Abstract
The reactions of 3-isoselenocyanato-2,2,5,5-tetramethylpyrrolidine-1-oxyl, 3-isoselenocyanatomethyl-2,2,5,5-tetramethyl-3-pyrrolidine-1-oxyl, and 4-isoselenocyanato-2,2,6,6-tetramethylpiperidine-1-oxyl with selected amines and alcohols give the corresponding novel nitroxyl selenoureas and selenocarbamates, all bearing a nitroxyl moiety. Synthesized selenoureas and selenocarbamates show significant activity against pathogenic fungi and bacteria. In contrast to piperidine nitroxides, pyrrolidine, five-membered nitroxyl selenoureas and selenocarbamates show excellent antifungal and antibacterial activity against pathogenic fungi and bacteria, respectively.
1. Introduction
In our previous paper [1], we synthesized nitroxyl radicals containing a tellurium atom and evaluated their antifungal activity. As a part of our continuing interest in the synthesis and evaluation of the biological activity of the compounds containing chalcogen atoms, the activity of organoselenium compounds bearing nitroxyl moieties is discussed in the present paper.
The role of organoselenium compounds in organic synthesis, their presence in living organisms, and application as bioactive compounds, have been recently discussed [2]. The biological activity of synthetic organoselenium compounds was reviewed [3,4,5,6,7,8,9].
Selenoureas showed free-radical scavenging [10,11,12], and enzyme inhibition [10,11,13,14,15,16] potential. They demonstrated anticancer [10,11,17], DNA binding [17,18,19,20], antioxidant [17,18,21,22,23], antibacterial [18], antifungal [18,24,25,26], and herbicidal [25] properties.
Selenourea derivatives were synthesized from the following starting compounds—sources of selenium: isoselenocyanates and primary or secondary amines [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] selenoamides and nitrile oxides (generated in situ) [45], hydrogen selenide [46,47,48], lithium aluminum hydride (LiAlH2Se) [12,32,36,49,50,51,52,53], bis(dimethylaluminum) selenide [54], tetraethylammonium tetraselenotungstate [14], elemental selenium with an isonitrile and an amine [55], elemental selenium with an amine and triethylorthoformate [56], and elemental selenium with a secondary amine, a base, and dihalomethan derivatives [57,58].
Selenoureas served as starting materials to the synthesis of further organoselenium derivatives [59]. Selenocarbamates showed antiproliferative [60], cytotoxic [61], antioxidant [22], pesticidal [24,26], and effective superoxide anion scavenger [62] activities. Selenocarbamates were obtained by addition reaction of isoselenocyanate with alcohols [54,63].
Methyl and ethyl nitroxyl selenocarbamates were obtained from 4-isoselenocyanato-2,2,6,6-tetramethylpiperidine-1-oxyl, by the reaction with either sodium methoxide or ethoxide, in methanol or ethanol, respectively [26]. The biological action of nitroxyl radicals was well documented [64,65,66,67]. Nitroxides showed antioxidant properties by scavenging free radical reactive oxygen species (ROS) and, in consequence, protecting cells against oxidative stress [64,65,66,67,68,69,70]. Antioxidant and antitumor activity of amides obtained from exactly the same nitroxyl amines (PROXYL-NH2, PROXYL-CH2NH2, and TEMPO-NH2) as used in this work have been recently described [71].
Nitroxides acted as the enzyme superoxide dismutase mimics, converting superoxide anion to oxygen and hydrogen peroxide using redox reactions involving a nitroxide, a corresponding hydroxylamine and an oxoammonium salt [65]. It was, however, stated that nitroxides were less active than superoxide dismutase itself [72].
Radioprotective effects of nitroxides in the presence of iron ions were detected [73]. Radioprotective effects in vivo of 4-hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxyl (TEMPOL) were studied in mice [74]. Ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one) derivatives modified with a nitroxyl radical fragment (see below) showed higher activity as glutathione peroxidase mimic than ebselene itself [75]. Antihypertensive effects observed for nitroxides (mainly TEMPOL) were reviewed [76]. The inhibitory effect of selected dinitroxides and polynitroxides on the growth of some species of bacteria, yeasts and fungi was described [77].
The role of nitroxides in cancer therapy connected with their antioxidant properties was reviewed [66,78]. Free nitroxyl radicals bearing an adamantyl moiety exhibited also the biological activity. Adamantyl derivatives of nitroxyl radicals showed antiparkinsonian activity [79]. In one of our previous work [26] fungicidal activities of six-membered nitroxyl selenoureas and selenocarbamates were presented. Herein, we would like to present the synthesis and pesticidal properties of five- and six-membered nitroxyl selenoureas and selenocarbamates.
2. Results and Discussion
2.1. Synthesis of Selenoureas 4–8 and Selenocarbamates 9, 10
Selenoureas 4a–4h and 5a–5h were synthesized by addition reaction of a series of amines 2a–2i to the five-membered nitroxyl isoselenocyanates: 3-isoselenocyanato-2,2,5,5-tetramethylpyrrolidine-1-oxyl (1a, PROXYL-NCSe), and 3-isoselenocyanatomethyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl (1b, PROXYL-CH2NCSe). The reactions occurred at room temperature (at approximately 0–10 °C in the case of volatile amines). Benzene was used as a solvent (Scheme 1).
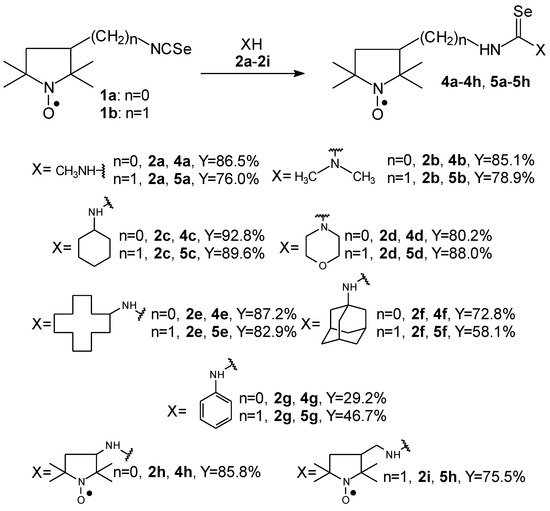
Scheme 1.
Selenoureas 4a–4h and 5a–5h; the reaction of 3-isoselenocyanato-2,2,5,5-tetramethylpyrrolidine-1-oxyl (1a, PROXYL-NCSe) and 3-isoselenocyanatomethyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl (1b, PROXYL-CH2NCSe) with a series of amines.
Selenoureas 6a and 6b were synthesized by addition reaction of methylamine (2a) and cyclododecylamine (2e) to the six-membered nitroxyl isoselenocyanate: 4-isoselenocyanato-2,2,6,6-tetramethylpiperidine-1-oxyl (1c, TEMPO-NCSe). Biradical selenourea 7 as well as selenoureas 8a and 8b were synthesized by addition reaction of the six-membered nitroxyl amine: 4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl (2j, TEMPO-NH2) to the five-membered nitroxyl isoselenocyanate: 3-isoselenocyanato-2,2,5,5-tetramethylpyrrolidine-1-oxyl (1a, PROXYL-NCSe) as well as adamantyl and 3-methylphenyl isoselenocyanates (1d and 1e, respectively). The reactions occurred at room temperature. Benzene was used as a solvent (Scheme 2).
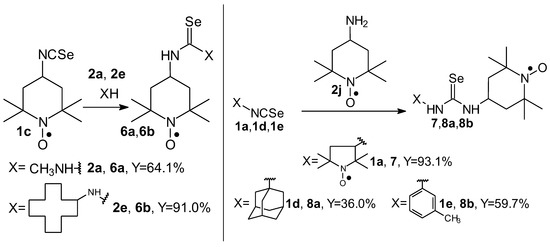
Scheme 2.
Selenoureas containing six-membered nitroxyl moiety 6a, 6b, 7, 8a, 8b; reaction of 4-isoselenocyanato-2,2,6,6-tetramethylpiperidine-1-oxyl (1c, TEMPO-NCSe), 3-isoselenocyanato-2,2,5,5-tetramethylpyrrolidine-1-oxyl (1a, PROXYL-NCSe) 1-adamantyl isoselenocyanate (1d), 3-methylphenyl isoselenocyanate (1e) with a series of amines.
Selenocarbamates 9a–9d were synthesized by addition reaction of methanol (3a) or ethanol (3b) to the five-membered isoselenocyanates: 3-isoselenocyanato-2,2,5,5-tetramethylpyrrolidine-1-oxyl (1a, PROXYL-NCSe), and 3-isoselenocyanatomethyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl (1b, PROXYL-CH2NCSe). The reaction occurred at room temperature. A corresponding alcohol was used as a solvent (Scheme 3).
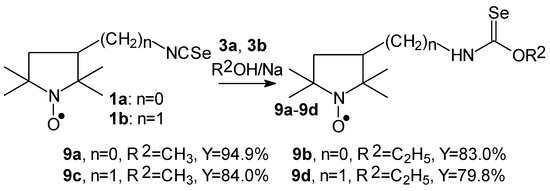
Scheme 3.
Selenocarbamates 9a–9d; the reaction of 3-isoselenocyanato-2,2,5,5-tetramethylpyrrolidine-1-oxyl (1a, PROXYL-NCSe) and 3-isoselenocyanatomethyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl (1b, PROXYL-CH2NCSe) with methanol and ethanol.
Selenocarbamates containing six-membered nitroxyl moiety 10a–10d were synthesized by addition reaction of nitroxyl secondary alcohol: 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (3c. TEMPOL) to the 1-adamantyl isoselenocyanate (1d) and aryl isoselenocyanates: 3-methylphenyl, 4-trifluoromethylphenyl, and 2-methyl-4-chlorophenyl isoselenocyanates (1e, 1f, 1g, respectively) in the presence of NaH as a base. The reaction occurred at room temperature. THF was used as a solvent (Scheme 4).
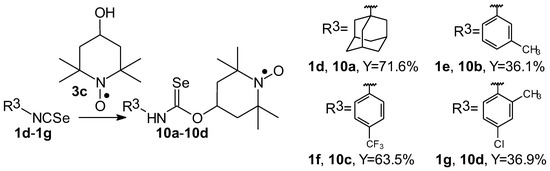
Scheme 4.
Selenocarbamates 10a–10d; the reaction of 1-adamantyl isoselenocyanate (1d), and aryl isoselenocyanates (1e–1g) with 4-hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxyl (3c, TEMPOL).
The predominant amount of synthesized nitroxyl selenoureas and selenocarbamates proved to be unstable at elevated temperatures. Compounds generally underwent decomposition upon heating during the melting temperature measurement. As a result of the decomposition, wide ranges of melting temperatures were observed. The purity of the synthesized compounds was evaluated by means of HPLC.
All the synthesized compounds were characterized using mass spectrometry (EI MS, ESI MS, HR MS) and IR spectroscopy (see the Supplementary Materials). The 1H NMR spectra were not performed due to the paramagnetic broadening, owing to the presence of the nitroxyl moieties [80,81,82,83,84]. EI MS showed the presence of the molecular mass peak for the almost all compounds under investigation (except six membered nitroxides 8a and 8b). The intensities of the molecular signals were diverse. The molecular signals were abundant for five-membered nitroxyl selenoureas 5b–5d and selenocarbamates 9a and 9c. The intensities of molecular signals were 100% for a six-membered nitroxyl selenourea 6a and a nitroxyl selenocarbamate 9d. Five-membered nitroxyl selenoureas 4f, 5f, 5h, six-membered nitroxyl selenoureas 6b, 8a, 8b, and nitroxyl selenocarbamates 10a–10c showed negligible molecular signals (for 8a, 8b no visible molecular signal were observed).
In order to confirm the molecular mass, ESI MS was performed for the almost all compounds under investigations (except of a selenourea 4b and selenocarbamates 9a and 10a–10c). The molecular masses were confirmed by the observation m/z M + 23 (100%) signals.
Exact molecular masses were confirmed by means of HR ESI MS (HR EI MS in the case of selenourea 4b and selenocarbamates 9a and 10a–10c).
IR spectra revealed strong absorption II amide band at ~1550 cm−1 characteristic for selenoureas [42].
2.2. Fungistatic and Bacteriostatic Activity of Selenoureas 4–8 and Selenocarbamates 9,10
All synthesized selenoureas 4–8 and selenocarbamates 9, 10 were tested for the herbicidal, insecticidal, acaricidal, antifungal, and antibacterial activities. No herbicidal, insecticidal, and acaricidal activities were observed. Significant fungistatic and bacteriostatic activities were found.
The investigated selenium containing nitroxides 4–8 and 9, 10 were tested in vitro against the basic set of phytopathogenic fungi: Botrytis cinerea, Fusarium culmorum, Phytophthora cactorum, and Rhizoctonia solani at the concentration of 200 mg/L, and for the selected, active compounds, at the concentration of 20 mg/L. The selenoureas 4–8 and selenocarbamates 9, 10 were also tested in vivo against Blumeria graminis as a phytopathogenic fungi, however, no tested compounds showed satisfactory activity against this species.
In order to enlarge the set of phytopathogenic fungi, the selected, active selenouraeas 4–8 and selenocarbamates 9, 10 were also tested at the concentration of 20 mg/L against the phytopathogenic fungi Alternaria alternata, Fusarium oxysporum, Phytophtora infestans, and against Ascosphaera apis (causing chalkbrood disease in honey bees).
The bacteriostatic activity of selenoureas 4–8 and selenocarbamates 9, 10 was tested for phytopathogenic bacteria Erwinia carotovora sub. atraseptica, Pseudomonas phaseolicola, Pseudomonas lachrymans, Pseudomonas syringae at concentration of 100 mg/L. The results of the fungistatic activity were presented in Table 1 and Table 2 The results of the bacteriostatic activity were presented in Table 3 and Table 4.

Table 1.
Fungistatic activities a of selenoureas 4–8 at 200 mg/L and 20 mg/L.

Table 2.
Fungistatic activities a of selenocarbamates 9, 10 at 200 mg/L and 20 mg/L.

Table 3.
Bacteriostatic activity MIC of selenoureas 4–8 at 100 mg/L.

Table 4.
Bacteriostatic activity MIC of selenocarbamates 9, 10 at 100 mg/L.
Almost all nitroxyl selenoureas 4–8 (except 4e—cyclododecyl derivative—see below) showed 100% activity against at least one fungus of the basic set of the tested fungi at the basic concentration of 200 mg/L.
-4b, 4d, 5b, 5c, 5d, 5h, 6b, 7 were active at 100% level against four basic fungi.
-4a, 4c, 4h, 5a, 5g, 6a, were active against three of four basic fungi at 100% level.
-4f, 4g, 8b were active against two of four basic fungi at 100% level.
-5e, 5f were active against one of four basic fungi at 100% level.
Especially, both five-membered nitroxyl selenourea series 4 i 5 showed the high activity against B.cinerea, P.cactorum, and R.solani at the concentration of 200 mg/L.
However, it was worthy to note that the cyclododecyl and adamantyl nitroxyl selenoureas revealed significantly lower activity:
-cyclododecyl and adamantyl nitroxyl selenoureas 4e, 4f, 5e, 5f against B. cinerea,
-cyclododecyl nitroxyl selenourea 4e i adamantyl nitroxyl selenourea 5f against P.cactorum,
-cyclododecyl nitroxyl selenoureas 4e i 5e against R.solani.
Nitroxyl carbamates 9a–9d were active at 100% level against all basic tested species B.cinerea, F.culmorum P.cactorum, and R.solani at the basic concentration of 200 mg/L. Nitroxyl selenocarbamates 10a–10d (TEMPOL (3c) derivatives) showed neither fungicidal nor bacteriostatic activity.
As noted above almost all “100%” compounds at the basic concentration of 200 mg/L were tested against the basic set of fungi (B. cinerea, F. culmorum, P. cactorum, and R. solani) at the concentration of 20 mg/L and against the additional set of fungi (A. alternata, F. oxysporum, P. infestans, and A. apis) also at the concentration of 20 mg/L. No tested nitroxyl compounds attained 100% in tests with the additional set of fungi. However, against the basic set of fungi significant amount of the tested nitroxyl selenoureas were active also at the concentration of 20 mg/L at the same 100% level. Nitroxyl selenourea 4c was active at the concentration of 20 mg/L at 100% level (MIC ≤ 20) against two species: B. cinerea and P. cactorum. Nitroxyl selenoureas 4d, 4h, 5a–5d, 5g, 5h, 7 were active at the concentration of 20 mg/L at 100% level (MIC ≤ 20) against P. cactorum.
The different size of alkyl and cycloalkyl fragments present in the nitroxyl selenoureas 4–8, prompted us to estimate the potential correlation between the observed fungicidal activity and the calculated octanol-water partition coefficient (clog P, HyperChem 7 software, Hypercube Inc., Gainesville, Fl, USA). Linear dependence between the average fungicidal activity (at 200 mg/L) vs. clog P was observed for the series of five-membered nitroxides 4a–4g (R2 = 0.95). Interestingly, the analogous dependence for the similar series 5a–5g was not observed.
Nitroxyl selenoureas 4c, 5b, 5c, 5h, 6a, 6b showed activity at the concentration of <100 mg/L for all four bacterial species.
5g showed activity at concentration of <100 mg/L against three of four bacteria species.
4a, 4d, 4g, 5f showed activity at concentration of <100 mg/L against two of four bacteria species.
4f showed activity at concentration of <100 mg/L against one of four bacteria species.
Nitroxyl selenocarbamate 9b showed activity at concentration of <100 mg/L against three of four tested bacteria species. Nitroxyl selenocarbamate 10d showed activity at concentration of <100 mg/L against one of four tested bacteria species.
3. Materials and Methods
3.1. General
The synthesis of the following nitroxyl, cycloalkyl and aryl isoselenocyanates: 3-isoselenocyanato-2,2,5,5-tetramethylpyrrolidine-1-oxyl (PROXYL-NCSe, 1a) and 3-isoselenocyanatomethyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl (PROXYL-CH2NCSe, 1b), 1-adamantyl isoselenocyanate (1d), 3-methylphenyl isoselenocyanate (1e), 4-trifluoromethylphenyl isoselenocyanate (1f), and 4-chloro-2-methylphenyl isoselenocyanate (1g) has been recently described [85], The synthesis of 4-isoselenocyanato-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO-NCSe, 1c) was described previously [26,85,86,87]. The references for the synthesis of the following nitroxyl amines: 3-amino-2,2,5,5-tetramethylpyrrolidine-1-oxyl, PROXYL-NH2 (2h), 3-aminomethyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl PROXYL-CH2-NH2 (2i), 4-amino-2,2,6,6-tetramethylppiperidine-1-oxyl, TEMPO-NH2 (2j) have been recently cited [85]. The references for the synthesis of 4-hydroxy-2,2,6,6-tetramethylppiperidine-1-oxyl (TEMPOL (3c)) was cited [88]. TLC was carried out on silica gel Merck Alurolle 5562 or Alufolien 5554; TLC visualization was achieved using UV 254 nm light and/or I2 vapor; visualization of selenium containing compounds: UV 254 nm and irradiation with UV lamp for 5–10 min (red spots) or spraying with 1% ethanolic PdCl2 (dark brown spots on pale beige background). Column chromatography was performed on silica gel 0.040–0.063 mm, 230–400 mesh: Merck 1.09385.1000 or Zeochem 60 hyd. HPLC conditions: C18, 5 μ, 150 × 4.6 mm, UV detector, λ = 220 nm; method a: mobil phase: acetonitrile:H2O 1:1, flow: 1 mL/min; method b: mobil phase: acetonitrile:H2O 1:3, flow: 1 mL/min; method c: mobil phase: acetonitrile:H2O 3:1, flow: 1.3 mL/min. EI-MS data (70 eV) were recorded on an AMD 604 and Agilent Technologies 5975 B mass spectrometers. HRMS-EI data were recorded by using an AMD 604 mass spectrometer. MS-ESI and HRMS-ESI (MeOH as a solvent) were recorded by using a Micromass LCT apparatus. IR spectra were recorded on an FT/IR Jasco 420 spectrophotometer.
3.2. 1,3-Substituted Nitroxyl Selenoureas 4a, 4b, 5a, 5b, 6a; Reaction of the Nitroxyl Isoselenocyanates 1a–1c with Volatile Amines 2a, 2b; a General Procedure
To a chilled and magnetically stirred solution of the corresponding nitroxyl isoselenocyanate (1a–1c, 0.001 mol) in benzene (5–6 mL), benzene solution of methylamine (2a) or dimethylamine (2b) (~1 mL) was added dropwise at about 5 °C. The reaction was carried out for 1 h at room temperature. The precipitate was filtered off and washed with hexane. The filtrate was evaporated, the residue was triturated with hexane to give the additional amount of the product (Scheme 1).
3.3. 1,3-Substituted Nitroxyl Selenoureas 4c–4h, 5c–5h, 6b, 7, 8a, 8b; Reaction of the Nitroxyl Isoselenocyanates 1a–1c with Liquid and Solid Amines (2c–2j); a General Procedure
A corresponding nitroxyl isoselenocyanate (1a–1c, 0.001 mol) was dissolved in benzene (5–6 mL). The corresponding amine (2c–2j, 0.0011 mol) was added using a syringe. The reagents were stirred for 1 h at room temperature. The formed precipitate was filtered off and washed with hexane. If no precipitate was formed, the solution was evaporated. The residue was either triturated with hexane and filtered off or chromatographed (Scheme 1 and Scheme 2).
1-(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)-3-methyl selenourea (4a). 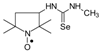 C10H20N3OSe, M = 278, 0.32 g, Yield: 86.5%, yellow glass, m.p. 141–144 °C (dec.), TLC: Rf = 0.14 benzene:methanol 9:1; purity (HPLC, method b): 98.2%; MS (EI, 70 eV, m/z, int [%]): 279 (13), 278 (20, M), 276 (12), 262 (10), 260 (43), 258 (22), 248 (25), 246 (24), 244 (11), 233 (10), 205 (50), 203 (29), 192 (25), 190 (14), 177 (22), 175 (11), 167 (12), 165 (35), 163 (20), 139 (13), 138 (10), 137 (10), 136 (12), 126 (30), 125 (21), 124 (38), 123 (15), 122 (28), 120 (14), 111 (92), 110 (74), 100 (27), 98 (54), 95 (59), 84 (100), 70 (33), 69 (29), 67 (21), 57 (66), 56 (59), 55 (28), 53 (12), 42 (30), 41 (39); MS (ESI, m/z, int [%]): 301 (100, M + 23), 299 (20); HR MS (ESI, m/z) for C10H20N3OSeNa: calcd: 301.0669, found: 301.0656; IR (ν, cm−1, KBr): 3420, 3280, 2971, 1564.
