A Redox-Switchable Colorimetric Probe for “Naked-Eye” Detection of Hypochlorous Acid and Glutathione
Abstract
1. Introduction
2. Results and Discussion
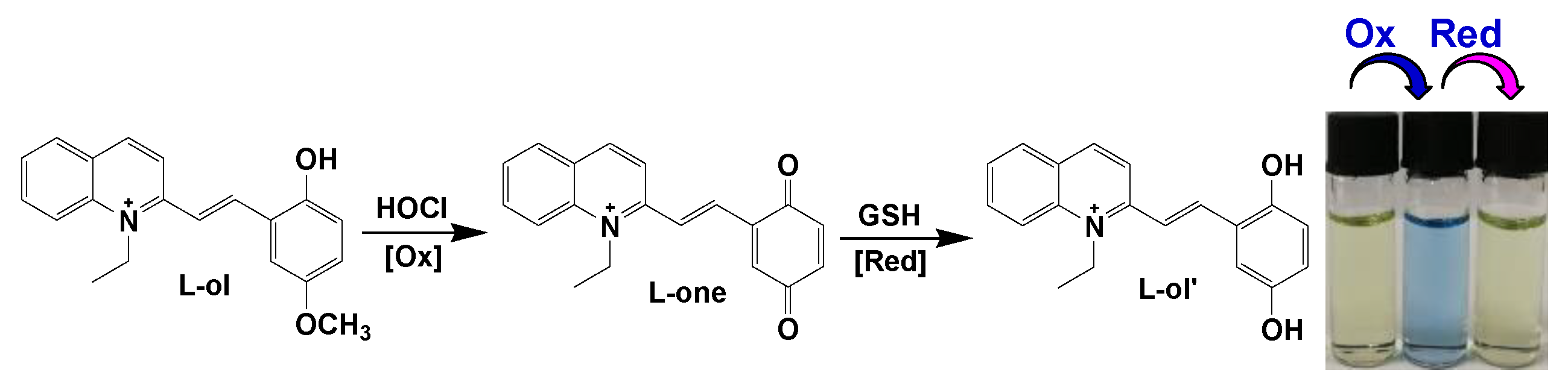
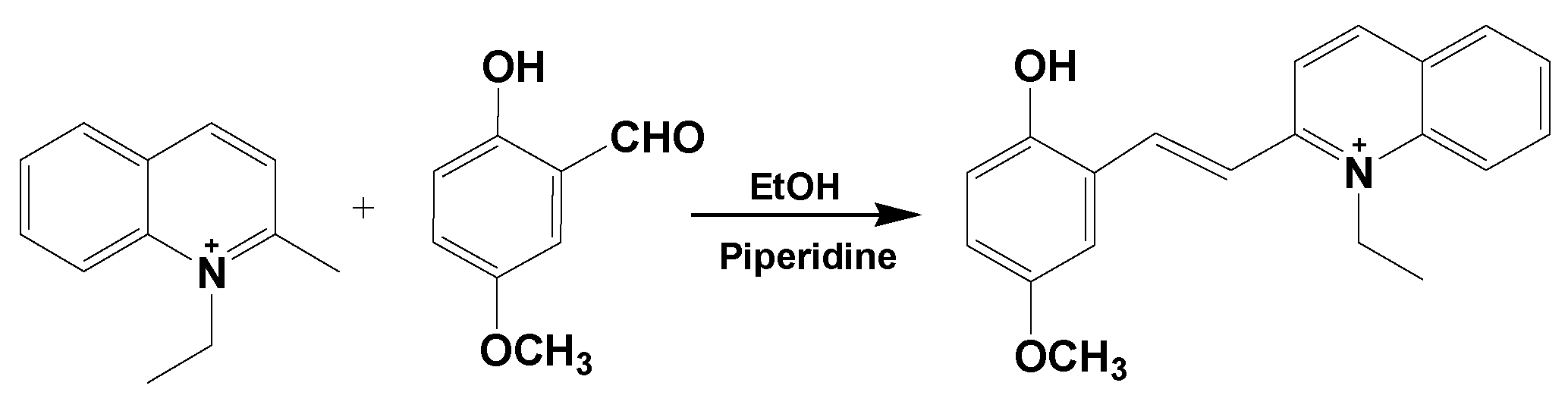
2.1. Design, Synthesis and Characterization of the Molecular Probe
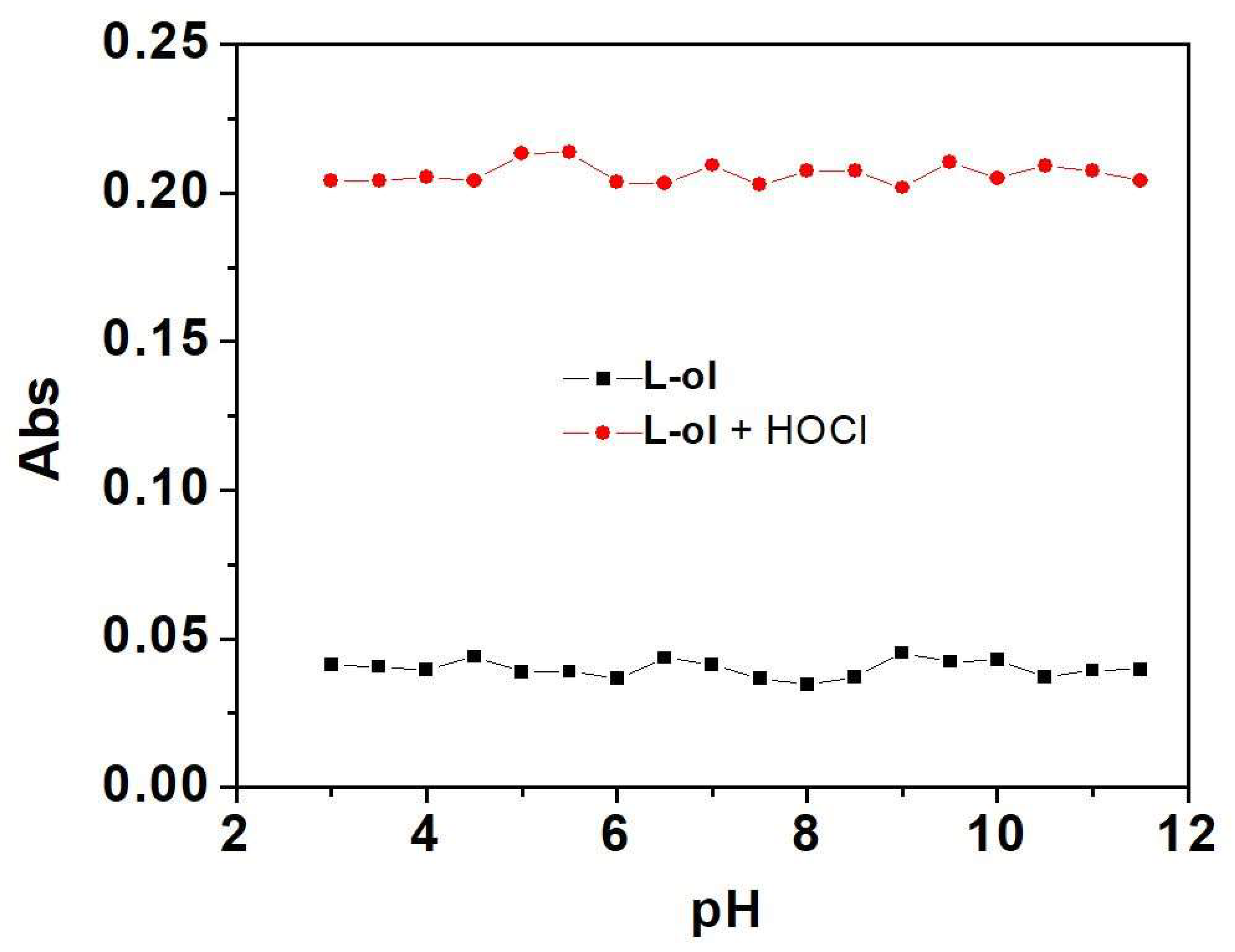
2.2. Oxidation of L-ol by HOCl and UV-vis Spectra Studies
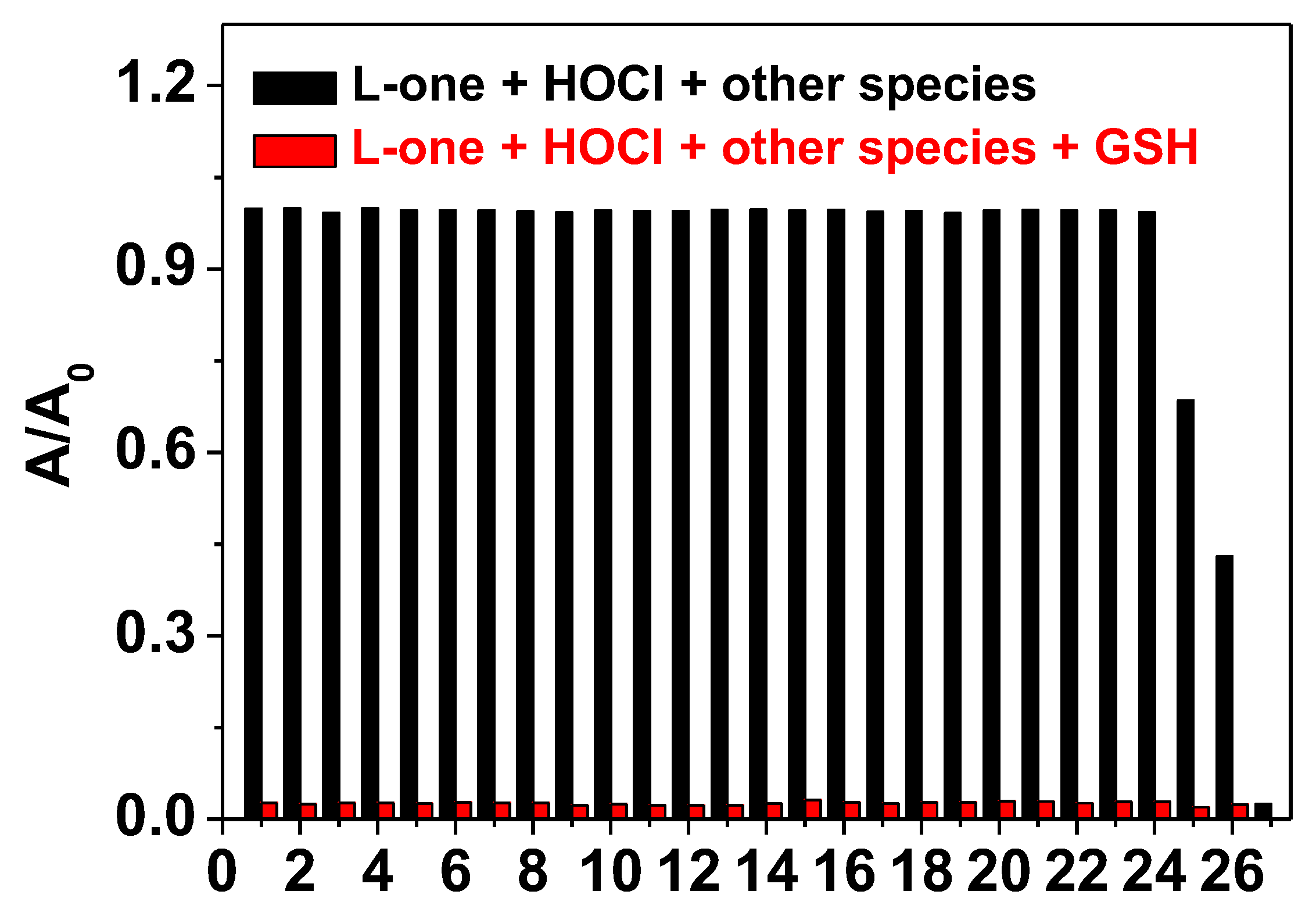
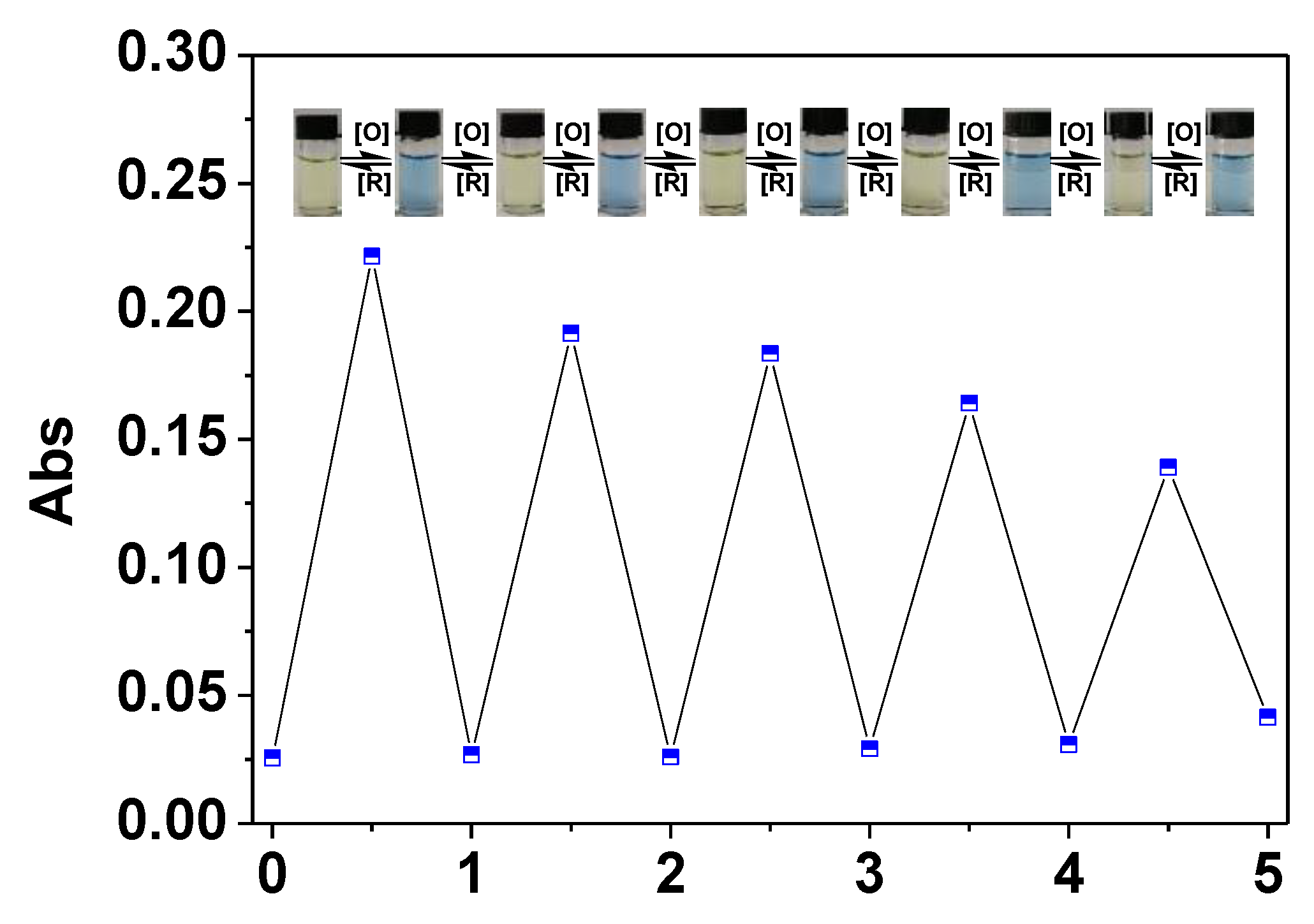
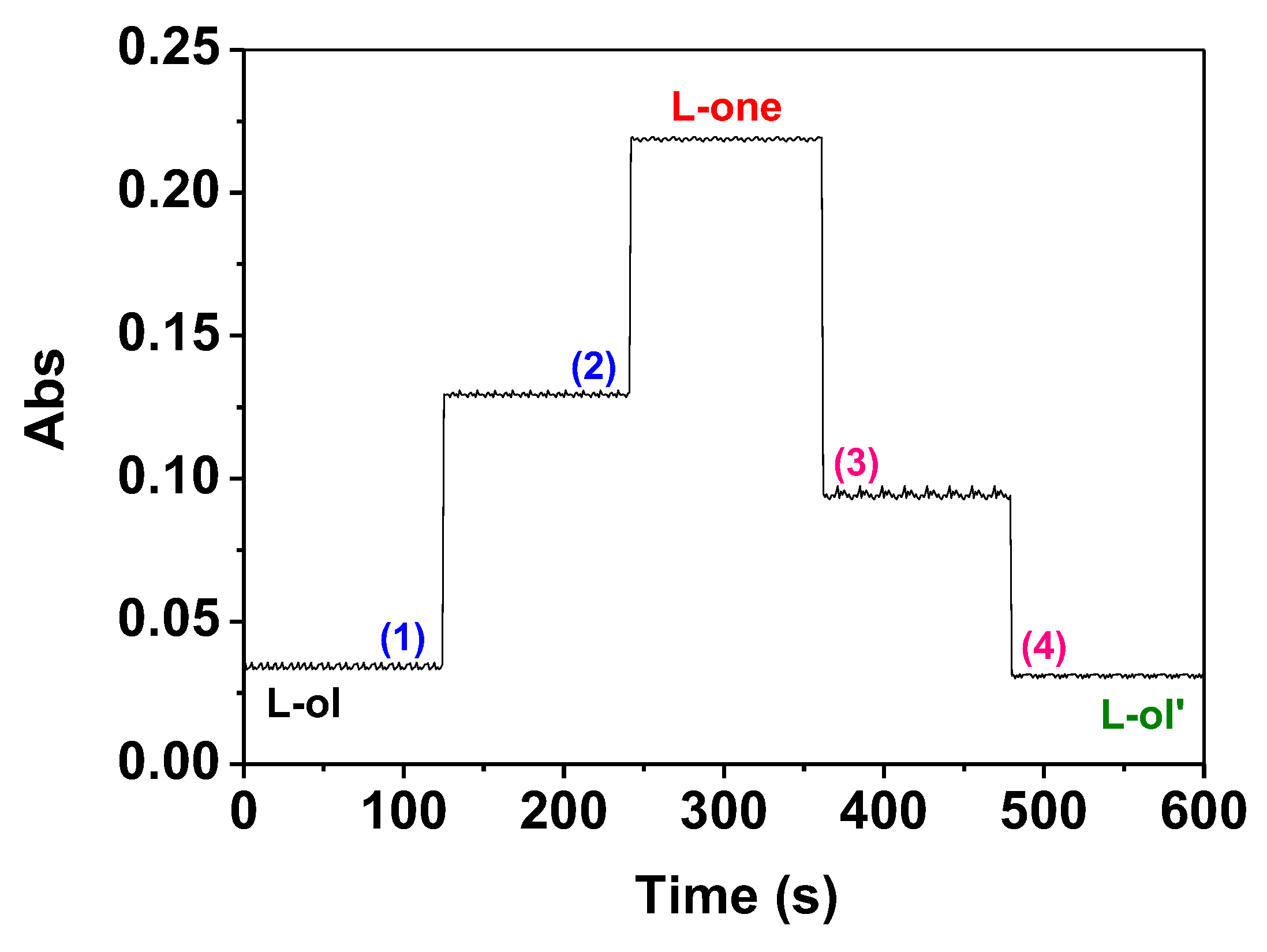
2.3. Reduction of L-one by GSH and UV-vis Spectra Studies
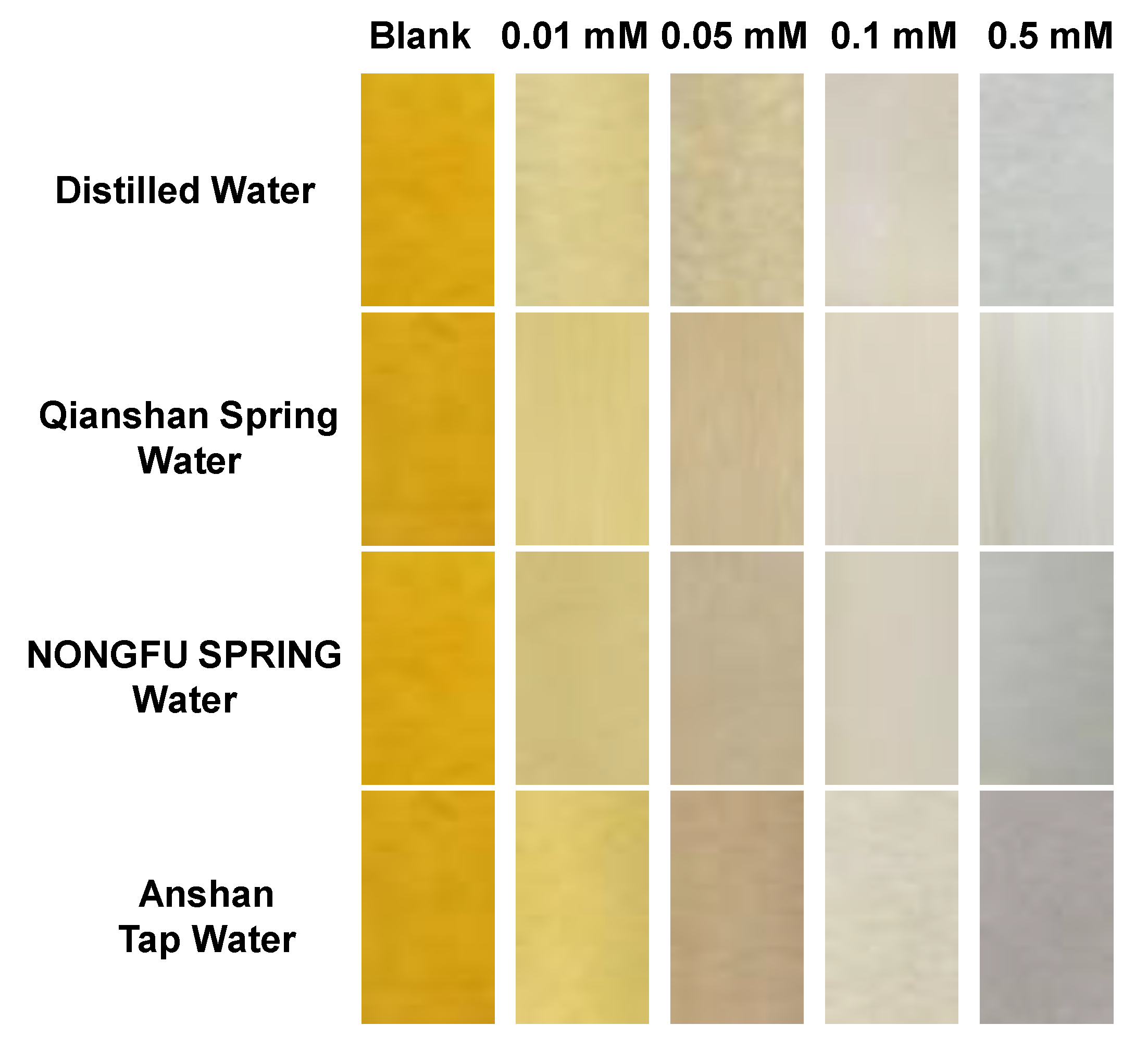
2.4. Application of L-ol-Loaded Chromatography Plates in the Quantitative Analysis of HOCl in Natural Water Samples
3. Experimental
3.1. Reagents and Materials
3.2. Apparatus
3.3. Synthesis and Characterization of L-ol
3.4. Preparation of Stock Solutions of Probes and Analyte
3.5. “Naked-Eye” Analysis of HOCl in Natural Water Samples by L-ol-Based Chromatography Plates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fruehauf, J.P.; Meyskens, F.L. Reactive oxygen species: A breath of life or death? Clin. Cancer Res. 2007, 13, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, P.; Yu, F.; Chen, J.; Qu, Z.; Han, K. A near-infrared reversible and ratiometric fluorescent probe based on Se-BODIPY for the redox cycle mediated by hypobromous acid and hydrogen sulfide in living cells. Chem. Commun. 2013, 49, 5790–5792. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.P.; Hicks, A.J.; Neidigh, J.W. Toxic effect of gestational exposure to nonylphenol on F1 male rats. Chem. Res. Toxicol. 