Preparation of SiO2-MnFe2O4 Composites via One-Pot Hydrothermal Synthesis Method and Microwave Absorption Investigation in S-Band
Abstract
1. Introduction
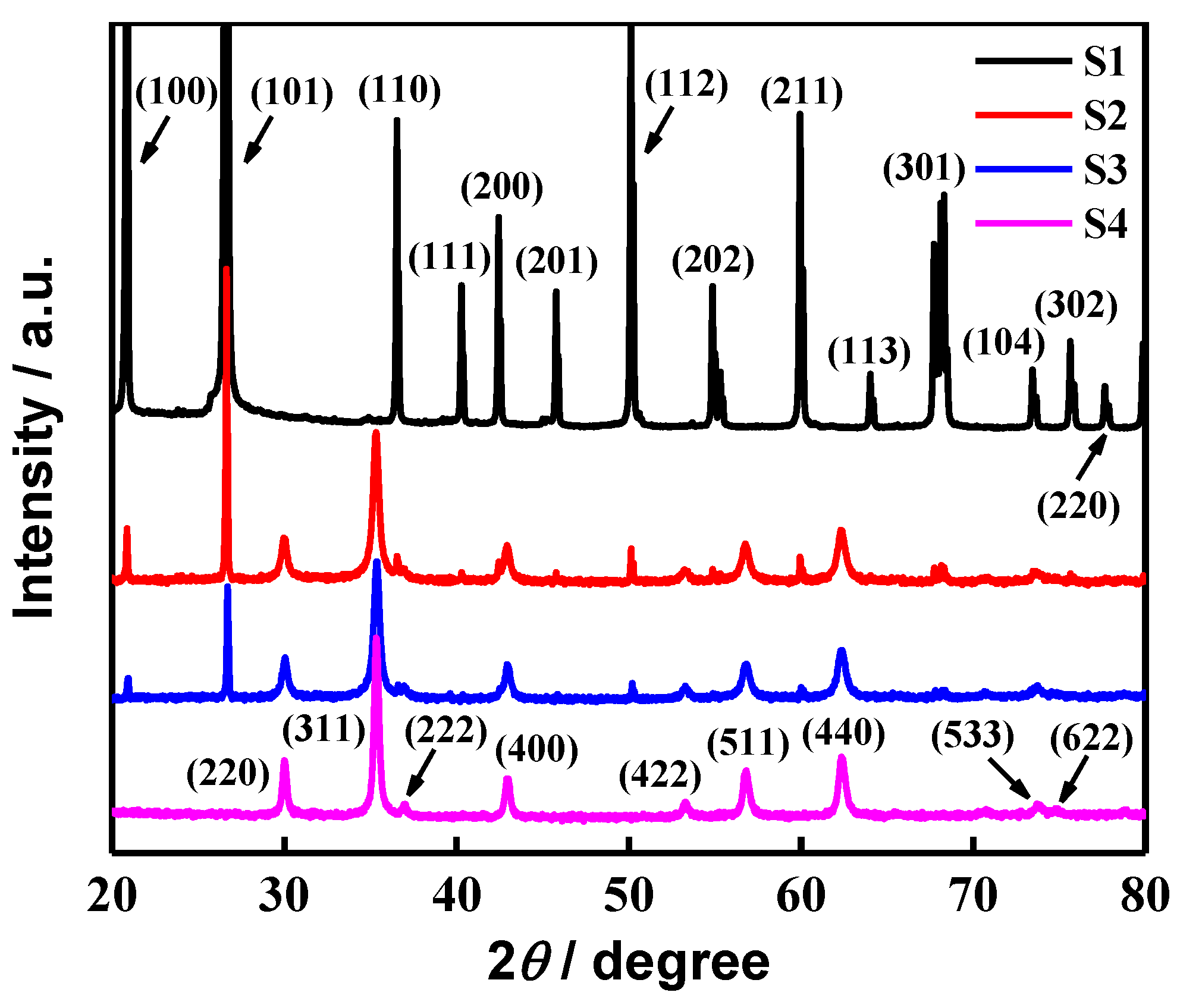
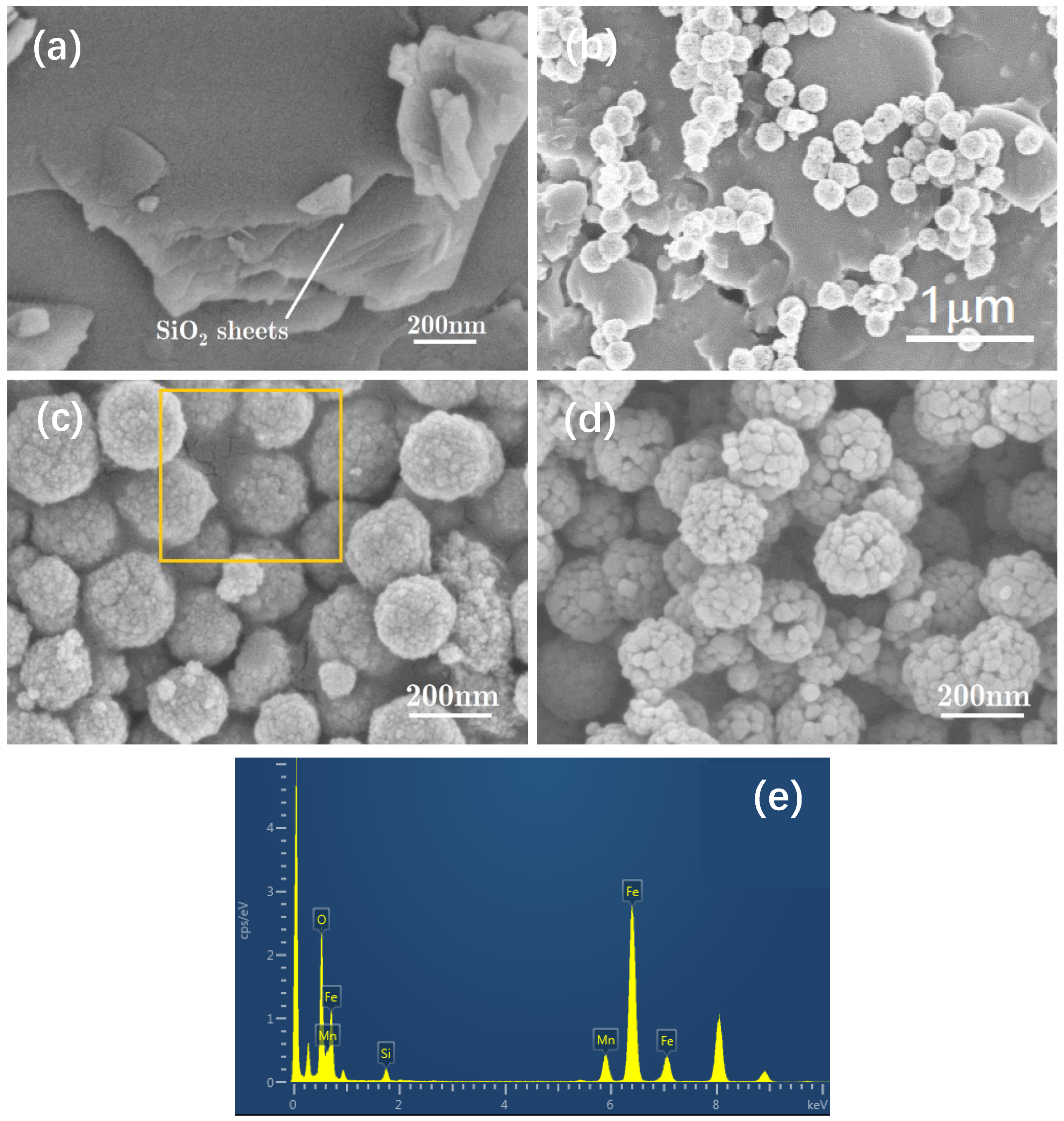
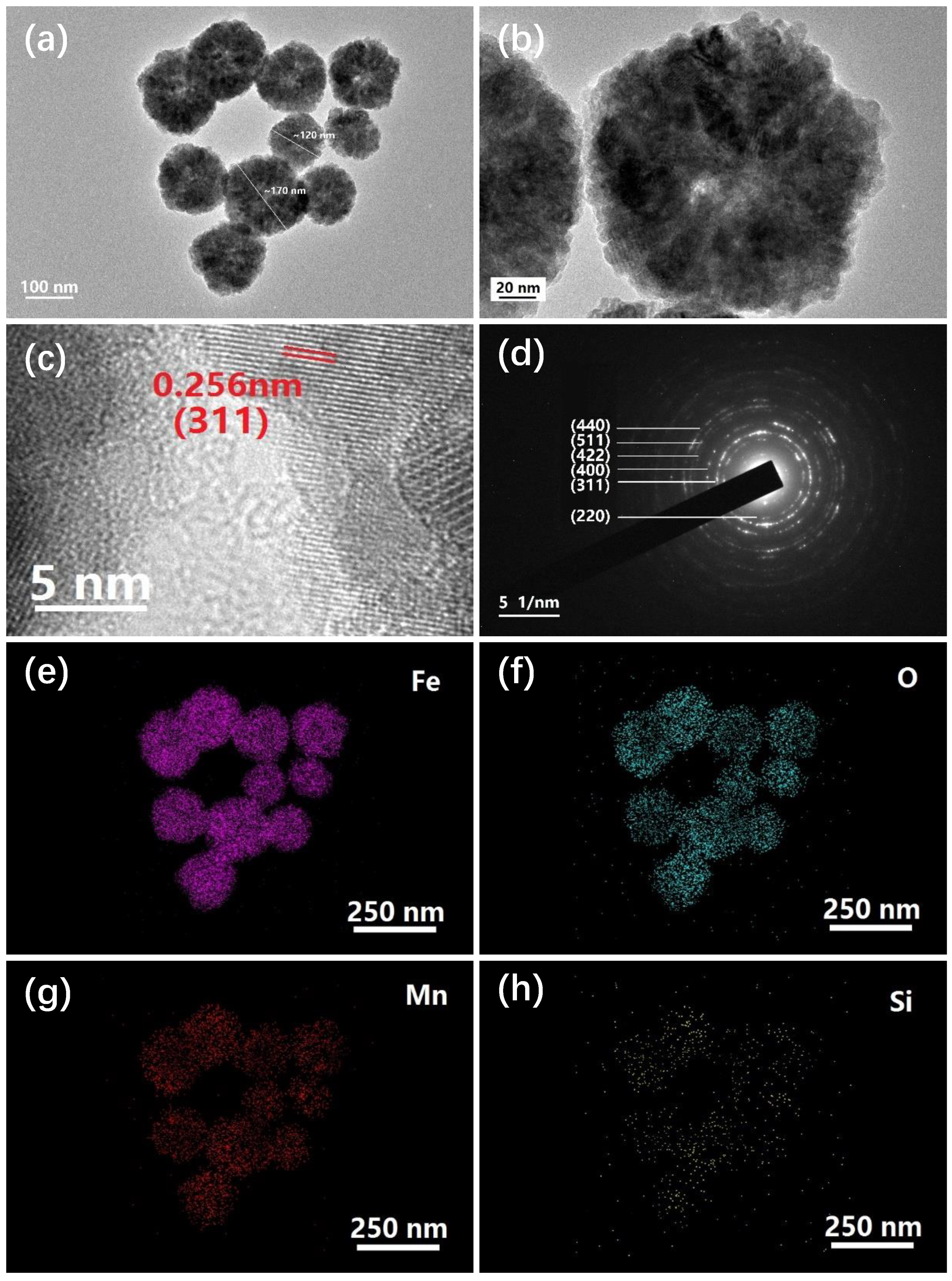
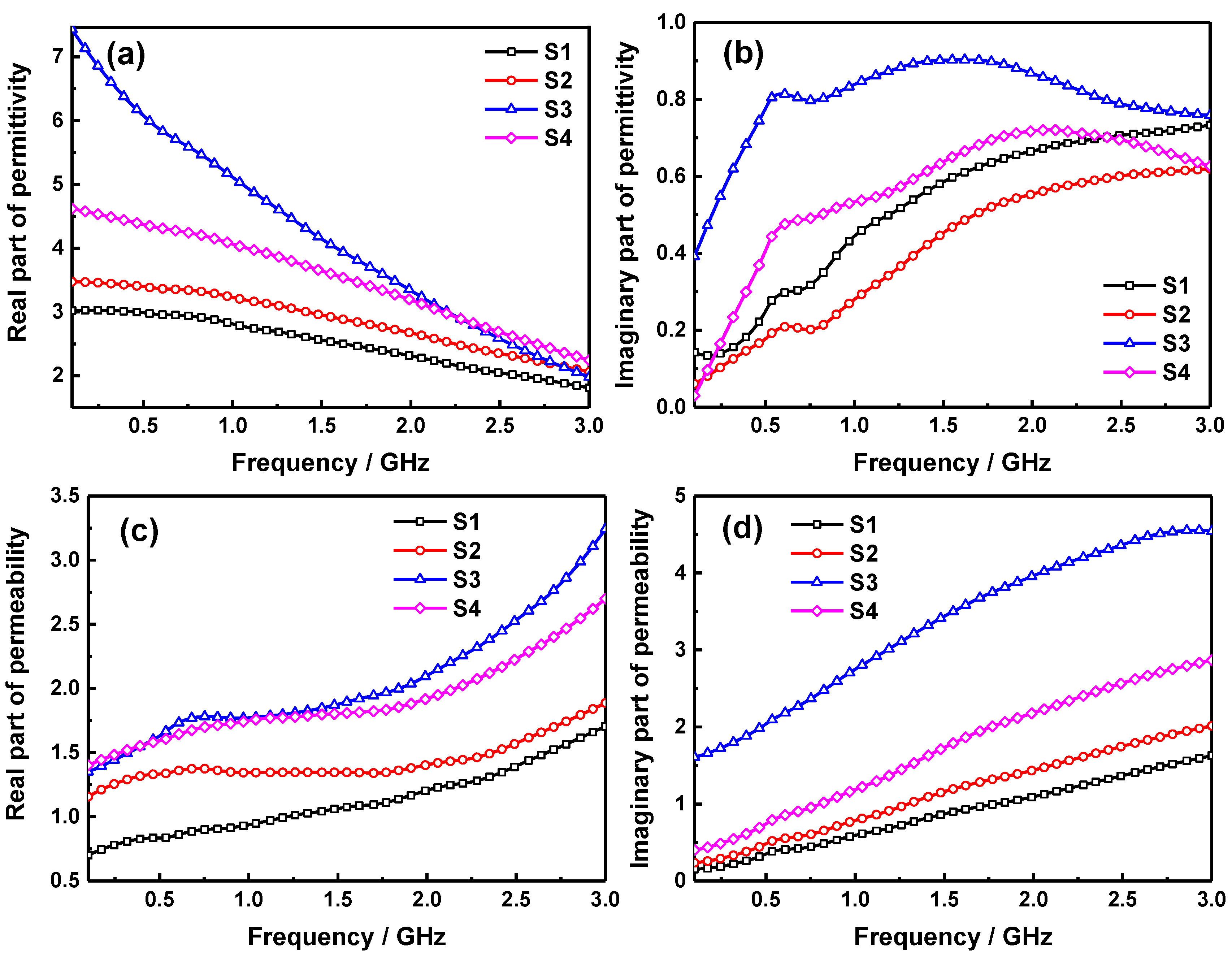
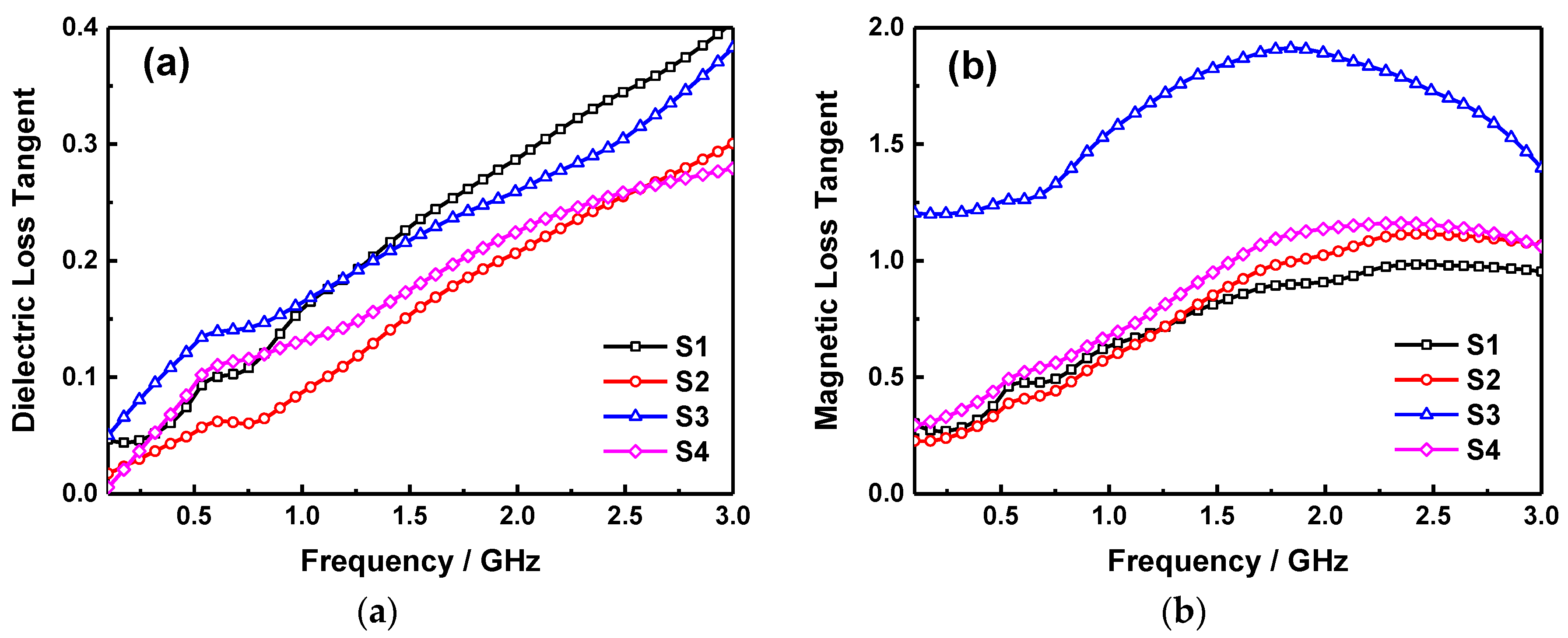
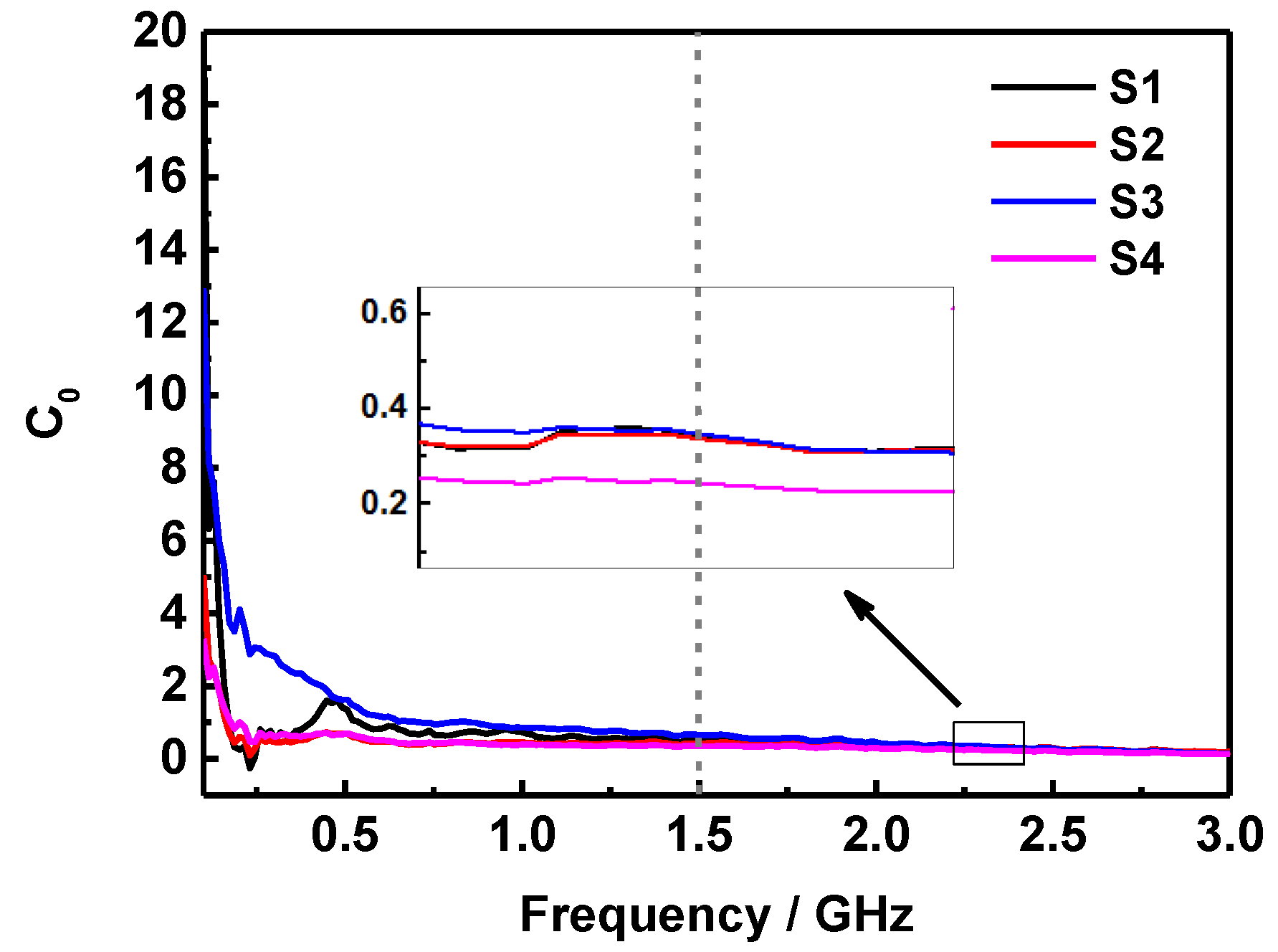
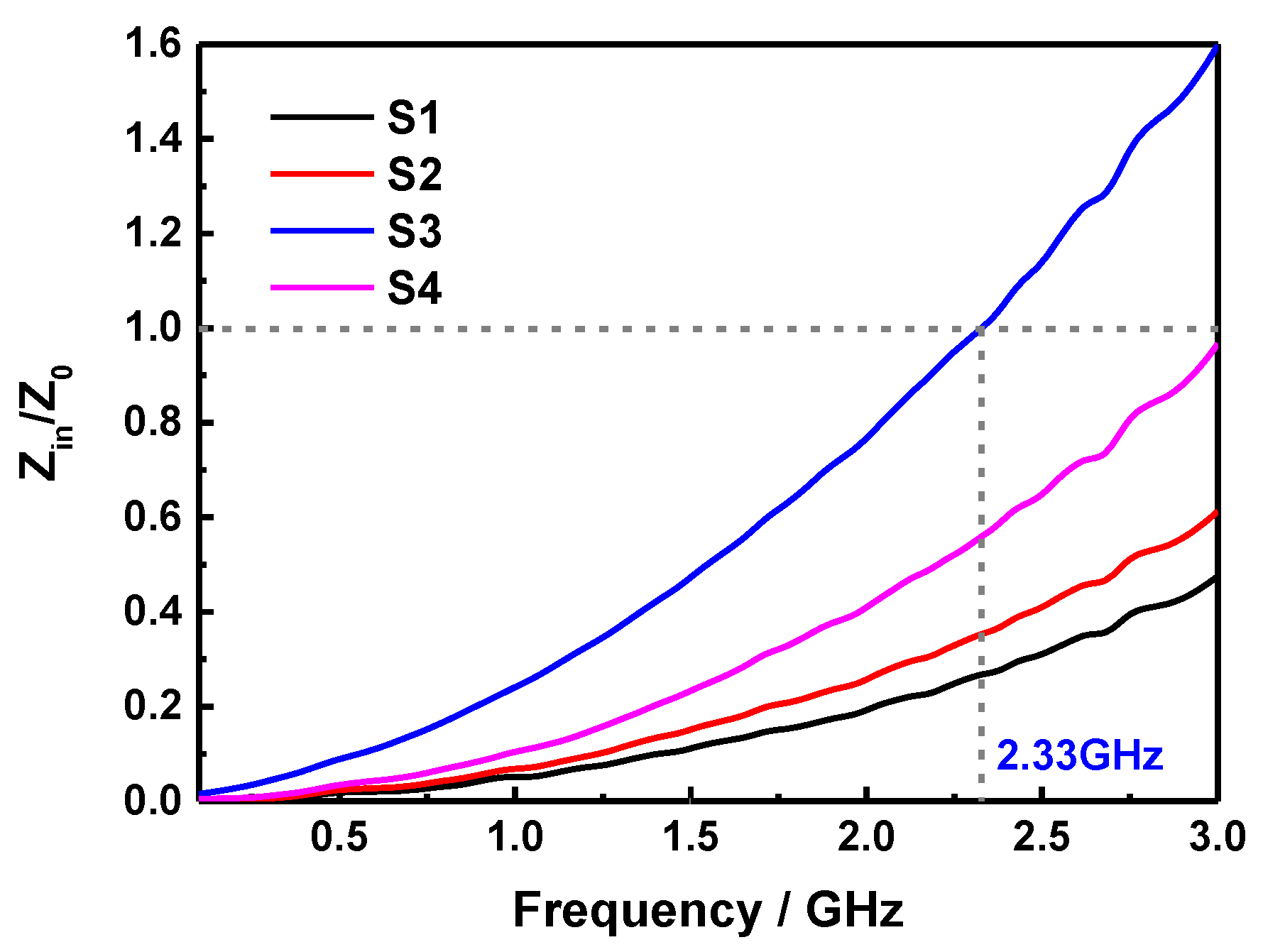
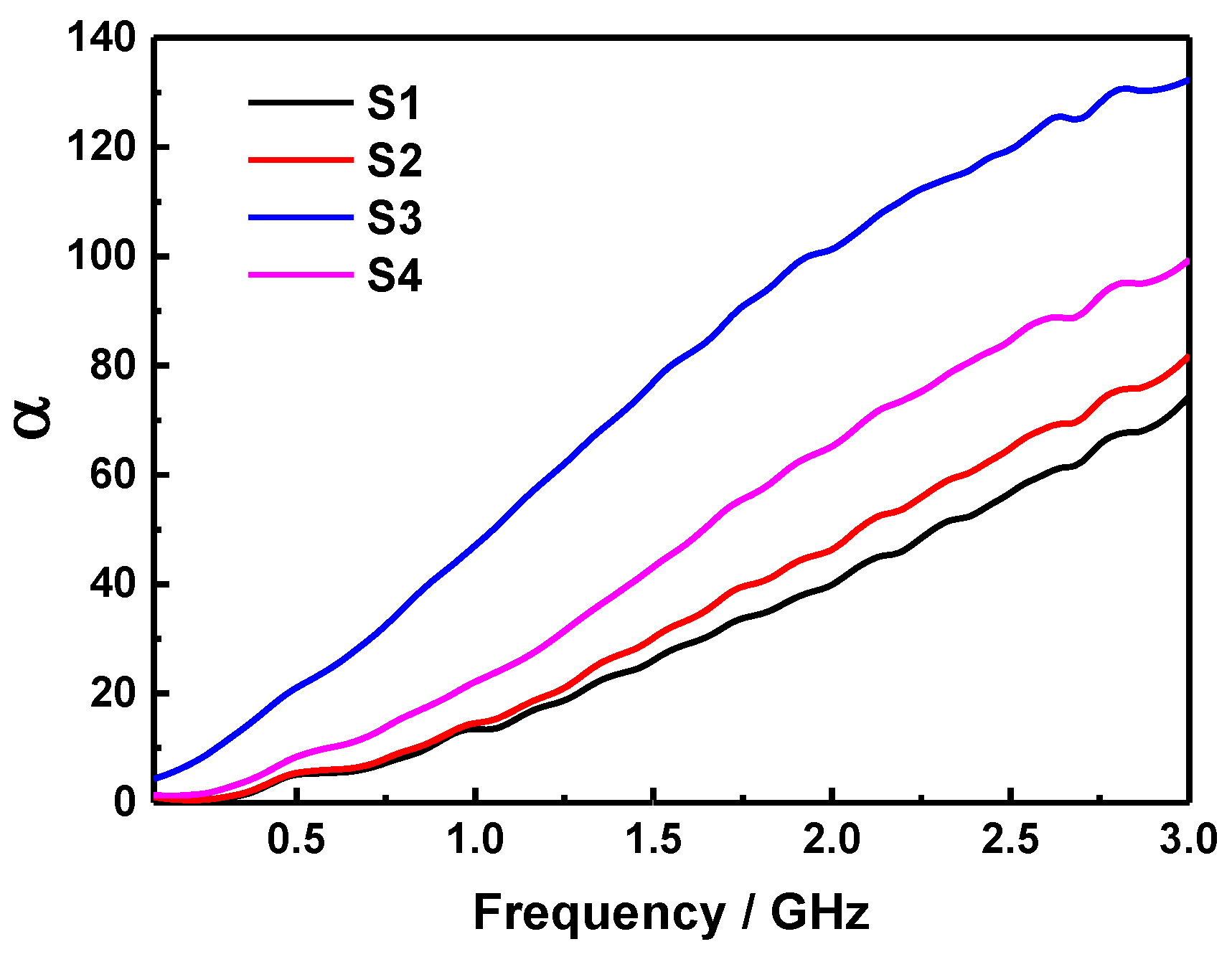
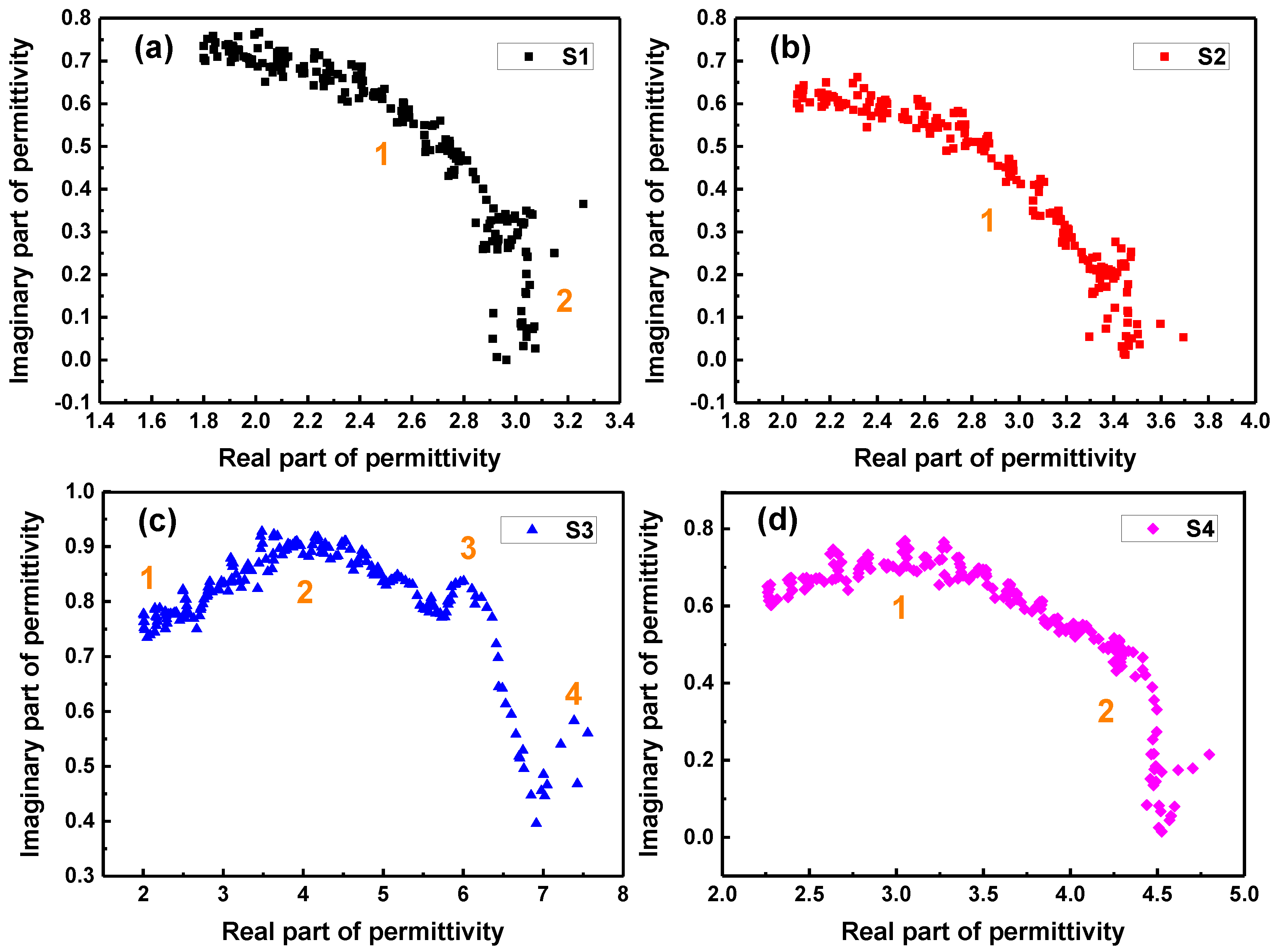
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Preparation of MnFe2O4 NPs and SiO2-MnFe2O4 Composites
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ling, J.; Zhai, W.; Feng, W.; Shen, B.; Zhang, J.; Zheng, W. Facile preparation of lightweight microcellular polyetherimide/graphene composite foams for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2013, 5, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meng, F.; Zhou, Z.; Tian, X.; Shan, L.; Zhu, S.; Xu, X.; Jiang, M.; Wang, L.; Hui, D.; et al. One-step synthesis of graphene/polyaniline hybrids by in situ intercalation polymerization and their electromagnetic properties. Nanoscale 2014, 6, 8140–8148. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Yin, X.; Yuan, X.; Zhang, Y.; Liu, X.; Cheng, L.; Zhang, L. Electromagnetic wave absorption properties of graphene modified with carbon nanotube/poly (dimethyl siloxane) composites. Carbon 2014, 73, 185–193. [Google Scholar] [CrossRef]
- Lan, D.; Qin, M.; Yang, R.; Chen, S.; Wu, H.; Fan, Y.; Fu, Q.; Zhang, F. Facile synthesis of hierarchical chrysanthemum-like copper cobaltate-copper oxide composites for enhanced microwave absorption performance. J. Colloid Inter. Sci. 2019, 533, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Khani, O.; Shoushtari, M.Z.; Ackland, K.; Stamenov, P. The structural, magnetic and microwave properties of spherical and flake shaped carbonyl iron particles as thin multilayer microwave absorbers. J. Magn. Magn. Mater. 