C10H20N3OSe, M = 278, 0.32 g, Yield: 86.5%, yellow glass, m.p. 141–144 °C (dec.), TLC: Rf = 0.14 benzene:methanol 9:1; purity (HPLC, method b): 98.2%; MS (EI, 70 eV, m/z, int [%]): 279 (13), 278 (20, M), 276 (12), 262 (10), 260 (43), 258 (22), 248 (25), 246 (24), 244 (11), 233 (10), 205 (50), 203 (29), 192 (25), 190 (14), 177 (22), 175 (11), 167 (12), 165 (35), 163 (20), 139 (13), 138 (10), 137 (10), 136 (12), 126 (30), 125 (21), 124 (38), 123 (15), 122 (28), 120 (14), 111 (92), 110 (74), 100 (27), 98 (54), 95 (59), 84 (100), 70 (33), 69 (29), 67 (21), 57 (66), 56 (59), 55 (28), 53 (12), 42 (30), 41 (39); MS (ESI, m/z, int [%]): 301 (100, M + 23), 299 (20); HR MS (ESI, m/z) for C10H20N3OSeNa: calcd: 301.0669, found: 301.0656; IR (ν, cm−1, KBr): 3420, 3280, 2971, 1564.
 C10H20N3OSe, M = 278, 0.32 g, Yield: 86.5%, yellow glass, m.p. 141–144 °C (dec.), TLC: Rf = 0.14 benzene:methanol 9:1; purity (HPLC, method b): 98.2%; MS (EI, 70 eV, m/z, int [%]): 279 (13), 278 (20, M), 276 (12), 262 (10), 260 (43), 258 (22), 248 (25), 246 (24), 244 (11), 233 (10), 205 (50), 203 (29), 192 (25), 190 (14), 177 (22), 175 (11), 167 (12), 165 (35), 163 (20), 139 (13), 138 (10), 137 (10), 136 (12), 126 (30), 125 (21), 124 (38), 123 (15), 122 (28), 120 (14), 111 (92), 110 (74), 100 (27), 98 (54), 95 (59), 84 (100), 70 (33), 69 (29), 67 (21), 57 (66), 56 (59), 55 (28), 53 (12), 42 (30), 41 (39); MS (ESI, m/z, int [%]): 301 (100, M + 23), 299 (20); HR MS (ESI, m/z) for C10H20N3OSeNa: calcd: 301.0669, found: 301.0656; IR (ν, cm−1, KBr): 3420, 3280, 2971, 1564.
C10H20N3OSe, M = 278, 0.32 g, Yield: 86.5%, yellow glass, m.p. 141–144 °C (dec.), TLC: Rf = 0.14 benzene:methanol 9:1; purity (HPLC, method b): 98.2%; MS (EI, 70 eV, m/z, int [%]): 279 (13), 278 (20, M), 276 (12), 262 (10), 260 (43), 258 (22), 248 (25), 246 (24), 244 (11), 233 (10), 205 (50), 203 (29), 192 (25), 190 (14), 177 (22), 175 (11), 167 (12), 165 (35), 163 (20), 139 (13), 138 (10), 137 (10), 136 (12), 126 (30), 125 (21), 124 (38), 123 (15), 122 (28), 120 (14), 111 (92), 110 (74), 100 (27), 98 (54), 95 (59), 84 (100), 70 (33), 69 (29), 67 (21), 57 (66), 56 (59), 55 (28), 53 (12), 42 (30), 41 (39); MS (ESI, m/z, int [%]): 301 (100, M + 23), 299 (20); HR MS (ESI, m/z) for C10H20N3OSeNa: calcd: 301.0669, found: 301.0656; IR (ν, cm−1, KBr): 3420, 3280, 2971, 1564.1-(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)-3,3-dimethyl selenourea (4b). 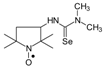 C11H22N3Ose, M = 292, Yield: 85.1%, yellow crystalline powder, m.p. 178–180 °C (dec.), TLC: Rf = 0.11 benzene:methanol 9:1; purity (HPLC, method b): 94.6%; MS (EI, 70 eV, m/z, int [%]): 292 (14, M), 290 (8), 262 (15), 260 (14), 247 (10), 245 (7), 221 (11), 219 (53), 217 (32), 206 (22.6), 205 (9), 204 (11), 203 (8), 193 (22), 191 (37), 189 (14), 179 (37), 177 (21), 153 (11), 138 (21), 136 (88), 134 (43), 126 (28), 125 (30), 124 (37), 111 (12), 110 (31), 100 (53), 98 (40), 95 (34), 82 (19), 71 (100), 69 (30), 67 (23), 56 (34), 55 (39), 44 (54), 42 (45), 41 (45); HR MS (EI, 70 eV, m/z) for C11H22N3OSe: calcd: 292.09281, found: 292.09388; IR (ν, cm−1, KBr): 3440, 3344, 2973, 1557, 1341.
C11H22N3Ose, M = 292, Yield: 85.1%, yellow crystalline powder, m.p. 178–180 °C (dec.), TLC: Rf = 0.11 benzene:methanol 9:1; purity (HPLC, method b): 94.6%; MS (EI, 70 eV, m/z, int [%]): 292 (14, M), 290 (8), 262 (15), 260 (14), 247 (10), 245 (7), 221 (11), 219 (53), 217 (32), 206 (22.6), 205 (9), 204 (11), 203 (8), 193 (22), 191 (37), 189 (14), 179 (37), 177 (21), 153 (11), 138 (21), 136 (88), 134 (43), 126 (28), 125 (30), 124 (37), 111 (12), 110 (31), 100 (53), 98 (40), 95 (34), 82 (19), 71 (100), 69 (30), 67 (23), 56 (34), 55 (39), 44 (54), 42 (45), 41 (45); HR MS (EI, 70 eV, m/z) for C11H22N3OSe: calcd: 292.09281, found: 292.09388; IR (ν, cm−1, KBr): 3440, 3344, 2973, 1557, 1341.
 C11H22N3Ose, M = 292, Yield: 85.1%, yellow crystalline powder, m.p. 178–180 °C (dec.), TLC: Rf = 0.11 benzene:methanol 9:1; purity (HPLC, method b): 94.6%; MS (EI, 70 eV, m/z, int [%]): 292 (14, M), 290 (8), 262 (15), 260 (14), 247 (10), 245 (7), 221 (11), 219 (53), 217 (32), 206 (22.6), 205 (9), 204 (11), 203 (8), 193 (22), 191 (37), 189 (14), 179 (37), 177 (21), 153 (11), 138 (21), 136 (88), 134 (43), 126 (28), 125 (30), 124 (37), 111 (12), 110 (31), 100 (53), 98 (40), 95 (34), 82 (19), 71 (100), 69 (30), 67 (23), 56 (34), 55 (39), 44 (54), 42 (45), 41 (45); HR MS (EI, 70 eV, m/z) for C11H22N3OSe: calcd: 292.09281, found: 292.09388; IR (ν, cm−1, KBr): 3440, 3344, 2973, 1557, 1341.
C11H22N3Ose, M = 292, Yield: 85.1%, yellow crystalline powder, m.p. 178–180 °C (dec.), TLC: Rf = 0.11 benzene:methanol 9:1; purity (HPLC, method b): 94.6%; MS (EI, 70 eV, m/z, int [%]): 292 (14, M), 290 (8), 262 (15), 260 (14), 247 (10), 245 (7), 221 (11), 219 (53), 217 (32), 206 (22.6), 205 (9), 204 (11), 203 (8), 193 (22), 191 (37), 189 (14), 179 (37), 177 (21), 153 (11), 138 (21), 136 (88), 134 (43), 126 (28), 125 (30), 124 (37), 111 (12), 110 (31), 100 (53), 98 (40), 95 (34), 82 (19), 71 (100), 69 (30), 67 (23), 56 (34), 55 (39), 44 (54), 42 (45), 41 (45); HR MS (EI, 70 eV, m/z) for C11H22N3OSe: calcd: 292.09281, found: 292.09388; IR (ν, cm−1, KBr): 3440, 3344, 2973, 1557, 1341.1-(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)-3,3-pentyleno selenourea (4c). 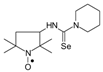 C14H26N3OSe, M = 332, 0.399 g, Yield: 92.8%, yellow crystalline powder, m.p. 154–156 °C (dec.), TLC: Rf = 0.25 benzene:methanol 9:1; purity (HPLC, method a): 98.8%; MS (EI, 70 eV, m/z, int [%]): 333 (6), 332 (9, M), 330 (5), 302 (11), 300 (19), 287 (10), 285 (6), 259 (76), 257 (39), 246 (18), 233 (33), 231 (32), 219 (19), 176 (95), 174 (47), 165 (55), 164 (31), 136 (12), 126 (14), 124 (32), 111 (58), 110 (30), 100 (27), 98 (47), 95 (21), 84 (100), 69 (61), 67 (19), 56 (41), 55 (40), 41 (63); MS (ESI, m/z, int [%]): 355 (100, M + Na), 353 (10); HR MS (ESI, m/z) for C14H26N3OSeNa: calcd: 355.1139, found: 355.1154; IR (ν, cm−1, KBr): 3359, 2960, 2938, 1550, 1331, 1239, 1132.
C14H26N3OSe, M = 332, 0.399 g, Yield: 92.8%, yellow crystalline powder, m.p. 154–156 °C (dec.), TLC: Rf = 0.25 benzene:methanol 9:1; purity (HPLC, method a): 98.8%; MS (EI, 70 eV, m/z, int [%]): 333 (6), 332 (9, M), 330 (5), 302 (11), 300 (19), 287 (10), 285 (6), 259 (76), 257 (39), 246 (18), 233 (33), 231 (32), 219 (19), 176 (95), 174 (47), 165 (55), 164 (31), 136 (12), 126 (14), 124 (32), 111 (58), 110 (30), 100 (27), 98 (47), 95 (21), 84 (100), 69 (61), 67 (19), 56 (41), 55 (40), 41 (63); MS (ESI, m/z, int [%]): 355 (100, M + Na), 353 (10); HR MS (ESI, m/z) for C14H26N3OSeNa: calcd: 355.1139, found: 355.1154; IR (ν, cm−1, KBr): 3359, 2960, 2938, 1550, 1331, 1239, 1132.
 C14H26N3OSe, M = 332, 0.399 g, Yield: 92.8%, yellow crystalline powder, m.p. 154–156 °C (dec.), TLC: Rf = 0.25 benzene:methanol 9:1; purity (HPLC, method a): 98.8%; MS (EI, 70 eV, m/z, int [%]): 333 (6), 332 (9, M), 330 (5), 302 (11), 300 (19), 287 (10), 285 (6), 259 (76), 257 (39), 246 (18), 233 (33), 231 (32), 219 (19), 176 (95), 174 (47), 165 (55), 164 (31), 136 (12), 126 (14), 124 (32), 111 (58), 110 (30), 100 (27), 98 (47), 95 (21), 84 (100), 69 (61), 67 (19), 56 (41), 55 (40), 41 (63); MS (ESI, m/z, int [%]): 355 (100, M + Na), 353 (10); HR MS (ESI, m/z) for C14H26N3OSeNa: calcd: 355.1139, found: 355.1154; IR (ν, cm−1, KBr): 3359, 2960, 2938, 1550, 1331, 1239, 1132.
C14H26N3OSe, M = 332, 0.399 g, Yield: 92.8%, yellow crystalline powder, m.p. 154–156 °C (dec.), TLC: Rf = 0.25 benzene:methanol 9:1; purity (HPLC, method a): 98.8%; MS (EI, 70 eV, m/z, int [%]): 333 (6), 332 (9, M), 330 (5), 302 (11), 300 (19), 287 (10), 285 (6), 259 (76), 257 (39), 246 (18), 233 (33), 231 (32), 219 (19), 176 (95), 174 (47), 165 (55), 164 (31), 136 (12), 126 (14), 124 (32), 111 (58), 110 (30), 100 (27), 98 (47), 95 (21), 84 (100), 69 (61), 67 (19), 56 (41), 55 (40), 41 (63); MS (ESI, m/z, int [%]): 355 (100, M + Na), 353 (10); HR MS (ESI, m/z) for C14H26N3OSeNa: calcd: 355.1139, found: 355.1154; IR (ν, cm−1, KBr): 3359, 2960, 2938, 1550, 1331, 1239, 1132.1-(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)-3,3-(3-oxapentyleno) selenourea (4d). 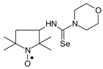 C13H24N3O2Se, M = 334, 0.348 g, Yield: 80.2%, yellow solid, m.p. 135–139 °C (dec.), TLC: Rf = 0.16 benzene:methanol 9:1; purity (HPLC, method b): 97.2%; MS (EI, 70 eV, m/z, int [%]): 335 (9), 334 (12, M), 332 (7), 304 (18), 302 (19), 289 (10), 261 (80), 259 (40), 248 (24), 235 (20), 233 (28), 231 (13), 221 (36), 219 (21), 178 (100), 176 (52), 167 (63), 134 (32), 132 (16), 126 (18), 124 (45), 113 (52), 111 (16), 100 (54), 98 (49), 95 (37), 86 (58), 69 (52), 56 (54), 55 (43), 42 (34), 41 (53), MS (ESI, m/z, int [%]): 357 (100, M + Na), 355 (20); HR MS (ESI, m/z) for C13H24N3O2SeNa: calcd: 357.0931, found: 357.0928; IR (ν, cm−1, KBr): 3358, 2973, 1543, 1339, 1231, 1220, 1121, 1025, 878.
C13H24N3O2Se, M = 334, 0.348 g, Yield: 80.2%, yellow solid, m.p. 135–139 °C (dec.), TLC: Rf = 0.16 benzene:methanol 9:1; purity (HPLC, method b): 97.2%; MS (EI, 70 eV, m/z, int [%]): 335 (9), 334 (12, M), 332 (7), 304 (18), 302 (19), 289 (10), 261 (80), 259 (40), 248 (24), 235 (20), 233 (28), 231 (13), 221 (36), 219 (21), 178 (100), 176 (52), 167 (63), 134 (32), 132 (16), 126 (18), 124 (45), 113 (52), 111 (16), 100 (54), 98 (49), 95 (37), 86 (58), 69 (52), 56 (54), 55 (43), 42 (34), 41 (53), MS (ESI, m/z, int [%]): 357 (100, M + Na), 355 (20); HR MS (ESI, m/z) for C13H24N3O2SeNa: calcd: 357.0931, found: 357.0928; IR (ν, cm−1, KBr): 3358, 2973, 1543, 1339, 1231, 1220, 1121, 1025, 878.
 C13H24N3O2Se, M = 334, 0.348 g, Yield: 80.2%, yellow solid, m.p. 135–139 °C (dec.), TLC: Rf = 0.16 benzene:methanol 9:1; purity (HPLC, method b): 97.2%; MS (EI, 70 eV, m/z, int [%]): 335 (9), 334 (12, M), 332 (7), 304 (18), 302 (19), 289 (10), 261 (80), 259 (40), 248 (24), 235 (20), 233 (28), 231 (13), 221 (36), 219 (21), 178 (100), 176 (52), 167 (63), 134 (32), 132 (16), 126 (18), 124 (45), 113 (52), 111 (16), 100 (54), 98 (49), 95 (37), 86 (58), 69 (52), 56 (54), 55 (43), 42 (34), 41 (53), MS (ESI, m/z, int [%]): 357 (100, M + Na), 355 (20); HR MS (ESI, m/z) for C13H24N3O2SeNa: calcd: 357.0931, found: 357.0928; IR (ν, cm−1, KBr): 3358, 2973, 1543, 1339, 1231, 1220, 1121, 1025, 878.
C13H24N3O2Se, M = 334, 0.348 g, Yield: 80.2%, yellow solid, m.p. 135–139 °C (dec.), TLC: Rf = 0.16 benzene:methanol 9:1; purity (HPLC, method b): 97.2%; MS (EI, 70 eV, m/z, int [%]): 335 (9), 334 (12, M), 332 (7), 304 (18), 302 (19), 289 (10), 261 (80), 259 (40), 248 (24), 235 (20), 233 (28), 231 (13), 221 (36), 219 (21), 178 (100), 176 (52), 167 (63), 134 (32), 132 (16), 126 (18), 124 (45), 113 (52), 111 (16), 100 (54), 98 (49), 95 (37), 86 (58), 69 (52), 56 (54), 55 (43), 42 (34), 41 (53), MS (ESI, m/z, int [%]): 357 (100, M + Na), 355 (20); HR MS (ESI, m/z) for C13H24N3O2SeNa: calcd: 357.0931, found: 357.0928; IR (ν, cm−1, KBr): 3358, 2973, 1543, 1339, 1231, 1220, 1121, 1025, 878.1-(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)-3-cyclododecyl selenourea (4e). 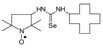 C21H40N3OSe, M = 430, 0.376 g, Yield: 87.2%, beige crystalline powder, m.p. 142–150 °C (dec.), TLC: Rf = 0.16 benzene:methanol 9:1; purity (HPLC, method c): 99.4%; MS (EI, 70 eV, m/z, int [%]): 431 (42), 430 (19, M), 429 (22), 414 (18), 412 (29), 410 (14), 398 (11), 357 (70), 355 (37), 344 (21), 318 (14), 317 (12), 315 (12), 291 (14), 289 (11). 277 (10), 275 (14), 263 (19), 182 (36), 168 (22), 151 (16), 149 (12), 126 (52), 124 (38), 112 (12), 111 (38), 110 (39), 99 (62), 98 (89), 84 (100), 69 (50), 67 (25), 56 (56), 55 (64), 43 (29), 41 (46); MS (ESI, m/z, int [%]): 453 (100, M + 23), 451 (20); HR MS (ESI, m/z) for C21H40N3OSeNa: calcd: 453.2234, found: 453.2254; IR (ν, cm−1, KBr): 3434, 2932, 1552, 1469, 1364.
C21H40N3OSe, M = 430, 0.376 g, Yield: 87.2%, beige crystalline powder, m.p. 142–150 °C (dec.), TLC: Rf = 0.16 benzene:methanol 9:1; purity (HPLC, method c): 99.4%; MS (EI, 70 eV, m/z, int [%]): 431 (42), 430 (19, M), 429 (22), 414 (18), 412 (29), 410 (14), 398 (11), 357 (70), 355 (37), 344 (21), 318 (14), 317 (12), 315 (12), 291 (14), 289 (11). 277 (10), 275 (14), 263 (19), 182 (36), 168 (22), 151 (16), 149 (12), 126 (52), 124 (38), 112 (12), 111 (38), 110 (39), 99 (62), 98 (89), 84 (100), 69 (50), 67 (25), 56 (56), 55 (64), 43 (29), 41 (46); MS (ESI, m/z, int [%]): 453 (100, M + 23), 451 (20); HR MS (ESI, m/z) for C21H40N3OSeNa: calcd: 453.2234, found: 453.2254; IR (ν, cm−1, KBr): 3434, 2932, 1552, 1469, 1364.
 C21H40N3OSe, M = 430, 0.376 g, Yield: 87.2%, beige crystalline powder, m.p. 142–150 °C (dec.), TLC: Rf = 0.16 benzene:methanol 9:1; purity (HPLC, method c): 99.4%; MS (EI, 70 eV, m/z, int [%]): 431 (42), 430 (19, M), 429 (22), 414 (18), 412 (29), 410 (14), 398 (11), 357 (70), 355 (37), 344 (21), 318 (14), 317 (12), 315 (12), 291 (14), 289 (11). 277 (10), 275 (14), 263 (19), 182 (36), 168 (22), 151 (16), 149 (12), 126 (52), 124 (38), 112 (12), 111 (38), 110 (39), 99 (62), 98 (89), 84 (100), 69 (50), 67 (25), 56 (56), 55 (64), 43 (29), 41 (46); MS (ESI, m/z, int [%]): 453 (100, M + 23), 451 (20); HR MS (ESI, m/z) for C21H40N3OSeNa: calcd: 453.2234, found: 453.2254; IR (ν, cm−1, KBr): 3434, 2932, 1552, 1469, 1364.
C21H40N3OSe, M = 430, 0.376 g, Yield: 87.2%, beige crystalline powder, m.p. 142–150 °C (dec.), TLC: Rf = 0.16 benzene:methanol 9:1; purity (HPLC, method c): 99.4%; MS (EI, 70 eV, m/z, int [%]): 431 (42), 430 (19, M), 429 (22), 414 (18), 412 (29), 410 (14), 398 (11), 357 (70), 355 (37), 344 (21), 318 (14), 317 (12), 315 (12), 291 (14), 289 (11). 277 (10), 275 (14), 263 (19), 182 (36), 168 (22), 151 (16), 149 (12), 126 (52), 124 (38), 112 (12), 111 (38), 110 (39), 99 (62), 98 (89), 84 (100), 69 (50), 67 (25), 56 (56), 55 (64), 43 (29), 41 (46); MS (ESI, m/z, int [%]): 453 (100, M + 23), 451 (20); HR MS (ESI, m/z) for C21H40N3OSeNa: calcd: 453.2234, found: 453.2254; IR (ν, cm−1, KBr): 3434, 2932, 1552, 1469, 1364.1-(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)-3-(1-adamantyl) selenourea (4f). 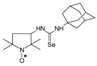 C19H32N3OSe, M = 398, 0.260 g, Yield: 72.8%, pale beige crystalline powder m.p. 140–145 °C, TLC: Rf = 0.21 benzene:methanol 9:1; purity (HPLC, method a): 99.5%; MS (EI, 70 eV, m/z, int [%]): 399 (3, M + 1), 398 (1, M), 397 (1), 396 (1), 135 (100), 110 (6), 107 (10), 99 (37), 98 (11), 94 (12), 93 (17), 84 (20), 79 (18), 71 (8), 67 (11), 56 (14), 55 (8), 41 (16); MS (ESI, m/z, int [%]): 421 (100, M + 23), 419 (20); HR MS (ESI, m/z) for C19H32N3OSeNa: calcd: 421.1608, found: 421.1592; IR (ν, cm−1, KBr): 3439, 2910, 1544.
