Abstract
Seven novel norcycloartane glycosides, maryloside A–G (1–7), were isolated from the leaves of Cymbidium Great Flower ‘Marylaurencin’, along with a known norcycloartane glycoside, cymbidoside (8). These structures were determined on the basis of mainly NMR experiments as well as chemical degradation and X-ray crystallographic analysis. The isolated compounds (1–6 and 8) were evaluated for the inhibitory activity on lipopolysaccharide (LPS) and interferon-γ (IFN-γ)-stimulated nitric oxide (NO) production in RAW 264.7 cells. Consequently, 1 and 3 exhibited moderate activity.
1. Introduction
Cymbidium Great Flower ‘Marylaurencin’ is a new breed produced by Kawano-Mericlone Corporation by mating. The seed is registered at the Ministry of Agriculture, Forestry and Fisheries of Japan. During our search for bioactive substances from C. Great Flower ‘Marylaurencin’, which is a cultivar, we have already reported some phenanthrene derivatives with antibacterial effect and cytotoxic activity, and some aromatic glycosides with antioxidant activity from its root, stem and flower [1,2,3,4]. The leaves of C. Great Flower ‘Marylaurencin’ are sold as an herb tea in this area. Therefore, we started exploring the active ingredients from the leaves of this plant to support peoples’ health. Here, we report the isolation and structural determination of seven novel 30-norcycloartane-type triterpene glycosides (1–7) along with one known compound, cymbidoside (8) [5], from the leaves of C. Great Flower ‘Marylaurencin’. Their structures were elucidated using high resolution (HR)-FAB-MS analyses, extensive two dimensional (2D)-heteronuclear NMR data interpretation and X-ray crystallographic analysis.
Nitric oxide (NO) plays important role as biophylaxis. On the other hand, it is involved in the overproduction of nitric oxide generated aggravation of inflammation and carcinogenesis. Nitric oxide synthase has three isoforms, eNOS (endothelial nitric oxide), nNOS (neuronal nitric oxide) and iNOS (inducible nitric oxide). Among them, especially activation of iNOS induced by such an inflammatory reaction is responsible for the overproduction of nitric oxide and contributes to DNA damage that leads to mutagenesis and carcinogenesis. We evaluated the anti-inflammatory activity of isolated compounds from C. Great Flower ‘Marylaurencin’ on the LPS and IFN-γ-induced NO production in mouse monocyte macrophage cell line, RAW264.7.
2. Results and Discussion
The leaves of C. Great Flower ‘Marylaurencin’ were extracted with 70% EtOH aq. at room temperature. The 70% EtOH aq. extract was partitioned into EtOAc-, BuOH- and H2O- soluble layers. The BuOH soluble layer was subjected to silica gel column chromatography and successively purified by reversed-phase high performance liquid chromatography (HPLC) to yield maryloside A–G (1–7) along with a known compound (8).
1D and 2D NMR data of these compounds (1–7) suggested triterpene saponin. Acid hydrolysis of these compounds (1–7) afforded D-glucose, whose absolute configuration was confirmed by the method using reversed-phase HPLC developed by Tanaka [6].
The molecular formula of maryloside A (1) was determined to be C36H60O10 from the molecular ion peak [M + Na]+ at m/z 675.4082 (calculated for m/z 675.4084) in the positive-ion HR-FAB-MS with 7 degrees of unsaturation. The IR spectrum showed the presence of carbonyl group (1705 cm−1) and hydroxyl group (3271 cm−1). The 1H-NMR and 13C-NMR spectra of 1 suggested the presence of sugar moiety in the molecule (Table 1 and Table 2). The hydrolysis of 1 by cellulase liberated an aglycone of 1 (1a). The 1H-NMR spectrum of 1a indicated characteristic signals of cyclopropane ring at δH 0.33 (doublet, d, J = 4.1 Hz) and 0.56 (d, J = 4.1 Hz), the presence of two methyl groups—signals at δH 0.86 (singlet, s) and 