2017, 428, 28–35. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, L.; Liang, Z.; Wang, X.; Li, X. A wide frequency absorbing material added CIPs using the fuse deposition modeling. J. Alloy. Compd. 2017, 704, 593–598. [Google Scholar] [CrossRef]
- Tang, N.; Yang, Y.; Lin, K.; Zhong, W.; Au, C.; Du, Y. Synthesis of plait-like carbon nanocoils in ultrahigh yield and their microwave absorption properties. J. Phys. Chem. C 2008, 112, 10061–10067. [Google Scholar] [CrossRef]
- Wu, H.; Qu, S.; Lin, K.; Qing, Y.; Wang, L.; Fan, Y.; Fu, Q.; Zhang, F. Enhanced low-frequency microwave absorbing property of SCFs@TiO2 composite. Powder Technol. 2018, 333, 153–159. [Google Scholar] [CrossRef]
- Luo, H.; Chen, W.; Zhou, W.; Long, L.; Deng, L.; Xiao, P.; Li, Y. Carbon fiber/Si3N4 composites with SiC nanofiber interphase for enhanced microwave absorption properties. Ceram. Int. 2017, 43, 12328–12332. [Google Scholar] [CrossRef]
- Yun, S.; Kirakosyan, A.; Surabhi, S.; Jeong, J.R.; Choi, J. Controlled morphology of MWCNTs driven by polymer-grafted nanoparticles for enhanced microwave absorption. J. Mater. Chem. C 2017, 5, 8436–8443. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Yu, D. Three-phase heterostructures f-NiFe2O4/PANI/PI EMI shielding fabric with high microwave absorption performance. Appl. Surf. Sci. 2017, 425, 518–525. [Google Scholar] [CrossRef]
- Wu, H.; Wu, G.; Ren, Y.; Yang, L.; Wang, L.; Li, X. Co2+/Co3+ ratio dependence of electromagnetic wave absorption in hierarchical NiCo2O4-CoNiO2 hybrids. J. Mater. Chem. C 2015, 3, 7677–7690. [Google Scholar] [CrossRef]
- Wu, H.; Wu, G.; Wang, L. Peculiar porous α-Fe2O3, γ-Fe2O3 and Fe3O4 nanospheres: Facile synthesis and electromagnetic properties. Powder Technol. 2015, 269, 443–451. [Google Scholar] [CrossRef]