C19H32N3OSe, M = 398, 0.260 g, Yield: 72.8%, pale beige crystalline powder m.p. 140–145 °C, TLC: Rf = 0.21 benzene:methanol 9:1; purity (HPLC, method a): 99.5%; MS (EI, 70 eV, m/z, int [%]): 399 (3, M + 1), 398 (1, M), 397 (1), 396 (1), 135 (100), 110 (6), 107 (10), 99 (37), 98 (11), 94 (12), 93 (17), 84 (20), 79 (18), 71 (8), 67 (11), 56 (14), 55 (8), 41 (16); MS (ESI, m/z, int [%]): 421 (100, M + 23), 419 (20); HR MS (ESI, m/z) for C19H32N3OSeNa: calcd: 421.1608, found: 421.1592; IR (ν, cm−1, KBr): 3439, 2910, 1544.
 C19H32N3OSe, M = 398, 0.260 g, Yield: 72.8%, pale beige crystalline powder m.p. 140–145 °C, TLC: Rf = 0.21 benzene:methanol 9:1; purity (HPLC, method a): 99.5%; MS (EI, 70 eV, m/z, int [%]): 399 (3, M + 1), 398 (1, M), 397 (1), 396 (1), 135 (100), 110 (6), 107 (10), 99 (37), 98 (11), 94 (12), 93 (17), 84 (20), 79 (18), 71 (8), 67 (11), 56 (14), 55 (8), 41 (16); MS (ESI, m/z, int [%]): 421 (100, M + 23), 419 (20); HR MS (ESI, m/z) for C19H32N3OSeNa: calcd: 421.1608, found: 421.1592; IR (ν, cm−1, KBr): 3439, 2910, 1544.
C19H32N3OSe, M = 398, 0.260 g, Yield: 72.8%, pale beige crystalline powder m.p. 140–145 °C, TLC: Rf = 0.21 benzene:methanol 9:1; purity (HPLC, method a): 99.5%; MS (EI, 70 eV, m/z, int [%]): 399 (3, M + 1), 398 (1, M), 397 (1), 396 (1), 135 (100), 110 (6), 107 (10), 99 (37), 98 (11), 94 (12), 93 (17), 84 (20), 79 (18), 71 (8), 67 (11), 56 (14), 55 (8), 41 (16); MS (ESI, m/z, int [%]): 421 (100, M + 23), 419 (20); HR MS (ESI, m/z) for C19H32N3OSeNa: calcd: 421.1608, found: 421.1592; IR (ν, cm−1, KBr): 3439, 2910, 1544.1-(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)-3-phenyl selenourea (4g). 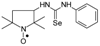 C15H22N3OSe, M = 340, 0.149 g, Yield: 29.2%, beige crystalline powder, m.p. 135–136 °C, TLC: Rf = 0.15 benzene:methanol 9:1; purity (HPLC, method a): 94.1%; MS (EI, 70 eV, m/z, int [%]): 341 (16), 340 (12, M), 339 (8), 338 (9), 322 (8), 310 (8), 308 (9), 295 (8), 267 (42), 265 (23), 254 (17), 244 (13), 243 (20), 239 (14), 229 (15), 228 (28), 227 (18), 201 (11), 199 (16), 188 (16), 187 (19), 186 (14), 185 (25), 184 (15), 173 (42), 171 (24), 159 (21), 158 (16), 157 (12), 156 (10), 145 (15), 144 (21), 126 (36), 124 (28), 119 (60), 118 (29), 111 (22), 110 (54), 108 (11), 104 (31), 99 (90), 98 (55), 96 (14), 95 (32), 94 (18), 93 (37), 92 (18), 91 (12), 84 (100), 82 (12), 81 (11), 78 (19), 77 (67), 71 (24), 70 (32), 69 (37), 68 (10), 67 (22), 65 (20), 58 (27), 57 (18), 56 (77), 55 (33), 53 (14), 51 (19), 43 (17), 42 (34), 41 (54); MS (ESI, m/z, int [%]): 363 (100, M + Na), 361 (15), 301 (20), 271 (5); HR MS (ESI, m/z) for C15H22N3OSeNa: calcd: 363.0826, found: 363.0831; IR (ν, cm−1, KBr): 3273, 2982, 1547, 1240, 692.
C15H22N3OSe, M = 340, 0.149 g, Yield: 29.2%, beige crystalline powder, m.p. 135–136 °C, TLC: Rf = 0.15 benzene:methanol 9:1; purity (HPLC, method a): 94.1%; MS (EI, 70 eV, m/z, int [%]): 341 (16), 340 (12, M), 339 (8), 338 (9), 322 (8), 310 (8), 308 (9), 295 (8), 267 (42), 265 (23), 254 (17), 244 (13), 243 (20), 239 (14), 229 (15), 228 (28), 227 (18), 201 (11), 199 (16), 188 (16), 187 (19), 186 (14), 185 (25), 184 (15), 173 (42), 171 (24), 159 (21), 158 (16), 157 (12), 156 (10), 145 (15), 144 (21), 126 (36), 124 (28), 119 (60), 118 (29), 111 (22), 110 (54), 108 (11), 104 (31), 99 (90), 98 (55), 96 (14), 95 (32), 94 (18), 93 (37), 92 (18), 91 (12), 84 (100), 82 (12), 81 (11), 78 (19), 77 (67), 71 (24), 70 (32), 69 (37), 68 (10), 67 (22), 65 (20), 58 (27), 57 (18), 56 (77), 55 (33), 53 (14), 51 (19), 43 (17), 42 (34), 41 (54); MS (ESI, m/z, int [%]): 363 (100, M + Na), 361 (15), 301 (20), 271 (5); HR MS (ESI, m/z) for C15H22N3OSeNa: calcd: 363.0826, found: 363.0831; IR (ν, cm−1, KBr): 3273, 2982, 1547, 1240, 692.
 C15H22N3OSe, M = 340, 0.149 g, Yield: 29.2%, beige crystalline powder, m.p. 135–136 °C, TLC: Rf = 0.15 benzene:methanol 9:1; purity (HPLC, method a): 94.1%; MS (EI, 70 eV, m/z, int [%]): 341 (16), 340 (12, M), 339 (8), 338 (9), 322 (8), 310 (8), 308 (9), 295 (8), 267 (42), 265 (23), 254 (17), 244 (13), 243 (20), 239 (14), 229 (15), 228 (28), 227 (18), 201 (11), 199 (16), 188 (16), 187 (19), 186 (14), 185 (25), 184 (15), 173 (42), 171 (24), 159 (21), 158 (16), 157 (12), 156 (10), 145 (15), 144 (21), 126 (36), 124 (28), 119 (60), 118 (29), 111 (22), 110 (54), 108 (11), 104 (31), 99 (90), 98 (55), 96 (14), 95 (32), 94 (18), 93 (37), 92 (18), 91 (12), 84 (100), 82 (12), 81 (11), 78 (19), 77 (67), 71 (24), 70 (32), 69 (37), 68 (10), 67 (22), 65 (20), 58 (27), 57 (18), 56 (77), 55 (33), 53 (14), 51 (19), 43 (17), 42 (34), 41 (54); MS (ESI, m/z, int [%]): 363 (100, M + Na), 361 (15), 301 (20), 271 (5); HR MS (ESI, m/z) for C15H22N3OSeNa: calcd: 363.0826, found: 363.0831; IR (ν, cm−1, KBr): 3273, 2982, 1547, 1240, 692.
C15H22N3OSe, M = 340, 0.149 g, Yield: 29.2%, beige crystalline powder, m.p. 135–136 °C, TLC: Rf = 0.15 benzene:methanol 9:1; purity (HPLC, method a): 94.1%; MS (EI, 70 eV, m/z, int [%]): 341 (16), 340 (12, M), 339 (8), 338 (9), 322 (8), 310 (8), 308 (9), 295 (8), 267 (42), 265 (23), 254 (17), 244 (13), 243 (20), 239 (14), 229 (15), 228 (28), 227 (18), 201 (11), 199 (16), 188 (16), 187 (19), 186 (14), 185 (25), 184 (15), 173 (42), 171 (24), 159 (21), 158 (16), 157 (12), 156 (10), 145 (15), 144 (21), 126 (36), 124 (28), 119 (60), 118 (29), 111 (22), 110 (54), 108 (11), 104 (31), 99 (90), 98 (55), 96 (14), 95 (32), 94 (18), 93 (37), 92 (18), 91 (12), 84 (100), 82 (12), 81 (11), 78 (19), 77 (67), 71 (24), 70 (32), 69 (37), 68 (10), 67 (22), 65 (20), 58 (27), 57 (18), 56 (77), 55 (33), 53 (14), 51 (19), 43 (17), 42 (34), 41 (54); MS (ESI, m/z, int [%]): 363 (100, M + Na), 361 (15), 301 (20), 271 (5); HR MS (ESI, m/z) for C15H22N3OSeNa: calcd: 363.0826, found: 363.0831; IR (ν, cm−1, KBr): 3273, 2982, 1547, 1240, 692.1,3-Bis(2,2,5,5-tetramethyl-1-oxyl-3-pyrrolidinyl) selenourea (4h). 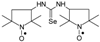 C17H32N4O2Se, M = 404, 0.343 g, Yield: 85.8%, m.p. 68–73 °C, TLC: Rf = 0.21 benzene:methanol 9:1, yellow, glassy solid; purity (HPLC, method a): 97.8%; MS (EI, 70 eV, m/z, int [%]): 406 (6), 405 (4), 404 (15, M), 402 (8), 386 (19), 374 (10), 372 (9), 340 (4), 322 (9), 250 (12), 245 (14), 177 (10), 142 (24), 126 (70), 124 (35), 111 (40), 110 (58), 98 (100), 95 (30), 84 (84), 70 (24), 69 (42), 67 (20), 58 (36), 56 (93), 55 (37), 43 (24), 42 (19), 41 (38); MS (ESI, m/z, int [%]): 427 (100, M + Na), 425 (10); HR MS (ESI, m/z) for C17H32N4O2SeNa: calcd: 427.1588, found: 427.1591; IR (ν, cm−1, KBr): 3437, 2973, 1547, 1462, 1365.
C17H32N4O2Se, M = 404, 0.343 g, Yield: 85.8%, m.p. 68–73 °C, TLC: Rf = 0.21 benzene:methanol 9:1, yellow, glassy solid; purity (HPLC, method a): 97.8%; MS (EI, 70 eV, m/z, int [%]): 406 (6), 405 (4), 404 (15, M), 402 (8), 386 (19), 374 (10), 372 (9), 340 (4), 322 (9), 250 (12), 245 (14), 177 (10), 142 (24), 126 (70), 124 (35), 111 (40), 110 (58), 98 (100), 95 (30), 84 (84), 70 (24), 69 (42), 67 (20), 58 (36), 56 (93), 55 (37), 43 (24), 42 (19), 41 (38); MS (ESI, m/z, int [%]): 427 (100, M + Na), 425 (10); HR MS (ESI, m/z) for C17H32N4O2SeNa: calcd: 427.1588, found: 427.1591; IR (ν, cm−1, KBr): 3437, 2973, 1547, 1462, 1365.
 C17H32N4O2Se, M = 404, 0.343 g, Yield: 85.8%, m.p. 68–73 °C, TLC: Rf = 0.21 benzene:methanol 9:1, yellow, glassy solid; purity (HPLC, method a): 97.8%; MS (EI, 70 eV, m/z, int [%]): 406 (6), 405 (4), 404 (15, M), 402 (8), 386 (19), 374 (10), 372 (9), 340 (4), 322 (9), 250 (12), 245 (14), 177 (10), 142 (24), 126 (70), 124 (35), 111 (40), 110 (58), 98 (100), 95 (30), 84 (84), 70 (24), 69 (42), 67 (20), 58 (36), 56 (93), 55 (37), 43 (24), 42 (19), 41 (38); MS (ESI, m/z, int [%]): 427 (100, M + Na), 425 (10); HR MS (ESI, m/z) for C17H32N4O2SeNa: calcd: 427.1588, found: 427.1591; IR (ν, cm−1, KBr): 3437, 2973, 1547, 1462, 1365.
C17H32N4O2Se, M = 404, 0.343 g, Yield: 85.8%, m.p. 68–73 °C, TLC: Rf = 0.21 benzene:methanol 9:1, yellow, glassy solid; purity (HPLC, method a): 97.8%; MS (EI, 70 eV, m/z, int [%]): 406 (6), 405 (4), 404 (15, M), 402 (8), 386 (19), 374 (10), 372 (9), 340 (4), 322 (9), 250 (12), 245 (14), 177 (10), 142 (24), 126 (70), 124 (35), 111 (40), 110 (58), 98 (100), 95 (30), 84 (84), 70 (24), 69 (42), 67 (20), 58 (36), 56 (93), 55 (37), 43 (24), 42 (19), 41 (38); MS (ESI, m/z, int [%]): 427 (100, M + Na), 425 (10); HR MS (ESI, m/z) for C17H32N4O2SeNa: calcd: 427.1588, found: 427.1591; IR (ν, cm−1, KBr): 3437, 2973, 1547, 1462, 1365.1-[(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)methyl]-3-methyl selenourea (5a). 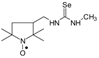 C11H22N3OSe, M = 292, 0.31 g, Yield: 76.0%, yellow crystalline powder, m.p. 117–122 °C (dec.), TLC: Rf = 0.5 benzene:acetone 1:1; purity (HPLC, method b): 97.3%; MS (EI, 70 eV, m/z, int [%]): 293 (16), 292 (31, M), 290 (16), 262 (13), 260 (11), 219 (13), 217 (7), 210 (7), 206 (9), 196 (13), 195 (10), 180 (95), 163 (19), 151 (28), 140 (51), 124 (70), 122 (18), 112 (16), 111 (13), 110 (20), 109 (25), 98 (27), 84 (12), 83 (17), 82 (20), 81 (17), 78 (55), 69 (100), 58 (27), 57 (34), 56 (22), 55 (32), 42 (32), 41 (51); MS (ESI, m/z, int [%]): 315 (100, M + Na), 313 (10); HR MS (ESI, m/z) for C11H22N3OSeNa: calcd: 315.0826, found: 315.0832; IR (ν, cm−1, KBr) 3434, 2971, 2930, 1567, 1460, 1364, 681.
C11H22N3OSe, M = 292, 0.31 g, Yield: 76.0%, yellow crystalline powder, m.p. 117–122 °C (dec.), TLC: Rf = 0.5 benzene:acetone 1:1; purity (HPLC, method b): 97.3%; MS (EI, 70 eV, m/z, int [%]): 293 (16), 292 (31, M), 290 (16), 262 (13), 260 (11), 219 (13), 217 (7), 210 (7), 206 (9), 196 (13), 195 (10), 180 (95), 163 (19), 151 (28), 140 (51), 124 (70), 122 (18), 112 (16), 111 (13), 110 (20), 109 (25), 98 (27), 84 (12), 83 (17), 82 (20), 81 (17), 78 (55), 69 (100), 58 (27), 57 (34), 56 (22), 55 (32), 42 (32), 41 (51); MS (ESI, m/z, int [%]): 315 (100, M + Na), 313 (10); HR MS (ESI, m/z) for C11H22N3OSeNa: calcd: 315.0826, found: 315.0832; IR (ν, cm−1, KBr) 3434, 2971, 2930, 1567, 1460, 1364, 681.
 C11H22N3OSe, M = 292, 0.31 g, Yield: 76.0%, yellow crystalline powder, m.p. 117–122 °C (dec.), TLC: Rf = 0.5 benzene:acetone 1:1; purity (HPLC, method b): 97.3%; MS (EI, 70 eV, m/z, int [%]): 293 (16), 292 (31, M), 290 (16), 262 (13), 260 (11), 219 (13), 217 (7), 210 (7), 206 (9), 196 (13), 195 (10), 180 (95), 163 (19), 151 (28), 140 (51), 124 (70), 122 (18), 112 (16), 111 (13), 110 (20), 109 (25), 98 (27), 84 (12), 83 (17), 82 (20), 81 (17), 78 (55), 69 (100), 58 (27), 57 (34), 56 (22), 55 (32), 42 (32), 41 (51); MS (ESI, m/z, int [%]): 315 (100, M + Na), 313 (10); HR MS (ESI, m/z) for C11H22N3OSeNa: calcd: 315.0826, found: 315.0832; IR (ν, cm−1, KBr) 3434, 2971, 2930, 1567, 1460, 1364, 681.
C11H22N3OSe, M = 292, 0.31 g, Yield: 76.0%, yellow crystalline powder, m.p. 117–122 °C (dec.), TLC: Rf = 0.5 benzene:acetone 1:1; purity (HPLC, method b): 97.3%; MS (EI, 70 eV, m/z, int [%]): 293 (16), 292 (31, M), 290 (16), 262 (13), 260 (11), 219 (13), 217 (7), 210 (7), 206 (9), 196 (13), 195 (10), 180 (95), 163 (19), 151 (28), 140 (51), 124 (70), 122 (18), 112 (16), 111 (13), 110 (20), 109 (25), 98 (27), 84 (12), 83 (17), 82 (20), 81 (17), 78 (55), 69 (100), 58 (27), 57 (34), 56 (22), 55 (32), 42 (32), 41 (51); MS (ESI, m/z, int [%]): 315 (100, M + Na), 313 (10); HR MS (ESI, m/z) for C11H22N3OSeNa: calcd: 315.0826, found: 315.0832; IR (ν, cm−1, KBr) 3434, 2971, 2930, 1567, 1460, 1364, 681.1-[(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)methyl]-3,3-dimethyl selenourea (5b). 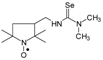 C12H24N3OSe, M = 306, 0.3 g, Yield: 78.9%, yellow crystalline powder, m.p. 162–170 °C, TLC: Rf = 0.31 benzene:acetone 1:1; purity (HPLC, method b): 99.1%; MS (EI, 70 eV, m/z, int [%]): 306 (84, M), 304 (41), 276 (44), 274 (37), 272 (16), 233 (75), 231 (39), 220 (26), 195 (16), 177 (82), 175 (41), 153 (19), 152 (15), 140 (24), 138 (27), 136 (100), 134 (48), 124 (24), 121 (14), 109 (26), 98 (17), 85 (15), 81 (14), 71 (85), 69 (38), 56 (19), 55 (25), 44 (29), 42 (24), 41 (50); MS (ESI, m/z, int [%]): 329 (100, M + Na), 327 (20); HR MS (ESI, m/z) for C12H24N3OSeNa: calcd: 329.0982, found: 329.0976; IR (ν, cm−1, KBr): 3440, 3293, 2969, 2929, 1552, 1459, 1373.
C12H24N3OSe, M = 306, 0.3 g, Yield: 78.9%, yellow crystalline powder, m.p. 162–170 °C, TLC: Rf = 0.31 benzene:acetone 1:1; purity (HPLC, method b): 99.1%; MS (EI, 70 eV, m/z, int [%]): 306 (84, M), 304 (41), 276 (44), 274 (37), 272 (16), 233 (75), 231 (39), 220 (26), 195 (16), 177 (82), 175 (41), 153 (19), 152 (15), 140 (24), 138 (27), 136 (100), 134 (48), 124 (24), 121 (14), 109 (26), 98 (17), 85 (15), 81 (14), 71 (85), 69 (38), 56 (19), 55 (25), 44 (29), 42 (24), 41 (50); MS (ESI, m/z, int [%]): 329 (100, M + Na), 327 (20); HR MS (ESI, m/z) for C12H24N3OSeNa: calcd: 329.0982, found: 329.0976; IR (ν, cm−1, KBr): 3440, 3293, 2969, 2929, 1552, 1459, 1373.
 C12H24N3OSe, M = 306, 0.3 g, Yield: 78.9%, yellow crystalline powder, m.p. 162–170 °C, TLC: Rf = 0.31 benzene:acetone 1:1; purity (HPLC, method b): 99.1%; MS (EI, 70 eV, m/z, int [%]): 306 (84, M), 304 (41), 276 (44), 274 (37), 272 (16), 233 (75), 231 (39), 220 (26), 195 (16), 177 (82), 175 (41), 153 (19), 152 (15), 140 (24), 138 (27), 136 (100), 134 (48), 124 (24), 121 (14), 109 (26), 98 (17), 85 (15), 81 (14), 71 (85), 69 (38), 56 (19), 55 (25), 44 (29), 42 (24), 41 (50); MS (ESI, m/z, int [%]): 329 (100, M + Na), 327 (20); HR MS (ESI, m/z) for C12H24N3OSeNa: calcd: 329.0982, found: 329.0976; IR (ν, cm−1, KBr): 3440, 3293, 2969, 2929, 1552, 1459, 1373.
C12H24N3OSe, M = 306, 0.3 g, Yield: 78.9%, yellow crystalline powder, m.p. 162–170 °C, TLC: Rf = 0.31 benzene:acetone 1:1; purity (HPLC, method b): 99.1%; MS (EI, 70 eV, m/z, int [%]): 306 (84, M), 304 (41), 276 (44), 274 (37), 272 (16), 233 (75), 231 (39), 220 (26), 195 (16), 177 (82), 175 (41), 153 (19), 152 (15), 140 (24), 138 (27), 136 (100), 134 (48), 124 (24), 121 (14), 109 (26), 98 (17), 85 (15), 81 (14), 71 (85), 69 (38), 56 (19), 55 (25), 44 (29), 42 (24), 41 (50); MS (ESI, m/z, int [%]): 329 (100, M + Na), 327 (20); HR MS (ESI, m/z) for C12H24N3OSeNa: calcd: 329.0982, found: 329.0976; IR (ν, cm−1, KBr): 3440, 3293, 2969, 2929, 1552, 1459, 1373.1-[(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)methyl]-3,3-pentyleno selenourea (5c). 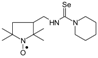 C15H28N3OSe, M = 346, 0.319 g, Yield: 89.6%, yellow crystalline powder, m.p. 165–171 °C, TLC: Rf = 0.17 benzene:methanol 9:1; purity (HPLC, method a): 97.7%; MS (EI, 70 eV, m/z, int [%]): 346 (76, M), 344 (39), 316 (31), 314 (31), 312 (14), 273 (100), 271 (52), 260 (23), 259 (20), 235 (21), 217 (91), 215 (46), 193 (14), 176 (71), 174 (35), 166 (8), 152 (8), 140 (15), 124 (15), 111 (76), 84 (63), 69 (71), 56 (28), 55 (38), 41 (67); MS (ESI, m/z, int [%]): 369 (100, M + Na), 367 (15); HR MS (ESI, m/z) for C15H28N3OSeNa: calcd: 369.1295, found: 369.1290; IR (ν, cm−1, KBr): 3352, 2926, 1553, 1332.