2011, 24, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.; Dawes, I.W. Redox control of cell proliferation. Trends Cell Biol. 2012, 22, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, H.; Tian, J.; Ding, B.; Xie, Y.; Qiang, M.; Tang, B. A near-infrared reversible fluorescent probe for peroxynitrite and imaging of redox cycles in living cells. Chem. Commun. 2011, 47, 9468–9470. [Google Scholar] [CrossRef] [PubMed]
- Dooley, C.T.; Dore, T.M.; Hanson, G.T.; Jackson, W.C.; Remington, S.J.; Tsien, R.Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004, 279, 22284–22293. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Nordberg, J.; Arner, E. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, Z.Q.; Meng, Q.T.; Jia, H.M.; Wang, Y.; Zhang, R. Rapid Response Fluorescence Probe Enabled in vivo Diagnosis and Assessing Treatment Response of Hypochlorous Acid Mediated Rheumatoid Arthritis. Adv. Sci. 2018, 5, 1800397. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.L.; Wang, J.Y.; Mu, H.Y.; Peng, X.J. An “Enhanced PET”-Based Fluorescent Probe with Ultrasensitivity for Imaging Basal and Elesclomol-Induced HClO in Cancer Cells. J. Am. Chem. Soc. 2014, 136, 12820–12823. [Google Scholar] [CrossRef]
- Prokopowicz, Z.M.; Arce, F.; Biedron, R.; Chiang, C.L.L.; Ciszek, M.D.; Katz, R.; Nowakowska, M.; Zapotoczny, S.; Marcinkiewicz, J.; Chain, B.M. Hypochlorous acid: A natural adjuvant that facilitates antigen processing, cross-priming, and the induction of adaptive immunity. J. Immunol. 2010, 184, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wang, F.; Wei, T.; Chen, X. Highly Sensitive and Selective Fluorescent Probes for the Detection of HOCl/OCl− Based on Fluorescein Derivatives. Ind. Eng. Chem. Res. 2017, 56, 3757–3764. [Google Scholar] [CrossRef]
