Anti-Multiple Myeloma Potential of Secondary Metabolites from Hibiscus sabdariffa
Abstract
1. Introduction
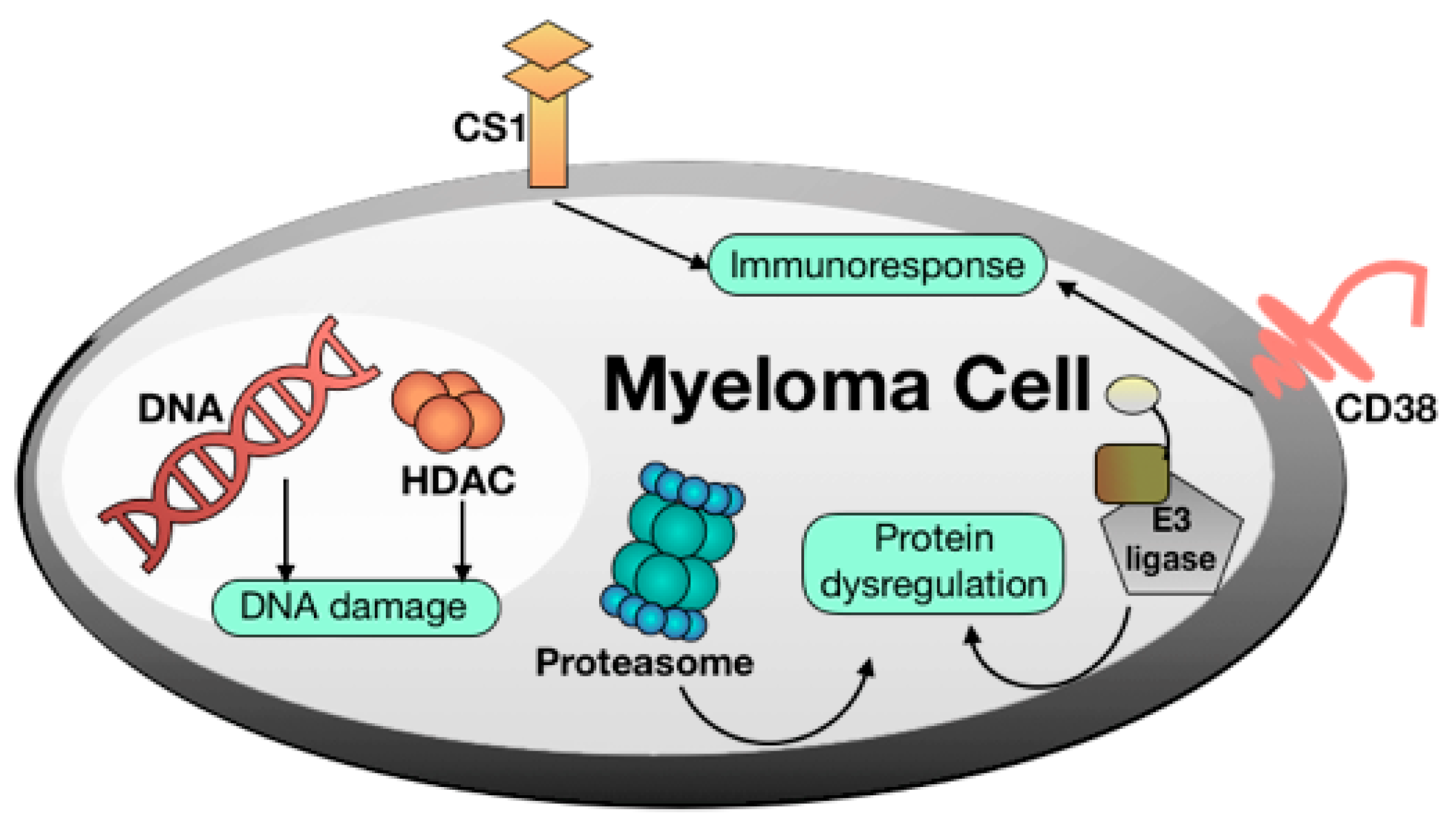
- DNA damaging agents. Alkylating agents such as melphalan and other DNA damaging agents such as doxorubicin as well as pan-HDAC inhibitors such as panobinostat are still important MM drugs [7].
- Proteasome inhibitors [8]. The proteasome is a multicatalytic target responsible for the degradation of 80–90% of proteins during cell life. Until now, three proteasome inhibitors have been approved for clinical use: Bortezomib, Carfilzomib, and Ixazomib. Particularly, Bortezomib was the first FDA-approved proteasome inhibitor and represents one of the most important discoveries for fighting MM of recent years. Of note, these agents can stimulate bone formation in MM patients [9]. Proteasome modulation can be also achieved by employing small molecules such as lenalidomide, structurally related to thalidomide and acting as E3-Ligasi inhibitors [10].
- Immunomodulators [11]. MM was among the first tumors wherein the therapeutic efficacy of blockade of inhibitory immune receptors, particularly the PD-1 axis, was demonstrated in preclinical models.
- Monoclonal antibodies [12]. There are only two FDA-approved monoclonal antibodies for the treatment of MM: daratumumab, targeting the CD38 pathway, and elotuzumab, targeting the SLAMF7 pathway. Despite the efficacy of this strategy in long term cancer remission, monoclonal antibodies still remain very expensive, thus limiting their diffusion.