1.04 (s), three methyl groups at δH 1.21 (d, J = 6.0 Hz), 1.22 (d, J = 7.0 Hz) and 1.25 (d, J = 7.0 Hz), two oxygen-bearing methylene protons at δH 3.96 (d, J = 11.0 Hz) and 4.39 (d, J = 11.0 Hz), 4.05 (d, J = 11.9 Hz), 4.06 (d, J = 11.9 Hz) and one oxygen-bearing methine proton at δH 4.61 (d, J = 9.9 Hz) (Table 3). All thirty carbons in 13C-NMR spectrum of 1a were clearly assigned on the basis of H-H COSY, HMQC and HMBC experiments. The 13C-NMR spectrum of 1a showed the presence of a carbonyl group at δC 211.9, a tertiary alcohol at δC 76.1, a secondary alcohol at δC 69.3 and two primary alcohols at δC 57.7 and 66.8 (Table 2). The H-H COSY and HMQC spectra showed that 1a had partial structures (a–f) –CH2–CH2– (a; C-1 and C-2), –CH–CH2– (b; C-4 and C-28), –CH–CH2–CH2–CH– (c; from C-5 to C-8), –CH2–CH2– (d; C-11 and C-12), –CH2–CH2–CH–CH(CH3)–CH–CH2– (e; from C-15 to C-23) and CH3–CH–CH3 (f; from C-26 to C-25 and from C-25 to C-27) (Figure 1). The HMBC spectrum showed correlations from H-1, H-2, H-5 and H-28 to C-3, and from H-19 to C-1, C-5, C-8, C-9, C-10 and C-11. These correlations established the connection between the partial structures a and d. Furthermore, the HMBC spectrum exhibited cross-peaks from H-18 to C-12, C-13, C-14 and C-17, and from H-29 to C-8, C-13, C-14 and C-15, which connected among partial structures c, d and e. In the HMBC spectrum, there were cross-peaks appearing from H-23 to C-24 and C-30, from H-25 to C-24 and C-30, and from H-26 and H-27 to C-24. Finally, all partial structures of a–f were connected. The relative configuration of 1a was determined by the NOESY experiment, coupling constant and X-ray crystallographic analysis. The NOESY spectrum between H-4β-axial and H-19, between H-8β-axial [δH 1.58 (dd, J = 4.9, 12.6 Hz)] and H-18, and between H-8β-axial and H-19 confirmed to be β-axial oriented protons at C-4, C-8 C-18 and C-19. On the other hand, the NOESY spectrum between H-1α-axial and H-5α-axial, between H-6α-equatorial and H-28, between H-7α-axial and H-29, between H-12α-axial and H-29, and between H-17α-axial and H-29 confirmed to be α-axial-oriented protons at C-5, C-17 and C-29 positions. The configuration at C-20 position of 1a was suggested as S* configuration according to NOE correlations between H-12β -equatorial and H-21, and between H-18 and H-20. Finally, 20S*, 22R* and 24R* were confirmed by X-ray crystallographic analysis (Figure 2).

Table 1.
1H-NMR (600 MHz) spectral data of compounds 1–7 [δ in ppm, coupling constants (J in Hz, in parenthesis)].

Table 2.
13C-NMR (150 MHz) spectral data of compounds 1–7, 1a, and 8a (δ in ppm).

Table 3.
1H-NMR (600 MHz) spectral data of compounds 1a and 8a [δ in ppm, coupling constants (J in Hz, in parenthesis)].
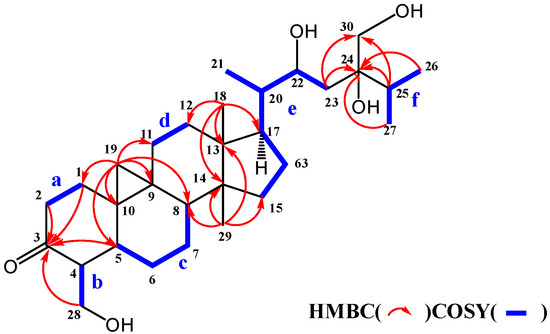
Figure 1.
Selected COSY and HMBC correlations of 1a.
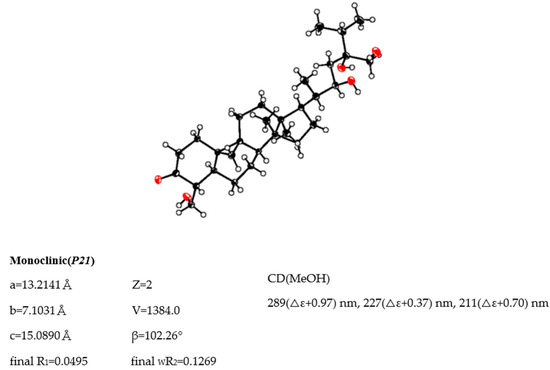
Figure 2.
ORTEP drawing and crystal data of 1a.
Sugar moiety of 1 was estimated as one D-glucose from acid hydrolysis with the method of Tanaka using reversed-phase HPLC [6] and chemical shift of 13C-NMR spectra. 1 was hydrolyzed by 0.5 M HCl. After drying in vacuo, the residue was dissolved in pyridine containing L-cysteine methyl ester hydrochloride and heated at 60 °C for 1 hr. The reaction mixture was directly analyzed by reversed-phase HPLC. The peak at 10.626 min was coincided with the derivative of D-glucose (10.607 min), whereas that of L-glucose was 10.142 min. The HMBC correlation of 1 from anomeric proton at H-1’ to C-28 indicated that glucose was connected to C-28 (Figure 3). The coupling constant of anomeric proton at H-1’ as J = 7.8 Hz revealed to be β-D-glucopyranosyl linkage. From the above data, structure of 1 was elucidated as 24-methyl-30-norcycloart-22R*, 24R*, 28, 30-tetraol-3-one-28-O-β-D-glucopyranoside (Figure 4).
