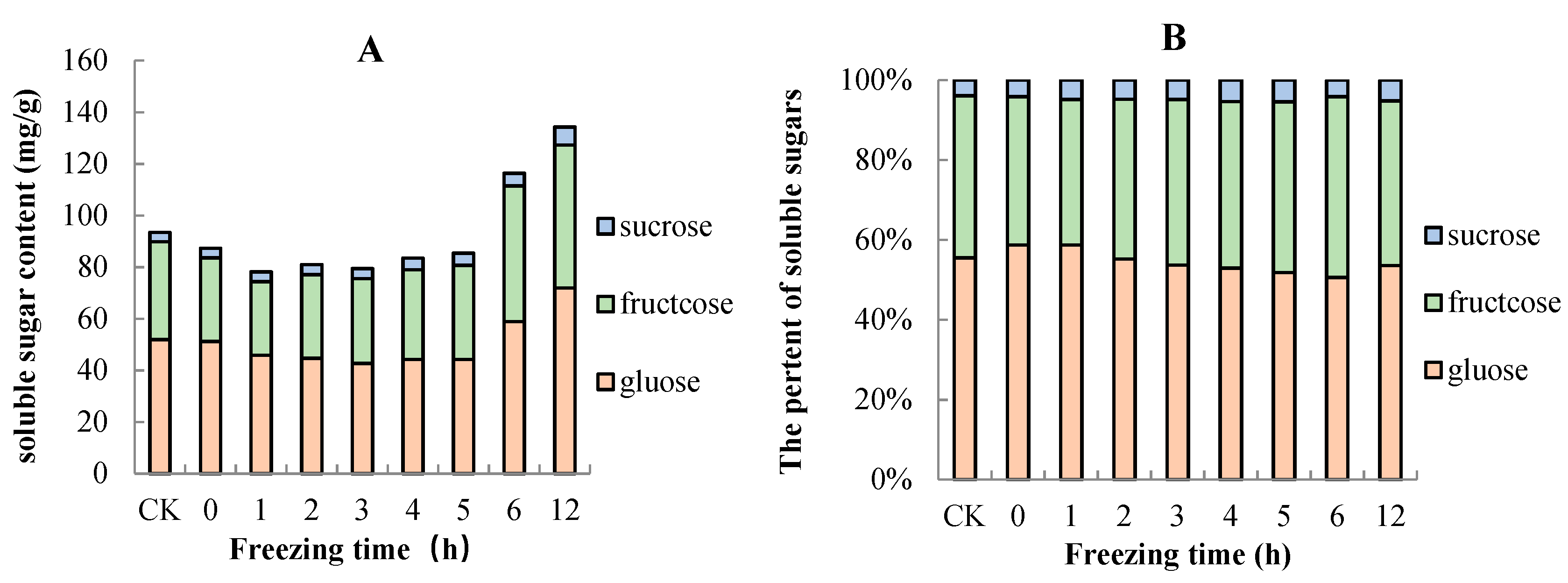
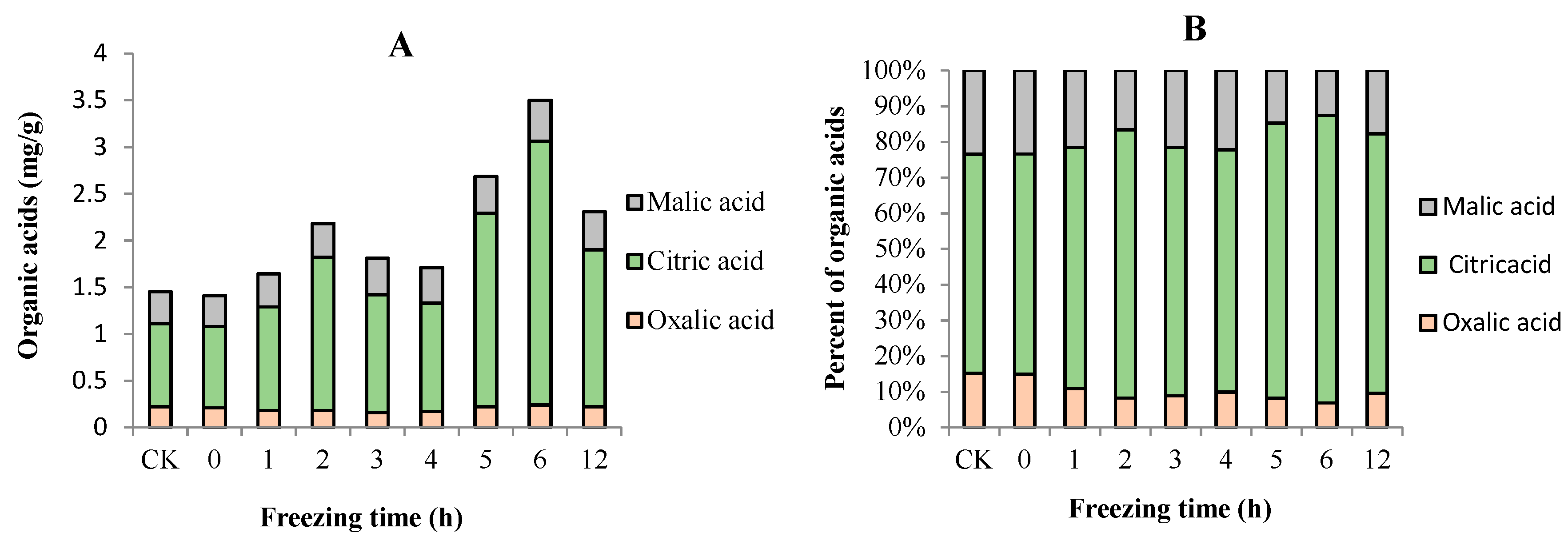
2.4. Sugar and Organic Acid Contents of “Ruaner” Pear
3. Discussion
3.1. Variation in Total Polyphenols and Total Flavonoids in “Ruaner” Pear
3.2. Variation in Firmness, Soluble Solid Content (SSC) and Titratable Acidity (TA) of “Ruaner” Pear
3.3. Soluble Sugars and Organic Acid Contents in “Ruaner” Pear
4. Materials and Methods
4.1. Plant Material
4.2. Pigment Extraction And Quantification
4.3. Total Polyphenol and Flavonoid Extraction and Quantification
4.4. Firmness, Water Content, Soluble Solid Content (SSC) and Titratable Acidity (TA) Determination
4.5. Extraction, Purification, and Isolation of the Sugars and Organic Acids
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bao, L.; Chen, K.; Zhang, D.Y.; Yamamoto, T.; Teng, Y. Genetic diversity and similarity of pear (Pyrus L.) cultivars native to East Asia revealed by SSR (simple sequence repeat) markers. Genet. Resour. Crop Evol. 2007, 54, 959. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, C.L.; Ding, R.J.; Bi, Y.; Wang, Y.; Wei, Y.B. Effects of different thawing methods on fruit quality and antioxidant compound contents of ‘Ruaner’. Food Sci. 2018, 39, 236–244. (In Chinese) [Google Scholar]
- Zhang, H.; Rui, Y. Nutritional composition of the specialty “frozen pear” from the Northeastern of China. J. Verbrauch Lebensm. 2010, 5, 345–346. [Google Scholar] [CrossRef]
- Cao, Y.; Tian, L.; Gao, Y.; Liu, F. Genetic diversity of cultivated and wild Ussurian Pear (Pyrus ussuriensis Maxim.) in China evaluated with M13-tailed SSR markers. Genet Resour Crop Ev. 2012, 59, 9–17. [Google Scholar] [CrossRef]