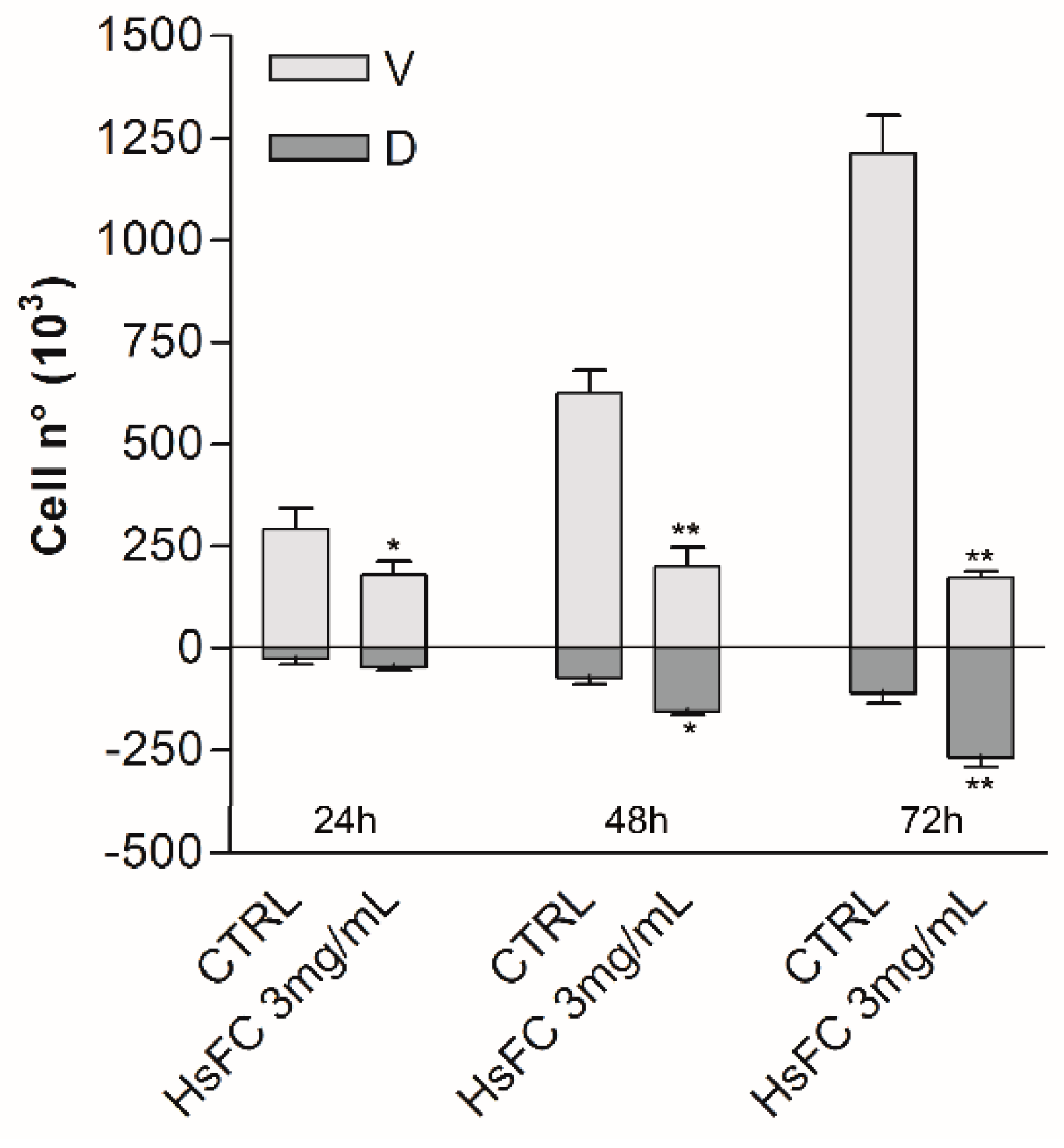
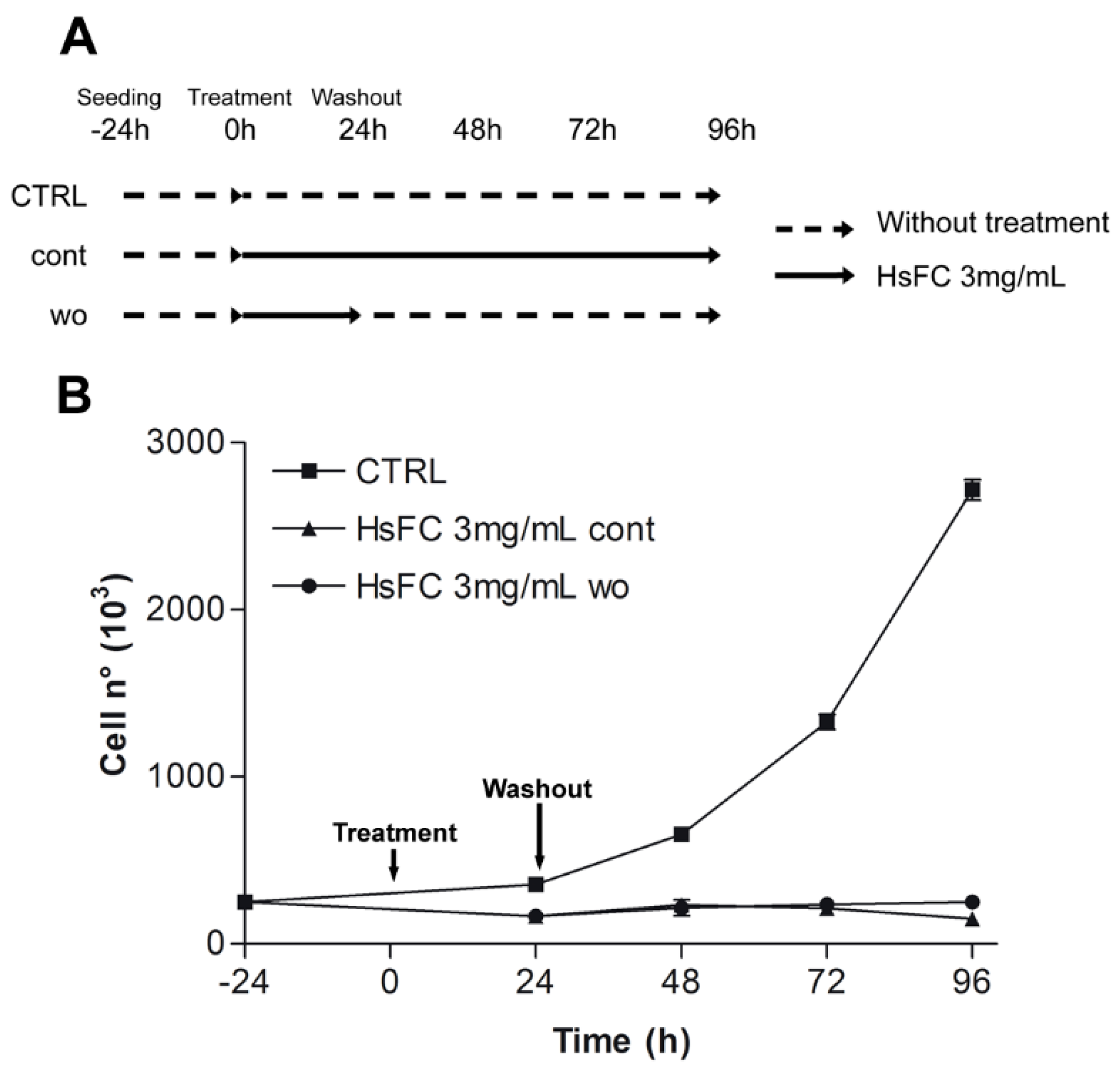
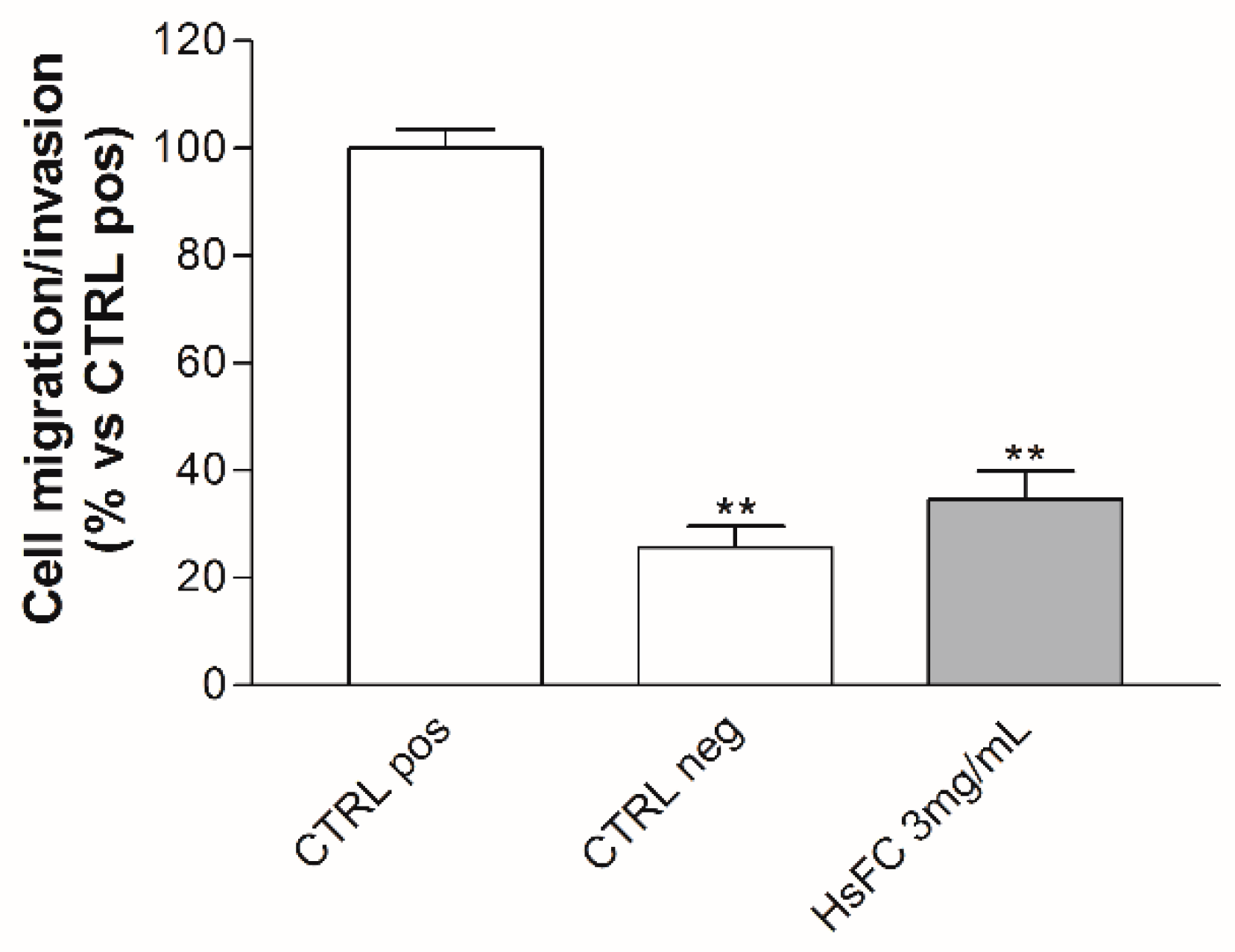
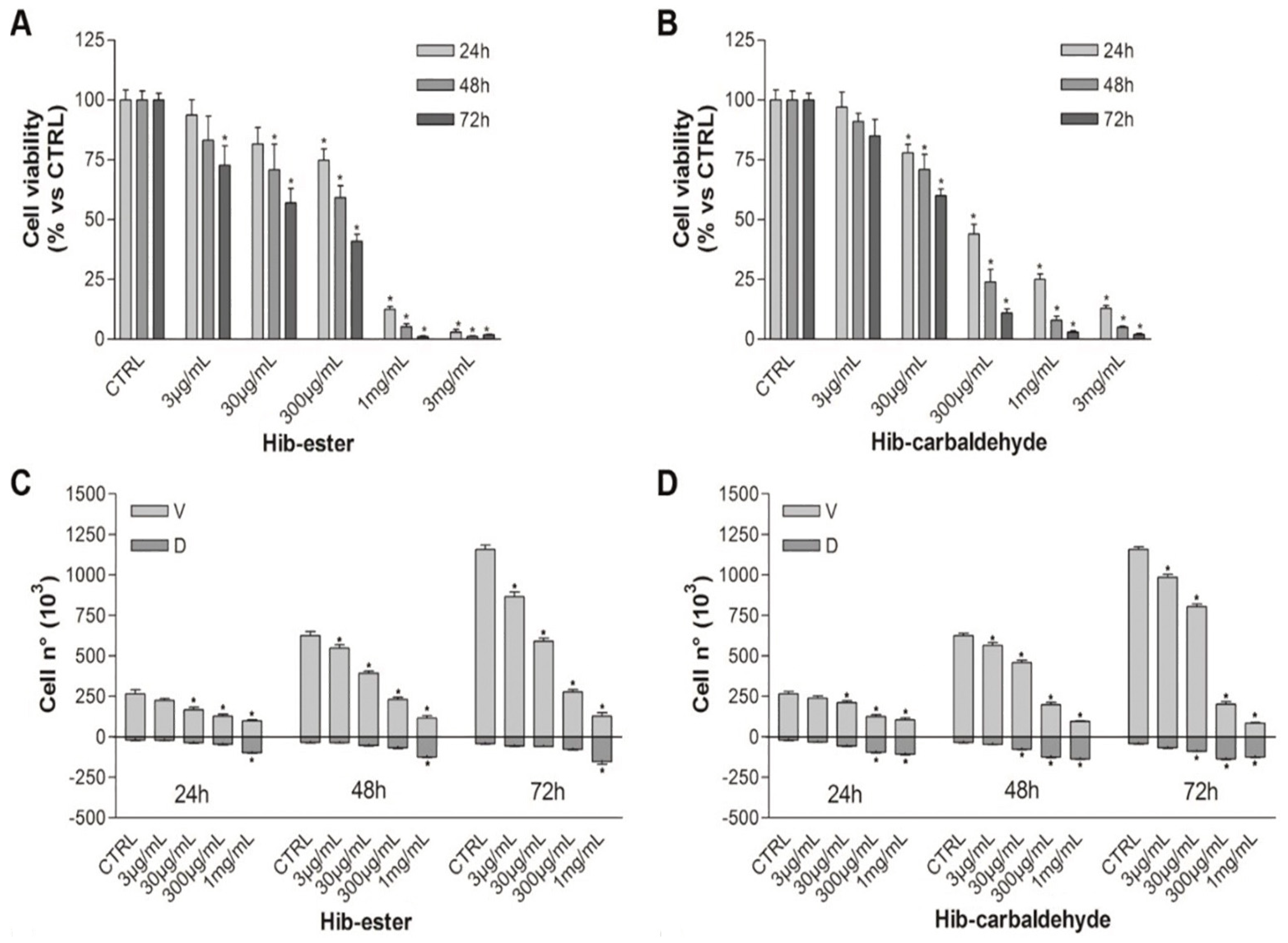
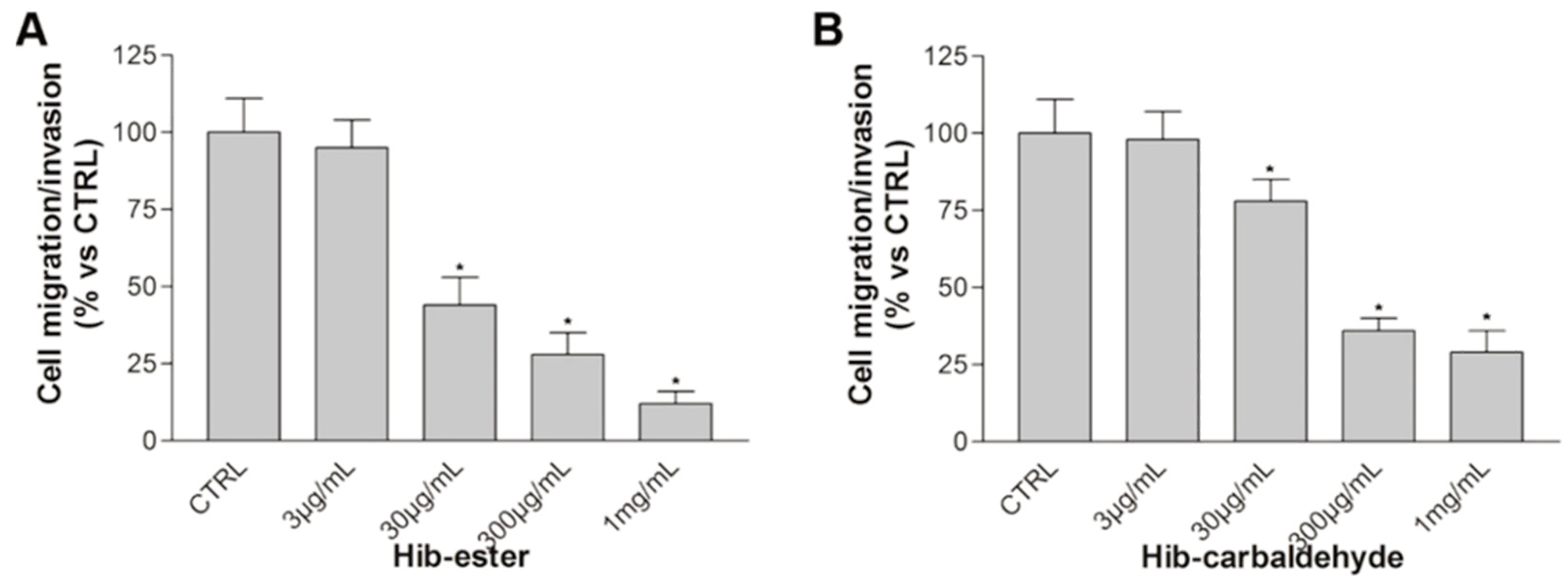
2. Results and discussion
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Standards
4.2. Instruments
4.3. Plant Material and Extraction Procedure
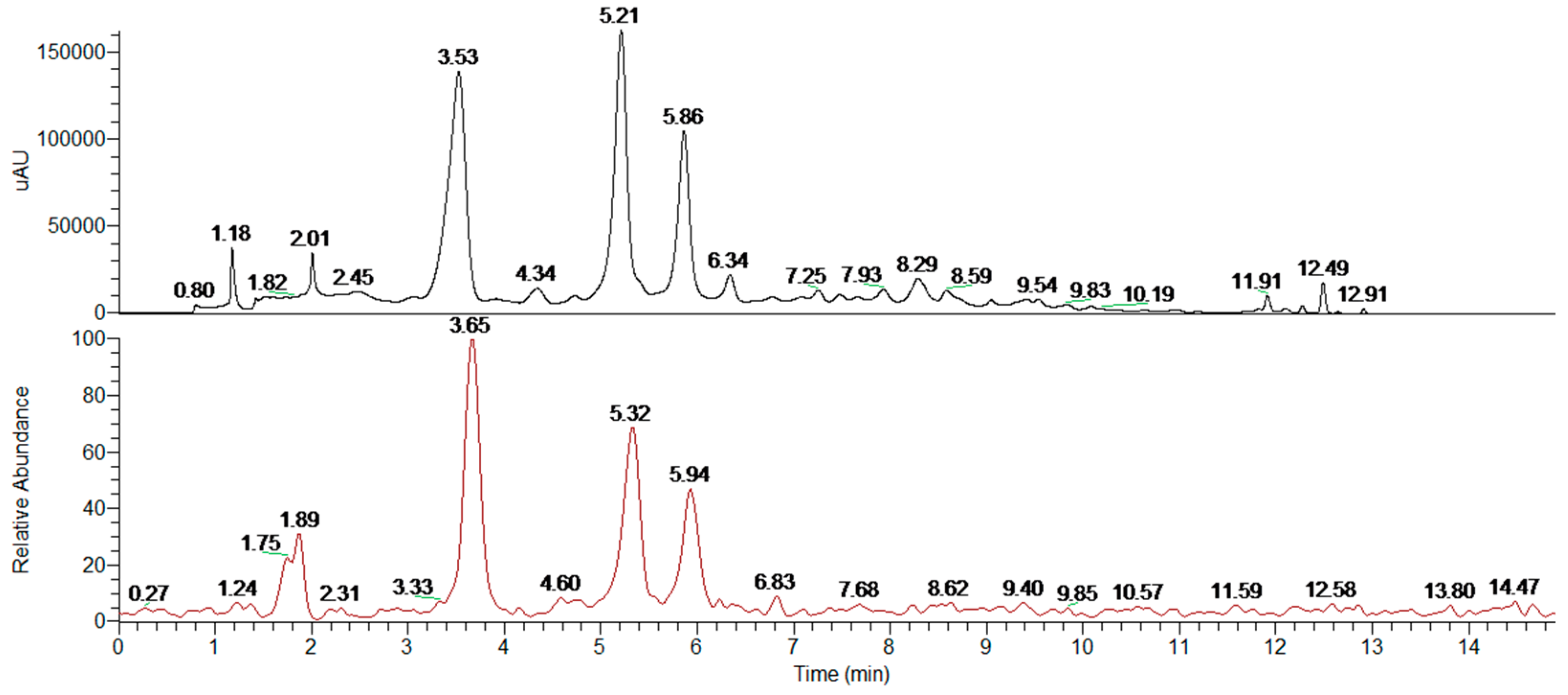
4.4. Extract Fractionation and Analysis
4.5. Cell Culture
4.6. Biological Assays
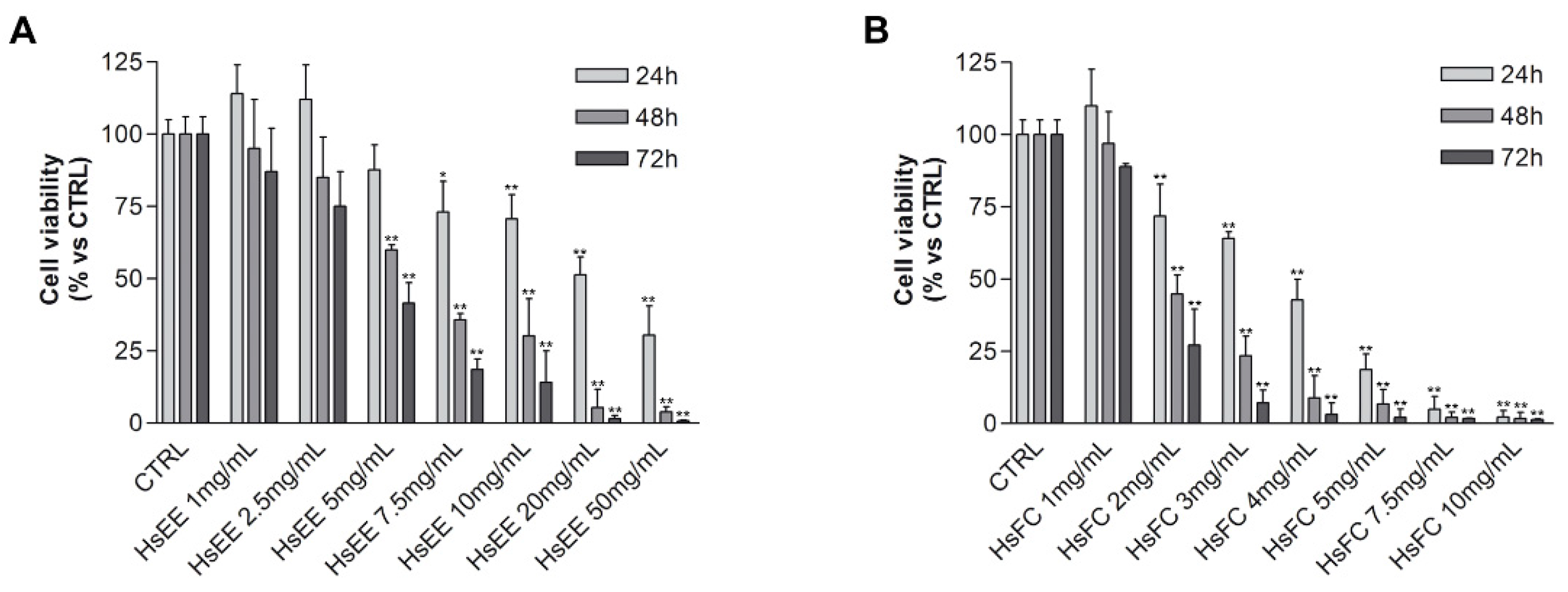
4.6.1. MTT Assay
4.6.2. Trypan Blue Vital Count
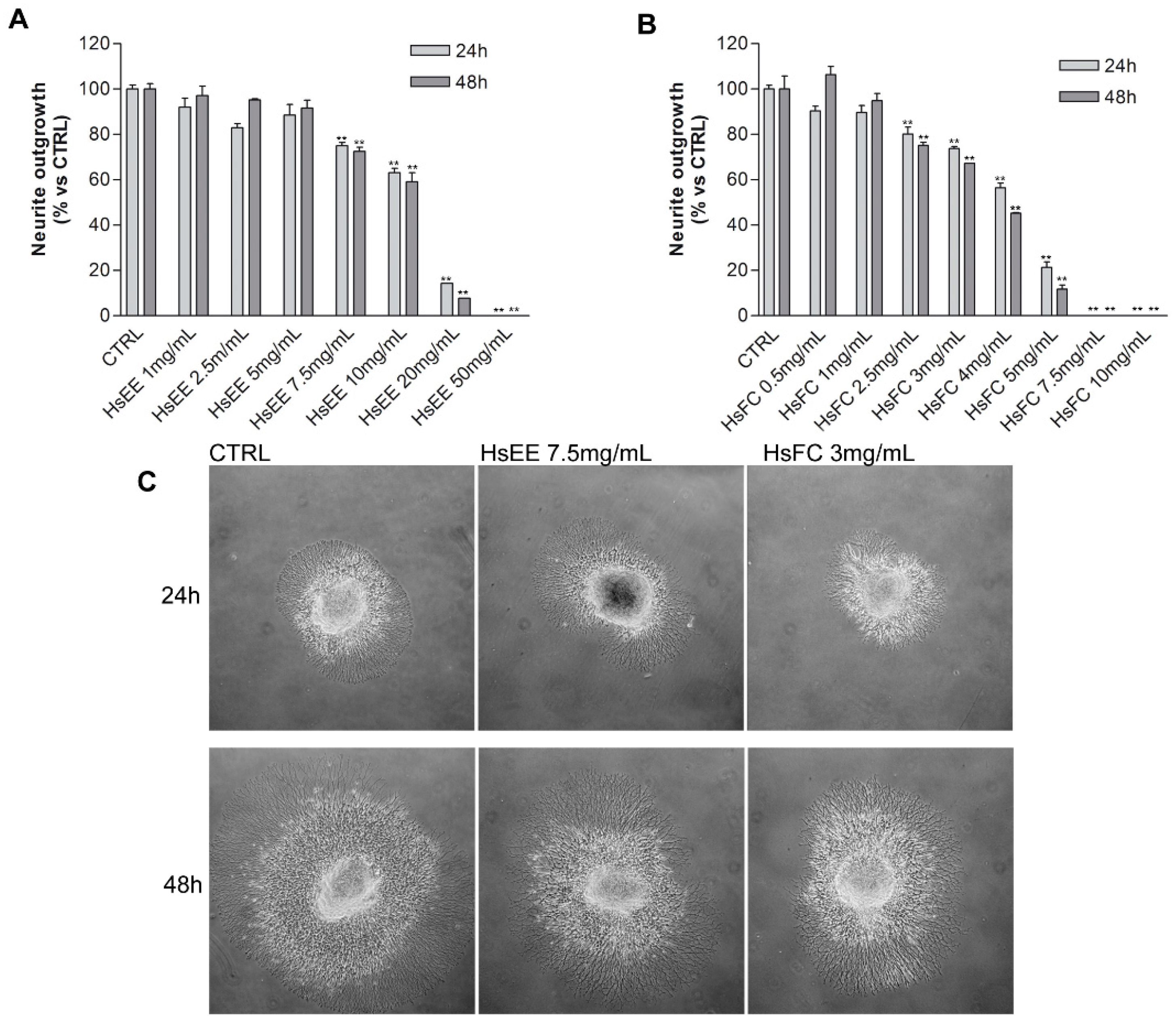
4.6.3. Boyden Chamber Assay
4.6.4. DRG Neurotoxicity Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergsagel, P.L.; Kuehl, W.M. Chromosome translocations in multiple myeloma. Oncogene 2001, 20, 5611–5622. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple Myeloma: 2016 update on Diagnosis, Risk-stratification and Management. Am. J. Hematol. 2016, 91, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, D. Multiple myeloma epidemiology and survival, a unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Moschetta, M.; Sacco, A.; Belotti, A.; Ribolla, R.; Chiarini, M.; Giustini, V.; Bertoli, D.; Sottini, A.; Valotti, M.; Ghidini, C.; et al. Bone Marrow Stroma and Vascular Contributions to Myeloma Bone Homing. Curr. Osteoporos. Rep. 2017, 15, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Shupp, A.B.; Kolb, A.D.; Mukhopadhyay, D.; Bussard, K.M. Cancer Metastases to Bone: Concepts, Mechanisms, and Interactions with Bone Osteoblasts. Cancers 2018, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Klausen, U.; Holmberg, S.; Holmström, M.O.; Jørgensen, N.G.D.; Grauslund, J.H.; Svane, I.M.; Andersen, M.H. Novel Strategies for Peptide-Based Vaccines in Hematological Malignancies. Front. Immunol. 