- Lan, Y.; Li, X.; Zong, Y.; Li, Z.; Sun, Y.; Tan, G.; Feng, J.; Ren, Z.; Zheng, X. In-situsynthesis of carbon nanotubes decorated by magnetite nanoclusters and their applications as highly efficient and enhanced microwave absorber. Ceram. Int. 2016, 42, 19110–19118. [Google Scholar] [CrossRef]
- Xiao, H.M.; Liu, X.M.; Fu, S.Y. Synthesis, magnetic and microwave absorbing properties of core-shell structured MnFe2O4/TiO2 nanocomposites. Compos. Sci. Technol. 2006, 66, 2003–2008. [Google Scholar] [CrossRef]
- Reddy, M.P.; Mohamed, A.M.A.; Ramana, M.V.; Zhou, X.B.; Huang, Q. Spark plasma sintering and microwave electromagnetic properties of MnFe2O4 ceramics. J. Magn. Magn. Mater. 2015, 395, 185–189. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Zhang, W.; Huang, S. Synthesis and electromagnetic absorption properties of Ag-coated reduced graphene oxide with MnFe2O4 particles. J. Magn. Magn. Mater. 2016, 404, 58–63. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, G.S.; Cao, W.Q.; Wei, Y.Z.; Liang, J.F.; Guo, L.; Cao, M.S. Enhanced microwave absorption property of reduced graphene oxide (RGO)-MnFe2O4 nanocomposites and polyvinylidene fluoride. ACS Appl. Mater. Interfaces 2014, 6, 7471–7478. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Moghimi, A.; Moloudi, M. Magnetic, conductive, and microwave absorption properties of polythiophene nanofibers layered on MnFe2O4/Fe3O4 core-shell structures. Mat. Sci. Semicon. Proc. 2014, 24, 272–277. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Zhang, W.; Huang, S. One-pot synthesis of MnFe2O4 nanoparticles decorated reduced graphene oxide for enhanced microwave absorption properties. Mater. Technol. 2017, 32, 32–37. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Bera, P.; Chakradhar, R.P.S.; Choudhury, B.; Pawar, S.P.; Bose, S.; Nair, R.U.; Barshilia, H.C. Enhanced microwave absorption properties of PMMA modified MnFe2O4-polyaniline nanocomposites. Phys. Chem. Chem. Phys. 2019, 21, 5068–5077. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, C.; Xiong, Y.; Yao, Q.; Chang, Q.; Chen, Y.; Jin, C.; Sun, Q. Solvothermal fabrication and growth behavior study of spherical MnFe2O4 through a bottom-up method on wood substrate with effective microwave absorption. RSC Adv. 2017, 7, 24764–24770. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Mohseni, S.H.; Asadnia, A.; Kerdari, H. Synthesis and microwave absorbing properties of polyaniline/MnFe2O4 nanocomposite. J. Alloy. Compd. 2011, 509, 4682–4687. [Google Scholar] [CrossRef]
- Han, M.; Liang, D.; Deng, L. Fabrication and electromagnetic wave absorption properties of amorphous Fe79Si16B5 microwires. Appl. Phys. Lett. 2011, 99, 082503. [Google Scholar]
- Wang, H.; Dai, Y.; Gong, W.; Geng, D.; Ma, S.; Li, D.; Liu, W.; Zhang, Z. Broadband microwave absorption of CoNi@C nanocapsules enhanced by dual dielectric relaxation and multiple magnetic resonances. Appl. Phys. Lett. 2013, 102, 223113. [Google Scholar] [CrossRef]
- Weng, X.; Li, B.; Zhang, Y.; Lv, X.; Gu, G. Synthesis of flake shaped carbonyl iron/reduced graphene oxide/polyvinyl pyrrolidone ternary nanocomposites and their microwave absorbing properties. J. Alloy. Compd. 2017, 695, 508–519. [Google Scholar] [CrossRef]
- Li, J.; Xie, Y.; Lu, W.; Chou, T.W. Flexible electromagnetic wave absorbing composite based on 3D rGO-CNT-Fe3O4 ternary films. Carbon 2018, 129, 76–84. [Google Scholar] [CrossRef]
- Chen, D.Z.; Wang, G.S.; He, S.; Liu, J.; Guo, L.; Cao, M.S. Controllable fabrication of mono-dispersed RGO-hematite nanocomposites and their enhanced wave absorption properties. J. Mater. Chem. A 2013, 1, 5996–6003. [Google Scholar] [CrossRef]
- Li, R.; Wang, T.; Tan, G.G.; Zuo, W.L.; Wei, J.Q.; Qiao, L.; Li, F.S. Microwave absorption properties of oriented Pr2Fe17N3-δ particles/paraffin composite with planar anisotropy. J. Alloy. Compd. 2014, 586, 239–243. [Google Scholar] [CrossRef]