C15H28N3OSe, M = 346, 0.319 g, Yield: 89.6%, yellow crystalline powder, m.p. 165–171 °C, TLC: Rf = 0.17 benzene:methanol 9:1; purity (HPLC, method a): 97.7%; MS (EI, 70 eV, m/z, int [%]): 346 (76, M), 344 (39), 316 (31), 314 (31), 312 (14), 273 (100), 271 (52), 260 (23), 259 (20), 235 (21), 217 (91), 215 (46), 193 (14), 176 (71), 174 (35), 166 (8), 152 (8), 140 (15), 124 (15), 111 (76), 84 (63), 69 (71), 56 (28), 55 (38), 41 (67); MS (ESI, m/z, int [%]): 369 (100, M + Na), 367 (15); HR MS (ESI, m/z) for C15H28N3OSeNa: calcd: 369.1295, found: 369.1290; IR (ν, cm−1, KBr): 3352, 2926, 1553, 1332.
 C15H28N3OSe, M = 346, 0.319 g, Yield: 89.6%, yellow crystalline powder, m.p. 165–171 °C, TLC: Rf = 0.17 benzene:methanol 9:1; purity (HPLC, method a): 97.7%; MS (EI, 70 eV, m/z, int [%]): 346 (76, M), 344 (39), 316 (31), 314 (31), 312 (14), 273 (100), 271 (52), 260 (23), 259 (20), 235 (21), 217 (91), 215 (46), 193 (14), 176 (71), 174 (35), 166 (8), 152 (8), 140 (15), 124 (15), 111 (76), 84 (63), 69 (71), 56 (28), 55 (38), 41 (67); MS (ESI, m/z, int [%]): 369 (100, M + Na), 367 (15); HR MS (ESI, m/z) for C15H28N3OSeNa: calcd: 369.1295, found: 369.1290; IR (ν, cm−1, KBr): 3352, 2926, 1553, 1332.
C15H28N3OSe, M = 346, 0.319 g, Yield: 89.6%, yellow crystalline powder, m.p. 165–171 °C, TLC: Rf = 0.17 benzene:methanol 9:1; purity (HPLC, method a): 97.7%; MS (EI, 70 eV, m/z, int [%]): 346 (76, M), 344 (39), 316 (31), 314 (31), 312 (14), 273 (100), 271 (52), 260 (23), 259 (20), 235 (21), 217 (91), 215 (46), 193 (14), 176 (71), 174 (35), 166 (8), 152 (8), 140 (15), 124 (15), 111 (76), 84 (63), 69 (71), 56 (28), 55 (38), 41 (67); MS (ESI, m/z, int [%]): 369 (100, M + Na), 367 (15); HR MS (ESI, m/z) for C15H28N3OSeNa: calcd: 369.1295, found: 369.1290; IR (ν, cm−1, KBr): 3352, 2926, 1553, 1332.1-[(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)methyl]-3,3-(3-oxapentyleno) selenourea (5d). 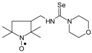 C14H26N3O2Se, M = 348, 0.315 g, Yield: 88.0%, m.p. 140–146 °C, TLC: Rf = 0.38 benzene:acetone 1:1, yellow solid; purity (HPLC, method b): 99.4%; MS (EI, 70 eV, m/z, int [%]): 348 (88, M), 346 (46), 318 (44), 316 (40), 314 (18), 275 (100), 273 (51), 262 (37), 261 (38), 260 (19), 259 (24), 237 (21), 219 (97), 217 (50), 167 (9), 152 (11), 140 (22), 134 (25), 124 (28), 113 (66), 109 (32), 98 (18), 86 (38), 83 (17), 81 (21), 69 (96), 67 (22), 57 (23), 56 (36), 55 (36), 41 (70); MS (ESI, m/z, int [%]): 371 (100, M + Na), 369 (25), 193 (20); HR MS (ESI, m/z) for C14H26N3O2SeNa: calcd: 371.1088, found: 371.1097; IR (ν, cm−1, KBr): 3364, 2970, 1547, 1462, 1420, 1363, 1334, 1273, 1250, 1212, 1119, 1022.
C14H26N3O2Se, M = 348, 0.315 g, Yield: 88.0%, m.p. 140–146 °C, TLC: Rf = 0.38 benzene:acetone 1:1, yellow solid; purity (HPLC, method b): 99.4%; MS (EI, 70 eV, m/z, int [%]): 348 (88, M), 346 (46), 318 (44), 316 (40), 314 (18), 275 (100), 273 (51), 262 (37), 261 (38), 260 (19), 259 (24), 237 (21), 219 (97), 217 (50), 167 (9), 152 (11), 140 (22), 134 (25), 124 (28), 113 (66), 109 (32), 98 (18), 86 (38), 83 (17), 81 (21), 69 (96), 67 (22), 57 (23), 56 (36), 55 (36), 41 (70); MS (ESI, m/z, int [%]): 371 (100, M + Na), 369 (25), 193 (20); HR MS (ESI, m/z) for C14H26N3O2SeNa: calcd: 371.1088, found: 371.1097; IR (ν, cm−1, KBr): 3364, 2970, 1547, 1462, 1420, 1363, 1334, 1273, 1250, 1212, 1119, 1022.
 C14H26N3O2Se, M = 348, 0.315 g, Yield: 88.0%, m.p. 140–146 °C, TLC: Rf = 0.38 benzene:acetone 1:1, yellow solid; purity (HPLC, method b): 99.4%; MS (EI, 70 eV, m/z, int [%]): 348 (88, M), 346 (46), 318 (44), 316 (40), 314 (18), 275 (100), 273 (51), 262 (37), 261 (38), 260 (19), 259 (24), 237 (21), 219 (97), 217 (50), 167 (9), 152 (11), 140 (22), 134 (25), 124 (28), 113 (66), 109 (32), 98 (18), 86 (38), 83 (17), 81 (21), 69 (96), 67 (22), 57 (23), 56 (36), 55 (36), 41 (70); MS (ESI, m/z, int [%]): 371 (100, M + Na), 369 (25), 193 (20); HR MS (ESI, m/z) for C14H26N3O2SeNa: calcd: 371.1088, found: 371.1097; IR (ν, cm−1, KBr): 3364, 2970, 1547, 1462, 1420, 1363, 1334, 1273, 1250, 1212, 1119, 1022.
C14H26N3O2Se, M = 348, 0.315 g, Yield: 88.0%, m.p. 140–146 °C, TLC: Rf = 0.38 benzene:acetone 1:1, yellow solid; purity (HPLC, method b): 99.4%; MS (EI, 70 eV, m/z, int [%]): 348 (88, M), 346 (46), 318 (44), 316 (40), 314 (18), 275 (100), 273 (51), 262 (37), 261 (38), 260 (19), 259 (24), 237 (21), 219 (97), 217 (50), 167 (9), 152 (11), 140 (22), 134 (25), 124 (28), 113 (66), 109 (32), 98 (18), 86 (38), 83 (17), 81 (21), 69 (96), 67 (22), 57 (23), 56 (36), 55 (36), 41 (70); MS (ESI, m/z, int [%]): 371 (100, M + Na), 369 (25), 193 (20); HR MS (ESI, m/z) for C14H26N3O2SeNa: calcd: 371.1088, found: 371.1097; IR (ν, cm−1, KBr): 3364, 2970, 1547, 1462, 1420, 1363, 1334, 1273, 1250, 1212, 1119, 1022.1-[(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)methyl]-3-cyclododecyl selenourea (5e). 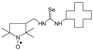 C22H42N3OSe, M = 444, 0.276 g, Yield: 82.9%, m.p. 145–150 °C, TLC: Rf = 0.16 benzene:methanol 9:1; yellow solid; purity (HPLC, method c): 97.6%; MS (EI, 70 eV, m/z, int [%]): 445 (20), 444 (21, M), 443 (10), 442 (12), 429 (3), 414 (7), 412 (9), 371 (34), 369 (18), 362 (18), 358 (19), 347 (15), 332 (100), 315 (23), 313 (12), 304 (7), 291 (14), 289 (9), 276 (14), 264 (7), 250 (5), 247 (5), 234 (4), 221 (9), 197 (11), 182 (61), 166 (12), 151 (8), 140 (49), 138 (16), 124 (34), 111 (23), 110 (19), 97 (25), 83 (33), 81 (23), 69 (51), 67 (22), 55 (61), 43 (24), 41 (51); MS (ESI, m/z, int [%]): 467 (100, M + Na), 465 (20); HR MS (ESI, m/z) for C22H42N3OSeNa: calcd: 467.2391, found: 467.2386; IR (ν, cm−1, KBr): 3308, 2931, 1550.
C22H42N3OSe, M = 444, 0.276 g, Yield: 82.9%, m.p. 145–150 °C, TLC: Rf = 0.16 benzene:methanol 9:1; yellow solid; purity (HPLC, method c): 97.6%; MS (EI, 70 eV, m/z, int [%]): 445 (20), 444 (21, M), 443 (10), 442 (12), 429 (3), 414 (7), 412 (9), 371 (34), 369 (18), 362 (18), 358 (19), 347 (15), 332 (100), 315 (23), 313 (12), 304 (7), 291 (14), 289 (9), 276 (14), 264 (7), 250 (5), 247 (5), 234 (4), 221 (9), 197 (11), 182 (61), 166 (12), 151 (8), 140 (49), 138 (16), 124 (34), 111 (23), 110 (19), 97 (25), 83 (33), 81 (23), 69 (51), 67 (22), 55 (61), 43 (24), 41 (51); MS (ESI, m/z, int [%]): 467 (100, M + Na), 465 (20); HR MS (ESI, m/z) for C22H42N3OSeNa: calcd: 467.2391, found: 467.2386; IR (ν, cm−1, KBr): 3308, 2931, 1550.
 C22H42N3OSe, M = 444, 0.276 g, Yield: 82.9%, m.p. 145–150 °C, TLC: Rf = 0.16 benzene:methanol 9:1; yellow solid; purity (HPLC, method c): 97.6%; MS (EI, 70 eV, m/z, int [%]): 445 (20), 444 (21, M), 443 (10), 442 (12), 429 (3), 414 (7), 412 (9), 371 (34), 369 (18), 362 (18), 358 (19), 347 (15), 332 (100), 315 (23), 313 (12), 304 (7), 291 (14), 289 (9), 276 (14), 264 (7), 250 (5), 247 (5), 234 (4), 221 (9), 197 (11), 182 (61), 166 (12), 151 (8), 140 (49), 138 (16), 124 (34), 111 (23), 110 (19), 97 (25), 83 (33), 81 (23), 69 (51), 67 (22), 55 (61), 43 (24), 41 (51); MS (ESI, m/z, int [%]): 467 (100, M + Na), 465 (20); HR MS (ESI, m/z) for C22H42N3OSeNa: calcd: 467.2391, found: 467.2386; IR (ν, cm−1, KBr): 3308, 2931, 1550.
C22H42N3OSe, M = 444, 0.276 g, Yield: 82.9%, m.p. 145–150 °C, TLC: Rf = 0.16 benzene:methanol 9:1; yellow solid; purity (HPLC, method c): 97.6%; MS (EI, 70 eV, m/z, int [%]): 445 (20), 444 (21, M), 443 (10), 442 (12), 429 (3), 414 (7), 412 (9), 371 (34), 369 (18), 362 (18), 358 (19), 347 (15), 332 (100), 315 (23), 313 (12), 304 (7), 291 (14), 289 (9), 276 (14), 264 (7), 250 (5), 247 (5), 234 (4), 221 (9), 197 (11), 182 (61), 166 (12), 151 (8), 140 (49), 138 (16), 124 (34), 111 (23), 110 (19), 97 (25), 83 (33), 81 (23), 69 (51), 67 (22), 55 (61), 43 (24), 41 (51); MS (ESI, m/z, int [%]): 467 (100, M + Na), 465 (20); HR MS (ESI, m/z) for C22H42N3OSeNa: calcd: 467.2391, found: 467.2386; IR (ν, cm−1, KBr): 3308, 2931, 1550.1-[(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)methyl]-3-(1-adamantyl) selenourea (5f). 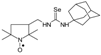 C20H34N3OSe, M = 412, 0.335 g, Yield: 58.1%, m.p. 78–82 °C, TLC: Rf = 0.26 benzene:methanol 9:1, yellow crystals; purity (HPLC, method a): 98.4%; MS (EI, 70 eV, m/z, int [%]): 413 (2), 412 (2, M), 330 (4), 300 (23), 135 (100), 124 (8), 94 (7), 93 (12), 79 (12), 55 (7), 41 (8); MS (ESI, m/z, int [%]): 435 (100, M + Na), 433 (10); HR MS (ESI, m/z) for C20H34N3OSeNa: calcd: 435.1765, found: 435.1767; IR (ν, cm−1, KBr): 3430, 2907, 1543, 683.
C20H34N3OSe, M = 412, 0.335 g, Yield: 58.1%, m.p. 78–82 °C, TLC: Rf = 0.26 benzene:methanol 9:1, yellow crystals; purity (HPLC, method a): 98.4%; MS (EI, 70 eV, m/z, int [%]): 413 (2), 412 (2, M), 330 (4), 300 (23), 135 (100), 124 (8), 94 (7), 93 (12), 79 (12), 55 (7), 41 (8); MS (ESI, m/z, int [%]): 435 (100, M + Na), 433 (10); HR MS (ESI, m/z) for C20H34N3OSeNa: calcd: 435.1765, found: 435.1767; IR (ν, cm−1, KBr): 3430, 2907, 1543, 683.
 C20H34N3OSe, M = 412, 0.335 g, Yield: 58.1%, m.p. 78–82 °C, TLC: Rf = 0.26 benzene:methanol 9:1, yellow crystals; purity (HPLC, method a): 98.4%; MS (EI, 70 eV, m/z, int [%]): 413 (2), 412 (2, M), 330 (4), 300 (23), 135 (100), 124 (8), 94 (7), 93 (12), 79 (12), 55 (7), 41 (8); MS (ESI, m/z, int [%]): 435 (100, M + Na), 433 (10); HR MS (ESI, m/z) for C20H34N3OSeNa: calcd: 435.1765, found: 435.1767; IR (ν, cm−1, KBr): 3430, 2907, 1543, 683.
C20H34N3OSe, M = 412, 0.335 g, Yield: 58.1%, m.p. 78–82 °C, TLC: Rf = 0.26 benzene:methanol 9:1, yellow crystals; purity (HPLC, method a): 98.4%; MS (EI, 70 eV, m/z, int [%]): 413 (2), 412 (2, M), 330 (4), 300 (23), 135 (100), 124 (8), 94 (7), 93 (12), 79 (12), 55 (7), 41 (8); MS (ESI, m/z, int [%]): 435 (100, M + Na), 433 (10); HR MS (ESI, m/z) for C20H34N3OSeNa: calcd: 435.1765, found: 435.1767; IR (ν, cm−1, KBr): 3430, 2907, 1543, 683.1-[(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)methyl]-3-phenyl selenourea (5g). 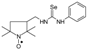 C16H24N3OSe, M = 354, 0.170 g, Yield: 46.7%, beige crystalline powder, m.p. 130–135 °C, TLC: Rf = 0.25 benzene:methanol 9:1; purity (HPLC, method a): 95.9%; MS (EI, 70 eV, m/z, int [%]): 355 (34), 354 (55, M), 352 (25), 339 (10), 337 (7), 324 (25), 322 (22), 320 (11), 281 (40), 279 (23), 272 (20), 268 (25), 260 (46), 258 (40), 257 (66), 243 (44), 242 (75), 226 (51), 225 (53), 223 (24), 213 (16), 204 (33), 203 (37), 201 (21), 199 (17), 184 (24), 183 (22), 174 (16), 173 (15), 167 (13), 157 (21), 140 (75), 133 (27), 131 (98), 124 (100), 119 (93), 118 (48), 110 (20), 109 (24), 99 (19), 98 (25), 93 (99), 81 (25), 77 (83), 69 (59), 67 (24), 65 (25), 58 (26), 56 (23), 55 (39), 51 (23), 41 (76); MS (ESI, m/z, int [%]): 377 (100, M + Na), 375 (30), 193 (20), 133 (10); HR MS (ESI, m/z) for C16H24N3OSeNa: calcd 377.0982, found: 377.0970; IR (ν, cm−1, KBr): 3161, 2967, 1552.
C16H24N3OSe, M = 354, 0.170 g, Yield: 46.7%, beige crystalline powder, m.p. 130–135 °C, TLC: Rf = 0.25 benzene:methanol 9:1; purity (HPLC, method a): 95.9%; MS (EI, 70 eV, m/z, int [%]): 355 (34), 354 (55, M), 352 (25), 339 (10), 337 (7), 324 (25), 322 (22), 320 (11), 281 (40), 279 (23), 272 (20), 268 (25), 260 (46), 258 (40), 257 (66), 243 (44), 242 (75), 226 (51), 225 (53), 223 (24), 213 (16), 204 (33), 203 (37), 201 (21), 199 (17), 184 (24), 183 (22), 174 (16), 173 (15), 167 (13), 157 (21), 140 (75), 133 (27), 131 (98), 124 (100), 119 (93), 118 (48), 110 (20), 109 (24), 99 (19), 98 (25), 93 (99), 81 (25), 77 (83), 69 (59), 67 (24), 65 (25), 58 (26), 56 (23), 55 (39), 51 (23), 41 (76); MS (ESI, m/z, int [%]): 377 (100, M + Na), 375 (30), 193 (20), 133 (10); HR MS (ESI, m/z) for C16H24N3OSeNa: calcd 377.0982, found: 377.0970; IR (ν, cm−1, KBr): 3161, 2967, 1552.
 C16H24N3OSe, M = 354, 0.170 g, Yield: 46.7%, beige crystalline powder, m.p. 130–135 °C, TLC: Rf = 0.25 benzene:methanol 9:1; purity (HPLC, method a): 95.9%; MS (EI, 70 eV, m/z, int [%]): 355 (34), 354 (55, M), 352 (25), 339 (10), 337 (7), 324 (25), 322 (22), 320 (11), 281 (40), 279 (23), 272 (20), 268 (25), 260 (46), 258 (40), 257 (66), 243 (44), 242 (75), 226 (51), 225 (53), 223 (24), 213 (16), 204 (33), 203 (37), 201 (21), 199 (17), 184 (24), 183 (22), 174 (16), 173 (15), 167 (13), 157 (21), 140 (75), 133 (27), 131 (98), 124 (100), 119 (93), 118 (48), 110 (20), 109 (24), 99 (19), 98 (25), 93 (99), 81 (25), 77 (83), 69 (59), 67 (24), 65 (25), 58 (26), 56 (23), 55 (39), 51 (23), 41 (76); MS (ESI, m/z, int [%]): 377 (100, M + Na), 375 (30), 193 (20), 133 (10); HR MS (ESI, m/z) for C16H24N3OSeNa: calcd 377.0982, found: 377.0970; IR (ν, cm−1, KBr): 3161, 2967, 1552.
C16H24N3OSe, M = 354, 0.170 g, Yield: 46.7%, beige crystalline powder, m.p. 130–135 °C, TLC: Rf = 0.25 benzene:methanol 9:1; purity (HPLC, method a): 95.9%; MS (EI, 70 eV, m/z, int [%]): 355 (34), 354 (55, M), 352 (25), 339 (10), 337 (7), 324 (25), 322 (22), 320 (11), 281 (40), 279 (23), 272 (20), 268 (25), 260 (46), 258 (40), 257 (66), 243 (44), 242 (75), 226 (51), 225 (53), 223 (24), 213 (16), 204 (33), 203 (37), 201 (21), 199 (17), 184 (24), 183 (22), 174 (16), 173 (15), 167 (13), 157 (21), 140 (75), 133 (27), 131 (98), 124 (100), 119 (93), 118 (48), 110 (20), 109 (24), 99 (19), 98 (25), 93 (99), 81 (25), 77 (83), 69 (59), 67 (24), 65 (25), 58 (26), 56 (23), 55 (39), 51 (23), 41 (76); MS (ESI, m/z, int [%]): 377 (100, M + Na), 375 (30), 193 (20), 133 (10); HR MS (ESI, m/z) for C16H24N3OSeNa: calcd 377.0982, found: 377.0970; IR (ν, cm−1, KBr): 3161, 2967, 1552.1,3-bis[(2,2,5,5-tetramethyl-1-oxyl-3-pyrrolidinyl)methyl] selenourea (5h). 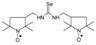 C19H36N4O2Se, M = 432, 0.249 g, Yield: 75.5%, m.p. 50–58 °C, TLC: Rf = 0.38 benzene:acetone 1:1, yellow glass; MS (EI, 70 eV, m/z, int [%]): 433 (4), 432 (4, M), 403 (3), 368 (5), 354 (11), 352 (15), 351 (10), 338 (26), 336 (31), 323 (10), 321 (14), 320 (12), 305 (24), 262 (32), 252 (13), 249 (15), 248 (13), 222 (14), 220 (13), 210 (12), 199 (23), 198 (25), 194 (29), 183 (46), 182 (54), 166 (21), 140 (69), 138 (40), 124 (100), 111 (20), 110 (24), 99 (22), 98 (22), 96 (20), 95 (16), 84 (22), 81 (20), 69 (30), 67 (19), 58 (26), 55 (31), 41 (38); MS (ESI, m/z, int [%]): 455 (100, M+Na), 453 (20); HR MS (ESI, m/z) for C19H36N4O2SeNa: calcd 455.1901, found: 455.1914; IR (ν, cm−1, KBr): 3432, 2971, 1557, 1462, 1364.