2018, 9, 2264. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, A.A.; González-Méndez, L.; San-Segundo, L.; Herrero, A.B.; Hernández-García, S.; Martín-Sánchez, M.; Gutiérrez, N.C.; Paíno, T.; Avilés, P.; Mateos, M.V.; et al. Synergistic DNA-damaging effect in multiple myeloma with the combination of zalypsis, bortezomib and dexamethasone. Haematologica 2017, 102, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Richardson, P.G.; Cavo, M.; Orlowski, R.Z.; Miguel, J.F.S.; Palumbo, A.; Harousseau, J.-L. Proteasome inhibitors in multiple myeloma: 10 years later. Blood 2012, 120, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, A.; Li, X.; Ling, W.; Khan, S.; Zangari, M.; Yaccoby, S. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am. J. Hematol. 2009, 84, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J. Expression of E3 Ubiquitin Ligases in Multiple Myeloma Patients after Treatment with the Proteasome Inhibitor Bortezomib. Cancer Transl. Med. 2015, 1, 153. [Google Scholar] [CrossRef]
- Costa, F.; Das, R.; Bailur, J.K.; Dhodapkar, K.; Dhodapkar, M.V. Checkpoint Inhibition in Myeloma: Opportunities and Challenges. Front. Immunol. 2018, 9, 2204. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T. Therapeutic antibodies for multiple myeloma. Jpn. J. Clin. Oncol. 2018, 48, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug resistance in multiple myeloma. Cancer Treat. Rev. 2018, 70, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Gay, F.; Palumbo, A. Multiple myeloma: Management of adverse events. Med. Oncol. 