Zn(II) Curcuminate Complexes with 2,2′-bipyridine and Carboxylates
Abstract
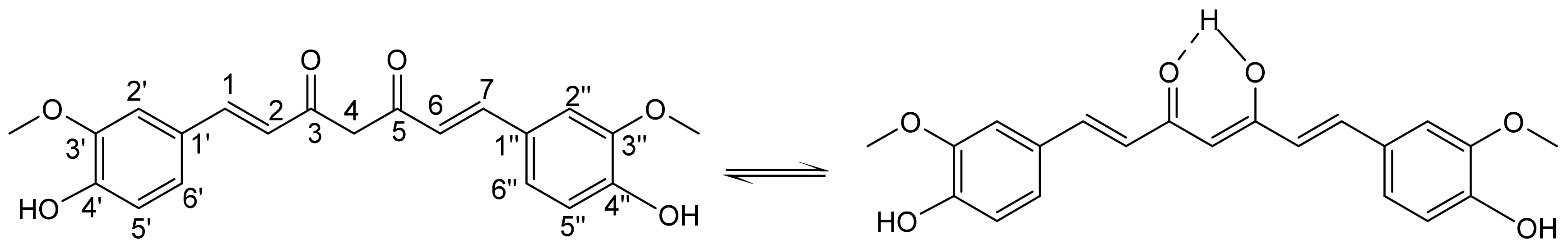
1. Introduction
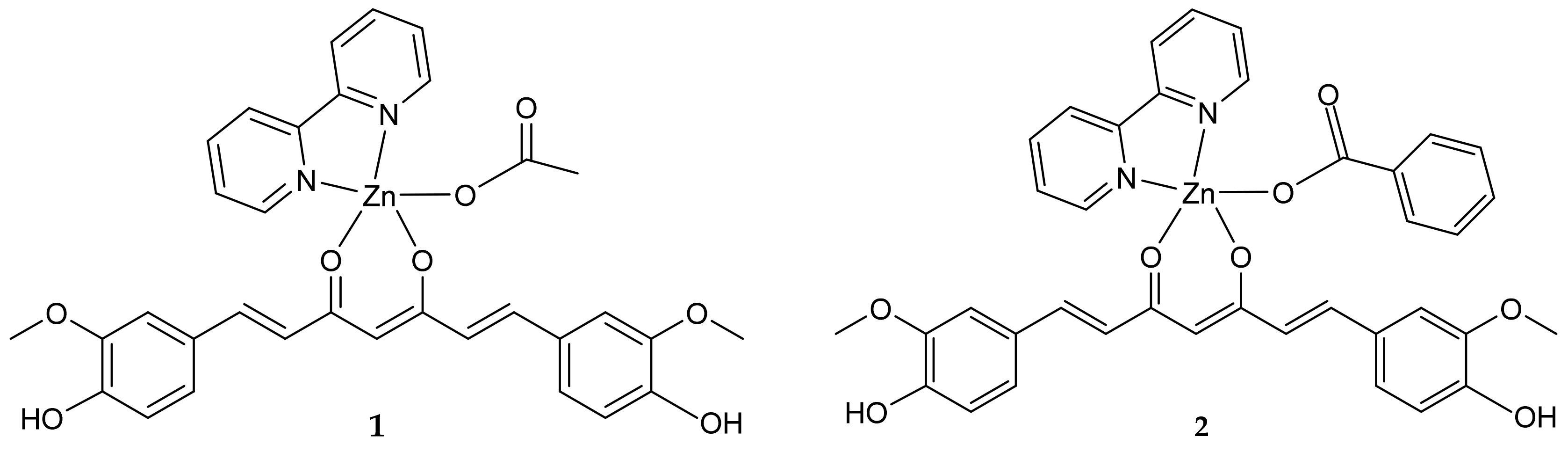
2. Results and Discussion
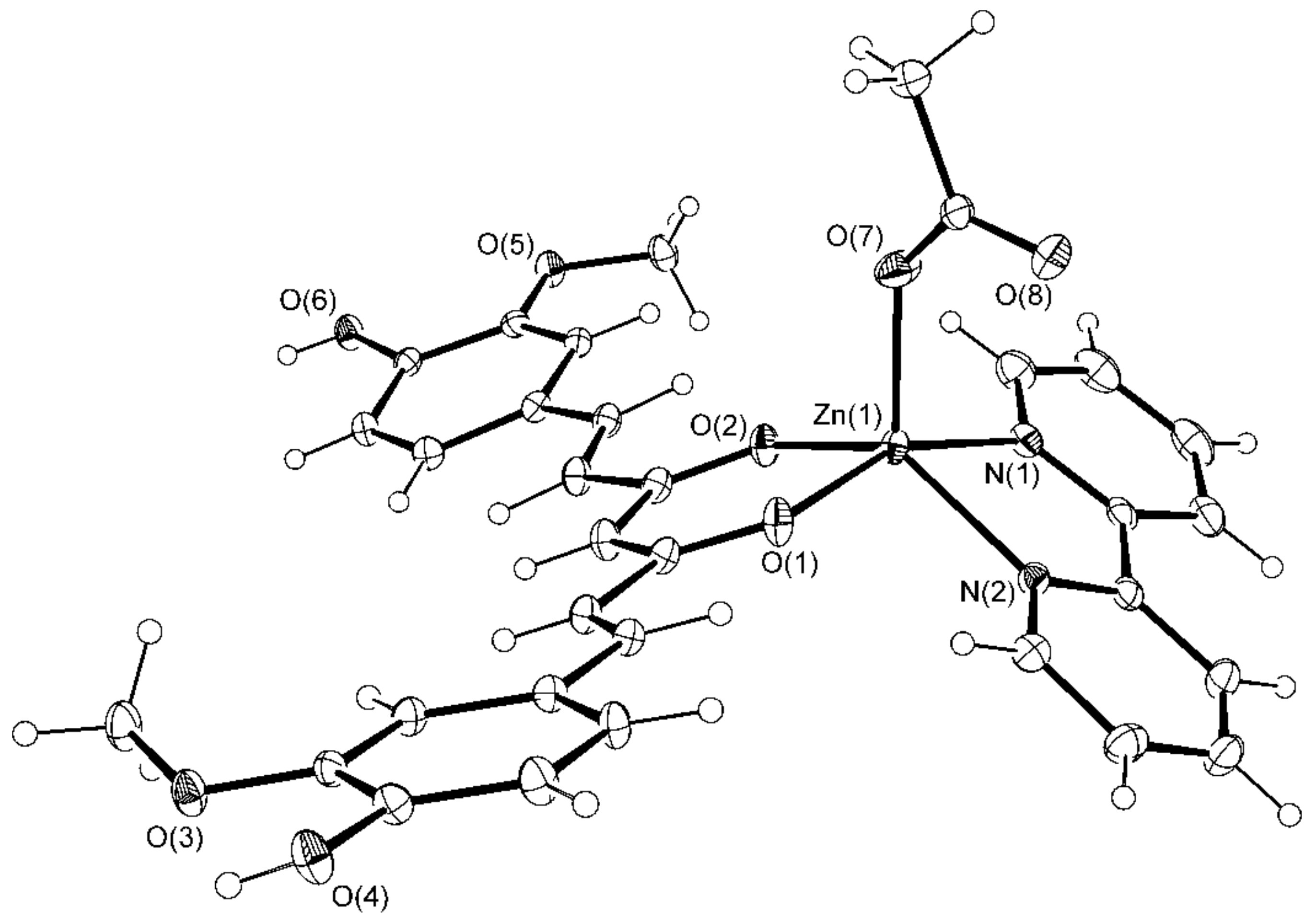
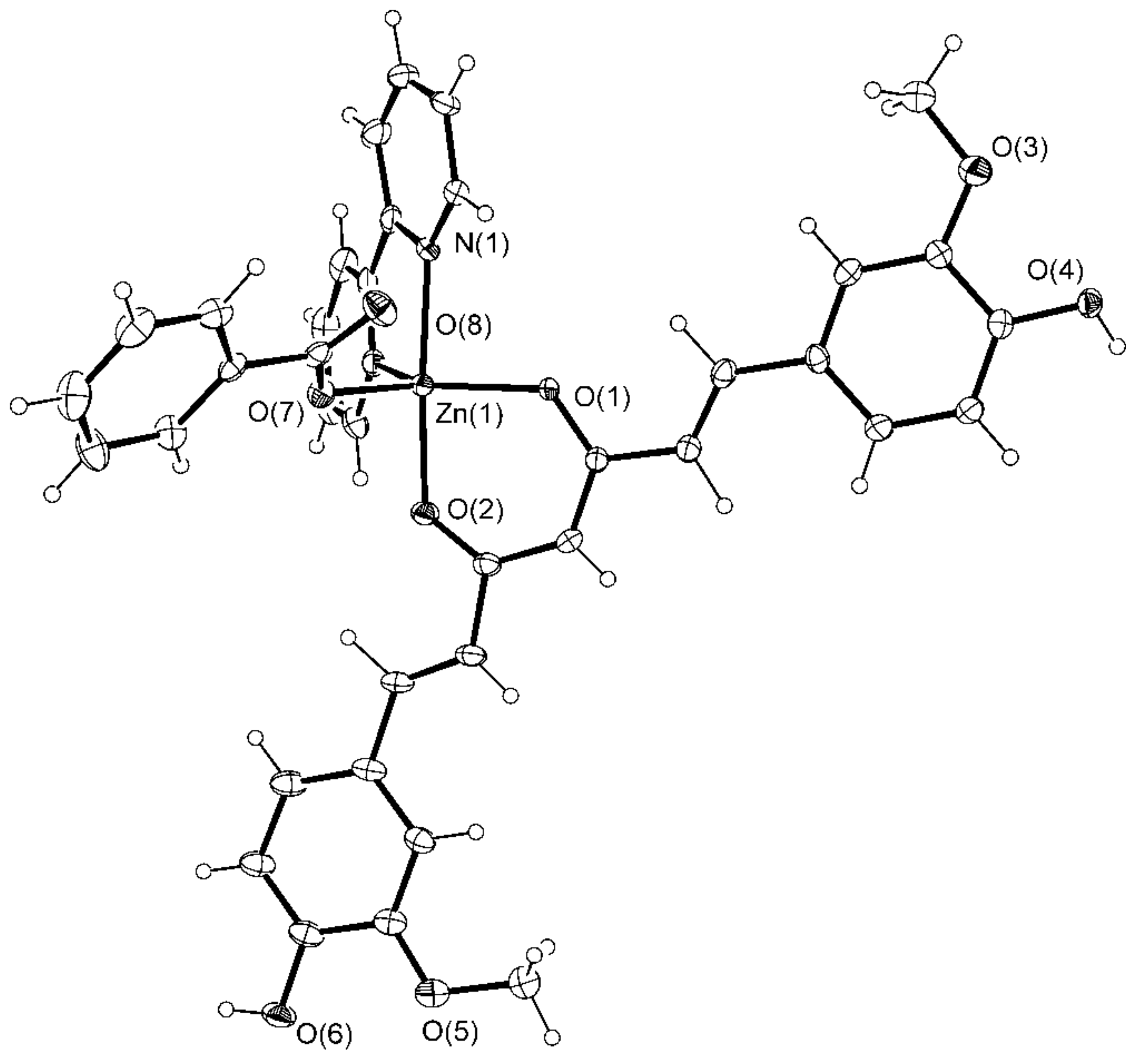
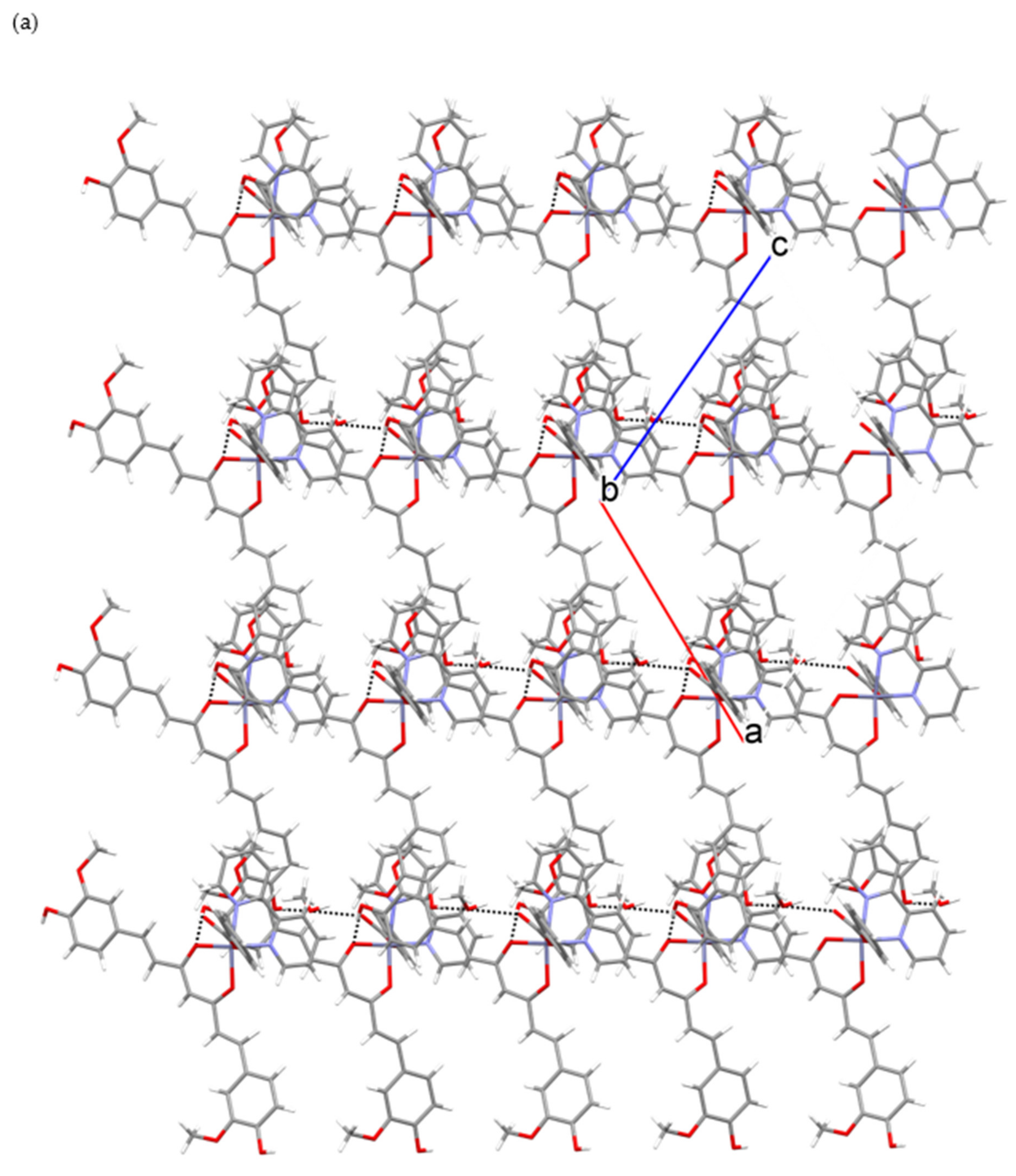
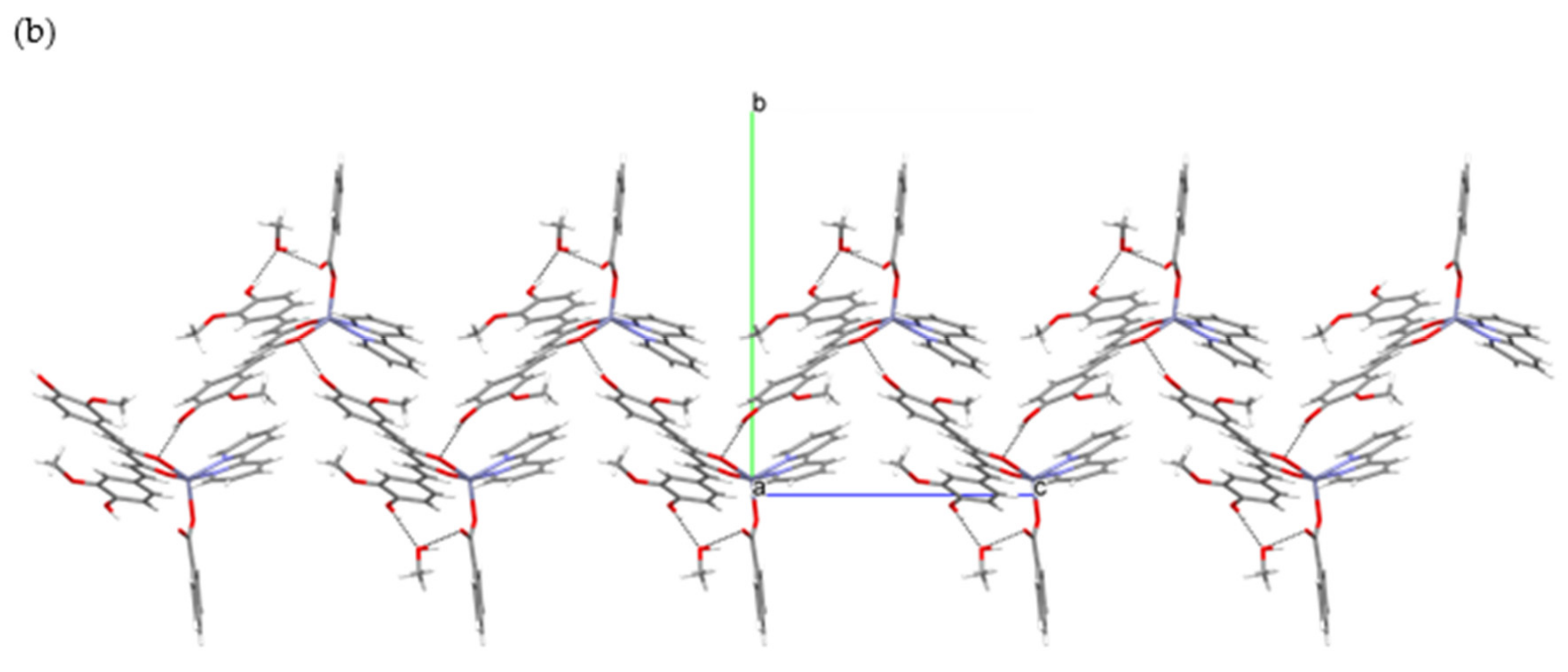
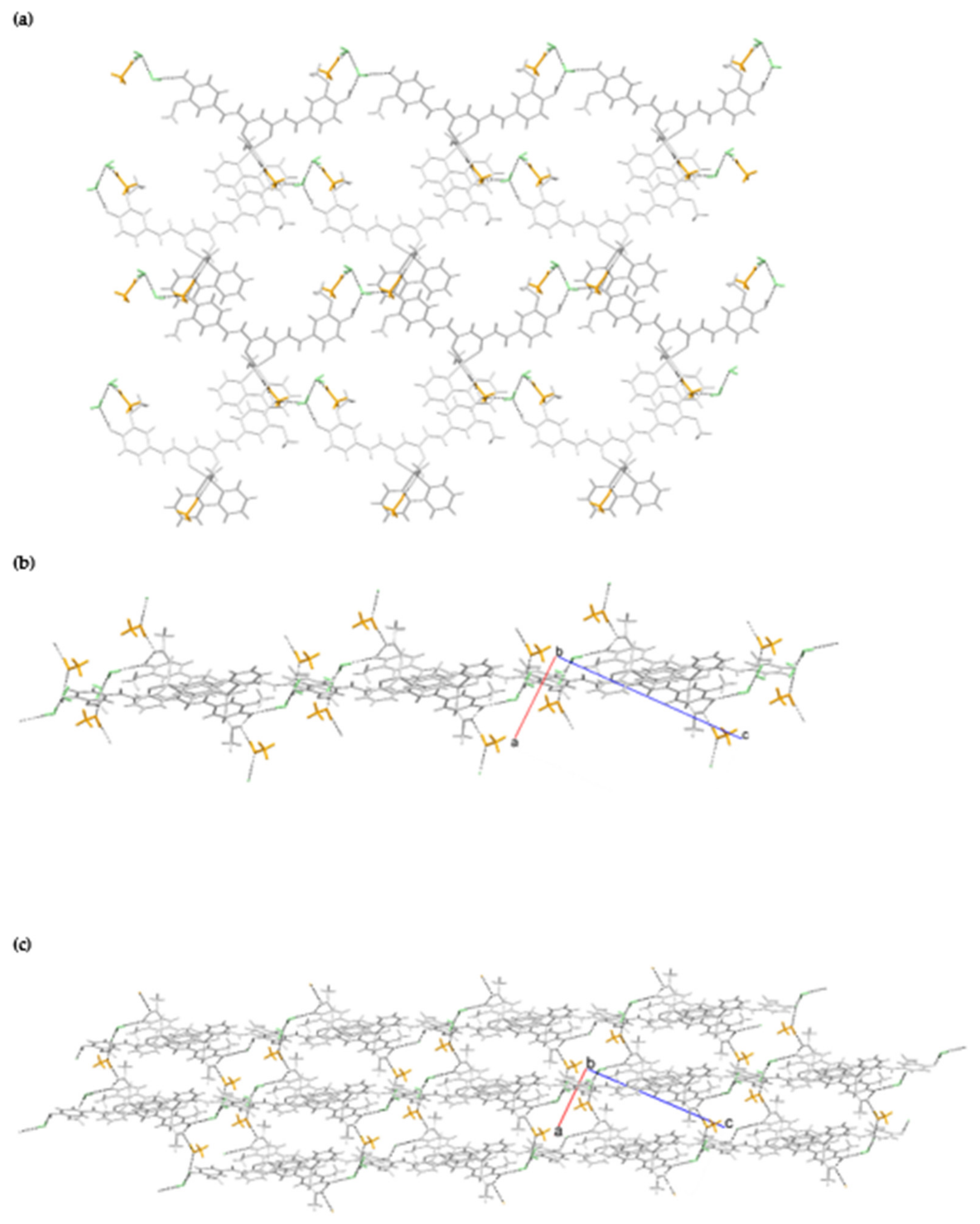
2.1. Crystallographic Study
2.2. Synthesis
2.3. Solubility and Stability
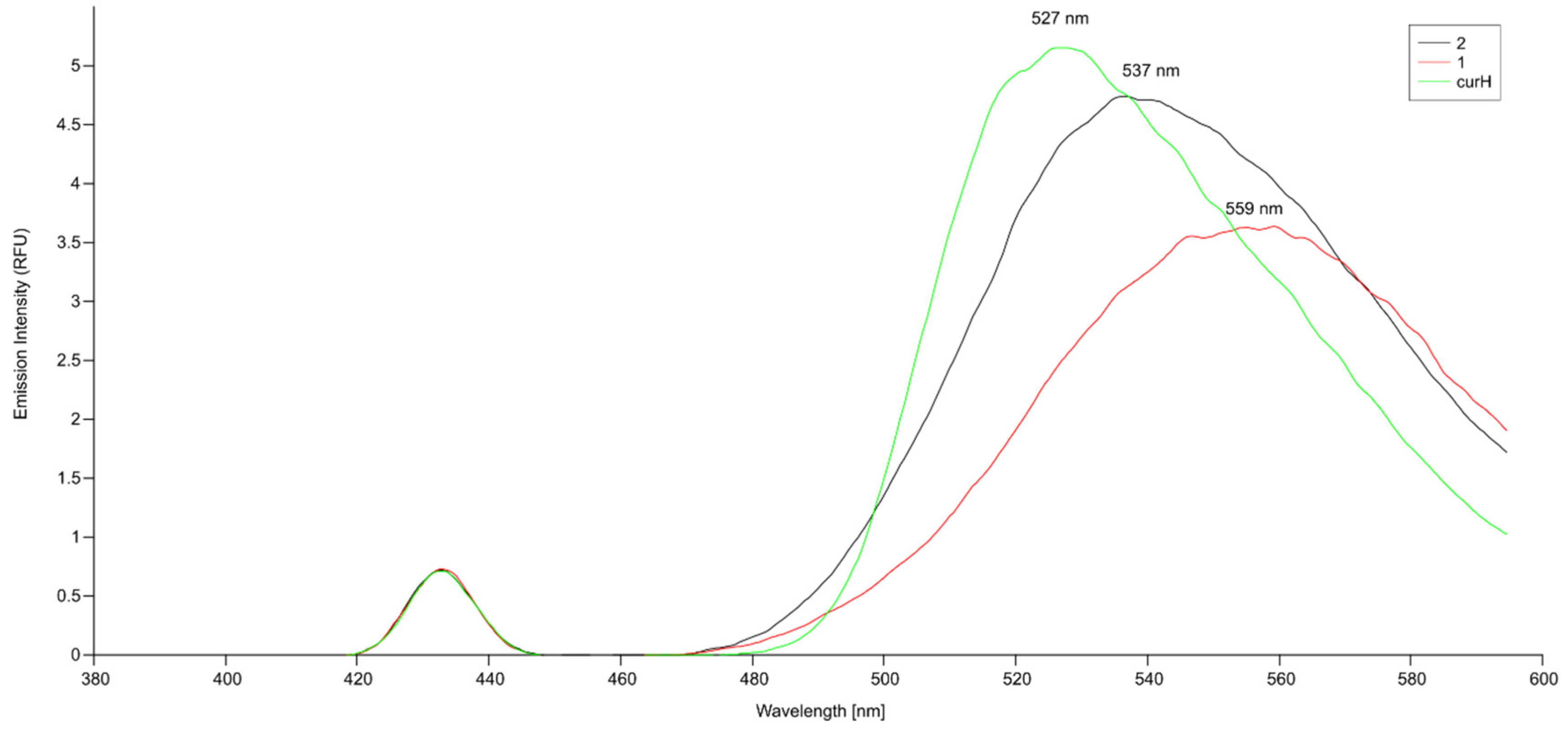
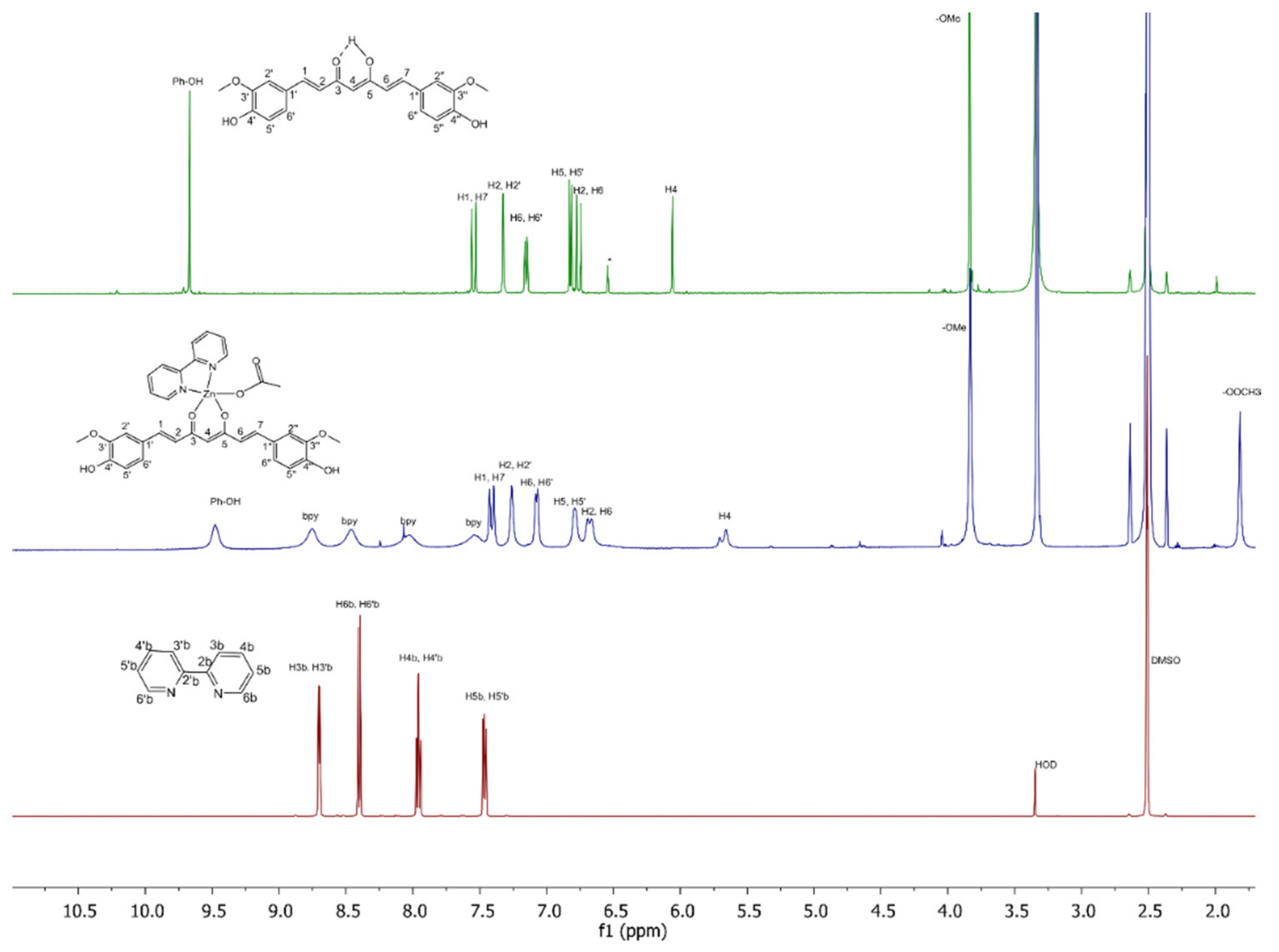
2.4. Spectroscopic Studies
3. Materials and Methods
3.1. General
3.2. Measurements
3.3. Syntheses
3.3.1. Synthesis of [Zn(PhCOO)2]
3.3.2. Synthesis of [Zn(CH3COO)(cur)(bpy)](1)·CH3OH·2H2O
3.3.3. Synthesis of [Zn(PhCOO)(cur)(bpy)](2) CH3OH
3.4. X-Ray Structure Determinations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aggarwal, B.B.; Ichikawa, H.; Garodia, P.; Weerasinghe, P.; Sethi, G.; Bhatt, I.D.; Pandey, M.K.; Shishodia, S.; Nair, M.G. From Traditional Ayurvedic Medicine to Modern Medicine: Identification of Therapeutic Targets for Suppression of Inflammation and Cancer. Expert Opin. Ther. Targets 2006, 10, 87–118. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases: Curcumin: From Kitchen to Clinic. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in Inflammatory Diseases. BioFactors 2013, 39, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, J.; Zheng, S.; Feng, Q.; Xiao, X. Curcumin, A Polyphenolic Curcuminoid With Its Protective Effects and Molecular Mechanisms in Diabetes and Diabetic Cardiomyopathy. Front. Pharmacol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Skin Regenerative Potentials of Curcumin. BioFactors 2013, 39, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin-From Molecule to Biological Function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Moustapha, A.; Pérétout, P.; Rainey, N.; Sureau, F.; Geze, M.; Petit, J.