- Eugene, A.P.; Husam, M.A.; Stanley, L.H. Myeloperoxidase-generated oxidants and atherosclerosis. Biol. Med. 2000, 28, 1717–1725. [Google Scholar]
- Pattison, D.I.; Davies, M.J. Evidence for Rapid Inter- and Intramolecular Chlorine Transfer Reactions of Histamine and Carnosine Chloramines: Implications for the Prevention of Hypochlorous-Acid-Mediated Damage. Biochemistry 2006, 45, 8152–8162. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Nukuna, B.; Brennan, M.L.; Sun, M.; Goormastic, M.; Settle, M.; Schmitt, D.; Fu, X.; Thomson, L.; Fox, P.L.; et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Investig. 2004, 114, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Winterbourna, C.C.; Kettle, A. Biomarkers of myeloperoxidase-derived hypochlorous acid. J. Biol. Med. 2000, 29, 403–410. [Google Scholar] [CrossRef]
- Winter, J.; Ilbert, M.; Graf, P.C.F.; Ӧzcelik, D.; Jakob, U. The relationship between basic need satisfaction and emotional eating. Cell 2008, 135, 691–701. [Google Scholar] [CrossRef]
- Wang, C.; Ji, H.; Li, M.; Cai, L.; Wang, Z.; Li, Q.; Li, Z. A highly sensitive and selective fluorescent probe for hypochlorite in pure water with aggregation induced emission characteristics. Faraday Discuss. 2017, 196, 427–438. [Google Scholar] [CrossRef]
- Mari, M.; Morales, A.; Colell, A.; Garcia-Ruiz, C.; Kaplowitz, N.; Fernandez-Checa, J.C. Mitochondrial glutathione: Features, regulation and role in disease. Biochim. Biophys. Acta 2013, 1830, 3317–3328. [Google Scholar] [CrossRef]