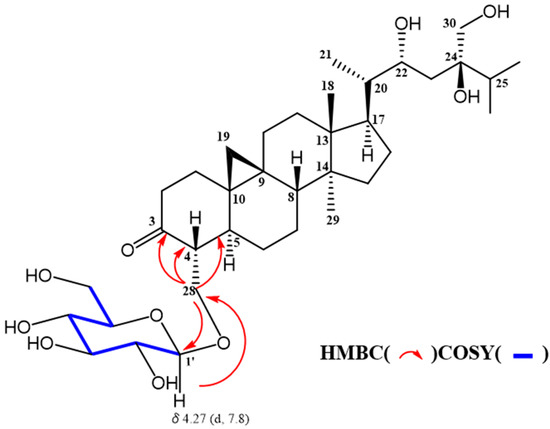
Figure 3.
Selected COSY and HMBC data of 1.
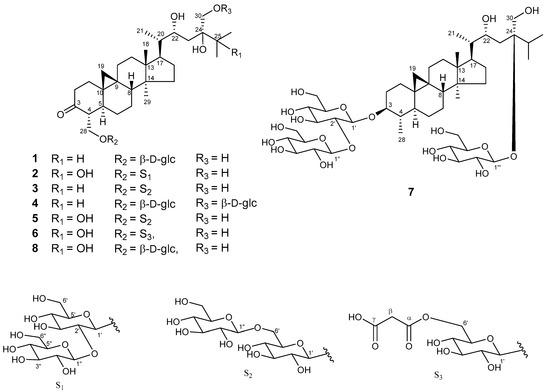
Figure 4.
Structures of 1–8.
The HR-FAB-MS of maryloside B (2) showed a molecular ion peak at m/z 853.4570 [M + Na]+, corresponding to the molecular formula C42H70O16. The IR spectrum of 2 showed the presence of carbonyl group (1697 cm−1) and hydroxyl group (3356 cm−1). The 1H and 13C-NMR spectra of 2 were very similar to those of 8 except for sugar moiety (Table 1). The 1H-NMR spectrum of 2 showed two anomeric proton doublets at δH 4.97 (H-1’) and δH 5.37 (H-1″) in the downfield region. The β-anomeric configuration of the D-glucose moieties were determined from their coupling constants of H-1’ (J = 7.7 Hz) and H-1″ (J = 7.7 Hz), respectively. Two glucose moieties (S1 in Figure 4) were determined to be connected to C-28 position by HMBC correlations from H-1″ to C-2’ and from H-1’ to C-28. The hydroxyl group at C-25 (δC 75.4) was determined by HMBC correlations from H-26 and H-27 to C-25. The X-ray crystallographic analysis of aglycone of known compound 8 was measured to determine the relative configuration of 2 because aglycone of both compounds have the same planner structure. The 1H and 13C-NMR values of aglycone of 2 were good agreement with those of aglycone of 8. Consequently, the structure of 2 was elucidated to be 24-methyl-30-norcycloart-22R*, 24S*, 25, 28, 30-pentaol-3-one-28-O-β-D-glucopyranosyl (1→2)-β-D-glucopyranoside (Figure 4).
The HR-FAB-MS analysis of maryloside C (3) displayed a pseudomolecular ion peak at m/z 813.4664 [M − H]-, indicating the molecular formula C42H70O15. The IR spectrum showed the presence of carbonyl group (1699 cm−1) and hydroxyl group (3340 cm−1). The 1H-NMR and 13C-NMR spectra of 3 were very similar to those of 1 except for the additional glucose moiety (Table 1 and Table 2). The 1H-NMR spectrum of 3 showed two anomeric proton doublets at δH 4.87 (J = 7.8 Hz) and δH 5.15 (J = 7.8 Hz) in the downfield region, indicating two β-linked sugar moieties. HMBC correlations from the anomeric proton of H-1’ to C-28 and from the anomeric proton of H-1″ to C’-6 indicated the presence of a disaccharide moiety (S2 in Figure 4) at C-28. Therefore, maryloside C (3) was identified as 24-methyl-30-norcycloart-22R* 24R*, 28, 30-tetraol-3-one-28-O-β-D-glucopyranosyl (1→6)-β-D-glucopyranoside (Figure 4).
The molecular formula of maryloside D (4), C42H70O15, was determined by HR-FAB-MS m/z 837.4648 [M + Na]+ and 13C-NMR data. The IR spectrum showed the presence of carbonyl group (1686 cm−1) and hydroxyl group (3393 cm−1). Detailed examination of 1D and 2D NMR spectra of 4 and comparison with those of 1 showed that the chemical shift values of 4 were very similar with those reported for 1, except for the additional extra sugar moiety linked to C-30 that was confirmed by the HMBC correlation from the anomeric proton H-1‴ at δH 5.00 (d, J = 7.8 Hz) to C-30 (Table 1 and Table 2). Thus, the structure of maryloside D (4) was established as 24-methyl-30-norcycloart-22R*, 24R*, 28, 30-tetraol-3-one-28-O-β-D-glucopyranosyl-30-O-β-D-glucopyranoside (Figure 4).