-M.; Dewailly, E.; Slomianny, C.; Petit, P. Curcumin Induces Crosstalk between Autophagy and Apoptosis Mediated by Calcium Release from the Endoplasmic Reticulum, Lysosomal Destabilization and Mitochondrial Events. Cell Death Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.M.; Catanzaro, M.; Rosini, M.; Racchi, M.; Lanni, C. Curcumin in Alzheimer’s Disease: Can We Think to New Strategies and Perspectives for This Molecule? Pharmacol. Res. 2017, 124, 146–155. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J.; Mostad, A. Structural Studies of Curcuminoids. I. The Crystal Structure of Curcumin. Acta Chem. Scand. Ser. B 1982. [Google Scholar] [CrossRef]
- Milobędzka, J.; Kostanecki, S.v.; Lampe, V. Zur Kenntnis des Curcumins. Ber. Dtsch. Chem. Ges. 1910, 43, 2163–2170. [Google Scholar] [CrossRef]
- Payton, F.; Sandusky, P.; Alworth, W.L. NMR Study of the Solution Structure of Curcumin. J. Nat. Prod. 2007, 70, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Bernabé-Pineda, M.; Ramıérez-Silva, M.T.; Romero-Romo, M.; González-Vergara, E.; Rojas-Hernández, A. Determination of Acidity Constants of Curcumin in Aqueous Solution and Apparent Rate Constant of Its Decomposition. Spectrochim. Acta A 2004, 60, 1091–1097. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of PH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.N.; Luis, P.B.; Sintim, H.O.; Schneider, C. Unraveling Curcumin Degradation. J. Biol. Chem. 2015, 290, 4817–4828. [Google Scholar] [CrossRef]
- Pröhl, M.; Schubert, U.S.; Weigand, W.; Gottschaldt, M. Metal Complexes of Curcumin and Curcumin Derivatives for Molecular Imaging and Anticancer Therapy. Coord. Chem. Rev. 2016, 307, 32–41. [Google Scholar] [CrossRef]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal Complexes of Curcumin–Synthetic Strategies, Structures and Medicinal Applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef]
- Kühlwein, F.; Polborn, K.; Beck, W. Metallkomplexe von Farbstoffen. VIII Übergangsmetallkomplexe des Curcumins und seiner Derivate. Z. Anorg. Allg. Chem. 1997, 623, 1211–1219. [Google Scholar] [CrossRef]
- Banerjee, S.; Chakravarty, A.R. Metal Complexes of Curcumin for Cellular Imaging, Targeting, and Photoinduced Anticancer Activity. Acc. Chem. Res. 2015, 48, 2075–2083. [Google Scholar] [CrossRef]