C19H36N4O2Se, M = 432, 0.249 g, Yield: 75.5%, m.p. 50–58 °C, TLC: Rf = 0.38 benzene:acetone 1:1, yellow glass; MS (EI, 70 eV, m/z, int [%]): 433 (4), 432 (4, M), 403 (3), 368 (5), 354 (11), 352 (15), 351 (10), 338 (26), 336 (31), 323 (10), 321 (14), 320 (12), 305 (24), 262 (32), 252 (13), 249 (15), 248 (13), 222 (14), 220 (13), 210 (12), 199 (23), 198 (25), 194 (29), 183 (46), 182 (54), 166 (21), 140 (69), 138 (40), 124 (100), 111 (20), 110 (24), 99 (22), 98 (22), 96 (20), 95 (16), 84 (22), 81 (20), 69 (30), 67 (19), 58 (26), 55 (31), 41 (38); MS (ESI, m/z, int [%]): 455 (100, M+Na), 453 (20); HR MS (ESI, m/z) for C19H36N4O2SeNa: calcd 455.1901, found: 455.1914; IR (ν, cm−1, KBr): 3432, 2971, 1557, 1462, 1364.
 C19H36N4O2Se, M = 432, 0.249 g, Yield: 75.5%, m.p. 50–58 °C, TLC: Rf = 0.38 benzene:acetone 1:1, yellow glass; MS (EI, 70 eV, m/z, int [%]): 433 (4), 432 (4, M), 403 (3), 368 (5), 354 (11), 352 (15), 351 (10), 338 (26), 336 (31), 323 (10), 321 (14), 320 (12), 305 (24), 262 (32), 252 (13), 249 (15), 248 (13), 222 (14), 220 (13), 210 (12), 199 (23), 198 (25), 194 (29), 183 (46), 182 (54), 166 (21), 140 (69), 138 (40), 124 (100), 111 (20), 110 (24), 99 (22), 98 (22), 96 (20), 95 (16), 84 (22), 81 (20), 69 (30), 67 (19), 58 (26), 55 (31), 41 (38); MS (ESI, m/z, int [%]): 455 (100, M+Na), 453 (20); HR MS (ESI, m/z) for C19H36N4O2SeNa: calcd 455.1901, found: 455.1914; IR (ν, cm−1, KBr): 3432, 2971, 1557, 1462, 1364.
C19H36N4O2Se, M = 432, 0.249 g, Yield: 75.5%, m.p. 50–58 °C, TLC: Rf = 0.38 benzene:acetone 1:1, yellow glass; MS (EI, 70 eV, m/z, int [%]): 433 (4), 432 (4, M), 403 (3), 368 (5), 354 (11), 352 (15), 351 (10), 338 (26), 336 (31), 323 (10), 321 (14), 320 (12), 305 (24), 262 (32), 252 (13), 249 (15), 248 (13), 222 (14), 220 (13), 210 (12), 199 (23), 198 (25), 194 (29), 183 (46), 182 (54), 166 (21), 140 (69), 138 (40), 124 (100), 111 (20), 110 (24), 99 (22), 98 (22), 96 (20), 95 (16), 84 (22), 81 (20), 69 (30), 67 (19), 58 (26), 55 (31), 41 (38); MS (ESI, m/z, int [%]): 455 (100, M+Na), 453 (20); HR MS (ESI, m/z) for C19H36N4O2SeNa: calcd 455.1901, found: 455.1914; IR (ν, cm−1, KBr): 3432, 2971, 1557, 1462, 1364.1-(2,2,6,6-Tetramethyl-1-oxyl-4-piperidynylyl)-3-methyl selenourea (6a). 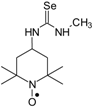 C11H22N3OSe, M = 292, 0.28 g, Yield: 64.1%, beige crystalline powder, m.p. 140 °C (dec.), TLC: Rf = 0.14 benzene:methanol 9:1; purity (HPLC, method b): 95.7%; MS (EI, 70 eV, m/z, int [%]): 294 (21), 293 (33), 292 (100, M), 290 (52), 289 (21), 288 (18), 260 (12), 258 (7), 219 (52), 217 (27), 180 (41), 163 (32), 140 (56), 139 (40), 124 (87), 109 (62), 98 (38), 97 (18), 96 (17), 84 (22), 83 (20), 82 (20), 81 (15), 69 (47), 67 (17), 58 (22), 57 (41), 56 (19), 55 (29), 42 (26), 41 (35); MS (ESI, m/z, int [%]): 315 (100, M + Na), 313 (25), 242 (60); HR MS (ESI, m/z) for C11H22N3OSeNa: calcd: 315.0826, found: 315.0823; IR (ν, cm−1, KBr): 3380, 3319, 1561.
C11H22N3OSe, M = 292, 0.28 g, Yield: 64.1%, beige crystalline powder, m.p. 140 °C (dec.), TLC: Rf = 0.14 benzene:methanol 9:1; purity (HPLC, method b): 95.7%; MS (EI, 70 eV, m/z, int [%]): 294 (21), 293 (33), 292 (100, M), 290 (52), 289 (21), 288 (18), 260 (12), 258 (7), 219 (52), 217 (27), 180 (41), 163 (32), 140 (56), 139 (40), 124 (87), 109 (62), 98 (38), 97 (18), 96 (17), 84 (22), 83 (20), 82 (20), 81 (15), 69 (47), 67 (17), 58 (22), 57 (41), 56 (19), 55 (29), 42 (26), 41 (35); MS (ESI, m/z, int [%]): 315 (100, M + Na), 313 (25), 242 (60); HR MS (ESI, m/z) for C11H22N3OSeNa: calcd: 315.0826, found: 315.0823; IR (ν, cm−1, KBr): 3380, 3319, 1561.
 C11H22N3OSe, M = 292, 0.28 g, Yield: 64.1%, beige crystalline powder, m.p. 140 °C (dec.), TLC: Rf = 0.14 benzene:methanol 9:1; purity (HPLC, method b): 95.7%; MS (EI, 70 eV, m/z, int [%]): 294 (21), 293 (33), 292 (100, M), 290 (52), 289 (21), 288 (18), 260 (12), 258 (7), 219 (52), 217 (27), 180 (41), 163 (32), 140 (56), 139 (40), 124 (87), 109 (62), 98 (38), 97 (18), 96 (17), 84 (22), 83 (20), 82 (20), 81 (15), 69 (47), 67 (17), 58 (22), 57 (41), 56 (19), 55 (29), 42 (26), 41 (35); MS (ESI, m/z, int [%]): 315 (100, M + Na), 313 (25), 242 (60); HR MS (ESI, m/z) for C11H22N3OSeNa: calcd: 315.0826, found: 315.0823; IR (ν, cm−1, KBr): 3380, 3319, 1561.
C11H22N3OSe, M = 292, 0.28 g, Yield: 64.1%, beige crystalline powder, m.p. 140 °C (dec.), TLC: Rf = 0.14 benzene:methanol 9:1; purity (HPLC, method b): 95.7%; MS (EI, 70 eV, m/z, int [%]): 294 (21), 293 (33), 292 (100, M), 290 (52), 289 (21), 288 (18), 260 (12), 258 (7), 219 (52), 217 (27), 180 (41), 163 (32), 140 (56), 139 (40), 124 (87), 109 (62), 98 (38), 97 (18), 96 (17), 84 (22), 83 (20), 82 (20), 81 (15), 69 (47), 67 (17), 58 (22), 57 (41), 56 (19), 55 (29), 42 (26), 41 (35); MS (ESI, m/z, int [%]): 315 (100, M + Na), 313 (25), 242 (60); HR MS (ESI, m/z) for C11H22N3OSeNa: calcd: 315.0826, found: 315.0823; IR (ν, cm−1, KBr): 3380, 3319, 1561.1-(2,2,6,6-Tetramethyl-1-oxyl-4-piperidynylyl)-3-(1-cyclododecyl) selenourea (6b). 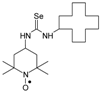 C22H42N3OSe, M = 444, 0.6 g, Yield: 91.0%, beige crystalline powder, m.p. 143–146 °C (dec.), TLC: Rf = 0.19 benzene:methanol 9:1; purity (HPLC, method c): 98.9%; MS (EI, 70 eV, m/z, int [%]): 447 (16), 446 (25), 445 (78, M+1), 444 (45, M), 443 (41), 442 (33), 441 (20), 429 (7), 415 (7), 412 (8), 371 (88), 369 (47), 332 (42), 315 (22), 313 (13), 291 (31), 289 (19), 182 (43), 157 (24), 155 (30), 140 (100), 124 (54), 109 (16), 98 (36), 83 (25), 74 (19), 69 (37), 55 (50), 43 (19), 41 (33); MS (ESI, m/z, int [%]): 467 (100, M + 23), 465 (20); HR MS (ESI, m/z) for C22H42N3OSeNa: calcd: 467.2391, found: 467.2383; IR (ν, cm−1, KBr): 3434, 3320, 2932, 1542.
C22H42N3OSe, M = 444, 0.6 g, Yield: 91.0%, beige crystalline powder, m.p. 143–146 °C (dec.), TLC: Rf = 0.19 benzene:methanol 9:1; purity (HPLC, method c): 98.9%; MS (EI, 70 eV, m/z, int [%]): 447 (16), 446 (25), 445 (78, M+1), 444 (45, M), 443 (41), 442 (33), 441 (20), 429 (7), 415 (7), 412 (8), 371 (88), 369 (47), 332 (42), 315 (22), 313 (13), 291 (31), 289 (19), 182 (43), 157 (24), 155 (30), 140 (100), 124 (54), 109 (16), 98 (36), 83 (25), 74 (19), 69 (37), 55 (50), 43 (19), 41 (33); MS (ESI, m/z, int [%]): 467 (100, M + 23), 465 (20); HR MS (ESI, m/z) for C22H42N3OSeNa: calcd: 467.2391, found: 467.2383; IR (ν, cm−1, KBr): 3434, 3320, 2932, 1542.
 C22H42N3OSe, M = 444, 0.6 g, Yield: 91.0%, beige crystalline powder, m.p. 143–146 °C (dec.), TLC: Rf = 0.19 benzene:methanol 9:1; purity (HPLC, method c): 98.9%; MS (EI, 70 eV, m/z, int [%]): 447 (16), 446 (25), 445 (78, M+1), 444 (45, M), 443 (41), 442 (33), 441 (20), 429 (7), 415 (7), 412 (8), 371 (88), 369 (47), 332 (42), 315 (22), 313 (13), 291 (31), 289 (19), 182 (43), 157 (24), 155 (30), 140 (100), 124 (54), 109 (16), 98 (36), 83 (25), 74 (19), 69 (37), 55 (50), 43 (19), 41 (33); MS (ESI, m/z, int [%]): 467 (100, M + 23), 465 (20); HR MS (ESI, m/z) for C22H42N3OSeNa: calcd: 467.2391, found: 467.2383; IR (ν, cm−1, KBr): 3434, 3320, 2932, 1542.
C22H42N3OSe, M = 444, 0.6 g, Yield: 91.0%, beige crystalline powder, m.p. 143–146 °C (dec.), TLC: Rf = 0.19 benzene:methanol 9:1; purity (HPLC, method c): 98.9%; MS (EI, 70 eV, m/z, int [%]): 447 (16), 446 (25), 445 (78, M+1), 444 (45, M), 443 (41), 442 (33), 441 (20), 429 (7), 415 (7), 412 (8), 371 (88), 369 (47), 332 (42), 315 (22), 313 (13), 291 (31), 289 (19), 182 (43), 157 (24), 155 (30), 140 (100), 124 (54), 109 (16), 98 (36), 83 (25), 74 (19), 69 (37), 55 (50), 43 (19), 41 (33); MS (ESI, m/z, int [%]): 467 (100, M + 23), 465 (20); HR MS (ESI, m/z) for C22H42N3OSeNa: calcd: 467.2391, found: 467.2383; IR (ν, cm−1, KBr): 3434, 3320, 2932, 1542.1-(2,2,5,5-Tetramethyl-1-oxyl-3-pyrrolidinyl)-3-(2,2,6,6-tetramethyl-1-oxyl-4-piperidynylyl) selenourea (7). 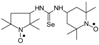 C18H34N4O2Se M = 417, 0.27 g; Yield: 93.1%, oil, TLC: Rf = 0.52 benzene:acetone 1:1, red oil; slow decomposition with evolving of selenium; purity (HPLC, method a): 99.8%; MS (EI, 70 eV, m/z, int [%]): 420 (13), 419 (16), 418 (43, M), 416 (22), 388 (4), 387 (6), 386 (7), 336 (24), 322 (11), 306 (17), 266 (11), 264 (20), 263 (11), 259 (16), 247 (16), 233 (13), 208 (22), 162 (12), 160 (32), 158 (27), 157 (11), 156 (19), 155 (16), 154 (20), 140 (89), 126 (49), 124 (100), 111 (43), 110 (46), 109 (50), 108 (19), 99 (44), 98 (91), 84 (86), 74 (47), 73 (28), 71 (22), 70 (46), 69 (68), 68 (17), 67 (29), 58 (58), 56 (99), 55 (67), 43 (73), 42 (34), 41 (67); MS (ESI, m/z, int [%]): 441(100, M + 23), 439 (10); HR MS (ESI, m/z) for C18H34N4O2SeNa: calcd: 441.1745, found: 441.1759; IR (ν, cm−1, film): 3316, 2973, 1545, 1461, 1364, 1330, 1242, 1180, 682.
C18H34N4O2Se M = 417, 0.27 g; Yield: 93.1%, oil, TLC: Rf = 0.52 benzene:acetone 1:1, red oil; slow decomposition with evolving of selenium; purity (HPLC, method a): 99.8%; MS (EI, 70 eV, m/z, int [%]): 420 (13), 419 (16), 418 (43, M), 416 (22), 388 (4), 387 (6), 386 (7), 336 (24), 322 (11), 306 (17), 266 (11), 264 (20), 263 (11), 259 (16), 247 (16), 233 (13), 208 (22), 162 (12), 160 (32), 158 (27), 157 (11), 156 (19), 155 (16), 154 (20), 140 (89), 126 (49), 124 (100), 111 (43), 110 (46), 109 (50), 108 (19), 99 (44), 98 (91), 84 (86), 74 (47), 73 (28), 71 (22), 70 (46), 69 (68), 68 (17), 67 (29), 58 (58), 56 (99), 55 (67), 43 (73), 42 (34), 41 (67); MS (ESI, m/z, int [%]): 441(100, M + 23), 439 (10); HR MS (ESI, m/z) for C18H34N4O2SeNa: calcd: 441.1745, found: 441.1759; IR (ν, cm−1, film): 3316, 2973, 1545, 1461, 1364, 1330, 1242, 1180, 682.
 C18H34N4O2Se M = 417, 0.27 g; Yield: 93.1%, oil, TLC: Rf = 0.52 benzene:acetone 1:1, red oil; slow decomposition with evolving of selenium; purity (HPLC, method a): 99.8%; MS (EI, 70 eV, m/z, int [%]): 420 (13), 419 (16), 418 (43, M), 416 (22), 388 (4), 387 (6), 386 (7), 336 (24), 322 (11), 306 (17), 266 (11), 264 (20), 263 (11), 259 (16), 247 (16), 233 (13), 208 (22), 162 (12), 160 (32), 158 (27), 157 (11), 156 (19), 155 (16), 154 (20), 140 (89), 126 (49), 124 (100), 111 (43), 110 (46), 109 (50), 108 (19), 99 (44), 98 (91), 84 (86), 74 (47), 73 (28), 71 (22), 70 (46), 69 (68), 68 (17), 67 (29), 58 (58), 56 (99), 55 (67), 43 (73), 42 (34), 41 (67); MS (ESI, m/z, int [%]): 441(100, M + 23), 439 (10); HR MS (ESI, m/z) for C18H34N4O2SeNa: calcd: 441.1745, found: 441.1759; IR (ν, cm−1, film): 3316, 2973, 1545, 1461, 1364, 1330, 1242, 1180, 682.
C18H34N4O2Se M = 417, 0.27 g; Yield: 93.1%, oil, TLC: Rf = 0.52 benzene:acetone 1:1, red oil; slow decomposition with evolving of selenium; purity (HPLC, method a): 99.8%; MS (EI, 70 eV, m/z, int [%]): 420 (13), 419 (16), 418 (43, M), 416 (22), 388 (4), 387 (6), 386 (7), 336 (24), 322 (11), 306 (17), 266 (11), 264 (20), 263 (11), 259 (16), 247 (16), 233 (13), 208 (22), 162 (12), 160 (32), 158 (27), 157 (11), 156 (19), 155 (16), 154 (20), 140 (89), 126 (49), 124 (100), 111 (43), 110 (46), 109 (50), 108 (19), 99 (44), 98 (91), 84 (86), 74 (47), 73 (28), 71 (22), 70 (46), 69 (68), 68 (17), 67 (29), 58 (58), 56 (99), 55 (67), 43 (73), 42 (34), 41 (67); MS (ESI, m/z, int [%]): 441(100, M + 23), 439 (10); HR MS (ESI, m/z) for C18H34N4O2SeNa: calcd: 441.1745, found: 441.1759; IR (ν, cm−1, film): 3316, 2973, 1545, 1461, 1364, 1330, 1242, 1180, 682.1-(1-Adamantyl)-3-(2,2,6,6-tetramethyl-1-oxyl-4-piperidynylyl) selenourea (8a). 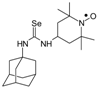 C20H34N3Ose, M = 412, 0.436 g, Yield: 36.0%, orange crystalline powder, m.p. 151–156 °C, TLC: Rf = 0.37 benzene:methanol 9:1; purity (HPLC, method a): 99.6%; MS (EI, 70 eV, m/z, int [%]): 318 (9), 300 (20), 177 (5), 160 (6), 151 (6), 140 (12), 135 (41), 124 (100), 98 (17), 94 (22), 79 (10), 67 (7), 58 (9), 57 (7), 56 (4), 42 (12), 41 (13); MS (ESI, m/z, int [%]): 435 (100, M + Na); HR MS (ESI): for C20H34N3OSeNa: calcd: 435.1765, found: 435.1746; IR (ν, cm−1, KBr): 3433, 2913, 1539.
C20H34N3Ose, M = 412, 0.436 g, Yield: 36.0%, orange crystalline powder, m.p. 151–156 °C, TLC: Rf = 0.37 benzene:methanol 9:1; purity (HPLC, method a): 99.6%; MS (EI, 70 eV, m/z, int [%]): 318 (9), 300 (20), 177 (5), 160 (6), 151 (6), 140 (12), 135 (41), 124 (100), 98 (17), 94 (22), 79 (10), 67 (7), 58 (9), 57 (7), 56 (4), 42 (12), 41 (13); MS (ESI, m/z, int [%]): 435 (100, M + Na); HR MS (ESI): for C20H34N3OSeNa: calcd: 435.1765, found: 435.1746; IR (ν, cm−1, KBr): 3433, 2913, 1539.
 C20H34N3Ose, M = 412, 0.436 g, Yield: 36.0%, orange crystalline powder, m.p. 151–156 °C, TLC: Rf = 0.37 benzene:methanol 9:1; purity (HPLC, method a): 99.6%; MS (EI, 70 eV, m/z, int [%]): 318 (9), 300 (20), 177 (5), 160 (6), 151 (6), 140 (12), 135 (41), 124 (100), 98 (17), 94 (22), 79 (10), 67 (7), 58 (9), 57 (7), 56 (4), 42 (12), 41 (13); MS (ESI, m/z, int [%]): 435 (100, M + Na); HR MS (ESI): for C20H34N3OSeNa: calcd: 435.1765, found: 435.1746; IR (ν, cm−1, KBr): 3433, 2913, 1539.
C20H34N3Ose, M = 412, 0.436 g, Yield: 36.0%, orange crystalline powder, m.p. 151–156 °C, TLC: Rf = 0.37 benzene:methanol 9:1; purity (HPLC, method a): 99.6%; MS (EI, 70 eV, m/z, int [%]): 318 (9), 300 (20), 177 (5), 160 (6), 151 (6), 140 (12), 135 (41), 124 (100), 98 (17), 94 (22), 79 (10), 67 (7), 58 (9), 57 (7), 56 (4), 42 (12), 41 (13); MS (ESI, m/z, int [%]): 435 (100, M + Na); HR MS (ESI): for C20H34N3OSeNa: calcd: 435.1765, found: 435.1746; IR (ν, cm−1, KBr): 3433, 2913, 1539.1-(3-Methylphenyl)-3-(2,2,6,6-tetramethyl-1-oxyl-4-piperidynylyl) selenourea (8b). 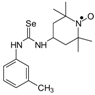 C17H26N3Ose, M = 368, 1.0334 g, Yield: 59.7%, brown crystalline powder, m.p. 140 °C, TLC: Rf = 0.20 benzene:methanol 9:1, purity (HPLC, method a): 95.6%; MS (EI, 70 eV, m/z, int [%]): 369 (0.5), 368 (0.5, M), 353 (1), 351 (0.5), 304 (1), 289 (3), 287 (3), 274 (15), 271 (16), 270 (8), 256 (37), 215 (10), 214 (12), 200 (19), 183 (7), 173 (5), 172 (5), 162 (12), 161 (8), 140 (18), 138 (6), 124 (100), 107 (18), 98 (33), 91 (18), 78 (98), 58 (23); MS (ESI, m/z, int [%]): 759 (5, 2*M + Na), 757 (5), 449 (7), 447 (7), 391 (85, M + Na), 389 (43), 370 (30), 369 (30, M + H), 368 (28), 367 (22), 366 (15), 365 (8), 354 (100), 352 (55), 327 (42), 290 (44); HR MS (ESI, m/z): for M + Na C17H26N3OSeNa, calcd.: 391.11333, found: 391.11179; IR (ν, cm−1, KBr): 3285, 3160, 2981, 1549.
C17H26N3Ose, M = 368, 1.0334 g, Yield: 59.7%, brown crystalline powder, m.p. 140 °C, TLC: Rf = 0.20 benzene:methanol 9:1, purity (HPLC, method a): 95.6%; MS (EI, 70 eV, m/z, int [%]): 369 (0.5), 368 (0.5, M), 353 (1), 351 (0.5), 304 (1), 289 (3), 287 (3), 274 (15), 271 (16), 270 (8), 256 (37), 215 (10), 214 (12), 200 (19), 183 (7), 173 (5), 172 (5), 162 (12), 161 (8), 140 (18), 138 (6), 124 (100), 107 (18), 98 (33), 91 (18), 78 (98), 58 (23); MS (ESI, m/z, int [%]): 759 (5, 2*M + Na), 757 (5), 449 (7), 447 (7), 391 (85, M + Na), 389 (43), 370 (30), 369 (30, M + H), 368 (28), 367 (22), 366 (15), 365 (8), 354 (100), 352 (55), 327 (42), 290 (44); HR MS (ESI, m/z): for M + Na C17H26N3OSeNa, calcd.: 391.11333, found: 391.11179; IR (ν, cm−1, KBr): 3285, 3160, 2981, 1549.