The molecular formula, C42H70O16 determined by HR-FAB-MS m/z 829.4549 [M−H]- and 13C-NMR data, of maryloside E (5) was the same as those of 2. The IR spectrum showed the presence of carbonyl group (1703 cm−1) and hydroxyl group (3370 cm−1). Detailed examination of 1D and 2D NMR spectra and HR-FAB-MS analysis of 5 showed that the chemical shift values of 5 were almost superimposable with those reported for 2 (Table 1 and Table 2). HMBC correlations from the anomeric proton signal at H-1″ at δH 5.15 (d, J = 7.8 Hz) to the carbon resonance at C-6’ and from H-1’ at δH 4.88 (d, J = 8.0 Hz) to C-28 indicated the presence of a disaccharide moiety (S2 in Figure 4) at C-28. Therefore, maryloside E (5) was identified as 24-methyl-30-norcycloart-22R*, 24S*, 25, 28, 30-pentaol-3-one-28-O-β-D-glucopyranosyl (1→6) -β-D-glucopyranoside (Figure 4).
The negative FAB-MS of maryloside F (6) gave a molecular ion peak at m/z 753.4033 [M − H]-, corresponding to the molecular formula C39H62O14. The IR spectrum showed the presence of carbonyl group (1711 cm−1) and hydroxyl group (3150 cm−1). The 1H-NMR and the 13C-NMR spectra of 6 were similar to those of 2, but a few differences were also recognized. The 13C-NMR spectrum of 6 showed the presence of two carboxylate esters at δC 169.6 and 168.1. The HMBC correlation from anomeric proton H-1’ at δH 4.90 (d, J = 7.8 Hz) to C-28 indicated that the glucose connected to C-28. The HMBC correlations from H-6’ to C-α and from H-β to C-α and C-γ indicated that malonyl moiety connected to C-6’. From the above data, the structure of maryloside F (6) was elucidated as 24-methyl-30-norcycloart-22R*, 24S*, 25, 28, 30-pentaol-3-one-28-O-(6′-O-malonyl) β-D-glucopyranoside (Figure 4).
The molecular formula of maryloside G (7) was determined to be C48H82O19 from the molecular ion peak [M − H]- at m/z 961.5522 (calcd. for m/z 961.5524). The IR spectrum showed the presence of hydroxyl group (3367 cm−1). The 1H-NMR and 13C-NMR spectra also suggested that 7 was the cycloartane-type saponin with three sugar moieties (Table 1 and Table 2). The 1H-NMR spectrum of 7 showed the presence of three anomeric protons at δH 4.98 (d, J = 7.7 Hz), 5.40 (d, J = 8,0 Hz) and 5.43 (d, J = 9.1 Hz), two methyl groups—signals at δH 0.85 (s) and 0.99 (s), four methyl groups at δH 1.13 (d, J = 6.9 Hz), 1.22 (d, J = 6.7 Hz), 1.27 (d, J = 6.9 Hz) and 1.41(d, J = 6.5 Hz), one oxygen-bearing methylene protons at δH 4.06 (d, J = 12.4 Hz) and 4.26 (d, J=12.4 Hz), and two oxygen-bearing methine protons at δH 3.45 (ddd, J = 10.6, 10.6, 4.6 Hz) and 4.56 (m). The 13C-NMR spectrum of 7 showed no carbonyl groups. HMBC correlations from a methyl group at δH 1.41 to C-3, C-4 and C-5 showed that this methyl connected to C-4 position (Figure 5). Moreover, an oxygen-bearing methine proton at δH 3.45 revealed to be at C-3 position from 1D and 2D NMR data. From the coupling pattern of H-3 at δH 3.45 (ddd, J = 10.6, 10.6, 4.6 Hz), the configurations of H-3 and H-4 found to be α-axial and β-axial protons, respectively. HMBC correlations from the anomeric proton at H-1’ to C-3, from the anomeric proton at H-1″to at C-2’ and from the anomeric proton at H-1‴ to at C-24 indicated that two glucose (S1 in Figure 4) attached to the C-3 position and another one glucose attached C-24 position. Furthermore, from the coupling constants of these anomeric protons, three sugar moieties were identified as all β-glucose (H-1’, δH 4.98, J = 7.7 Hz), (H-1″, δH 5.43, J = 9.1 Hz) and (H-1‴, δH 5.40, J = 8.0 Hz). From above data, structure of maryloside G (7) was elucidated as to be 24-methyl-30-norcycloart-3, 22R*, 24R*, 30-tetraol-3-O-β-D-glucopyranosyl (1→2)-β-D-glucopyranosyl-24-O-β-D-glucopyranoside (Figure 4).
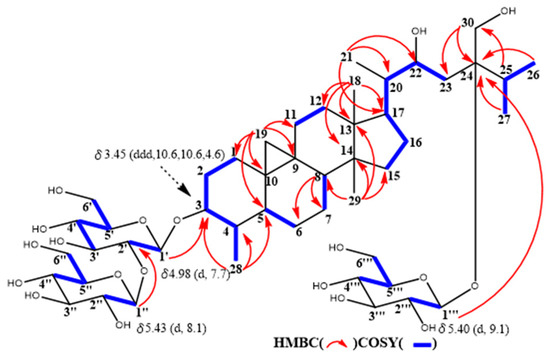
Figure 5.
Selected COSY and HMBC correlations of 7.