- Liu, X.F.; Cui, X.R.; Chen, Y.X.; Zhang, X.J.; Yu, R.H.; Wang, G.S.; Ma, H. Modulation of electromagnetic wave absorption by carbon shell thickness in carbon encapsulated magnetite nanospindles-poly (vinylidene fluoride) composites. Carbon 2015, 95, 870–878. [Google Scholar] [CrossRef]
- Song, W.L.; Cao, M.S.; Lu, M.M.; Liu, J.; Yuan, J.; Fan, L.Z. Improved dielectric properties and highly efficient and broadened bandwidth electromagnetic attenuation of thickness-decreased carbon nanosheet/wax composites. J. Mater. Chem. C 2013, 1, 1846–1854. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, W.; Zhao, J.; Xiao, J.; Lu, L.; Fan, H. Synthesis and characterization of TiO2/polyaniline/graphene oxide bouquet-like composites for enhanced microwave absorption performance. J. Alloy. Compd. 2017, 710, 717–724. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Zhang, X.J.; Wang, S.W.; Wang, G.S.; Yin, P.G. Enhanced microwave absorption material of ternary nanocomposites based on MnFe2O4@SiO2, polyaniline and polyvinylidene fluoride. RSC Adv. 2016, 6, 88104–88109. [Google Scholar] [CrossRef]
- Fang, J.; Liu, T.; Chen, Z.; Wang, Y.; Wei, W.; Yue, X.; Jiang, Z. A wormhole-like porous carbon/magnetic particles composite as an efficient broadband electromagnetic wave absorber. Nanoscale 2016, 8, 8899–8909. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yao, Z.; Lin, H.; Zhou, J.; Haidry, A.A.; Liu, P. The effect of polymerization temperature and reaction time on microwave absorption properties of Co-doped ZnNi ferrite/polyaniline composites. RSC Adv. 2018, 8, 29344–29355. [Google Scholar] [CrossRef]
- Long, Q.; Xu, Z.; Xiao, H.; Xie, K. A facile synthesis of a cobalt nanoparticle-graphene nanocomposite with high-performance and triple-band electromagnetic wave absorption properties. RSC Adv. 2018, 8, 1210–1217. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, X.; Zhang, Q.; Li, T.; Chen, H.; Chen, X. Preparation and microwave absorption properties of asphalt carbon coated reduced graphene oxide/magnetic CoFe2O4 hollow particles modified multi-wall carbon nanotube composites. J. Alloy. Compd. 