 C17H26N3Ose, M = 368, 1.0334 g, Yield: 59.7%, brown crystalline powder, m.p. 140 °C, TLC: Rf = 0.20 benzene:methanol 9:1, purity (HPLC, method a): 95.6%; MS (EI, 70 eV, m/z, int [%]): 369 (0.5), 368 (0.5, M), 353 (1), 351 (0.5), 304 (1), 289 (3), 287 (3), 274 (15), 271 (16), 270 (8), 256 (37), 215 (10), 214 (12), 200 (19), 183 (7), 173 (5), 172 (5), 162 (12), 161 (8), 140 (18), 138 (6), 124 (100), 107 (18), 98 (33), 91 (18), 78 (98), 58 (23); MS (ESI, m/z, int [%]): 759 (5, 2*M + Na), 757 (5), 449 (7), 447 (7), 391 (85, M + Na), 389 (43), 370 (30), 369 (30, M + H), 368 (28), 367 (22), 366 (15), 365 (8), 354 (100), 352 (55), 327 (42), 290 (44); HR MS (ESI, m/z): for M + Na C17H26N3OSeNa, calcd.: 391.11333, found: 391.11179; IR (ν, cm−1, KBr): 3285, 3160, 2981, 1549.
C17H26N3Ose, M = 368, 1.0334 g, Yield: 59.7%, brown crystalline powder, m.p. 140 °C, TLC: Rf = 0.20 benzene:methanol 9:1, purity (HPLC, method a): 95.6%; MS (EI, 70 eV, m/z, int [%]): 369 (0.5), 368 (0.5, M), 353 (1), 351 (0.5), 304 (1), 289 (3), 287 (3), 274 (15), 271 (16), 270 (8), 256 (37), 215 (10), 214 (12), 200 (19), 183 (7), 173 (5), 172 (5), 162 (12), 161 (8), 140 (18), 138 (6), 124 (100), 107 (18), 98 (33), 91 (18), 78 (98), 58 (23); MS (ESI, m/z, int [%]): 759 (5, 2*M + Na), 757 (5), 449 (7), 447 (7), 391 (85, M + Na), 389 (43), 370 (30), 369 (30, M + H), 368 (28), 367 (22), 366 (15), 365 (8), 354 (100), 352 (55), 327 (42), 290 (44); HR MS (ESI, m/z): for M + Na C17H26N3OSeNa, calcd.: 391.11333, found: 391.11179; IR (ν, cm−1, KBr): 3285, 3160, 2981, 1549.3.4. Nitroxyl Selenocarbamates 9a–9d; Reaction of the Nitroxyl Isoselenocyanates 1a, 1b with either Sodium Methoxide or Sodium Ethoxide; a General Procedure
A sodium methoxide solution was prepared by dissolving metallic sodium (0.050 g, 0.00217 mol) in either methanol or ethanol (5 mL). The sodium methoxide (ethoxide) solution (3.5 mL, 0.0015 mol), respectively, was added dropwise to the solution of the isoselenocyanate 1a or 1b (0.001 mol) in methanol or ethanol (5 mL). The reagents were stirred for 1 h at room temperature. The solvent was evaporated under reduced pressure. The residue was purified by column chromatography using benzene:methanol 9:1 or benzene:ethanol 9:1, respectively, as a mobile phase. A chromatographically purified product was triturated with hexane. A crystalline precipitate was filtered off to give the appropriate nitroxyl selenocarbamate 9a–9d (Scheme 3).
Methyl N-(2,2,5,5-tetramethyl-1-oxyl-3-pyrrolidinyl) selenonocarbamate (9a). 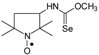 C10H19N2O2Se, M = 279, 0.37 g, Yield: 94.9%, yellow crystalline powder, m.p. 133–136 °C, TLC: Rf = 0.33 benzene:methanol 9:1; purity (HPLC, method b): 99.6%; MS (EI, 70 eV, m/z, int [%]): 280 (28), 279 (60, M), 277 (35), 249 (21), 247 (29), 245 (15), 234 (7), 222 (12), 220 (7), 206 (95), 204 (40), 203 (16), 202 (18), 193 (35), 191 (19), 178 (38), 176 (22), 168 (17), 166 (100), 164 (51), 163 (18), 162 (19), 149 (40), 147 (21), 136 (15), 126 (32), 125 (20), 124 (16), 123 (30), 112 (43), 111 (17), 110 (66), 100 (87), 98 (35), 95 (33), 82 (10), 81 (10), 69 (29), 67 (25), 58 (58), 56 (56), 55 (24), 42 (25), 41 (33); HR MS (EI, 70 eV, m/z) for C10H19N2O2Se: calcd: 279.06117, found: 279.06205; IR (ν, cm−1, KBr): 3420, 3235, 2973, 1537, 1458, 1386, 1208, 1138, 997.
C10H19N2O2Se, M = 279, 0.37 g, Yield: 94.9%, yellow crystalline powder, m.p. 133–136 °C, TLC: Rf = 0.33 benzene:methanol 9:1; purity (HPLC, method b): 99.6%; MS (EI, 70 eV, m/z, int [%]): 280 (28), 279 (60, M), 277 (35), 249 (21), 247 (29), 245 (15), 234 (7), 222 (12), 220 (7), 206 (95), 204 (40), 203 (16), 202 (18), 193 (35), 191 (19), 178 (38), 176 (22), 168 (17), 166 (100), 164 (51), 163 (18), 162 (19), 149 (40), 147 (21), 136 (15), 126 (32), 125 (20), 124 (16), 123 (30), 112 (43), 111 (17), 110 (66), 100 (87), 98 (35), 95 (33), 82 (10), 81 (10), 69 (29), 67 (25), 58 (58), 56 (56), 55 (24), 42 (25), 41 (33); HR MS (EI, 70 eV, m/z) for C10H19N2O2Se: calcd: 279.06117, found: 279.06205; IR (ν, cm−1, KBr): 3420, 3235, 2973, 1537, 1458, 1386, 1208, 1138, 997.
 C10H19N2O2Se, M = 279, 0.37 g, Yield: 94.9%, yellow crystalline powder, m.p. 133–136 °C, TLC: Rf = 0.33 benzene:methanol 9:1; purity (HPLC, method b): 99.6%; MS (EI, 70 eV, m/z, int [%]): 280 (28), 279 (60, M), 277 (35), 249 (21), 247 (29), 245 (15), 234 (7), 222 (12), 220 (7), 206 (95), 204 (40), 203 (16), 202 (18), 193 (35), 191 (19), 178 (38), 176 (22), 168 (17), 166 (100), 164 (51), 163 (18), 162 (19), 149 (40), 147 (21), 136 (15), 126 (32), 125 (20), 124 (16), 123 (30), 112 (43), 111 (17), 110 (66), 100 (87), 98 (35), 95 (33), 82 (10), 81 (10), 69 (29), 67 (25), 58 (58), 56 (56), 55 (24), 42 (25), 41 (33); HR MS (EI, 70 eV, m/z) for C10H19N2O2Se: calcd: 279.06117, found: 279.06205; IR (ν, cm−1, KBr): 3420, 3235, 2973, 1537, 1458, 1386, 1208, 1138, 997.
C10H19N2O2Se, M = 279, 0.37 g, Yield: 94.9%, yellow crystalline powder, m.p. 133–136 °C, TLC: Rf = 0.33 benzene:methanol 9:1; purity (HPLC, method b): 99.6%; MS (EI, 70 eV, m/z, int [%]): 280 (28), 279 (60, M), 277 (35), 249 (21), 247 (29), 245 (15), 234 (7), 222 (12), 220 (7), 206 (95), 204 (40), 203 (16), 202 (18), 193 (35), 191 (19), 178 (38), 176 (22), 168 (17), 166 (100), 164 (51), 163 (18), 162 (19), 149 (40), 147 (21), 136 (15), 126 (32), 125 (20), 124 (16), 123 (30), 112 (43), 111 (17), 110 (66), 100 (87), 98 (35), 95 (33), 82 (10), 81 (10), 69 (29), 67 (25), 58 (58), 56 (56), 55 (24), 42 (25), 41 (33); HR MS (EI, 70 eV, m/z) for C10H19N2O2Se: calcd: 279.06117, found: 279.06205; IR (ν, cm−1, KBr): 3420, 3235, 2973, 1537, 1458, 1386, 1208, 1138, 997.Ethyl N-(2,2,5,5-tetramethyl-1-oxyl-3-pyrrolidinyl) selenonocarbamate (9b). 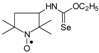 C11H21N2O2Se, M = 293, 0.365 g, Yield: 83.0%, yellow crystalline powder, m.p. 120–125 °C, TLC: Rf = 0.38 benzene:methanol 9:1; purity (HPLC, method b): 99.4%; MS (EI, 70 eV, m/z, int [%]): 293 (13, M), 291 (7), 263 (7), 261 (9), 236 (5), 234 (7), 220 (22), 218 (11), 207 (13), 205 (7), 192 (22), 190 (11), 182 (11), 180 (47), 178 (35), 176 (16), 154 (10), 152 (23), 150 (17), 149 (19), 126 (32), 125 (16), 124 (18), 110 (64), 100 (50), 98 (68), 95 (47), 84 (41), 70 (32), 69 (31), 67 (23), 58 (42), 57 (22), 56 (100), 55 (35), 43 (22), 42 (18), 41 (39); MS (ESI, m/z, int [%]): 316 (100, M + Na), 314 (15); HR MS (ESI, m/z) for C11H21N2O2SeNa: calcd: 316.0666, found: 316.0656; IR (ν, cm−1, KBr): 3420, 3225, 1541, 1463, 1379, 1208, 1020.
C11H21N2O2Se, M = 293, 0.365 g, Yield: 83.0%, yellow crystalline powder, m.p. 120–125 °C, TLC: Rf = 0.38 benzene:methanol 9:1; purity (HPLC, method b): 99.4%; MS (EI, 70 eV, m/z, int [%]): 293 (13, M), 291 (7), 263 (7), 261 (9), 236 (5), 234 (7), 220 (22), 218 (11), 207 (13), 205 (7), 192 (22), 190 (11), 182 (11), 180 (47), 178 (35), 176 (16), 154 (10), 152 (23), 150 (17), 149 (19), 126 (32), 125 (16), 124 (18), 110 (64), 100 (50), 98 (68), 95 (47), 84 (41), 70 (32), 69 (31), 67 (23), 58 (42), 57 (22), 56 (100), 55 (35), 43 (22), 42 (18), 41 (39); MS (ESI, m/z, int [%]): 316 (100, M + Na), 314 (15); HR MS (ESI, m/z) for C11H21N2O2SeNa: calcd: 316.0666, found: 316.0656; IR (ν, cm−1, KBr): 3420, 3225, 1541, 1463, 1379, 1208, 1020.
 C11H21N2O2Se, M = 293, 0.365 g, Yield: 83.0%, yellow crystalline powder, m.p. 120–125 °C, TLC: Rf = 0.38 benzene:methanol 9:1; purity (HPLC, method b): 99.4%; MS (EI, 70 eV, m/z, int [%]): 293 (13, M), 291 (7), 263 (7), 261 (9), 236 (5), 234 (7), 220 (22), 218 (11), 207 (13), 205 (7), 192 (22), 190 (11), 182 (11), 180 (47), 178 (35), 176 (16), 154 (10), 152 (23), 150 (17), 149 (19), 126 (32), 125 (16), 124 (18), 110 (64), 100 (50), 98 (68), 95 (47), 84 (41), 70 (32), 69 (31), 67 (23), 58 (42), 57 (22), 56 (100), 55 (35), 43 (22), 42 (18), 41 (39); MS (ESI, m/z, int [%]): 316 (100, M + Na), 314 (15); HR MS (ESI, m/z) for C11H21N2O2SeNa: calcd: 316.0666, found: 316.0656; IR (ν, cm−1, KBr): 3420, 3225, 1541, 1463, 1379, 1208, 1020.
C11H21N2O2Se, M = 293, 0.365 g, Yield: 83.0%, yellow crystalline powder, m.p. 120–125 °C, TLC: Rf = 0.38 benzene:methanol 9:1; purity (HPLC, method b): 99.4%; MS (EI, 70 eV, m/z, int [%]): 293 (13, M), 291 (7), 263 (7), 261 (9), 236 (5), 234 (7), 220 (22), 218 (11), 207 (13), 205 (7), 192 (22), 190 (11), 182 (11), 180 (47), 178 (35), 176 (16), 154 (10), 152 (23), 150 (17), 149 (19), 126 (32), 125 (16), 124 (18), 110 (64), 100 (50), 98 (68), 95 (47), 84 (41), 70 (32), 69 (31), 67 (23), 58 (42), 57 (22), 56 (100), 55 (35), 43 (22), 42 (18), 41 (39); MS (ESI, m/z, int [%]): 316 (100, M + Na), 314 (15); HR MS (ESI, m/z) for C11H21N2O2SeNa: calcd: 316.0666, found: 316.0656; IR (ν, cm−1, KBr): 3420, 3225, 1541, 1463, 1379, 1208, 1020.Methyl N-((2,2,5,5-tetramethyl-1-oxyl-3-pyrrolidinyl)methyl) selenonocarbamate (9c). 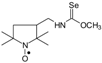 C11H21N2O2Se, M = 293, 0.246 g, Yield: 84.0%, beige crystalline powder, m.p. 140–145 °C, TLC: Rf = 0.31 benzene:methanol 9:1; purity (HPLC, method b): 99.2%; MS (EI, 70 eV, m/z, int [%]): 295 (18), 294 (15), 293 (96, M), 291 (48), 290 (17), 289 (18), 263 (73), 261 (59), 259 (25), 220 (49), 218 (24), 207 (32), 205 (16), 182 (35), 164 (55), 162 (27), 152 (44), 150 (25), 149 (25), 140 (44), 139 (49), 126 (29), 124 (39), 123 (49), 113 (28), 109 (69), 95 (19), 81 (38), 74 (18), 69 (100), 67 (33), 58 (26), 56 (28), 55 (42), 53 (21), 44 (62), 41 (96); MS (ESI, m/z, int [%]): 316 (100, M + Na), 314 (25), 304 (35), 227 (20); HR MS (ESI, m/z) for C11H21N2O2SeNa: calcd: 316.0666, found: 316.0672; IR (ν, cm−1, KBr): 3200, 2972, 1552, 1205, 973, 566.
C11H21N2O2Se, M = 293, 0.246 g, Yield: 84.0%, beige crystalline powder, m.p. 140–145 °C, TLC: Rf = 0.31 benzene:methanol 9:1; purity (HPLC, method b): 99.2%; MS (EI, 70 eV, m/z, int [%]): 295 (18), 294 (15), 293 (96, M), 291 (48), 290 (17), 289 (18), 263 (73), 261 (59), 259 (25), 220 (49), 218 (24), 207 (32), 205 (16), 182 (35), 164 (55), 162 (27), 152 (44), 150 (25), 149 (25), 140 (44), 139 (49), 126 (29), 124 (39), 123 (49), 113 (28), 109 (69), 95 (19), 81 (38), 74 (18), 69 (100), 67 (33), 58 (26), 56 (28), 55 (42), 53 (21), 44 (62), 41 (96); MS (ESI, m/z, int [%]): 316 (100, M + Na), 314 (25), 304 (35), 227 (20); HR MS (ESI, m/z) for C11H21N2O2SeNa: calcd: 316.0666, found: 316.0672; IR (ν, cm−1, KBr): 3200, 2972, 1552, 1205, 973, 566.
 C11H21N2O2Se, M = 293, 0.246 g, Yield: 84.0%, beige crystalline powder, m.p. 140–145 °C, TLC: Rf = 0.31 benzene:methanol 9:1; purity (HPLC, method b): 99.2%; MS (EI, 70 eV, m/z, int [%]): 295 (18), 294 (15), 293 (96, M), 291 (48), 290 (17), 289 (18), 263 (73), 261 (59), 259 (25), 220 (49), 218 (24), 207 (32), 205 (16), 182 (35), 164 (55), 162 (27), 152 (44), 150 (25), 149 (25), 140 (44), 139 (49), 126 (29), 124 (39), 123 (49), 113 (28), 109 (69), 95 (19), 81 (38), 74 (18), 69 (100), 67 (33), 58 (26), 56 (28), 55 (42), 53 (21), 44 (62), 41 (96); MS (ESI, m/z, int [%]): 316 (100, M + Na), 314 (25), 304 (35), 227 (20); HR MS (ESI, m/z) for C11H21N2O2SeNa: calcd: 316.0666, found: 316.0672; IR (ν, cm−1, KBr): 3200, 2972, 1552, 1205, 973, 566.
C11H21N2O2Se, M = 293, 0.246 g, Yield: 84.0%, beige crystalline powder, m.p. 140–145 °C, TLC: Rf = 0.31 benzene:methanol 9:1; purity (HPLC, method b): 99.2%; MS (EI, 70 eV, m/z, int [%]): 295 (18), 294 (15), 293 (96, M), 291 (48), 290 (17), 289 (18), 263 (73), 261 (59), 259 (25), 220 (49), 218 (24), 207 (32), 205 (16), 182 (35), 164 (55), 162 (27), 152 (44), 150 (25), 149 (25), 140 (44), 139 (49), 126 (29), 124 (39), 123 (49), 113 (28), 109 (69), 95 (19), 81 (38), 74 (18), 69 (100), 67 (33), 58 (26), 56 (28), 55 (42), 53 (21), 44 (62), 41 (96); MS (ESI, m/z, int [%]): 316 (100, M + Na), 314 (25), 304 (35), 227 (20); HR MS (ESI, m/z) for C11H21N2O2SeNa: calcd: 316.0666, found: 316.0672; IR (ν, cm−1, KBr): 3200, 2972, 1552, 1205, 973, 566.Ethyl N-((2,2,5,5-tetramethyl-1-oxyl-3-pyrrolidinyl)methyl) selenonocarbamate (9d). 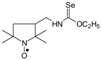 C12H23N2O2Se, M = 307, 0.225 g, Yield: 79.8%, beige crystalline powder, m.p. 120–130 °C, TLC: Rf = 0.31 benzene:methanol 9:1; purity (HPLC, method b): 99.5%; MS (EI, 70 eV, m/z, int [%]): 309 (19), 308 (17), 307 (100, M), 305 (52), 304 (19), 303 (20), 277 (65), 275 (49), 261 (5), 247 (10), 234 (42), 232 (20), 221 (32), 219 (16), 206 (12), 196 (28), 192 (26), 190 (13), 183 (22), 178 (51), 176 (25), 166 (20), 164 (13), 154 (30), 149 (21), 140 (35), 139 (33), 138 (47), 127 (22), 126 (30), 124 (40), 123 (22), 109 (64), 98 (13), 96 (11), 95 (15), 84 (23), 83 (17), 82 (21), 81 (36), 74 (19), 69 (94), 67 (30), 58 (36), 56 (32), 55 (47), 53 (19), 41 (92); MS (ESI, m/z, int [%]): 330 (100, M + Na), 328 (25); HR MS (ESI, m/z) for C12H23N2O2SeNa: calcd 330.0822, found: 330.0808; IR (ν, cm−1, KBr): 3200, 2971, 1556, 1414, 1201, 1019.
C12H23N2O2Se, M = 307, 0.225 g, Yield: 79.8%, beige crystalline powder, m.p. 120–130 °C, TLC: Rf = 0.31 benzene:methanol 9:1; purity (HPLC, method b): 99.5%; MS (EI, 70 eV, m/z, int [%]): 309 (19), 308 (17), 307 (100, M), 305 (52), 304 (19), 303 (20), 277 (65), 275 (49), 261 (5), 247 (10), 234 (42), 232 (20), 221 (32), 219 (16), 206 (12), 196 (28), 192 (26), 190 (13), 183 (22), 178 (51), 176 (25), 166 (20), 164 (13), 154 (30), 149 (21), 140 (35), 139 (33), 138 (47), 127 (22), 126 (30), 124 (40), 123 (22), 109 (64), 98 (13), 96 (11), 95 (15), 84 (23), 83 (17), 82 (21), 81 (36), 74 (19), 69 (94), 67 (30), 58 (36), 56 (32), 55 (47), 53 (19), 41 (92); MS (ESI, m/z, int [%]): 330 (100, M + Na), 328 (25); HR MS (ESI, m/z) for C12H23N2O2SeNa: calcd 330.0822, found: 330.0808; IR (ν, cm−1, KBr): 3200, 2971, 1556, 1414, 1201, 1019.
 C12H23N2O2Se, M = 307, 0.225 g, Yield: 79.8%, beige crystalline powder, m.p. 120–130 °C, TLC: Rf = 0.31 benzene:methanol 9:1; purity (HPLC, method b): 99.5%; MS (EI, 70 eV, m/z, int [%]): 309 (19), 308 (17), 307 (100, M), 305 (52), 304 (19), 303 (20), 277 (65), 275 (49), 261 (5), 247 (10), 234 (42), 232 (20), 221 (32), 219 (16), 206 (12), 196 (28), 192 (26), 190 (13), 183 (22), 178 (51), 176 (25), 166 (20), 164 (13), 154 (30), 149 (21), 140 (35), 139 (33), 138 (47), 127 (22), 126 (30), 124 (40), 123 (22), 109 (64), 98 (13), 96 (11), 95 (15), 84 (23), 83 (17), 82 (21), 81 (36), 74 (19), 69 (94), 67 (30), 58 (36), 56 (32), 55 (47), 53 (19), 41 (92); MS (ESI, m/z, int [%]): 330 (100, M + Na), 328 (25); HR MS (ESI, m/z) for C12H23N2O2SeNa: calcd 330.0822, found: 330.0808; IR (ν, cm−1, KBr): 3200, 2971, 1556, 1414, 1201, 1019.