Marylosides A–G (1–7) and cymbidoside (8) were evaluated for the inhibitory activity of NO production in the LPS and IFN-γ-stimulated RAW 264.7 cells. Furthermore, 1 and 3 showed the inhibitory activity in dose dependently, the IC50 value was calculated as 17.8 ± 2.3 and 83.9 ± 4.8 µM, respectively, while IC50 of AG was calculated as 81.4 ± 2.6 µM (Figure 6). Other compounds showed weak inhibitory activities (IC50 values were ≧100 µM). The result of MTT cell viability assay revealed that all compounds hardly affect to cell viability. Considering these results, the hydroxy group at position 25 reduced the activity. Additionally, the comparison of 1 and 4 suggests the presence of glucose moiety at position 30 which also reduced the NO producing inhibitory activity.
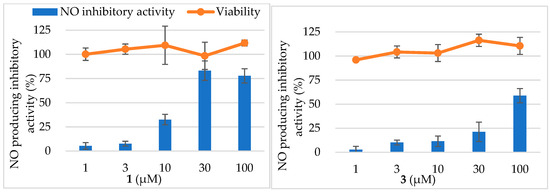
Figure 6.
Nitric oxide (NO) production inhibitory activities and cell viability of 1 and 3.
3. Materials and Methods
3.1. General Procedures
Melting points were determined with a Yanagimoto micro melting point apparatus (Yanaco, Kyoto, Japan). Optical rotation was performed on JASCO DIP-1000 polarimeter (JASCO, Tokyo, Japan). IR spectra were measured on a JASCO FT/IR-5300. NMR spectra were obtained on a Varian UNITY 600 NMR spectrometer (Varian, CA, USA) with deuterated solvents (MeOH-d4 and pyridine-d5), and the solvent chemical shifts were taken as the internal standard. The chemical shifts are given in δ (ppm), and coupling constants are reported in Hz. HR-FAB-MS spectra were obtained on a JEOL JMS-700 instrument (JEOL, Tokyo, Japan). Kieselgel 60 (230–400 mesh, Merck) and Sephadex LH-20 (GE Healthcare Life Sciences, IL, USA) were used for column chromatography. HPLC separation was performed on a JASCO PU 1580 pump with a JASCO UV-970 detector with a CAPCELL PAK C18 AQ (SHISEIDO, Tokyo, Japan, 20 mm i.d. × 250 mm).
3.2. Plant Material
Plant growth conditions of inside a plastic greenhouse of Kawano-Mericlone Corporation in Tokushima Prefecture, Japan. Insolation; Cymbidium was grown in a plastic greenhouse. A 50% shaded net was used in summer. The average temperature was 12–30 °C. The average humidity was 40–90%.
The powder of leaves of C. Great Flower ‘Marylaurencin’ were supplied by Kawano-Mericlone Corporation in Tokushima Prefecture, Japan. C. Great Flower ‘Marylaurencin’ (Ministry of Agriculture, Forestry and Fisheries of Japan, seed registration No. 2841) was cultivated and harvested in November 2008 at Kawano-Mericlone Co., Ltd. and were identified by one of the authors (Dr. S. Kawano). A voucher specimen (TB 5430) has been deposited in the Herbarium of Faculty of Pharmaceutical Sciences, Tokushima Bunri University, Tokushima, Japan.
3.3. Extraction and Isolation
The leaves were dried after harvest and made into powder and stored at −20 degrees until we started the research. The powder of leaves of C. Great Flower ‘Marylaurencin’ (2.0 kg) were extracted with 70% EtOH aq. at room temperature for 3 months. The 70% EtOH aq. extract was dried in vacuo and partitioned into EtOAc-, BuOH- and H2O-soluble layers and concentrated in vacuo. The BuOH-soluble portion (42.5 g) was subjected to flush silica gel column chromatography by gradually raising the polarity with isopropylether-MeOH-H2O (25:6:0.1–0:80:20) to afford six fractions. Each fraction was repeatedly subjected to silica gel column chromatography using increasing concentrations of MeOH and H2O in chloroform as eluent. Further purification was carried out by chromatography on Sephadex LH-20 using MeOH and by repeated gradient HPLC (MeOH/H2O solvent system; 60–80% MeOH for 1, 45–75% MeOH for 2–8, flow rate: 3 mL/min,; UV wave length: 205 nm to afford 1 (87.5 mg), 2 (440.5 mg), 3 (66.1 mg), 4 (34.0 mg), 5 (52.3 mg), 6 (36.1 mg), 7 (14.5 mg) and 8 (106.0 mg). Their structures were elucidated by the analysis of HR-FAB-MS and 1H and 13C-NMR spectra, including 2D NMR experiments and X-ray crystallographic analysis.