2017, 723, 912–921. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Y.; Zong, M.; Ding, X.; Li, S.; Wang, M. Synthesis of ZnS quantum dots and CoFe2O4 nanoparticles co-loaded with graphene nanosheets as an efficient broad band EM wave absorber. Chem. Eng. J. 2017, 308, 214–221. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, X.Q.; Zhao, W.Y.; Deng, J.H.; Fan, B.B.; Shao, G.; Bai, Z.Y.; Zhang, R. Facile synthesis of yolk-shell Ni@void@SnO2(Ni3Sn2) ternary composites via galvanic replacement/Kirkendall effect and their enhanced microwave absorption properties. Nano Res. 2017, 10, 331–343. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, D.; Wang, X.; Luo, F. Influence of silicon carbide fiber (SiCf) type on the electromagnetic microwave absorbing properties of SiCf/epoxy composites. Compos. Part. A 2017, 93, 10–17. [Google Scholar] [CrossRef]
- Du, Y.; Liu, W.; Qiang, R.; Wang, Y.; Han, X.; Ma, J.; Xu, P. Shell Thickness-Dependent Microwave Absorption of Core-Shell Fe3O4@C Composites. ACS Appl. Mater. Interfaces 2014, 6, 12997–13006. [Google Scholar] [CrossRef] [PubMed]
- Alanagh, F.M.; Khiabani, A.B.; Salimkhani, H. Improvement in magnetic and microwave absorption properties of nano-Fe3O4@CFs composites using a modified multi-step EPD process. Appl. Surf. Sci. 2017, 420, 726–739. [Google Scholar] [CrossRef]
- Heidari, B.; Ansari, M.; Hoseinabadi, A.; Jiriaee, H.; Heidary, F. The effect of ZnO, Fe3O4 and graphene oxide nanostructures on the microwave absorbing properties of polystyrene composites. J. Mater. Sci. Mater. Electron. 2017, 28, 1028–1037. [Google Scholar] [CrossRef]