C12H23N2O2Se, M = 307, 0.225 g, Yield: 79.8%, beige crystalline powder, m.p. 120–130 °C, TLC: Rf = 0.31 benzene:methanol 9:1; purity (HPLC, method b): 99.5%; MS (EI, 70 eV, m/z, int [%]): 309 (19), 308 (17), 307 (100, M), 305 (52), 304 (19), 303 (20), 277 (65), 275 (49), 261 (5), 247 (10), 234 (42), 232 (20), 221 (32), 219 (16), 206 (12), 196 (28), 192 (26), 190 (13), 183 (22), 178 (51), 176 (25), 166 (20), 164 (13), 154 (30), 149 (21), 140 (35), 139 (33), 138 (47), 127 (22), 126 (30), 124 (40), 123 (22), 109 (64), 98 (13), 96 (11), 95 (15), 84 (23), 83 (17), 82 (21), 81 (36), 74 (19), 69 (94), 67 (30), 58 (36), 56 (32), 55 (47), 53 (19), 41 (92); MS (ESI, m/z, int [%]): 330 (100, M + Na), 328 (25); HR MS (ESI, m/z) for C12H23N2O2SeNa: calcd 330.0822, found: 330.0808; IR (ν, cm−1, KBr): 3200, 2971, 1556, 1414, 1201, 1019.3.5. Nitroxyl Selenocarbamates 10a–10d; Reaction of the Isoselenocyanates 1d–1g with 4-hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxyl (3c); a General Procedure
4-Hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxyl (3c, 0.69 g, 0.004 mola) was dissolved in anhydrous THF (15 mL). Sodium hydride as 60% dispersion in mineral oil (0.2 g, 0.005 mol) was added. The reagents were refluxed for 1 h. The reaction mixture was allowed to cool to room temperature. Isoselenocyanate 1d–1g (0.004 mola) was added. The reagents were stirred for 1 h at room temperature. Methylene chloride (15 mL) and water (15 mL) were added to the reaction mixture. The layers were separated. The organic layer was dried over anhydrous magnesium sulfate and concentrated to a constant mass. To the residue either the mixture of benzene (1 mL) and hexane (1 mL) or pure hexane was added. The mixture was stirred for 5 min. The precipitate was filtered off to give nitroxyl selenocarbamate 10a–10d (Scheme 4).
2,2,6,6-Tetramethyl-1-oxyl-4-piperidinyl N-(1-adamantyl) selenonocarbamate (10a). 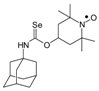 C20H33N2O2Se, M = 413, 1.18 g, Yield: 71.6%, orange crystalline powder, m.p. 198–203 °C, TLC: Rf = 0.51 benzene:ethyl acetate 4:1; purity (HPLC, method c): 99.3%; MS (EI, 70 eV, m/z, int [%]): 413 (5, M), 411 (3), 260 (8), 258 (4), 177 (16), 154 (53), 140 (15), 135 (100), 124 (98), 120 (40), 109 (45), 94 (37), 93 (17), 83 (8), 82 (10), 81 (16), 79 (19), 77 (10), 74 (18), 71 (6), 69 (14), 67 (20), 55 (28), 41 (33); HR MS (EI, 70 eV, m/z) for C20H33N2O2Se: calcd: 413.17072, found: 413.17190; IR (ν, cm−1, KBr): 3434, 3200, 2913, 1534, 1198, 1156.
C20H33N2O2Se, M = 413, 1.18 g, Yield: 71.6%, orange crystalline powder, m.p. 198–203 °C, TLC: Rf = 0.51 benzene:ethyl acetate 4:1; purity (HPLC, method c): 99.3%; MS (EI, 70 eV, m/z, int [%]): 413 (5, M), 411 (3), 260 (8), 258 (4), 177 (16), 154 (53), 140 (15), 135 (100), 124 (98), 120 (40), 109 (45), 94 (37), 93 (17), 83 (8), 82 (10), 81 (16), 79 (19), 77 (10), 74 (18), 71 (6), 69 (14), 67 (20), 55 (28), 41 (33); HR MS (EI, 70 eV, m/z) for C20H33N2O2Se: calcd: 413.17072, found: 413.17190; IR (ν, cm−1, KBr): 3434, 3200, 2913, 1534, 1198, 1156.
 C20H33N2O2Se, M = 413, 1.18 g, Yield: 71.6%, orange crystalline powder, m.p. 198–203 °C, TLC: Rf = 0.51 benzene:ethyl acetate 4:1; purity (HPLC, method c): 99.3%; MS (EI, 70 eV, m/z, int [%]): 413 (5, M), 411 (3), 260 (8), 258 (4), 177 (16), 154 (53), 140 (15), 135 (100), 124 (98), 120 (40), 109 (45), 94 (37), 93 (17), 83 (8), 82 (10), 81 (16), 79 (19), 77 (10), 74 (18), 71 (6), 69 (14), 67 (20), 55 (28), 41 (33); HR MS (EI, 70 eV, m/z) for C20H33N2O2Se: calcd: 413.17072, found: 413.17190; IR (ν, cm−1, KBr): 3434, 3200, 2913, 1534, 1198, 1156.
C20H33N2O2Se, M = 413, 1.18 g, Yield: 71.6%, orange crystalline powder, m.p. 198–203 °C, TLC: Rf = 0.51 benzene:ethyl acetate 4:1; purity (HPLC, method c): 99.3%; MS (EI, 70 eV, m/z, int [%]): 413 (5, M), 411 (3), 260 (8), 258 (4), 177 (16), 154 (53), 140 (15), 135 (100), 124 (98), 120 (40), 109 (45), 94 (37), 93 (17), 83 (8), 82 (10), 81 (16), 79 (19), 77 (10), 74 (18), 71 (6), 69 (14), 67 (20), 55 (28), 41 (33); HR MS (EI, 70 eV, m/z) for C20H33N2O2Se: calcd: 413.17072, found: 413.17190; IR (ν, cm−1, KBr): 3434, 3200, 2913, 1534, 1198, 1156.2,2,6,6-Tetramethyl-1-oxyl-4-piperidinyl N-(3-methylphenyl) selenonocarbamate (10b). 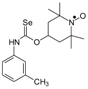 C17H25N2O2Se, M = 369, 0.4606 g, Yield: 36.1%, m.p. 48–51 °C, TLC: Rf = 0.45 benzene:ethyl acetate 9:1 Rf = 0.65 benzene:methanol 9:1, red glass; MS (EI, 70 eV, m/z, int [%]): 370 (4), 369 (6, M), 368 (2), 367 (3), 366 (2), 248 (1), 243 (2), 216 (3), 215 (2), 214 (3), 197 (19), 195 (9), 194 (4), 156 (18), 155 (29), 154 (75), 140 (39), 133 (22), 134 (25), 124 (100), 109 (83), 107 (20), 106 (18), 100 (13), 98 (11), 91 (27), 74 (20), 55 (25), 41 (24). HR MS (EI, 70 eV, m/z): for C17H25N2O2Se, calcd.: 369.10812, found: 369.10737, IR (ν, cm−1, film): 1634, 1531, 1364, 1179, 1140.
C17H25N2O2Se, M = 369, 0.4606 g, Yield: 36.1%, m.p. 48–51 °C, TLC: Rf = 0.45 benzene:ethyl acetate 9:1 Rf = 0.65 benzene:methanol 9:1, red glass; MS (EI, 70 eV, m/z, int [%]): 370 (4), 369 (6, M), 368 (2), 367 (3), 366 (2), 248 (1), 243 (2), 216 (3), 215 (2), 214 (3), 197 (19), 195 (9), 194 (4), 156 (18), 155 (29), 154 (75), 140 (39), 133 (22), 134 (25), 124 (100), 109 (83), 107 (20), 106 (18), 100 (13), 98 (11), 91 (27), 74 (20), 55 (25), 41 (24). HR MS (EI, 70 eV, m/z): for C17H25N2O2Se, calcd.: 369.10812, found: 369.10737, IR (ν, cm−1, film): 1634, 1531, 1364, 1179, 1140.
 C17H25N2O2Se, M = 369, 0.4606 g, Yield: 36.1%, m.p. 48–51 °C, TLC: Rf = 0.45 benzene:ethyl acetate 9:1 Rf = 0.65 benzene:methanol 9:1, red glass; MS (EI, 70 eV, m/z, int [%]): 370 (4), 369 (6, M), 368 (2), 367 (3), 366 (2), 248 (1), 243 (2), 216 (3), 215 (2), 214 (3), 197 (19), 195 (9), 194 (4), 156 (18), 155 (29), 154 (75), 140 (39), 133 (22), 134 (25), 124 (100), 109 (83), 107 (20), 106 (18), 100 (13), 98 (11), 91 (27), 74 (20), 55 (25), 41 (24). HR MS (EI, 70 eV, m/z): for C17H25N2O2Se, calcd.: 369.10812, found: 369.10737, IR (ν, cm−1, film): 1634, 1531, 1364, 1179, 1140.
C17H25N2O2Se, M = 369, 0.4606 g, Yield: 36.1%, m.p. 48–51 °C, TLC: Rf = 0.45 benzene:ethyl acetate 9:1 Rf = 0.65 benzene:methanol 9:1, red glass; MS (EI, 70 eV, m/z, int [%]): 370 (4), 369 (6, M), 368 (2), 367 (3), 366 (2), 248 (1), 243 (2), 216 (3), 215 (2), 214 (3), 197 (19), 195 (9), 194 (4), 156 (18), 155 (29), 154 (75), 140 (39), 133 (22), 134 (25), 124 (100), 109 (83), 107 (20), 106 (18), 100 (13), 98 (11), 91 (27), 74 (20), 55 (25), 41 (24). HR MS (EI, 70 eV, m/z): for C17H25N2O2Se, calcd.: 369.10812, found: 369.10737, IR (ν, cm−1, film): 1634, 1531, 1364, 1179, 1140. 2,2,6,6-Tetramethyl-1-oxyl-4-piperidinyl N-(4-(trifluoromethyl)phenyl) selenonocarbamate (10c). 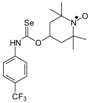 C17H22F3N2O2Se, M = 423, 0.670 g, Yield: 63.5%, beige crystalline powder, m.p. 119–123 °C (dec.), TLC: Rf = 0.59 hexane:ethyl acetate 9:1; purity (HPLC, method a): 99.6%; MS (EI, 70 eV, m/z, int [%]): 424 (5), 423 (4, M), 422 (3), 421 (3), 251 (12), 249 (6), 188 (12), 187 (20), 156 (37), 155 (65), 154 (42), 145 (27), 140 (45), 124 (71), 109 (54), 100 (48), 98 (22), 83 (32), 82 (20), 81 (23), 74 (100), 70 (6), 68 (10), 56 (42), 55 (75), 41 (59); HR MS (EI, 70 eV, m/z): for C17H22N2O2F3Se, calcd.: 423.07986, found: 423.08047; IR (ν, cm−1, KBr): 3436, 1618, 1525, 1324, 1166, 1120, 1068, 840.
C17H22F3N2O2Se, M = 423, 0.670 g, Yield: 63.5%, beige crystalline powder, m.p. 119–123 °C (dec.), TLC: Rf = 0.59 hexane:ethyl acetate 9:1; purity (HPLC, method a): 99.6%; MS (EI, 70 eV, m/z, int [%]): 424 (5), 423 (4, M), 422 (3), 421 (3), 251 (12), 249 (6), 188 (12), 187 (20), 156 (37), 155 (65), 154 (42), 145 (27), 140 (45), 124 (71), 109 (54), 100 (48), 98 (22), 83 (32), 82 (20), 81 (23), 74 (100), 70 (6), 68 (10), 56 (42), 55 (75), 41 (59); HR MS (EI, 70 eV, m/z): for C17H22N2O2F3Se, calcd.: 423.07986, found: 423.08047; IR (ν, cm−1, KBr): 3436, 1618, 1525, 1324, 1166, 1120, 1068, 840.
 C17H22F3N2O2Se, M = 423, 0.670 g, Yield: 63.5%, beige crystalline powder, m.p. 119–123 °C (dec.), TLC: Rf = 0.59 hexane:ethyl acetate 9:1; purity (HPLC, method a): 99.6%; MS (EI, 70 eV, m/z, int [%]): 424 (5), 423 (4, M), 422 (3), 421 (3), 251 (12), 249 (6), 188 (12), 187 (20), 156 (37), 155 (65), 154 (42), 145 (27), 140 (45), 124 (71), 109 (54), 100 (48), 98 (22), 83 (32), 82 (20), 81 (23), 74 (100), 70 (6), 68 (10), 56 (42), 55 (75), 41 (59); HR MS (EI, 70 eV, m/z): for C17H22N2O2F3Se, calcd.: 423.07986, found: 423.08047; IR (ν, cm−1, KBr): 3436, 1618, 1525, 1324, 1166, 1120, 1068, 840.
C17H22F3N2O2Se, M = 423, 0.670 g, Yield: 63.5%, beige crystalline powder, m.p. 119–123 °C (dec.), TLC: Rf = 0.59 hexane:ethyl acetate 9:1; purity (HPLC, method a): 99.6%; MS (EI, 70 eV, m/z, int [%]): 424 (5), 423 (4, M), 422 (3), 421 (3), 251 (12), 249 (6), 188 (12), 187 (20), 156 (37), 155 (65), 154 (42), 145 (27), 140 (45), 124 (71), 109 (54), 100 (48), 98 (22), 83 (32), 82 (20), 81 (23), 74 (100), 70 (6), 68 (10), 56 (42), 55 (75), 41 (59); HR MS (EI, 70 eV, m/z): for C17H22N2O2F3Se, calcd.: 423.07986, found: 423.08047; IR (ν, cm−1, KBr): 3436, 1618, 1525, 1324, 1166, 1120, 1068, 840.2,2,6,6-Tetramethyl-1-oxyl-4-piperidinyl N-(4-chloro-2-methylphenyl) selenonocarbamate (10d). 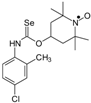 C17H24ClN2O2Se, M = 403, 0.595 g, Yield: 36.9%, yellow crystalline powder, m.p. 115–122 °C, TLC: Rf = 0.49 benzene:ethyl acetate 4:1; MS (EI, 70 eV, m/z, int [%]): 406 (5), 405 (7), 404 (10), 403 (16, M), 402 (6), 401 (9), 250 (5), 248 (3), 233 (13), 231 (24), 229 (10), 196 (4), 169 (14), 168 (14), 167 (47), 155 (43), 154 (60), 141 (35), 140 (44), 132 (22), 124 (100), 116 (17), 109 (75), 106 (18), 100 (21), 98 (27), 89 (18), 83 (18), 82 (3), 81 (20), 78 (26), 77 (25), 74 (46), 71 (25), 69 (34), 67 (37), 57 (50), 56 (52), 55 (59), 53 (10), 52 (12), 51 (16), 50 (13), 43 (43), 42 (33), 41 (92), 39 (35); MS (ESI, m/z, int [%]): 827 (10), 428 (20), 426 (100, M + 23), 424 (30); HR MS (ESI, 70 eV, m/z) for C17H24ClN2O2NaSe: calcd: 426.0589, found: 426.0574; IR (ν, cm−1, KBr): 3434, 1634, 1510, 1363, 1200, 1149.
C17H24ClN2O2Se, M = 403, 0.595 g, Yield: 36.9%, yellow crystalline powder, m.p. 115–122 °C, TLC: Rf = 0.49 benzene:ethyl acetate 4:1; MS (EI, 70 eV, m/z, int [%]): 406 (5), 405 (7), 404 (10), 403 (16, M), 402 (6), 401 (9), 250 (5), 248 (3), 233 (13), 231 (24), 229 (10), 196 (4), 169 (14), 168 (14), 167 (47), 155 (43), 154 (60), 141 (35), 140 (44), 132 (22), 124 (100), 116 (17), 109 (75), 106 (18), 100 (21), 98 (27), 89 (18), 83 (18), 82 (3), 81 (20), 78 (26), 77 (25), 74 (46), 71 (25), 69 (34), 67 (37), 57 (50), 56 (52), 55 (59), 53 (10), 52 (12), 51 (16), 50 (13), 43 (43), 42 (33), 41 (92), 39 (35); MS (ESI, m/z, int [%]): 827 (10), 428 (20), 426 (100, M + 23), 424 (30); HR MS (ESI, 70 eV, m/z) for C17H24ClN2O2NaSe: calcd: 426.0589, found: 426.0574; IR (ν, cm−1, KBr): 3434, 1634, 1510, 1363, 1200, 1149.
 C17H24ClN2O2Se, M = 403, 0.595 g, Yield: 36.9%, yellow crystalline powder, m.p. 115–122 °C, TLC: Rf = 0.49 benzene:ethyl acetate 4:1; MS (EI, 70 eV, m/z, int [%]): 406 (5), 405 (7), 404 (10), 403 (16, M), 402 (6), 401 (9), 250 (5), 248 (3), 233 (13), 231 (24), 229 (10), 196 (4), 169 (14), 168 (14), 167 (47), 155 (43), 154 (60), 141 (35), 140 (44), 132 (22), 124 (100), 116 (17), 109 (75), 106 (18), 100 (21), 98 (27), 89 (18), 83 (18), 82 (3), 81 (20), 78 (26), 77 (25), 74 (46), 71 (25), 69 (34), 67 (37), 57 (50), 56 (52), 55 (59), 53 (10), 52 (12), 51 (16), 50 (13), 43 (43), 42 (33), 41 (92), 39 (35); MS (ESI, m/z, int [%]): 827 (10), 428 (20), 426 (100, M + 23), 424 (30); HR MS (ESI, 70 eV, m/z) for C17H24ClN2O2NaSe: calcd: 426.0589, found: 426.0574; IR (ν, cm−1, KBr): 3434, 1634, 1510, 1363, 1200, 1149.
C17H24ClN2O2Se, M = 403, 0.595 g, Yield: 36.9%, yellow crystalline powder, m.p. 115–122 °C, TLC: Rf = 0.49 benzene:ethyl acetate 4:1; MS (EI, 70 eV, m/z, int [%]): 406 (5), 405 (7), 404 (10), 403 (16, M), 402 (6), 401 (9), 250 (5), 248 (3), 233 (13), 231 (24), 229 (10), 196 (4), 169 (14), 168 (14), 167 (47), 155 (43), 154 (60), 141 (35), 140 (44), 132 (22), 124 (100), 116 (17), 109 (75), 106 (18), 100 (21), 98 (27), 89 (18), 83 (18), 82 (3), 81 (20), 78 (26), 77 (25), 74 (46), 71 (25), 69 (34), 67 (37), 57 (50), 56 (52), 55 (59), 53 (10), 52 (12), 51 (16), 50 (13), 43 (43), 42 (33), 41 (92), 39 (35); MS (ESI, m/z, int [%]): 827 (10), 428 (20), 426 (100, M + 23), 424 (30); HR MS (ESI, 70 eV, m/z) for C17H24ClN2O2NaSe: calcd: 426.0589, found: 426.0574; IR (ν, cm−1, KBr): 3434, 1634, 1510, 1363, 1200, 1149.3.6. Antifungal Activity Assays
Fungitoxicity of the tested compounds against phytopathogenic fungi was assessed in vitro using agar growth medium poison technique. PDA media in 100 mm Petri plates containing the acetone solutions of the tested compounds in the defined concentrations were infected with agar disks with thin mycelium of fungi cultures and allowed the solvent to evaporate. Linear growth of each colony was determined after 3–5 days. The effect of each compound on mycelial growth was assessed by calculating the percentage of growth reduction, where: percentage of linear growth reduction = [(colony diameter of the control plate – colony diameter of the tested plate)/(colony diameter of the control plate)] × 100.
3.7. Antibacterial Activity Assays
Antibacterial tests were performed by dilution method on a solid support. The test results were read after 48 h incubation of the plates at 25 °C with bacterial strains. The antibacterial activity of the compounds was expressed in terms of the minimum growth inhibitory concentration of the test strain (MIC) in mg/L. Plant pathogenic strains: Erwinia carotovora, Pseudomonas phaseolicola Pseudomonas lachrymans, Pseudomonas syringae, were used.
4. Conclusions
Selenourea and selenocarbamate nitroxides 4–10 were synthesized in the reaction of corresponding isoselenocyanates and either amines or alcohols, respectively. The investigated selenium compounds 4–10 were tested in vitro against nine pathogenic fungi, and against four phytopathogenic bacteria. Significant fungistatic and bacteriostatic activities of the investigated compounds were found. Ten nitroxide selenoureas 4c, 4d, 4h, 5a–5d, 5g, 5h, and 7 were shown the fungistatic activity at the concentration of 20 mg/L at 100% level (MIC ≤ 20). Twelve nitroxide selenoureas 4a, 4c, 4d, 4f, 4g, 5b, 5c, 5g, 5h, 6a, 6b, and 8a, and two nitroxide selenocarbamates 9b, and 10d, have shown bacteriostatic activity at the concentration of 100 mg/L (MIC ≤ 100).
Supplementary Materials
The supplementary materials are available online. Mass spectrometry EI MS, ESI MS, and IR spectroscopy of the synthesized compounds.
Author Contributions
J.Z.: concept of the research, planning and performing of the experiments (synthesis), writing and preparing of the manuscript; B.H.: concept of the research, planning and performing of the experiments (synthesis); A.K.: performing of the MS spectra; M.K.: planning and performing of the biological assays; J.H.: HPLC analysis; K.J.: preparing of the supplementary material.
Funding
This work was financially supported by a Grant N N209 044739, National Science Centre, Poland.