maryloside A (1): m.p. 145.5–146.5 °C.; [α + 21.9 (c 1.1, MeOH); FT-IR (film) 3271, 1705, 1090 cm−1; 1H-NMR (600 MHz, CD3OD): See Table 1; 13C-NMR (150 MHz, CD3OD): See Table 2; HR-FAB-MS m/z [M + Na]+ 675.4082 (Calcd. for C36H60O10Na, 675.4084)
maryloside B (2): m.p. 151.4–152.7 °C; [α + 12.3 (c 1.1, MeOH); FT-IR (film): 3356, 2922, 1697, 1090 cm−1; 1H-NMR (600 MHz, C5D5N): See Table 1; 13C-NMR (150 MHz, C5D5N): See Table 2; HR-FAB-MS m/z [M + Na]+ 853.4570 (calcd. for C42H70O16Na, 853.4561)
maryloside C (3): m.p. 135.5–136.7 °C; [α + 11.8 (c 1.7, MeOH); FT-IR (film): 3340, 1699, 1094 cm−1; 1H-NMR (600 MHz, C5D5N): See Table 1; 13C-NMR (150 MHz, C5D5N): See Table 2; HR-FAB-MS m/z [M − H]- 813.4664 (calcd. for C42H69O15, 813.4636)
maryloside D (4): m.p. 133.0–134.3 °C; [α + 10.9 (c 1.3, MeOH); FT-IR (film): 3393, 1686, 1086 cm−1; 1H-NMR (600 MHz, C5D5N): See Table 1; 13C-NMR (150 MHz, C5D5N): See Table 2; HR-FAB-MS m/z [M + Na]+ 837.4648 (calcd. for C42H70O15Na, 837.4613)
maryloside E (5): m.p. 123.6–125.2 °C; [α + 6.5 (c 2.2, MeOH). FT-IR (film) 3370, 1703, 1078, 1042 cm−1; 1H-NMR (600 MHz, C5D5N): See Table 1; 13C-NMR (150 MHz, C5D5N): See Table 2; HR-FAB-MS m/z [M − H]– 829.4549 (calcd. for C42H69O16, 829.4586)
maryloside F (6): m.p. 119.2–121.7 °C; [α + 19.4 (c 1.9, MeOH); FT-IR (film) 3150, 1744, 1711 cm−1; 1H-NMR (600 MHz, C5D5N): See Table 1; 13C-NMR (150 MHz, C5D5N): See Table 2; HR-FAB-MS m/z [M − H]- 753.4033 (calcd. for C39H61O14: 753.4062)
maryloside G (7): m.p. 219.2–220.8 °C; [α + 17.9 (c 0.8, MeOH); FT-IR (film): 3367, 1074, 1040 cm−1; 1H-NMR (600 MHz, C5D5N): See Table 3; 13C-NMR (150 MHz, C5D5N): See Table 3; HR-FAB-MS: m/z [M−H]- 961.5522 (calcd. for C48H81O19: 961.5524)
cymbidoside (8): [α + 18.9 (c 0.5, MeOH); 13C-NMR (150 MHz, C5D5N): 210.2 (C-3), 105.3 (C-1’), 78.5 (C-3’), 78.5 (C-5’), 77.6 (C-24), 75.4 (C-25), 75.4 (C-2’), 71.7 (C-4′), 69.5 (C-22), 66.2 (C-30), 65.6 (C-28), 62.8 (C-6’), 56.0 (C-4), 49.6 (C-17), 48.6 (C-14), 47.6 (C-8), 45.9 (C-13,) 43.7 (C-20), 41.2 (C-2), 41.1 (C-5), 35.8 (C-15), 34.2 (C-23), 33.0 (C-12), 32.1 (C-1), 28.9 (C-10), 27.7 (C-16), 27.2 (C-11), 26.9 (C-19), 26.11 (C-26), 26.07 (C-27), 25.8 (C-6), 25.4 (C-7), 24.8 (C-9), 19.6 (C-29), 18.3 (C-18) and 12.6 (C-21)
MALDI-TOF-MS m/z [M + Na]+ 691.4028 (calcd. for C36H60O11Na: 691.3033)
3.4. Determination of Nitric Oxide (NO) Production and MTT Cell Viability Assay
The concentration of nitrite in the medium was measured as indicator of NO production according to the Griess method [7]. Cells were cultured in F-12 HAM medium with 10% fetal bovine serum, 100 U/mL penicillin G and 0.1 mg/mL streptomycin. Cells were seeded on 96-well plate at 2.4 × 105 cell/well and incubated at 37 °C for 2 h. Then, medium was changed to one with LPS (final concentration: 100 ng/mL), IFN-γ (final concentration: 0.33 µg/mL) in each sample. After 24 hr of incubation at 37 °C, supernatants were collected, and NO concentrations were measured by using the Griess method. Aminoguanidine was used as positive control (final concentration: 100 µM). Cell viability was assessed by MTT assay [8]. After removal of supernatant for measurement of NO concentration, 10 µL of MTT solution (5 mg/mL in phosphate buffered saline) was added. MTT-formazan was measured by the following procedure. Data represent the mean value ± standard error of mean of experiments performed in triplicated.