- Cao, M.S.; Yang, J.; Song, W.L.; Zhang, D.Q.; Wen, B.; Jin, H.B.; Hou, Z.L.; Yuan, J. Ferroferric oxide/multiwalled carbon nanotube vs. polyaniline/ferroferric oxide/multiwalled carbon nanotube multiheterostructures for highly effective microwave absorption. ACS Appl. Mater. Inter. 2012, 4, 6949–6956. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Jiao, Q.; Hao, L.; Ni, X.; Wang, Y.; Li, H.; Wu, Q.; Zhao, Y. Preparation of core-shell Zn-doped CoFe2O4 cubes@CNT composites and their absorbing performances. Micro Nano Lett. 2017, 12, 227–230. [Google Scholar] [CrossRef]
- Hou, C.L.; Li, T.H.; Zhao, T.K.; Liu, H.G.; Liu, L.H.; Zhang, W.J. Electromagnetic wave absorbing properties of multi-wall carbon nanotube/Fe3O4 hybrid materials. New Carbon Mater. 2013, 28, 184–190. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Or, S.W.; Ho, S.L. Exchange coupling and microwave absorption in core/shell-structured hard/soft ferrite-based CoFe2O4/NiFe2O4 nanocapsules. AIP Adv. 2017, 7, 056403. [Google Scholar] [CrossRef]
- Li, G.; Sheng, L.; Yu, L.; An, K.; Ren, W.; Zhao, X. Electromagnetic and microwave absorption properties of single-walled carbon nanotubes and CoFe2O4 nanocomposites. Mater. Sci. Eng. B 2015, 193, 153–159. [Google Scholar] [CrossRef]
- Guan, Z.J.; Jiang, J.T.; Chen, N.; Gong, Y.X.; Zhen, L. Carbon-coated CoFe-CoFe2O4 composite particles with high and dual-band electromagnetic wave absorbing properties. Nanotechnology 2018, 29, 305604. [Google Scholar] [CrossRef]
- Lia, J.; Feng, Y.; Wu, Y.; Yuan, Y. Fiber-guided and particle-localized microwave absorption of nanoscale CoFe2O4 derived from citric acid-based precursor. Phys. B 2019, 561, 16–22. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.L.; Peng, Q.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 117, 2842–2845. [Google Scholar] [CrossRef]
- Nicolson, A.M.; Ross, G.F. Measurement of the intrinsic properties of materials by time-domain techniques. IEEE T. Instrum. Meas. 1970, 19, 377–382. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds SiO2-MnFe2O4 are available from the authors. |

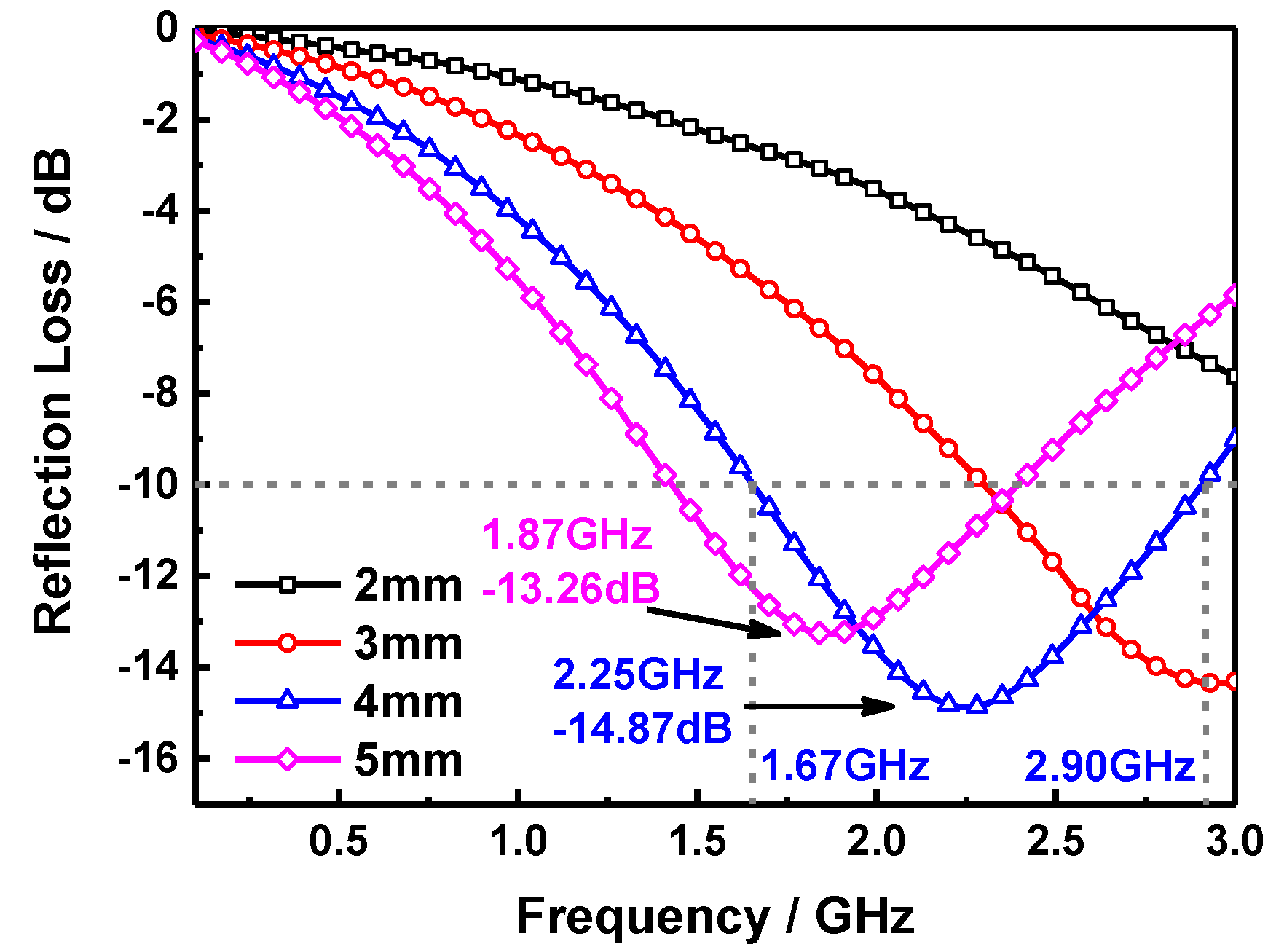