- Ben-Arie, R.; Kislev, N. Ultrastructural changes in the cell walls of ripening apple and pear fruit. Plant Physiol. 1979, 64, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, F.; Cheng, L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PloS ONE 2012, 7, e33055. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, T.; Wang, H.; Huang, X.; Qin, Y.; Hu, G. Patterns of enzyme activities and gene expressions in sucrose metabolism in relation to sugar accumulation and composition in the aril of Litchi chinensis Sonn. J. Plant Physiol. 2013, 170, 731–740. [Google Scholar] [CrossRef]
- Mesa, K.; Serra, S.; Masia, A.; Gagliardi, F.; Bucci, D.; Musacchi, S. Seasonal trends of starch and soluble carbohydrates in fruits and leaves of ‘Abbé Fétel’ pear trees and their relationship to fruit quality parameters. Sci. Hortic 2016, 211, 60–69. [Google Scholar] [CrossRef]
- Yang, X.T.; Song, J.; Campbell-Palmer, L.; Fillmore, S.; Zhang, Z.Q. Effect of ethylene and 1-MCP on expression of genes involved in ethylene biosynthesis and perception during ripening of apple fruit. Postharvest Biol. Technol. 2013, 78, 55–66. [Google Scholar] [CrossRef]
- Yoshioka, H.; Aoba, K.; Kashimura, Y. Molecular weight and degree of methoxylation in cell wall polyuronide during softening in pear and apple fruit. J. Am. Soc. Hortic Sci. 1992, 117, 600–606. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, X.J.; Zhao, Z.Y. Effects of fruit bagging on anthocyanins, sugars, organic acids and color properties of ‘Granny Smith’ and ‘Golden Delicious’ during fruit maturation. Eur. Food Res. Technol.ol. 2013, 236, 329–339. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, N.; Ma, Z.; Che, F.; Mao, J.; Chen, B. The changes in color, soluble sugars, organic acids, anthocyanins and aroma components in “Starkrimson” during the ripening period in China. Molecules 2016, 21, 812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, P.M.; Cheng, L.L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Yao, G.F.; Yang, Z.J.; Zang, S.L.; Cao, Y.F.; Liu, J.; Wu, J. Characteristics of components and contents of organic acid in pear fruits from different cultivated species. Acta Hortic. Sin. 2014, 41, 755–764. [Google Scholar]
- Walker, R.P.; Famiani, F. Organic acids in fruits: Metabolism, functions and contents. Hortic Rev. 2018. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Doty, T.E. Fructose sweetness: A new dimension. Cereal Foods World 1976, 21, 62–63. [Google Scholar]
- Bordonaba, J.G.; Terry, L.A. Manipulating the taste-related composition of strawberry fruits (Fragaria ananassa) from different cultivars using deficit irrigation. Food Chem. 2010, 122, 1020–1026. [Google Scholar] [CrossRef]
- Li, G.P.; Jia, H.J.; Li, J.H.; Li, H.X.; Teng, Y.W. Effects of 1-MCP on volatile production and transcription of ester biosynthesis related genes under cold storage in ‘Ruanerli’pear fruit (Pyrus ussuriensis Maxim.). Postharvest Biol. Technol.ol. 2016, 111, 168–174. [Google Scholar] [CrossRef]
- Wang, W.H.; Tong, W.; Jia, X.H. Investigation and analyses of the frozen pear production history, present situation and the industrial problems. Storage Process 2015, 15, 1–6. (In Chinese). Available online: http://www.bxyjg.com/ch/reader/view_abstract.aspx?file_no=201506001&flag=1 (accessed on 10 November 2015).