2010, 27, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Meregalli, C. An Overview of Bortezomib-Induced Neurotoxicity. Toxics 2015, 3, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Tacchetti, P.; Terragna, C.; Galli, M.; Zamagni, E.; Petrucci, M.T.; Pezzi, A.; Montefusco, V.; Martello, M.; Tosi, P.; Baldini, L.; et al. Bortezomib- and thalidomide-induced peripheral neuropathy in multiple myeloma: Clinical and molecular analyses of a phase 3 study. Am. J. Hematol. 2014, 89, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Cavaletti, G.; Bruna, J.; Kyritsis, A.P.; Kalofonos, H.P. Bortezomib-induced peripheral neurotoxicity: An update. Arch. Toxicol. 2014, 88, 1669–1679. [Google Scholar] [CrossRef]
- Meregalli, C.; Chiorazzi, A.; Carozzi, V.A.; Canta, A.; Sala, B.; Colombo, M.; Oggioni, N.; Ceresa, C.; Foudah, D.; La Russa, F.; et al. Evaluation of tubulin polymerization and chronic inhibition of proteasome as citotoxicity mechanisms in bortezomib-induced peripheral neuropathy. Cell Cycle 2014, 13, 612–621. [Google Scholar] [CrossRef]
- Cavaletti, G.; Jakubowiak, A.J. Peripheral neuropathy during bortezomib treatment of multiple myeloma: a review of recent studies. Leuk. Lymphoma 2010, 51, 1178–1187. [Google Scholar] [CrossRef]
- Malacrida, A.; Maggioni, D.; Cassetti, A.; Nicolini, G.; Cavaletti, G.; Miloso, M. Antitumoral Effect of Hibiscus sabdariffa on Human Squamous Cell Carcinoma and Multiple Myeloma Cells. Nutr. Cancer 2016, 68, 1–10. [Google Scholar] [CrossRef]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Fernández, J.; Pineda, M.; Aguilar, M. Roselle (Hibiscus sabdariffa) Seed Oil Is a Rich Source of? Tocopherol. J. Food Sci. 2007, 72, S207–S211. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, N.; Shivali; Kamboj, P. Hibiscus sabdariffa Linn.-An overview. Nat. Prod. Radiance 2009, 8, 77–83. [Google Scholar]