3.5. X-ray Crystallographic Analysis
Single crystals of aglycone of maryloside A (1a), obtained from MeOH solution were selected and fitted onto a glass fiber and measured at −173 °C with a Bruker Apex II ultra-diffractometer using MoKα radiation. Data correction and reduction were performed with the crystallographic package Apex II. The structure was solved by direct methods using SHELXS-97 and refined by means of full matrix least-squares based on F2 using SHELXL-97 (Sheldrick, 1997). All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were positioned geometrically. A total of 325 parameters were finally considered. Final disagreement indices were R1 = 0.0495, wR2 = 0.1269 (I > 2 sigma(I)). The ORTEP plot was obtained by the program PLATON (A.L. Spek, 2009).
Crystal data: C30H50O5, MW = 490, Monoclinic, space group P2(1), Z = 2, a = 13.2141(15) Å, b = 7.1031(8)Å, c = 15.0890(17)Å, β = 102.2600(10)°, V = 1384.0(3)Å3, GOF = 1.031.
Single crystals of aglycone of 8 (8a), obtained from MeOH solution were selected and fitted onto a glass fiber and measured at −173 °C with an Apex II ultra-diffractometer (Bruker, MA, USA) using MoKα radiation by the same method as 1a. A total parameter was finally considered. Final disagreement indices were R1 = 0.0527, wR2 = 0.1358 (I > 2 sigma(I)).
Crystal data: C30H50O6, MW = 506, Monoclinic, space group P2(1), Z = 4, a = 11.498(2) Å, b = 7.3583(14)Å, c = 31.322(6)Å, β=93.294(3)°, V = 2645.6(9)Å3, GOF = 1.034
Crystallographic data (excluding structure factors) for the structures of 1a and 8a have been deposited with the Cambridge Crystallographic Data Centre as Supplementary publication numbers CCDC1901424 and CCDC1457199, respectively. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ UK (Fax: +44(0)-1223-336033 or email: deposit@ccdc.cam.ac.uk).
4. Conclusions
Seven novel 30-norcycloartane-type triterpene glycosides (1–7) along with one known compound (8) were isolated from the leaves of C. Great Flower ‘Marylaurencin’. Although their relative structures were estimated by using NOE correlations and J values, it was difficult to determined relative configuration from them regarding side chains of these compounds. The configuration at the C-20 position of these compounds was suggested as S* configuration according to biosynthetic pathway [9,10,11]. The configuration at C-20 position of 1a was suggested as S* configuration according to NOE correlations between H-12β-equatorial and H-21, and between H-18 and H-20 and the relative configuration of side chain of 1 was definitely determined as 20S*, 22R* and 24R* by X-ray crystallographic analysis of 1a, eventually. The relative structure of the side chain from C-20 to C-30 of 3 was fully the same as 1. The chemical shift values of 13C-NMR of 3 and 4 almost superimposable with those reported for 1. Therefore, the relative configuration of side chain of 3 and 4 was decided the same as 1. The same thing as 3 and 4 could be said for 2, 5 and 6 compared with 8. The relative configuration of 8 was determined as 20S*, 22R* and 24S* by the X-ray crystallographic analysis of aglycon of 8 (Figure 7 and Figure 8). The chemical shift values of 13C-NMR of 2, 5 and 6 almost superimposable with those of 8. Therefore, configuration of side chain of 2, 5 and 6 was decided as 20S*, 22R* and 24S*.
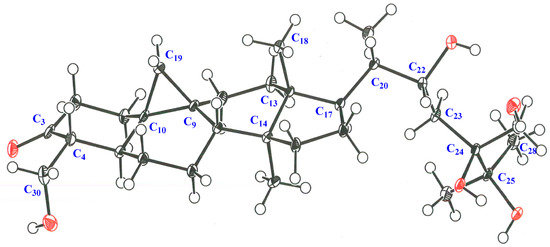
Figure 7.
ORTEP drawing for the X-ray crystal structure of 8a.
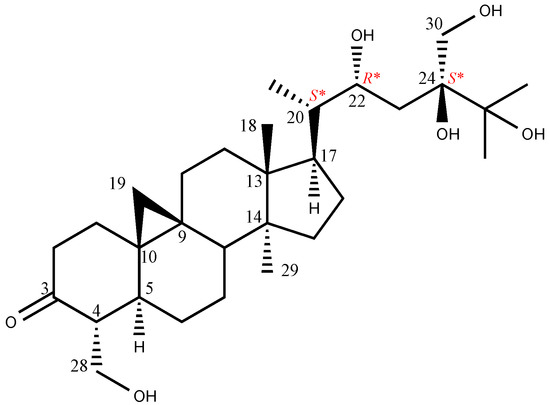
Figure 8.
Relative configuration of 8a.
In NO production inhibitory activity assay, 1 and 3 exhibited moderate activity whereas the result of the MTT cell viability assay revealed that all compounds hardly affect cell viability. To the best of our knowledge, this is the first report of the anti-inflammatory effect of norcycloartane glycosides while norcycloartanes are reported to show anti-bacterial activity against Micrococcus luteus and Bacillus subtilis or anti-human immunodeficiency virus activity [12,13]. Inflammation by over-production of NO was related to tissue toxicity, aging and occurrence of adenocarcinoma. Therefore, continuous drinking of tea of C. Great Flower ‘Marylaurencin’ may contribute to a human’s health life. Taking into consideration of all other bioactivities those we already reported about C. Great Flower ‘Marylaurencin’, it is worth to study C. Great Flower ‘Marylaurencin’ further.