Acknowledgments
We would like to thank Bogusława Wantusiak and Barbara Zdunek for assistance in the syntheses of all described compounds. We are grateful to Hanna Konopka for performing the IR measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zakrzewski, J.; Huras, B.; Kiełczewska, A.; Krawczyk, M. Reactions of nitroxides 16. First nitroxides containing tellurium atom. RSC Adv. 2016, 6, 98829–98834. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Banerjee, B.; Koketsu, M. Recent developments in the synthesis of biologically relevant selenium-containing scaffolds (Review). Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Hassan, W.; Oliveira, C.S.; Noreen, H.; Kamdem, J.P.; Nogueira, C.W.; Rocha, J.B.T. Organoselenium compounds as potential neuroprotective therapeutic agents. Curr. Org. Chem. 2016, 20, 218–231. [Google Scholar] [CrossRef]
- Puntel, R.L.; Ávila, D.S.; Roos, D.H.; Pinton, S. Mitochondrial effects of organoselenium and organotellurium compounds. Curr. Org. Chem. 2016, 20, 198–210. [Google Scholar] [CrossRef]
- Santi, C.; Tidei, C.; Scalera, C.; Piroddi, M.; Galli, F. Selenium containing compounds from poison to drug candidates: A review on the GPx-like activity. Curr. Chem. Biol. 2013, 7, 25–36. [Google Scholar] [CrossRef]
- Moroder, L. Isosteric replacement of sulfur with other chalcogens in peptides and protein. J. Peptide Sci. 2005, 11, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.-W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Xu, J.; Lin, A.; Wu, X.; Wu, L.; Xie, W. Recent advances for the synthesis of selenium-containing small molecules as potent antitumor agents. Curr. Med. Chem. 2018, 25, 2009–2033. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.A.; Badshah, A.; Shah, A. Synthesis and biological applications of selenoureas. Appl. Organomet. Chem. 2014, 28, 61–73. [Google Scholar] [CrossRef]
- Takahashi, H.; Nishina, A.; Fukumoto, R.-H.; Kimura, H.; Koketsu, M.; Ishihara, H. Selenoureas and thioureas are effective superoxide radical scavengers in vitro. Life Sci. 2005, 76, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, J.; Huras, B.; Hupko, J. Nowe pochodne selenoorganiczne i ich zastosowanie. Polish Patent 226530, 23 December 2013. [Google Scholar]
- Sivapriya, K.; Suguna, P.; Banerjee, A.; Saravanan, V.; Rao, D.N.; Chandrasekaran, S. Facile one-pot synthesis of thio and selenourea derivatives: A new class of potent urease inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 6387–6391. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, P.N.; Roy, G.; Mugesh, G. Effect of thione–thiol tautomerism on the inhibition of lactoperoxidase by anti-thyroid drugs and their analogues. J. Chem. Sci. 2008, 120, 143–154. [Google Scholar] [CrossRef]
- Ha, S.K.; Koketsu, M.; Lee, K.; Choi, S.Y.; Park, J.-H.; Ishihara, H.; Kim, S.Y. Inhibition of Tyrosinase Activity by N,N-Unsubstituted Selenourea Derivatives. Biol. Pharm. Bull. 2005, 28, 838–840. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.A.; Badshah, A.; Pezzuto, J.M.; Ahmed, N.; Kondratyuk, T.P.; Eun-Jung Park, E.-J. Ferrocene incorporated selenoureas as anticancer agents. J. Photochem. Photobiol. B: Biol. 2015, 148, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.A.; Badshah, A.; Tahir, M.N.; Hassan, T.-U.; Bano, A. Synthesis, Chemical Characterization, DNA Binding, Antioxidant, Antibacterial, and Antifungal Activities of Ferrocence Incorporated Selenoureas. J. Biochem. Mol. Toxicol. 2014, 28, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.A.; Badshah, A.; Sohail, M.; Lal, B.; Altaf, A.A. Synthesis, chemical characterization, DNA interaction and antioxidant studies of ortho, meta and para fluoro substituted ferrocene incorporated selenoureas. Inorg. Chim. Acta 2013, 402, 133–139. [Google Scholar] [CrossRef]
- Hussain, R.A.; Badshah, A.; Tahir, M.N.; Lal, B.; Khan, I.A. Synthesis, Chemical Characterisation, and DNA Binding; Studies of Ferrocene-Incorporated Selenoureas. Aust. J. Chem. 2013, 66, 626–634. [Google Scholar] [CrossRef]
- Neganova, M.E.; Proshin, A.N.; Redkozubova, O.M.; Serkov, I.V.; Serkova, T.P.; Dubova, L.G.; Shevtsova, E.F. N,N’-Substituted Selenoureas as Polyfunctional Antioxidants. Bull. Exp. Biol. Med. Pharmacol. Tox. 2016, 160, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Merino-Montiel, P.; Maza, S.; Martos, S.; López, O.; Maya, I.; Fernández-Bolaños, J.G. Synthesis and antioxidant activity of O-alkyl selenocarbamates, selenoureas and selenohydantoins. Eur. J. Pharm. Sci. 2013, 48, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, H.; Koketsu, M.; Kato, M.; Kurabayashi, M.; Nishina, A.; Kimura, H. Superoxide radical-scavenging effects from polymorphonuclear leukocytes and toxicity in human cell lines of newly synthesized organic selenium compounds. FEBS J. 2007, 274, 6046–6054. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, J.; Huras, B.; Krawczyk, M.; Hupko, J.; Wantusiak, B.; Zdunek, B.; Michalczyk, A.; Cieniecka-Rosłonkiewicz, A.; Świech, K.; Morytz, B.; et al. Nowe Pochodne Selenoorganiczne Oraz ich Zastosowanie. Polish Patent 226529, 23 December 2013. [Google Scholar]
- Zakrzewski, J.; Krawczyk, M. Synthesis and Pesticidal Properties of Thio and Seleno Analogs of Some Common Urea Herbicides. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 1880–1903. [Google Scholar] [CrossRef]
- Zakrzewski, J.; Krawczyk, M. Reactions of Nitroxides, Part 7: Synthesis of Novel Nitroxide Selenoureas. Heteroat. Chem. 2008, 19, 549–556. [Google Scholar] [CrossRef]
- Casula, A.; Begines, P.; Bettoschi, A.; Fernandez-Bolańos, J.G.; Isaia, F.; Lippolis, V.; López, Ó.; Picci, G.; Scorciapino, M.A.; Caltagironea, C. Selenoureas for anion binding as molecular logic gates. Chem. Commun. 2017, 53, 11869–11872. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, W.-D.; Ahlers, K.-D.; Falodun, A.; Villinger, A.; Langer, P. Synthesis and Spectroscopic Characterization of Arylated Selenoureas. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 324–332. [Google Scholar] [CrossRef]
- Koketsu, M. Thioamides, Thioureas, and Related Selenium and Tellurium Compounds. In Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium Volume 1, 2nd ed.; Devillanova, F., Du Mont, W.-W., Eds.; RSC Publishing: Stratford-upon-Avon, UK, 2013; Chapter 2.2; pp. 94–117. [Google Scholar]
- Chennakrishnareddy, G.; Nagendra, G.; Hemantha, H.P.; Sureshbabu, V.V.; Das, U.; Guru Row, T.N. Isoselenocyanates derived from Boc/Z-amino acids: Synthesis, isolation, characterization, and application to the efficient synthesis of unsymmetrical selenoureas and selenoureidopeptidomimetics. Tetrahedron 2010, 66, 6718–6724. [Google Scholar] [CrossRef]
- Hemantha, H.P.; Sureshbabu, V.V. Isoselenocyanates derived from amino acid esters: An expedient synthesis and application to the assembly of selenoureidopeptidomimetics, unsymmetrical Selenoureas and selenohydantoins. J. Pept. Sci. 2010, 16, 644–651. [Google Scholar] [CrossRef]
- Ninomiya, M.; Garud, D.R.; Koketsu, M. Selenium-Containing Heterocycles Using Selenoamides, Selenoureas, Selenazadienes, and Isoselenocyanates. Heterocycles 2010, 81, 2027–2055. [Google Scholar]
- Lopez, O.; Maza, S.; Ulgar, V.; Maya, I.; Fernandez-Bolanos, J.G. Synthesis of sugar-derived isoselenocyanates, selenoureas, and selenazoles. Tetrahedron 2009, 65, 2556–2566. [Google Scholar] [CrossRef]
- Garud, D.R.; Koketsu, M.; Ishihara, H. Isoselenocyanates: A Powerful Tool for the Synthesis of Selenium-Containing Heterocycles. Molecules 2007, 12, 504–535. [Google Scholar] [CrossRef] [PubMed]
- Koketsu, M.; Ishihara, H. Thiourea and Selenourea and Their Applications. Curr. Org. Synth. 2006, 439–455. [Google Scholar] [CrossRef]
- Koketsu, M.; Ishihara, H. Thioamides, Thioureas and Related Selenium and Tellurium Compounds. In Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium; Devillanova, F., Ed.; RSC: Stratford-upon-Avon, UK, 2007; Chapter 2.4; pp. 145–194. [Google Scholar]
- Braverman, S.; Cherkinsky, M.; Birsa, M.L. Science of Synthesis; Knight, J.G., Ed.; Geog Thieme Verlag: Stuttgart, Germany, 2005; Volume 18, pp. 65–320. [Google Scholar]
- Fernandez-Bolanos, J.G.; Lopez, O.; Ulgar, V.; Maya, I.; Fuentes, J. Synthesis of O-unprotected glycosyl selenoureas. A new access to bicyclic sugar isoureas. Tetrahedron Lett. 2004, 45, 4081–4084. [Google Scholar] [CrossRef]
- Atanassov, P.K.; Zhou, Y.; Linden, A.; Heimgartner, H. Synthesis of Bis(2,4-diarylimidazol-5-yl) Diselenides from N-Benzylbenzimidoyl Isoselenocyanates. Helv. Chim. Acta 2002, 8, 1102–1117. [Google Scholar] [CrossRef]
- Blum, T.; Ermert, J.; Coenen, H.H. No-carrier-added (n.c.a.) synthesis of asymmetric [73,75Se]selenoethers with isonitriles. Appl. Radiat. Isot. 2002, 57, 51–56. [Google Scholar] [CrossRef]
- Jensen, K.A.; Nielsen, P.H. Infrared spectra of Thioamides and Selenoamides. Acta Chem. Scand. 1966, 20, 597–629. [Google Scholar] [CrossRef]
- Collard-Charon, C.; Renson, M. Etude infra-rouge des substances possedant une liaison C=Se adajacente a un ou plusieurs atomes d’azote. I. Selenourees. Bull. Soc. Chim. Belg. 1963, 72, 149–165. [Google Scholar]
- Collard-Charon, C.; Huls, R.; Renson, M. Synthese des Selenosemicarbazides Substituees, II. Synthese des des Selenosemicarbazides Substituees en. Bull. Soc. Chim. Belg. 1962, 71, 541–553. [Google Scholar] [CrossRef]
- Collard-Charon, C.; Renson, M. Synthese des Selenosemicarbazides Substituees, I. Synthese des Estaers Isoselenocyaniques. Bull. Soc. Chim. Belg. 1962, 71, 531–540. [Google Scholar] [CrossRef]
- Koketsu, M.; Suzuki, N.; Ishihara, H. Preparation of Isoselenocyanate and Synthesis of Carbodiimide by Oxidation of Selenourea. J. Org. Chem. 1999, 64, 6473–6475. [Google Scholar] [CrossRef]
- Cohen, V.I. A Convenient Synthesis of Mono, N,N′-Di, and Trisubstituted Selenoureas from Methyl Carbamimidothioates (S-Methylpsedothioureas). Synthesis 1980, 1, 60–63. [Google Scholar] [CrossRef]
- Klayman, D.L.; Shine, R.J. A New Synthesis of Selenoureas and Selenothiocarbamic Esters from Thioureas. J. Org. Chem. 1969, 34, 3549–3551. [Google Scholar] [CrossRef] [PubMed]
- Klayman, D.L.; Chase, C.; Shine, R.J. Secret. Army; Method of Synthesizing Selenoureas from Thioureas. US Patent 3,597,444, 3 August 1971. [Google Scholar]
- Basavaprabhu, K.M.S.; Prabhu, G.; Panduranga, V.; Sureshbabu, V.V. A facile one-pot synthesis of selenoureidopeptides employing LiAlHSeH through staudinger aza-wittig-type reaction. Synthesis (Germany) 2015, 47, 801–806. [Google Scholar]
- Koketsu, M.; Ishihara, H. (Gifu University), Selenating Reagent. U.S. Patent 7,033,564, 1 April 2004. [Google Scholar]
- Koketsu, M.; Takakura, N.; Ishihara, H. Efficient Synthesis of Selenoureas from the Corresponding Carbodiimides. Synth. Commun. 2002, 32, 3075–3079. [Google Scholar] [CrossRef]
- Koketsu, M.; Fukuta, Y.; Ishihara, H. Reaction of N,N-Dimethylselenocarbamoyl Chloride with Nucleophiles. Preparation of Diselenocarbamates, Selenothiocarbamates, and Selenoureas. J. Org. Chem. 2002, 67, 1008–1011. [Google Scholar] [CrossRef]
- Ishihara, H.; Koketsu, M.; Fukuta, Y.; Nada, F. Reaction of Lithium Aluminum Hydride with Elemental Selenium: Its Application as a Selenating Reagent into Organic Molecules. J. Am. Chem. Soc. 2001, 123, 8408–8409. [Google Scholar] [CrossRef]
- Maeda, H.; Takashima, M.; Sakata, K.; Watanabe, T.; Honda, M.; Segi, M. One-pot synthesis of selenoureas and selenocarbamates via selenation of isocyanates with bis(dimethylaluminum) selenide. Tetrahedron Lett. 2011, 52, 415–417. [Google Scholar] [CrossRef]
- Campos, M.P.; Hendricks, M.P.; Beecher, A.N.; Walravens, W.; Swain, R.A.; Cleveland, G.T.; Hens, Z.; Sfeir, M.Y.; Owen, J.S. A Library of Selenourea Precursors to PbSe Nanocrystals with Size Distributions near the Homogeneous Limit. J. Am. Chem. Soc. 2017, 139, 2296–2305. [Google Scholar] [CrossRef]
- Zhou, Y.; Denk, M.K. Synthesis and reactivity of subvalent compounds. Part 13: Reaction of triethyl orthoformate with amines and selenium—A convenient one-step three-component synthesis for selenoureas. Tetrahedron Lett. 2003, 44, 1295–1299. [Google Scholar] [CrossRef]
- Shimada, K.; Yamaguchi, M.; Sasaki, T.; Ohnishi, K.; Takikawa, Y. A Willgerodt-Kindler Type Selenation of Dihalomethane Derivatives, Chloroform, and Sodium Trichloroacetate by Trating with a Base, Elemental Selenium, and an Amine. Bull. Chem. Soc. Jpn. 1996, 69, 2235–2242. [Google Scholar] [CrossRef]
- Takikawa, Y.; Yamaguchi, M.; Sasaki, T.; Ohnishi, K.; Shimada, K. Convenient Synthesis of N,N,N’,N’-Tetraalkylselenoureas by Treating Terminal gem-Dihaloalkanes, Chloroform, or Sodium Trichloroacetate with a Base, Elemental Selenium, and Amines. Chem. Lett. 1994, 23, 2105–2108. [Google Scholar] [CrossRef]
- Hua, G.; Cordes, D.B.; Du, J.; Slawin, A.M.Z.; Derek Woollins, J. Diverse derivatives of selenoureas: A synthetic and single crystal structural study. Molecules 2018, 23, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Font, M.; Encío, I.; Palop, J.A.; Sanmartín, C. Synthesis and antiproliferative activity of novel methylselenocarbamates. Eur. J. Med. Chem. 2014, 83, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Plano, D.; Encío, I.; Palop, J.A.; Sanmartín, C. In vitro radical scavenging and cytotoxic activities of novel hybrid selenocarbamates. Bioorg. Med. Chem. 2015, 23, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Nishina, A.; Fukumoto, R.-H.; Kimura, H.; Koketsu, M.; Ishihara, H. Selenocarbamates are effective superoxide anion scavengers in vitro. Eur. J. Pharm. Sci. 2005, 24, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.H.R.; Parekh, S.I.; Tajbakhsh, M.; Theodorakis, E.A.; Tse, C.L. A Convenient and High Yielding Procedure for the Preparation of Isoselenocyanates. Synthesis and Reactivity of O-Alkylselenocarbamates. Tetrahedron 1994, 50, 639–654. [Google Scholar] [CrossRef]
- Beigrezaei, S.; Nasri, H. Tempol as an antioxidant; an updated review on current knowledge. Ann. Res. Antioxid. 2017, 2, e01. [Google Scholar]
- Prescott, C.; Bottle, S.E. Biological Relevance of Free Radicals and Nitroxides. Cell Biochem. Biophys. 2017, 75, 227–240. [Google Scholar] [CrossRef]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as antioxidants and anticancer drugs. Int. J. Mol. Sci. 2017, 18, 2490–2515. [Google Scholar] [CrossRef]
- Hideg, K.; Kálai, T.; Sár, C.P. Recent results in chemistry and biology of nitroxides. J. Heterocycl. Chem. 2005, 42, 437–450. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Xu, J.; Zhang, H.; Tian, M. Using a TEMPO-based fluorescent probe for monitoring oxidative stress in living cells. Analyst 2011, 136, 4316–4320. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; DeGraff, W.; Hankovszky, O.H.; Sar, C.P.; Kalai, T.; Jeko, J.; Russo, A.; Mitchell, J.B.; Hideg, K. Studies of Structure-Activity Relationship of Nitroxide Free Radicals and Their Precursors as Modifiers Against Oxidative Damage. J. Med. Chem. 1998, 41, 3477–3492. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Russo, A.; Mitchell, J.B.; Goldstein, S.; Dafni, H.; Samuni, A. Do Nitroxide Antioxidants Act as Scavengers of O2. or as SOD Mimics? J. Biol. Chem. 1996, 271, 26026–26031. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, O.D.; Frolova, T.S.; Yushkova, Y.V.; Chernyak, E.I.; Pokrovsky, A.G.; Pokrovsky, M.A.; Morozov, S.V.; Sinitsina, O.I.; Grigoriev, I.A.; Nevinsky, G.A. Antioxidant and antitumor activity of trolox, trolox succinate, and α-tocopheryl succinate conjugates with nitroxides. Eur. J. Med. Chem. 2016, 122, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Samuni, A.; Hideg, K.; Merenyi, G. Structure-Activity Relationship of Cyclic Nitroxides as SOD Mimics and Scavengers of Nitrogen Dioxide and Carbonate Radicals. J. Phys. Chem. A 2006, 110, 3679–3685. [Google Scholar] [CrossRef] [PubMed]
- Metodiewa, D.; Skolimowski, J.; Karolczak, S. Tempace and troxyl-novel synthesized 2,2,6,6-tetramethylpiperidine derivatives as antioxidants and radioprotectors. Biochem. Mol. Biol. Int. 1996, 40, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.M.; Tochner, Z.; Krishna, C.M.; Glass, J.; Wilson, L.; Samuni, A.; Sprague, M.; Venzon, D.; Glatstein, E.; Mitchell, J.B.; et al. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992, 52, 1750–1753. [Google Scholar] [PubMed]
- Kálai, T.; Mugesh, G.; Roy, G.; Sies, H.; Berente, Z.; Hideg, K. Combining benzo[d]isoselenazol-3-ones with sterically hindered, alicyclic amines and nitroxides: Enhanced activity as glutathione, peroxidase mimics. Org. Biomol. Chem. 2005, 3, 3564–3569. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S.; Pearlman, A. Chemistry and Antihypertensive Effects of Tempol and Other Nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Poliak, P.; Kavala, M.; Barbieriková, Z.; Zalibera, M.; Fronc, M.; Švorc, Ľ.; Vihonská, Z.; Olejníková, P.; Lušpai, K.; et al. Polyradical PROXYL/TEMPO-Derived Amides: Synthesis, Physicochemical Studies, DFT Calculations, and Antimicrobial Activity. ChemPlusChem 2017, 82, 1326–1340. [Google Scholar] [CrossRef]
- Emanuel, N.M.; Konovalova, N.P. Nitroxyl Radicals for Cancer Chemotherapy. In Bioactive Spin Labels; Zhdanov, R.I., Ed.; Springer: Berlin, Germany, 1992; pp. 439–460. [Google Scholar]
- Skolimowski, J.; Kochman, A.; Gębicka, L.; Metodiewa, D. Synthesis and Antioxidant Activity Evaluation of Novel Antiparkinsonian Agents, Aminoadamantane Derivatives of Nitroxyl Free Radical. Bioorg. Med. Chem. 2003, 11, 3529–3539. [Google Scholar] [CrossRef]
- Einhorn, J.; Einhorn, C.; Ratajczak, F.; Durif, A.; Averbuch, M.-T.; Pierre, J.-L. Synthesis and resolution of a chiral analogue of 2,2,6,6-tetramethylpiperidine and of its corresponding nitroxide. Tetrahedron Lett. 1998, 39, 2565–2568. [Google Scholar] [CrossRef]
- Barrett, A.G.M.; Hanson, G.R.; White, A.J.P.; Williams, D.J.; Micallef, A.S. Synthesis of nitroxide-functionalized phthalocyanines. Tetrahedron 2007, 63, 5244–5250. [Google Scholar] [CrossRef]
- Gubskaya, V.P.; Berezhnaya, L.Sh.; Gubaidullin, A.T.; Faingold, I.I.; Kotelnikova, R.A.; Konovalova, N.P.; Morozov, V.I.; Litvinov, I.A.; Nuretdinov, I.A. Synthesis, structure and biological activity of nitroxide malonate methanofullerenes. Org. Biomol. Chem. 2007, 5, 976–981. [Google Scholar] [CrossRef]
- Yi, S.; Mileo, E.; Kaifer, A.E. EPR and NMR investigation on the interactions of nitroxide probes with resorcin [4]arene molecular capsules. Org. Lett. 2009, 11, 5690–5693. [Google Scholar] [CrossRef]
- Fairfull-Smith, K.E.; Debele, E.A.; Allen, J.P.; Pfrunder, M.C.; McMurtrie, J.C. Direct iodination of isoindolines and isoindoline nitroxides as precursors to functionalized nitroxides. Eur. J. Org. Chem. 2013, 22, 4829–4835. [Google Scholar] [CrossRef]
- Zakrzewski, J.; Huras, B.; Kiełczewska, A. Synthesis of isoselenocyanates. Synthesis 2016, 48, 85–96. [Google Scholar] [CrossRef]
- Zakrzewski, J.; Jezierska, J.; Hupko, J. 4-Isocyano-2,2,6,6-tetramethylpiperidin-1-oxyl: A Valuable Precursor for the Synthesis of New Nitroxides. Org. Lett. 2004, 6, 695–697. [Google Scholar] [CrossRef]
- Zakrzewski, J.; Krawczyk, M. Reactions of Nitroxides. Part X: Antifungal activity of selected sulfur and selenium derivatives of 2,2,6,6-tetramethylpiperidine. Bioorg. Med. Chem. Lett. 2011, 21, 21–514. [Google Scholar] [CrossRef]
- Huras, B.; Zakrzewski, J.; Krawczyk, M. Reactions of Nitroxides, Part XI: O-Aryl Phenylselenophosphonates Bearing a Nitroxyl Moiety. Heteroat. Chem. 2011, 22, 137–147. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).