| Materials | Thickness | Minimum RL | Position | RL < −10 dB | Refs. |
|---|---|---|---|---|---|
| SiO2-MnFe2O4 | 4 mm | −14.87 dB | 2.25 GHz | 1.67~2.9 GHz | This work |
| ZnO/Fe3O4/GO | 2 mm | −7.2 dB | / | 6.4~8 GHz | [43] |
| PANI/Fe3O4/MWCNT | 4 mm | −16 dB | / | 8~15 GHz | [44] |
| Zn-doped CoFe2O4 cubes@CNT | 2 mm | −9.98 dB | 9.33 GHz | / | [45] |
| MWCNTs/Fe3O4 | 2 mm | −18.22 dB | / | 10.9~12.4 GHz | [46] |
| CoFe2O4/NiFe2O4 | 4.5 mm | −20.1 dB | 9.7 GHz | 7.8~16.2 GHz | [47] |
| CoFe2O4/LPA-SWCNT | 2 mm | −30.7 dB | 12.9 GHz | 10.1~17.3 GHz | [48] |
| Carbon-coated CoFe-CoFe2O4 | 3.93 | −60 dB | 5.5 GHz | 4.1~7.8 GHz | [49] |
| CoFe2O4 fiber | 5 mm | −36.5 dB | 6.0 GHz | / | [50] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, P.; Zhang, L.; Wang, J.; Feng, X.; Zhao, L.; Rao, H.; Wang, Y.; Dai, J. Preparation of SiO2-MnFe2O4 Composites via One-Pot Hydrothermal Synthesis Method and Microwave Absorption Investigation in S-Band. Molecules 2019, 24, 2605. https://doi.org/10.3390/molecules24142605
Yin P, Zhang L, Wang J, Feng X, Zhao L, Rao H, Wang Y, Dai J. Preparation of SiO2-MnFe2O4 Composites via One-Pot Hydrothermal Synthesis Method and Microwave Absorption Investigation in S-Band. Molecules. 2019; 24(14):2605. https://doi.org/10.3390/molecules24142605
Chicago/Turabian StyleYin, Pengfei, Limin Zhang, Jian Wang, Xing Feng, Liang Zhao, Hanbing Rao, Yanying Wang, and Jianwu Dai. 2019. "Preparation of SiO2-MnFe2O4 Composites via One-Pot Hydrothermal Synthesis Method and Microwave Absorption Investigation in S-Band" Molecules 24, no. 14: 2605. https://doi.org/10.3390/molecules24142605
APA StyleYin, P., Zhang, L., Wang, J., Feng, X., Zhao, L., Rao, H., Wang, Y., & Dai, J. (2019). Preparation of SiO2-MnFe2O4 Composites via One-Pot Hydrothermal Synthesis Method and Microwave Absorption Investigation in S-Band. Molecules, 24(14), 2605. https://doi.org/10.3390/molecules24142605