- Tahir, H.E.; Xiaobo, Z.; Jiyong, S.; Mariod, A.A.; Wiliam, T. Rapid Determination of Antioxidant Compounds and Antioxidant Activity of Sudanese Karkade (Hibiscus sabdariffa L.) Using Near Infrared Spectroscopy. Food Anal. Methods 2016, 9, 1228–1236. [Google Scholar] [CrossRef]
- Duh, P.-D.; Yen, G.-C. Antioxidative activity of three herbal water extracts. Food Chem. 1997, 60, 639–645. [Google Scholar] [CrossRef]
- Hajifaraji, M.; Tarkhani, A.H. The effect of sour tea (Hibiscus sabdariffa) on essential hypertension. J. Ethnopharmacol. 1999, 65, 231–236. [Google Scholar] [CrossRef]
- Peng, C.-H.; Yang, Y.-S.; Chan, K.-C.; Wang, C.-J.; Chen, M.-L.; Huang, C.-N. Hibiscus sabdariffa Polyphenols Alleviate Insulin Resistance and Renal Epithelial to Mesenchymal Transition: A Novel Action Mechanism Mediated by Type 4 Dipeptidyl Peptidase. J. Agric. Food Chem. 2014, 62, 9736–9743. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Berchová, K.; Majerová, M.; Pokorná, M.; Švajdlenka, E. In vitro synergistic effect of Hibiscus sabdariffa aqueous extract in combination with standard antibiotics against Helicobacter pylori clinical isolates. Pharm. Biol. 2016, 54, 1736–1740. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hsu, J.-D.; Wang, S.-F.; Chiang, H.-C.; Yang, M.-Y.; Kao, E.-S.; Ho, Y.-C.; Wang, C.-J. Hibiscus sabdariffaExtract Inhibits the Development of Atherosclerosis in Cholesterol-Fed Rabbits. J. Agric. Food Chem. 2003, 51, 5472–5477. [Google Scholar] [CrossRef]
- Khaghani, S.; Razi, F.; Yajloo, M.M.; Paknejad, M.; Shariftabrizi, A.; Pasalar, P. Selective Cytotoxicity and Apoptogenic Activity of Hibiscus Sabdariffa Aqueous Extract Against MCF-7 Human Breast Cancer Cell Line. J. Cancer Ther. 2011, 2, 394–400. [Google Scholar] [CrossRef]