- Banerjee, S.; Prasad, P.; Hussain, A.; Khan, I.; Kondaiah, P.; Chakravarty, A.R. Remarkable Photocytotoxicity of Curcumin in HeLa Cells in Visible Light and Arresting Its Degradation on Oxovanadium(IV) Complex Formation. Chem. Commun. 2012. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Somyajit, K.; Banik, B.; Banerjee, S.; Nagaraju, G.; Chakravarty, A.R. Enhancing the Photocytotoxic Potential of Curcumin on Terpyridyl Lanthanide(III) Complex Formation. Dalton Trans. 2012, 42, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Banerjee, S.; Hussain, A. Remarkable Visible Light-Triggered Cytotoxicity of Mitochondria Targeting Mixed-Ligand Cobalt(III) Complexes of Curcumin and Phenanthroline Bases Binding to Human Serum Albumin. RSC Adv. 2015, 5, 16641–16653. [Google Scholar] [CrossRef]
- Sarkar, T.; Banerjee, S.; Mukherjee, S.; Hussain, A. Mitochondrial Selectivity and Remarkable Photocytotoxicity of a Ferrocenyl Neodymium(iii) Complex of Terpyridine and Curcumin in Cancer Cells. Dalton Trans. 2016, 45, 6424–6438. [Google Scholar] [CrossRef] [PubMed]
- Valentini, A.; Conforti, F.; Crispini, A.; De Martino, A.; Condello, R.; Stellitano, C.; Rotilio, G.; Ghedini, M.; Federici, G.; Bernardini, S.; et al. Synthesis, Oxidant Properties, and Antitumoral Effects of a Heteroleptic Palladium(II) Complex of Curcumin on Human Prostate Cancer Cells. J. Med. Chem. 2009, 52, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Rossi, M.; Benson, A.; Opazo, C.; Freedman, D.; Monti, E.; Gariboldi, M.B.; Shaulky, J.; Marchetti, F.; Pettinari, R.; et al. Ruthenium–Arene Complexes of Curcumin: X-Ray and Density Functional Theory Structure, Synthesis, and Spectroscopic Characterization, in Vitro Antitumor Activity, and DNA Docking Studies of (p -Cymene)Ru(Curcuminato)Chloro. J. Med. Chem. 2012, 55, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraju, S.; la Cour Poulsen, B.; Arisa, T.; Umadevi, D.; Dalton, H.L.; Hawes, C.S.; Estalayo-Adrián, S.; Savyasachi, A.J.; Watson, G.W.; Williams, D.C.; et al. Synthesis, Structural Characterisation and Antiproliferative Activity of a New Fluorescent 4-Amino-1,8-Naphthalimide Tröger’s Base–Ru(ii)–Curcumin Organometallic Conjugate. Chem. Commun. 2018, 54, 4120–4123. [Google Scholar] [CrossRef] [PubMed]