- Soni, D.; Gangada, S.; Duvva, N.; Roy, T.K.; Nimesh, S.; Arya, G.; Giribabu, L.; Chitta, R. Hypochlorite-promoted inhibition of photo-induced electron transfer in phenothiazine–borondipyrromethene donor–acceptor dyad: A cost-effective and metal-free “turn-on” fluorescent chemosensor for hypochlorite. New J. Chem. 2017, 41, 5322–5333. [Google Scholar] [CrossRef]
- Mari, M.; Morales, A.; Colell, A.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Li, P.; Sun, X.; Yang, S.; Wang, B.; Han, K. A fluorescent probe for rapid detection of thiols and imaging of thiols reducing repair and H2O2 oxidative stress cycles in living cells. Chem. Commun. 2013, 49, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, Y.; Chen, J.; Zhao, M.; Chen, H.; Song, X.; Matzuk, A.J.; Carroll, S.L.; Tan, X.; Sizovs, A.; et al. Quantitative Imaging of Glutathione in Live Cells Using a Reversible Reaction-Based Ratiometric Fluorescent Probe. ACS Chem. Biol. 2015, 10, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Banerjee, R. Redox outside the box: Linking extracellular redox remodeling with intracellular redox metabolism. J. Biol. Chem. 2012, 287, 4397–4402. [Google Scholar] [CrossRef] [PubMed]
- Lomaestro, B.M.; Malone, M. Glutathione in health and disease: Pharmacotherapeutic issues. Ann. Pharmacother. 1995, 29, 1263–1273. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, Y.; Dong, B.; Gou, Z.; Yang, T.; Lin, W. Binding Reaction Sites to Polysiloxanes: Unique Fluorescent Probe for Reversible Detection of ClO−/GSH Pair and the in situ Imaging in Live Cells and Zebrafish. Anal. Chem. 2019, 91, 1719–1723. [Google Scholar] [CrossRef]
- Lou, Z.; Li, P.; Han, K. Redox-Responsive Fluorescent Probes with Different Design Strategies. Acc. Chem. Res. 2015, 48, 1358–1368. [Google Scholar] [CrossRef]