- Akim, A.M.; Ling, L.C.; Rahmat, A. Antioxidant and anti-proliferative activities of Roselle juice on Caov-3, MCF-7, MDA-MB-231 and HeLa cancer cell lines. African J. Pharm. Pharmacol. 2011, 5, 957–965. [Google Scholar]
- Olvera-García, V.; Castaño-Tostado, E.; Rezendiz-Lopez, R.I.; Reynoso-Camacho, R.; González de Mejía, E.; Elizondo, G.; Loarca-Piña, G. Hibiscus sabdariffa L. extracts inhibit the mutagenicity in microsuspension assay and the proliferation of HeLa cells. J. Food Sci. 2008, 73, T75–T81. [Google Scholar] [CrossRef] [PubMed]
- Adanlawo, I.; Ajibade, V. Nutritive Value of the Two Varieties of Roselle (Hibiscus Sabdariffa) Calyces and Soaked with Wood Ash. Pak. J. Nutr. 2007, 1, 38–46. [Google Scholar]
- Chang, Y.-C.; Huang, H.-P.; Hsu, J.-D.; Yang, S.-F.; Wang, C.-J. Hibiscus anthocyanins rich extract-induced apoptotic cell death in human promyelocytic leukemia cells. Toxicol. Appl. Pharmacol. 2004, 205, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Amri, B.; Martino, E.; Vitulo, F.; Corana, F.; Ben-Kaâb, L.B.; Rui, M.; Rossi, D.; Mori, M.; Rossi, S.; Collina, S. Marrubium vulgare L. leave extract: Phytochemical composition, antioxidant and wound healing properties. Molecules 2017, 22, 1851. [Google Scholar] [CrossRef]
- Martino, E.; Collina, S.; Rossi, D.; Bazzoni, D.; Gaggeri, R.; Bracco, F.; Azzolina, O. Influence of the extraction mode on the yield of hyperoside, vitexin and vitexin-2-O-rhamnoside from Crataegus monogyna Jacq. (Hawthorn). Phytochem. Anal. 2008, 19, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Ramaiola, I.; Urbano, M.; Bracco, F.; Collina, S. Microwave-assisted extraction of coumarin and related compounds from Melilotus officinalis (L.) Pallas an alternative to Soxhlet and ultrasound-assisted extraction. J. Chromatogr. A 2006, 1125, 147–151. [Google Scholar] [CrossRef]