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol.ol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Merzlyak, M.N. Modeling pigment contributions to spectral reflection of apple fruit. Photoch. Photobiol. Sci. 2006, 5, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Charoenchongsuk, N.; Ikeda, K.; Itai, A.; Oikawa, A.; Murayama, H. Comparison of the expression of chlorophyll-degradation-related genes during ripening between stay-green and yellow-pear cultivars. Sci. Hortic. 2015, 181, 89–94. [Google Scholar] [CrossRef]
- Kou, X.H.; Guo, W.L.; Guo, R.Z.; Li, X.Y.; Xue, Z.H. Effects of chitosan, calcium chloride, and pullulan coating treatments on antioxidant activity in pear cv. “Huang guan” during storage. Food Bioprocess Technol. 2014, 7, 671–681. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5. Available online: https://doi.org/10.1017/jns.2016.41 (accessed on 29 December 2016). [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Sheng, L.; Zhou, X.; Liu, Z.Y.; Wang, J.W.; Zhou, Q.; Wang, L.; Zhang, Q.; Ji, S.J. Changed activities of enzymes crucial to membrane lipid metabolism accompany pericarp browning in ‘Nanguo’ pears during refrigeration and subsequent shelf life at room temperature. Postharvest Biol. Technol. 2016, 117, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Zhou, Q.; Cheng, S.; Wei, B.; Ji, S. Low temperature conditioning alleviates peel browning by modulating energy and lipid metabolisms of ‘Nanguo’ pears during shelf life after cold storage. Postharvest Biol. Technol. 2017, 131, 10–15. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Lin, Y.; Zhang, S.; Chen, Y.; Jiang, X. The roles of metabolism of membrane lipids and phenolics in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest. Biol. Technol. 2016, 111, 53–61. [Google Scholar] [CrossRef]
- Nath, S.; Choudhury, M.D.; Roychoudhury, S.; Talukdar, A.D.; Sirotkin, A.V.; Baková, Z.; Kádasi, A.; Maruniaková, N.; Kolesárová, A. Restorative aspect of castor plant on mammalian physiology: A review. J. Microbiol. Biotechnol. Food Sci. 2011, 1, 236. [Google Scholar]
- Zhang, H.P.; Wu, J.Y.; Qin, G.H.; Yao, G.F.; Qi, K.J.; Wang, L.F.; Zhang, S.L. The role of sucrose-metabolizing enzymes in pear fruit that differ in sucrose accumulation. Acta Physiol. Plant. 2014, 36, 71–77. [Google Scholar] [CrossRef]
- Wingenter, K.; Schulz, A.; Wormit, A.; Wic, S.; Trentmann, O.; Hoermiller, I.I.; Heyer, A.G.; Marten, I.; Hedrich, R.; NeuHATs, H.E. Increased activity of the vacuolar monosaccharide transporter TMT1 alters cellular sugar partitioning, sugar signaling, and seed yield in Arabidopsis. Plant. Physiol. 2010, 154, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Li, J.; Wu, J.; Zhang, S. Characteristics of organic acids in the fruit of different pear species. Afr. J. Agric. Res. 2011, 6, 2403–2410. [Google Scholar]
- Chen, J.X.; Wang, X.F. Experimental Guidance for Plant Physiology; South China University of Technol.ology Press: Guangzhou, China, 2002; pp. 35–36. [Google Scholar]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agri Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Sekhon-Loodu, S.; Warnakulasuriya, S.N.; Rupasinghe, H.V.; Shahidi, F. Antioxidant ability of fractionated apple peel phenolics to inhibit fish oil oxidation. Food Chem. 2013, 140, 189–196. [Google Scholar] [CrossRef]
- Amtliche Sammlung von Untersuchungsverfahren nach §35 LMBG jetzt auch online. Available online: https://www.bfr.bund.de/de/presseinformation/2002/21/amtliche_sammlung_von_untersuchungsverfahren_nach_paragraph35_lmbg_jetzt_auch_online-1600.html (accessed on 18 April 2000).
Sample Availability: Samples of the compounds are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wu, Y.; Che, F.; Zhang, Z.; Chen, B. Physical–Chemical Composition and Quality Related Changes in “Ruaner” Pear (Pyrus ussuriensis) During Freezing–Thawing Period. Molecules 2019, 24, 2611. https://doi.org/10.3390/molecules24142611
Liu Y, Wu Y, Che F, Zhang Z, Chen B. Physical–Chemical Composition and Quality Related Changes in “Ruaner” Pear (Pyrus ussuriensis) During Freezing–Thawing Period. Molecules. 2019; 24(14):2611. https://doi.org/10.3390/molecules24142611
Chicago/Turabian StyleLiu, Yulian, Yuxia Wu, Fei Che, Zhimin Zhang, and Baihong Chen. 2019. "Physical–Chemical Composition and Quality Related Changes in “Ruaner” Pear (Pyrus ussuriensis) During Freezing–Thawing Period" Molecules 24, no. 14: 2611. https://doi.org/10.3390/molecules24142611
APA StyleLiu, Y., Wu, Y., Che, F., Zhang, Z., & Chen, B. (2019). Physical–Chemical Composition and Quality Related Changes in “Ruaner” Pear (Pyrus ussuriensis) During Freezing–Thawing Period. Molecules, 24(14), 2611. https://doi.org/10.3390/molecules24142611