- Mitra, K.; Gautam, S.; Kondaiah, P.; Chakravarty, A.R. The Cis-Diammineplatinum(II) Complex of Curcumin: A Dual Action DNA Crosslinking and Photochemotherapeutic Agent. Angew. Chem. Int. Ed. 2015, 54, 13989–13993. [Google Scholar] [CrossRef]
- Mitra, K.; Gautam, S.; Kondaiah, P.; Chakravarty, A.R. Platinum(II) Complexes of Curcumin Showing Photocytotoxicity in Visible Light. Eur. J. Inorg. Chem. 2017, 2017, 1753–1763. [Google Scholar] [CrossRef]
- Sumanont, Y.; Murakami, Y.; Tohda, M.; Vajragupta, O.; Watanabe, H.; Matsumoto, K. Effects of Manganese Complexes of Curcumin and Diacetylcurcumin on Kainic Acid-Induced Neurotoxic Responses in the Rat Hippocampus. Biol. Pharm. Bull. 2007, 30, 1732–1739. [Google Scholar] [CrossRef]
- Barik, A.; Mishra, B.; Shen, L.; Mohan, H.; Kadam, R.M.; Dutta, S.; Zhang, H.-Y.; Priyadarsini, K.I. Evaluation of a New Copper(II)–Curcumin Complex as Superoxide Dismutase Mimic and Its Free Radical Reactions. Free Radical Biol. Med. 2005, 39, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Mishra, B.; Kunwar, A.; Kadam, R.M.; Shen, L.; Dutta, S.; Padhye, S.; Satpati, A.K.; Zhang, H.-Y.; Indira Priyadarsini, K. Comparative Study of Copper(II)–Curcumin Complexes as Superoxide Dismutase Mimics and Free Radical Scavengers. Eur. J. Med. Chem. 2007, 42, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Gupta, R.; Saraf, S.A.; Saraf, S.K. Zinc: The Metal of Life. Compr. Rev. Food Sci. Food Saf. 2014, 13, 358–376. [Google Scholar] [CrossRef]
- Pucci, D.; Crispini, A.; Mendiguchía, B.S.; Pirillo, S.; Ghedini, M.; Morelli, S.; De Bartolo, L. Improving the Bioactivity of Zn(Ii)-Curcumin Based Complexes. Dalton Trans. 2013. [Google Scholar] [CrossRef]
- Pucci, D.; Bellini, T.; Crispini, A.; D’Agnano, I.; Liguori, P.F.; Garcia-Orduna, P.; Pirillo, S.; Valentini, A.; Zanchetta, G. DNA Binding and Cytotoxicity of fluorescent Curcumin-Based Zn(II) Complexes. Med. Chem. Commun. 2012, 3, 462–468. [Google Scholar] [CrossRef]
- Ricciardi, L.; Pucci, D.; Pirillo, S.; La Deda, M. Emission Solvatochromic Behavior of a Pentacoordinated Zn(II) Complex: A Viable Tool for Studying the Metallodrug–Protein Interaction. J. Lumin. 2014, 151, 138–142. [Google Scholar] [CrossRef]