- Lv, X.; Yuan, X.; Wang, Y.; Guo, W. A naphthalimide based fast and selective fluorescent probe for hypochlorous acid/hypochlorite and its application for bioimaging. New J. Chem. 2018, 42, 15105–15110. [Google Scholar] [CrossRef]
- Liu, S.-R.; Wu, S.-P. Hypochlorous Acid Turn-on Fluorescent Probe Based on Oxidation of Diphenyl Selenide. Org. Lett. 2013, 15, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Manjare, S.T.; Kim, J.; Lee, Y.; Churchill, D.G. Facile meso-BODIPY Annulation and Selective Sensing of Hypochlorite in Water. Org. Lett. 2014, 16, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Li, P.; Pan, Q.; Han, K. A reversible fluorescent probe for detecting hypochloric acid in living cells and animals: Utilizing a novel strategy for effectively modulating the fluorescence of selenide and selenoxide. Chem. Commun. 2013, 49, 2445–24472. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gao, Y.; Wang, J.; Sun, S. Reversible and selective luminescent determination of ClO–/H2S redox cycle in vitro and in vivo based on a ruthenium trisbipyridyl probe. Analyst 2014, 139, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, X.; Zhang, R.; Liu, Y.; Ren, X.; Xian, M.; Ye, Y.; Zhao, Y. Lysosomal-Targeted Two-Photon Fluorescent Probe to Sense Hypochlorous Acid in Live Cells. Anal. Chem. 2017, 89, 10384–10390. [Google Scholar] [CrossRef]
- Yu, F.; Li, P.; Wang, B.; Han, K. Reversible Near-Infrared Fluorescent Probe Introducing Tellurium to Mimetic Glutathione Peroxidase for Monitoring the Redox Cycles between Peroxynitrite and Glutathione in vivo. J. Am. Chem. Soc. 2013, 135, 7674–7680. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Li, P.; Kang, J.; Wang, J.; Wang, H.; Tang, B. Reversible two-photon fluorescent probe for imaging of hypochlorous acid in live cells and in vivo. Chem. Commun. 2015, 51, 10150–10153. [Google Scholar] [CrossRef]