- Rossi, D.; Ahmed, K.M.; Gaggeri, R.; Volpe, S.D.; Maggi, L.; Mazzeo, G.; Longhi, G.; Abbate, S.; Corana, F.; Martino, E.; et al. (R)-(-)-Aloesaponol III 8-methyl ether from Eremurus persicus: A novel compound against leishmaniosis. Molecules 2017, 22, 519. [Google Scholar] [CrossRef]
- Martino, E.; Della Volpe, S.; Cavalloro, V.; Amri, B.; Kaab, L.B.B.; Marrubini, G.; Rossi, D.; Collina, S. The use of a microwave-assisted solvent extraction coupled with HPLC-UV/PAD to assess the quality of Marrubium vulgare L. (white horehound) herbal raw material. Phytochem. Anal. 2019, 30, 377–384. [Google Scholar] [CrossRef]
- Scuteri, A.; Nicolini, G.; Miloso, M.; Bossi, M.; Cavaletti, G.; Windebank, A.J.; Tredici, G. Paclitaxel toxicity in post-mitotic dorsal root ganglion (DRG) cells. Anticancer. Res. 2006, 26, 1065–1070. [Google Scholar]
- Polavarapu, P.L.; Donahue, E.A.; Shanmugam, G.; Scalmani, G.; Hawkins, E.K.; Rizzo, C.; Ibnusaud, I.; Thomas, G.; Habel, D.; Sebastian, D. A Single Chiroptical Spectroscopic Method May Not Be Able To Establish the Absolute Configurations of Diastereomers: Dimethylesters of Hibiscus and Garcinia Acids. J. Phys. Chem. A 2011, 115, 5665–5673. [Google Scholar] [CrossRef] [PubMed]
- Almahy, H.A.; Abdel-Razik, H.H.; El-Badry, Y.A.; Ibrahim, E.M. Ultrasonic extraction of anthocyanin’s natural dyes from Hibiscus sabdariffa (Karkade) and its application on dying foodstuff and beverages in kingdom of Saudi Arabia. Am. J. Biol. Pharm. Res. 2015, 2, 168–174. [Google Scholar]
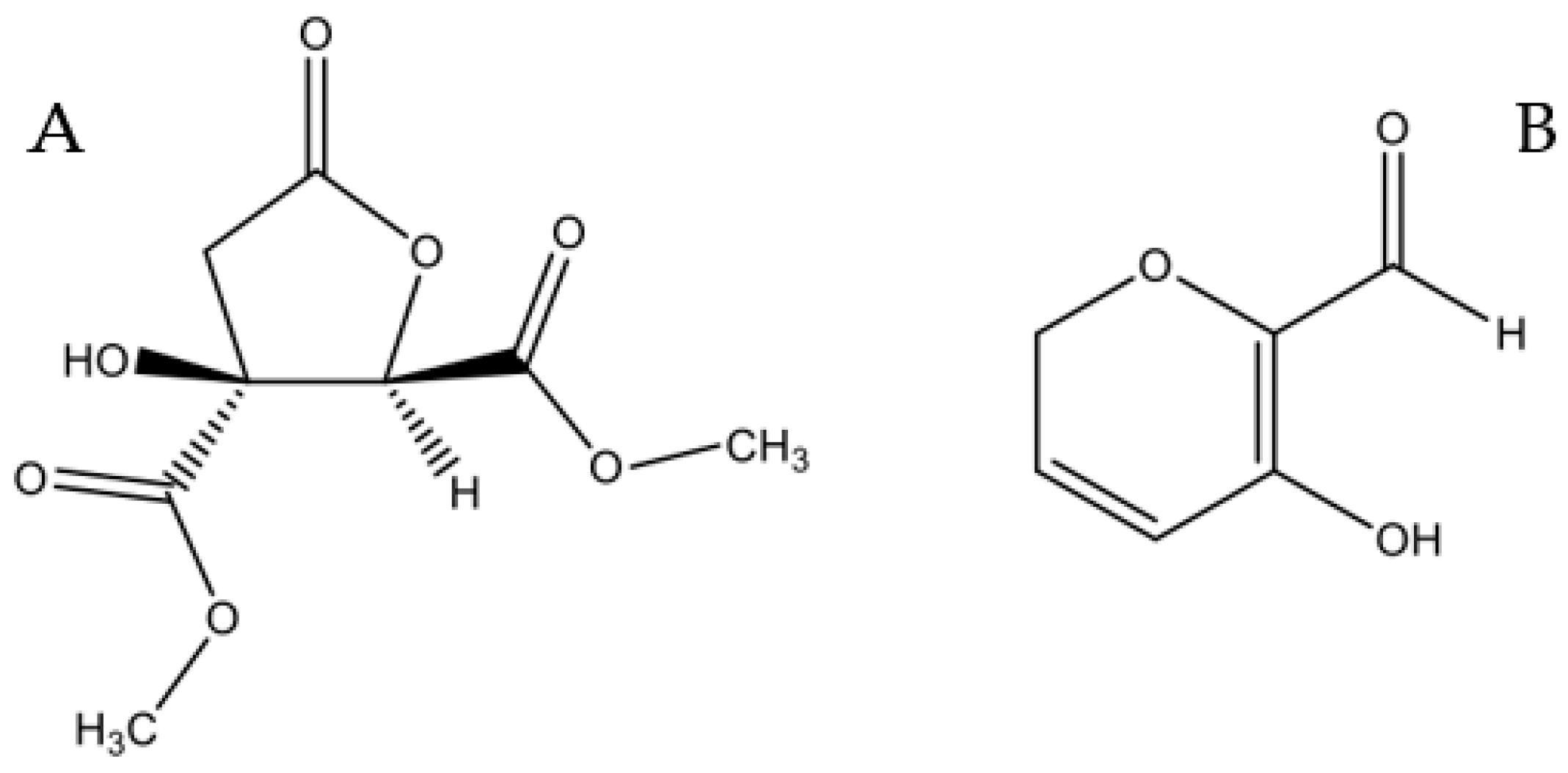
Samples of the compounds Hib-ester and Hib-carbaldeyde are available from the authors. |
| Drug | Trade Name | Target |
|---|---|---|
| Melphalan | Alkeran® | DNA |
| Bendamustine | Levact®, Ribomustin®, Treanda® | DNA |
| Doxorubicin | Adriamycin® | DNA |