- Al-Ali, K.; Abdel Fatah, H.S.; El-Badry, Y.A.-M. Dual Effect of Curcumin–Zinc Complex in Controlling Diabetes Mellitus in Experimentally Induced Diabetic Rats. Biol. Pharm. Bull. 2016, 39, 1774–1780. [Google Scholar] [CrossRef]
- Halevas, E.; Papadopoulos, T.A.; Swanson, C.H.; Smith, G.C.; Hatzidimitriou, A.; Katsipis, G.; Pantazaki, A.; Sanakis, I.; Mitrikas, G.; Ypsilantis, K.; et al. In-Depth Synthetic, Physicochemical and in Vitro Biological Investigation of a New Ternary V(IV) Antioxidant Material Based on Curcumin. J. Inorg. Biochem. 2019, 191, 94–111. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Z.; Zhang, C.; Li, S.; Zhang, L.; Zhou, G.; Wang, S.; Zhang, J. Synthesis, Characterization and ROS-Mediated Antitumor Effects of Palladium(II) Complexes of Curcuminoids. Eur. J. Med. Chem. 2018, 144, 662–671. [Google Scholar] [CrossRef]
- Ma, E.S.F.; Bates, W.D.; Edmunds, A.; Kelland, L.R.; Fojo, T.; Farrell, N. Enhancement of Aqueous Solubility and Stability Employing a Trans Acetate Axis in Trans Planar Amine Platinum Compounds While Maintaining the Biological Profile. J. Med. Chem. 2005, 48, 5651–5654. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, N.; Savino, S.; Marzano, C.; Pacifico, C.; Hoeschele, J.D.; Gandin, V.; Natile, G. Cytotoxicity-Boosting of Kiteplatin by Pt(IV) Prodrugs with Axial Benzoate Ligands. J. Inorg. Biochem. 2016, 160, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984. [Google Scholar] [CrossRef]
- Brahma, S.; Sachin, H.P.; Shivashankar, S.A.; Narasimhamurthy, T.; Rathore, R.S. Adducts of Bis(Acetylacetonato)Zinc(II) with 1,10-Phenanthroline and 2,2′-Bipyridine. Acta Cryst. C Cryst. Struct. Commun. 2008. [Google Scholar] [CrossRef] [PubMed]
- Granifo, J.; Gaviño, R.; Freire, E.; Baggio, R. Monodentate and Bridging Behaviour of the Sulfur-Containing Ligand 4′-[4-(Methylsulfanyl)Phenyl]-4,2′:6′,4′′-Terpyridine in Two Discrete Zinc(II) Complexes with Acetylacetonate. Acta Cryst. C Cryst. Struct. Commun. 2012. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, R.; Marchetti, F.; Condello, F.; Pettinari, C.; Lupidi, G.; Scopelliti, R.; Mukhopadhyay, S.; Riedel, T.; Dyson, P.J. Ruthenium(II)–Arene RAPTA Type Complexes Containing Curcumin and Bisdemethoxycurcumin Display Potent and Selective Anticancer Activity. Organometallics 2014, 33, 3709–3715. [Google Scholar] [CrossRef]