- Venkatesan, P.; Wu, S.-P. A turn-on fluorescent probe for hypochlorous acid based on the oxidation of diphenyl telluride. Analyst 2015, 140, 1349–1355. [Google Scholar] [CrossRef]
- Yue, Y.; Huo, F.; Yin, C.; Escobedoc, J.O.; Strongin, R.M. Recent progress in chromogenic and fluorogenic chemosensors for hypochlorous acid. Analyst 2016, 141, 1859–1873. [Google Scholar] [CrossRef]
- Sun, Z.-N.; Liu, F.-Q.; Chen, Y.; Tam, P.K.H.; Yang, D. A highly specific BODIPY-based fluorescent probe for the detection of hypochlorous acid. Org. Lett. 2008, 10, 2171–2174. [Google Scholar] [CrossRef]
- Kenmoku, S.; Urano, Y.; Kojima, H.; Nagano, T. Development of a highly specific rhodamine-based fluorescence probe for hypochlorous acid and its application to real-time imaging of phagocytosis. J. Am. Chem. Soc. 2007, 129, 7313–7329. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Wong, N.-K.; Gu, Q.; Bai, X.; Ye, S.; Yang, D. HKOCl-2 Series of Green BODIPY-Based Fluorescent Probes for Hypochlorous Acid Detection and Imaging in Live Cells. Org. Lett. 2014, 16, 3544–3547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Song, B.; Yuan, J. Bioanalytical methods for hypochlorous acid detection: Recent advances and challenges. Trends Anal. Chem. 2018, 99, 1–33. [Google Scholar] [CrossRef]