| Carmustine | BiCNU® | DNA |
| Cyclophosphamide | Cytoxan®, Neosar® | DNA |
| Panobinostat | Faridak® | HDAC |
| Elotuzumab | Empliciti® | CS1 |
| Daratumumab | Darzalex® | CD38 |
| Bortezomib | Velcade® | Proteasome |
| Ixazomib | Ninlaro® | Proteasome |
| Carfilzomib | Kyprolis® | Proteasome |
| Thalidomide | Thalidomide Celgene® | E3 ligase |
| Lenalidomide | Revlimid® | E3 ligase |
| Pomalidomide | Imnovid® | E3 ligase |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malacrida, A.; Cavalloro, V.; Martino, E.; Cassetti, A.; Nicolini, G.; Rigolio, R.; Cavaletti, G.; Mannucci, B.; Vasile, F.; Giacomo, M.D.; et al. Anti-Multiple Myeloma Potential of Secondary Metabolites from Hibiscus sabdariffa. Molecules 2019, 24, 2500. https://doi.org/10.3390/molecules24132500
Malacrida A, Cavalloro V, Martino E, Cassetti A, Nicolini G, Rigolio R, Cavaletti G, Mannucci B, Vasile F, Giacomo MD, et al. Anti-Multiple Myeloma Potential of Secondary Metabolites from Hibiscus sabdariffa. Molecules. 2019; 24(13):2500. https://doi.org/10.3390/molecules24132500
Chicago/Turabian StyleMalacrida, Alessio, Valeria Cavalloro, Emanuela Martino, Arianna Cassetti, Gabriella Nicolini, Roberta Rigolio, Guido Cavaletti, Barbara Mannucci, Francesca Vasile, Marcello Di Giacomo, and et al. 2019. "Anti-Multiple Myeloma Potential of Secondary Metabolites from Hibiscus sabdariffa" Molecules 24, no. 13: 2500. https://doi.org/10.3390/molecules24132500
APA StyleMalacrida, A., Cavalloro, V., Martino, E., Cassetti, A., Nicolini, G., Rigolio, R., Cavaletti, G., Mannucci, B., Vasile, F., Giacomo, M. D., Collina, S., & Miloso, M. (2019). Anti-Multiple Myeloma Potential of Secondary Metabolites from Hibiscus sabdariffa. Molecules, 24(13), 2500. https://doi.org/10.3390/molecules24132500