- Pettinari, R.; Marchetti, F.; Pettinari, C.; Condello, F.; Petrini, A.; Scopelliti, R.; Riedel, T.; Dyson, P.J. Organometallic Rhodium(iii) and Iridium(iii) Cyclopentadienyl Complexes with Curcumin and Bisdemethoxycurcumin Co-Ligands. Dalton Trans. 2015, 44, 20523–20531. [Google Scholar] [CrossRef]
- Triantis, C.; Tsotakos, T.; Tsoukalas, C.; Sagnou, M.; Raptopoulou, C.; Terzis, A.; Psycharis, V.; Pelecanou, M.; Pirmettis, I.; Papadopoulos, M. Synthesis and Characterization of Fac-[M(CO)3(P)(OO)] and Cis-Trans.-[M(CO)2(P)2(OO)] Complexes (M = Re, 99mTc) with Acetylacetone and Curcumin as OO Donor Bidentate Ligands. Inorg. Chem. 2013, 52, 12995–13003. [Google Scholar] [CrossRef]
- Garai, A.; Pant, I.; Banerjee, S.; Banik, B.; Kondaiah, P.; Chakravarty, A.R. Photorelease and Cellular Delivery of Mitocurcumin from Its Cytotoxic Cobalt(III) Complex in Visible Light. Inorg. Chem. 2016, 55, 6027–6035. [Google Scholar] [CrossRef]
- Pettinari, R.; Condello, F.; Marchetti, F.; Pettinari, C.; Smoleński, P.; Riedel, T.; Scopelliti, R.; Dyson, P.J. Dicationic Ruthenium(II)-Arene-Curcumin Complexes Containing Methylated 1,3,5-Triaza-7-Phosphaadamantane: Synthesis, Structure, and Cytotoxicity. Eur. J. Inorg. Chem. 2017, 2017, 2905–2910. [Google Scholar] [CrossRef]
- Bhattacharyya, U.; Kumar, B.; Garai, A.; Bhattacharyya, A.; Kumar, A.; Banerjee, S.; Kondaiah, P.; Chakravarty, A.R. Curcumin “Drug” Stabilized in Oxidovanadium(IV)-BODIPY Conjugates for Mitochondria-Targeted Photocytotoxicity. Inorg. Chem. 2017, 56, 12457–12468. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Douglas, B.B.; McDaniel, D.H.; Alexander, J.J. Concepts and Models of Inorganic Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Janiak, C. A Critical Account on π–π Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. J. Chem. Soc. Dalton Trans. 2000. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, Photochemistry and Photobiology of Curcumin: Studies from Organic Solutions, Bio-Mimetics and Living Cells. J. Photochem. Photobiol. 2009, 81–95. [Google Scholar] [CrossRef]