- McCord, J.M. Free radicals and inflammation: Protection of synovial fluid by superoxide dismutase. Science 1974, 185, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Zhang, D.; Zhang, G.; Zhu, D. A highly selective naked-eye probe for hypochlorite with the p-methoxyphenol-substituted aniline compound. Tetrahedron Lett. 2010, 51, 6052–6055. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, C.; Liu, L.; Qin, J.; Yang, C. Naked-eye visible and fluorometric dual-signaling chemodosimeter for hypochlorous acid based on water-soluble p-methoxyphenol derivative. Org. Biomol. Chem. 2011, 9, 5560–5563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, C.; Chang, J.; Lu, Y.; He, S.; Zhao, L.; Zeng, X. Novel water soluble styrylquinolinium boronic acid as a ratiometric reagent for the rapid detection of hypochlorite ion. Dyes Pigments 2013, 99, 733–739. [Google Scholar] [CrossRef]
- Feng, H.; Meng, Q.T.; Wang, Y.; Duan, C.C.; Wang, C.P.; Jia, H.M.; Zhang, Z.Q.; Zhang, R. Responsive Fluorescence Probe for Selective and Sensitive Detection of Hypochlorous Acid in Live Cells and Animals. Chem. Asian J. 2018, 13, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Sultanbawa, Y.; Feng, H.; Wang, Y.L.; Meng, Q.T.; Wang, Y.; Zhang, Z.Q.; Zhang, R. A New Red-emitting Fluorescence Probe for Rapid and Effective Visualisation of Bisulfite in Food Samples and Live Animals. J. Agric. Food Chem. 2019, 67, 4375–4383. [Google Scholar] [CrossRef]
- Zhang, R.; Ye, Z.; Wang, G.; Zhang, W.; Yuan, J. Development of a Ruthenium (II) Complex Based Luminescent Probe for Imaging Nitric Oxide Production in Living Cells. Chem. Eur. J. 2010, 16, 6884–6891. [Google Scholar] [CrossRef]