Supplementary Materials
Crystallographic data (excluding structure factors) for the structures of 1a and 8a have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication numbers CCDC1901424 and CCDC1457199, respectively. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ UK (Fax: +44(0)-1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).
Author Contributions
Conceptualization, T.H. and A.U.; validation, H.I., M.B., Y.K., T.Y. (Tadahiro Yahagi) and K.M.; formal analysis, T.Y. (Tatsuro Yoneyama) and K.I.; investigation, T.Y. (Tatsuro Yoneyama) and M.N.; resources, S.K.; data curation, K.I.; writing—original draft preparation, A.U.; writing—review and editing, A.U.; visualization, T.Y. (Tatsuro Yoneyama); supervision, A.U.; project administration, T.Y. (Tatsuro Yoneyama); funding acquisition, A.U.
Funding
This work was supported in part by a grant-in-aid from Tokushima Kawano Orchid Promotion Foundation (Tokushima Prefecture, Japan) where one of the authors (S.K.) belongs.
Acknowledgments
We are indebted to M. Tanaka for measurements of the 600 MHz NMR spectra and Y. Okamoto for measurements of mass spectra, Faculty of Pharmaceutical Sciences of Tokushima Bunri University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yoshikawa, K.; Ito, T.; Iseki, K.; Baba, C.; Imagawa, H.; Yagi, Y.; Morita, H.; Asakawa, Y.; Kawano, S.; Hashimoto, T. Phenanthrene Derivatives from Cymbidium Great Flower Marie Laurencin and Their Biological Activities. J. Nat. Prod. 2012, 75, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Otsu, M.; Ito, T.; Asakawa, Y.; Kawano, S.; Hashimoto, T. Aromatic constituents of Cymbidium Great Flower Marie Laurencin and their antioxidative activity. J. Nat. Med. 2013, 67, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Baba, C.; Iseki, K.; Ito, T.; Asakawa, Y.; Kawano, S.; Hashimoto, T. Phenanthrene and phenylpropanoid constituents from the roots of Cymbidium Great Flower ‘Marylaurencin’ and their antimicrobial activity. J. Nat. Med. 2014, 68, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Okahuji, M.; Iseki, K.; Ito, T.; Asakawa, Y.; Kawano, S.; Hashimoto, T. Two novel aromatic glucosides, marylaurencinosides D and E, from the fresh flowers of Cymbidium Great Flower ‘Marylaurencin’. J. Nat. Med. 2014, 68, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Dahmén, J.; Leander, K. A new triterpene glucoside from Cymbidium giganteum. Phytochemistry 1978, 17, 1978. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, T.; Yakura, N.; Matsuzaki, K.; Kitanaka, S. Inhibitory effect of chemical constituents from Artemisia scoparia Waldst. et Kit. on triglyceride accumulation in 3T3-L1 cells and nitric oxide production in RAW 264.7 cells. J. Nat. Med. 2014, 68, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.-K.; Min, H.-Y.; Han, A.-R.; Seo, E.-K.; Lee, S.K. Suppression of lipopolysaccharide-induced expression of inducible nitric oxide synthase by brazilin in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2005, 513, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Virgil, S.C. An Experimental Demonstration of the Stereochemistry of Enzymic Cyclization of 2,3-Oxidosqualene to the Protosterol System, Forerunner of Lanosterol and Cholesterol. J. Am. Chem. Soc. 1991, 113, 4025–4026. [Google Scholar] [CrossRef]
- Corey, E.J.; Virgil, S.C.; Sarshar, S. New Mechanistic and Stereochemical Insights on the Biosynthesis of Sterols from 2,3-Oxidosqualene. J. Am. Chem. Soc. 1991, 113, 8171–8172. [Google Scholar] [CrossRef]
- Corey, E.J.; Virgil, S.C.; David, R.L.; Sarshar, S. The Methyl Group at C (10) of 2,3-Oxidosqualene Is Crucial to the Correct Folding of This Substrate in the Cyclization-Rearrangement Step of Sterol Biosynthesis. J. Am. Chem. Soc. 1992, 114, 1524–1525. [Google Scholar] [CrossRef]
- Wang, H.; Ning, R.; Shen, Y.; Chen, Z.; Li, J.; Zhang, R.; Leng, Y.; Zhao, W. Lithocarpic Acids A-N, 3, 4-seco-Cycloartane Derivatives from the Cupules of Lithocarpus polystachyus. J. Nat. Prod. 2014, 77, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-T.; Han, Q.-B.; Zheng, Y.-T.; Wang, R.-R.; Yang, L.-M.; Lu, Y.; Sang, S.-Q.; Zheng, Q.-T.; Zhao, Q.S.; Sun, H.-D. Structure and anti-HIV activity of micrandilactones B and C, new nortriterpenoids possessing a unique skeleton from Schisandra micrantha. Chem. Commun. 2005, 23, 2936–2938. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).