- Hotze, A.C.G.; van der Geer, E.P.L.; Kooijman, H.; Spek, A.L.; Haasnoot, J.G.; Reedijk, J. Characterization by NMR Spectroscopy, X-Ray Analysis and Cytotoxic Activity of the Ruthenium(II) Compounds [RuL3](PF6)2(L = 2-Phenylazopyridine Oro-Tolylazopyridine) and [RuL′2L″](PF6)2(L′, L″ = 2-Phenylazopyridine, 2,2′-Bipyridine). Eur. J. Inorg. Chem. 2005, 2005, 2648–2657. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and Experimental Studies of the Structure and Vibrational Spectra of Curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Bhanoth, S.; Nangia, A. New Polymorphs of Curcumin. Chem. Commun. 2011, 47, 5013. [Google Scholar] [CrossRef]
- Zeleňák, V.; Vargová, Z.; Györyová, K. Correlation of Infrared Spectra of Zinc(II) Carboxylates with Their Structures. Spectrochim. Acta A 2007, 66, 262–272. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Part. B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 64–67. [Google Scholar] [CrossRef]
- Krackov, M.H.; Bellis, H.E. Process for the Synthesis of Curcumin-Related Compounds. U.S. Patent 5,679,864, 21 October 1997. [Google Scholar]
- Phosphate-Buffered Saline (PBS). Available online: http://cshprotocols.cshlp.org/content/2006/1/pdb.rec8247 (accessed on 2 June 2019).
- CrysAlis PRO. Version 1.171.35.11; Agilent Technologies: Yarnton, Oxfordshire, UK, 2011.
- Sheldrick, G.M. SHELXS97 and SHELXL97.Program for Crystal Structure Solution and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windowa–A Version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- CrystalMaker® X. Version 10.4.0; CrystalMaker Software Ltd.: Oxfordshire, UK, 2019.
Sample Availability: Samples of the compounds are not available from the authors. |
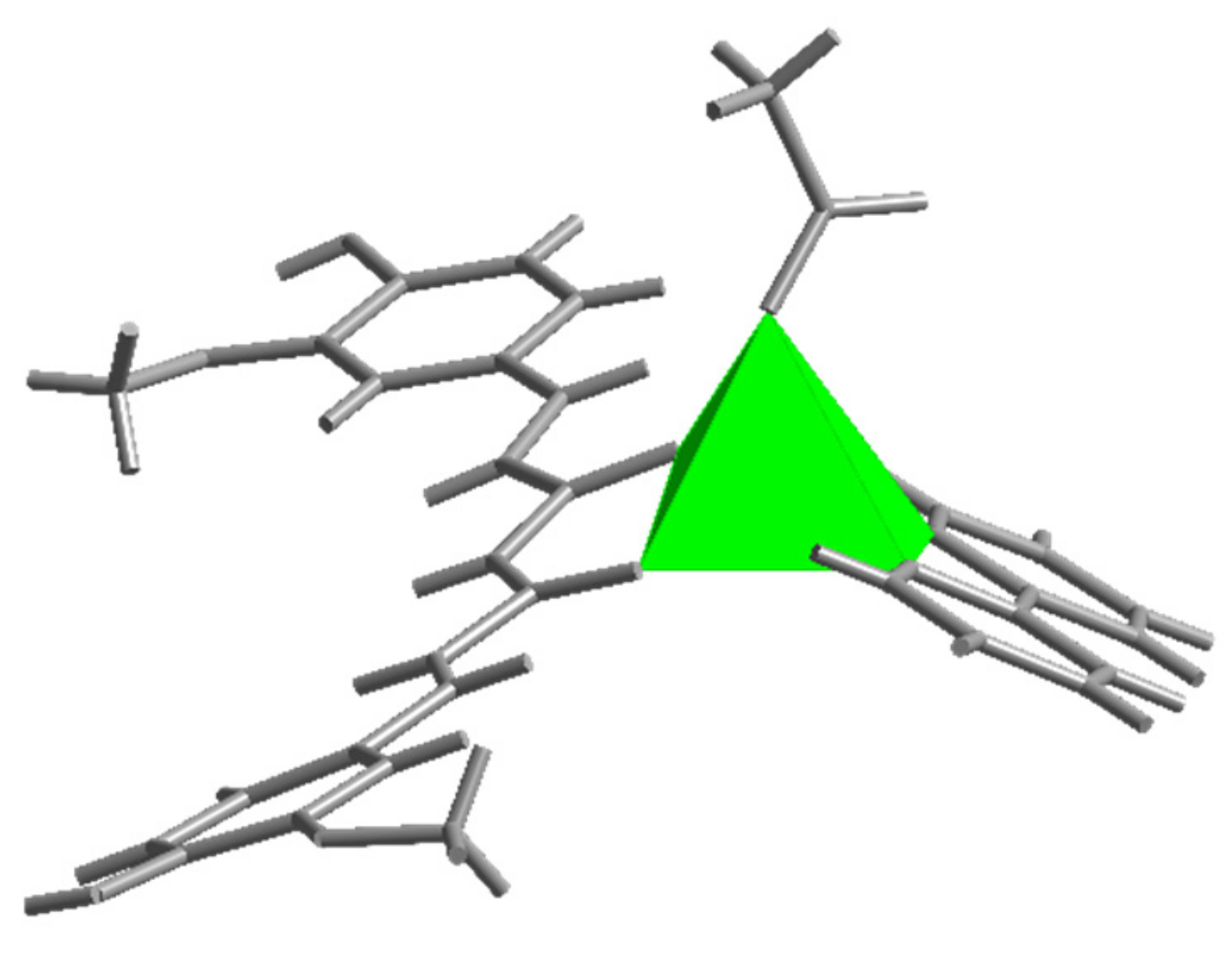
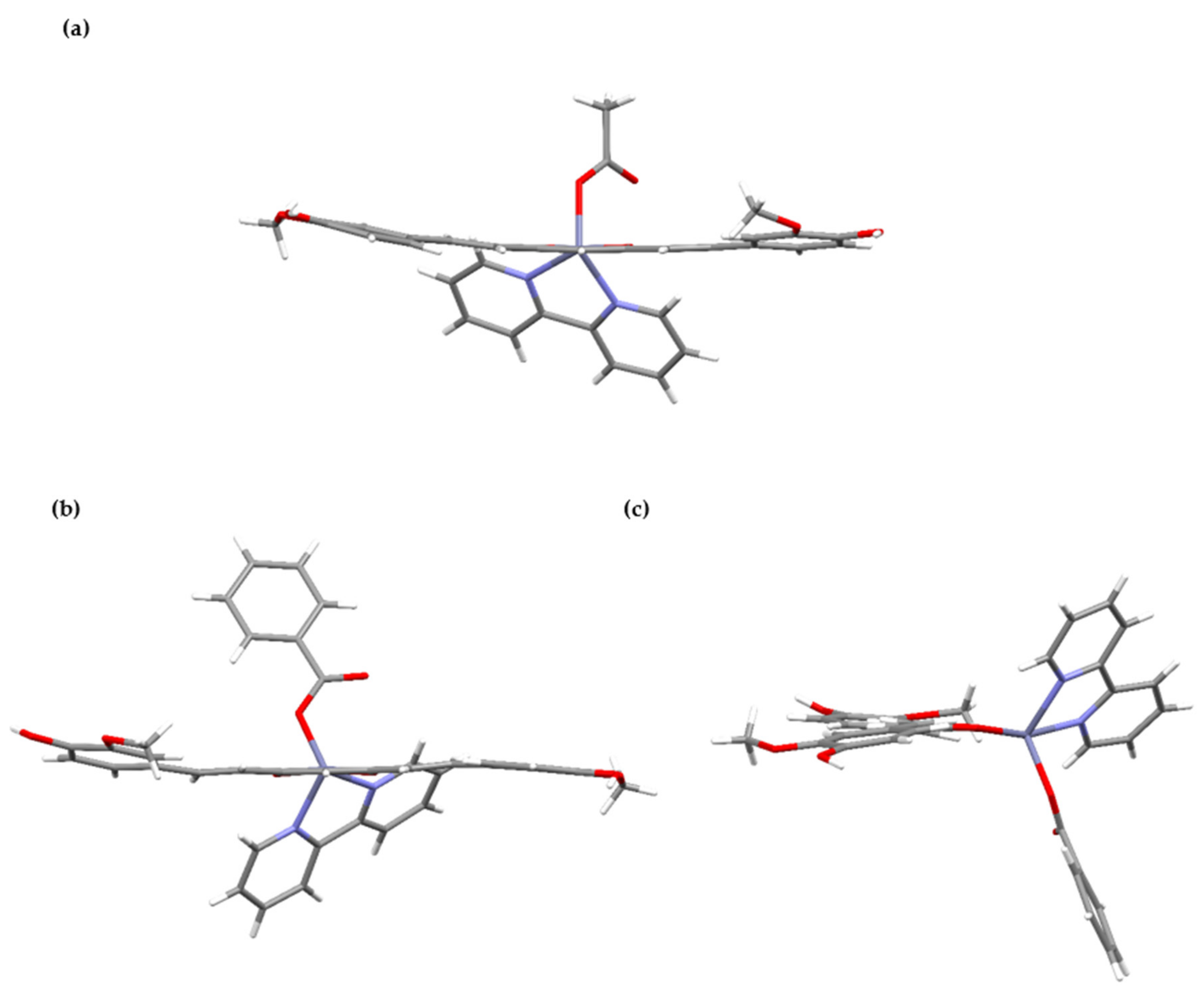


| 1 | 2 | ||
|---|---|---|---|
| Zn(1)–O(7) | 1.973(1) | Zn(1)–O(7) | 1.987(3) |
| Zn(1)–O(1) | 2.016(1) | Zn(1)–O(1) | 2.007(3) |
| Zn(1)–O(2) | 2.001(1) | Zn(1)–O(2) | 2.051(3) |