- Setsukinai, K.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of Novel Fluorescence Probes That Can Reliably Detect Reactive Oxygen Species and Distinguish Specific Species. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, R.; Ye, Z.; Dai, Z.; An, H.; Yuan, J. Lanthanide complex-based luminescent probes for highly sensitive time-gated luminescence detection of hypochlorous acid. Anal. Chem. 2012, 84, 10785–10792. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Wong, N.-K.; Ye, S.; Chen, X.; Lu, M.-Y.; Zhao, A.Q.; Guo, Y.; Ma, A.C.-H.; Leung, A.Y.-H.; Shen, J.; et al. Fluorescent Probe HKSOX-1 for Imaging and Detection of Endogenous Superoxide in Live Cells and in vivo. J. Am. Chem. Soc. 2015, 137, 6837–6843. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Molecular probe L-ol is not available from the authors. |




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Zhou, F.; Wang, Y.; Feng, H.; Meng, Q.; Zhang, Z.; Zhang, R. A Redox-Switchable Colorimetric Probe for “Naked-Eye” Detection of Hypochlorous Acid and Glutathione. Molecules 2019, 24, 2455. https://doi.org/10.3390/molecules24132455
Han Q, Zhou F, Wang Y, Feng H, Meng Q, Zhang Z, Zhang R. A Redox-Switchable Colorimetric Probe for “Naked-Eye” Detection of Hypochlorous Acid and Glutathione. Molecules. 2019; 24(13):2455. https://doi.org/10.3390/molecules24132455
Chicago/Turabian StyleHan, Qian, Fang Zhou, Yue Wang, Huan Feng, Qingtao Meng, Zhiqiang Zhang, and Run Zhang. 2019. "A Redox-Switchable Colorimetric Probe for “Naked-Eye” Detection of Hypochlorous Acid and Glutathione" Molecules 24, no. 13: 2455. https://doi.org/10.3390/molecules24132455
APA StyleHan, Q., Zhou, F., Wang, Y., Feng, H., Meng, Q., Zhang, Z., & Zhang, R. (2019). A Redox-Switchable Colorimetric Probe for “Naked-Eye” Detection of Hypochlorous Acid and Glutathione. Molecules, 24(13), 2455. https://doi.org/10.3390/molecules24132455