| Zn(1)–N(1) | 2.162(2) | Zn(1)–N(1) | 2.121(4) |
| Zn(1)–N(2) | 2.123(1) | Zn(1)–N(2) | 2.112(4) |
| Zn(1)–O(8) | 3.303(2) | Zn(1)–O(8) | 3.127(3) |
| C(8)–O(7) | 1.263(2) | C(31)–O(7) | 1.266(5) |
| C(8)–O(8) | 1.243(2) | C(31)–O(8) | 1.242(5) |
| O(7)–Zn(1)–O(1) | 100.79(6) | N(1)–Zn(1)–O(2) | 162.9(1) |
| O(7)–Zn(1)–O(2) | 101.48(6) | N(1)–Zn(1)–N(2) | 77.0(2) |
| O(7)–Zn(1)–N(1) | 99.73(6) | N(1)–Zn(1)–O(1) | 90.5(1) |
| O(7)–Zn(1)–N(2) | 123.32(6) | N(1)–Zn(1)–O(7) | 98.6(1) |
| O(1)–Zn(1)–N(1) | 158.86(6) | O(1)–Zn(1)–O(7) | 127.3(1) |
| O(2)–Zn(1)–N(2) | 134.18(6) | O(7)–Zn(1)–N(2) | 94.8(1) |
| O(1)–Zn(1)–O(2) | 92.22(5) | N(2)–Zn(1)–O(1) | 124.8(1) |
| O(2)–Zn(1)–N(1) | 88.61(5) | O(2)–Zn(1)–O(1) | 89.75(1) |
| N(1)–Zn(1)–N(2) | 75.82(6) | O(2)–Zn(1)–O(7) | 94.8(1) |
| N(2)–Zn(1)–O(1) | 88.66(5) | O(2)–Zn(1)–N(2) | 88.9(1) |
| Groups Engaged in Interaction (b) | Donor Atom Acceptor (c) | Length (Å) |
|---|---|---|
| OH·CH3OH | O(6)···O(9) i | 2.715(5) |
| OH···O(cur−) | O(4)···O(1) ii | 2.788(4) |
| CH3OH···COO− | O(9)···O(8) | 2.709(5) |
| Groups Engaged in Interaction (b) | Donor Atom Acceptor (c) | Length (Å) |
|---|---|---|
| OH···COO− | O(6)···O(8) i | 2.653(2) |
| OH···H2O | O(4)···O(12) ii | 2.729(2) |
| OH···H2O | O(6)···O(12) iii | 3.012(2) |
| CH3OH···COO− | O(10)···O(8) i | 2.787(3) |
| CH3OH···H2O | O(10)···O(11) iv | 2.702(3) |
| H2O···OCH3 | O(12)···O(3) ii | 3.026(2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabner, S.; Modec, B. Zn(II) Curcuminate Complexes with 2,2′-bipyridine and Carboxylates. Molecules 2019, 24, 2540. https://doi.org/10.3390/molecules24142540
Grabner S, Modec B. Zn(II) Curcuminate Complexes with 2,2′-bipyridine and Carboxylates. Molecules. 2019; 24(14):2540. https://doi.org/10.3390/molecules24142540
Chicago/Turabian StyleGrabner, Sabina, and Barbara Modec. 2019. "Zn(II) Curcuminate Complexes with 2,2′-bipyridine and Carboxylates" Molecules 24, no. 14: 2540. https://doi.org/10.3390/molecules24142540
APA StyleGrabner, S., & Modec, B. (2019). Zn(II) Curcuminate Complexes with 2,2′-bipyridine and Carboxylates. Molecules, 24(14), 2540. https://doi.org/10.3390/molecules24142540







