Intracellular Accumulation as an Indicator of Cytotoxicity to Screen Hepatotoxic Components of Chelidonium majus L. by LC–MS/MS
Abstract
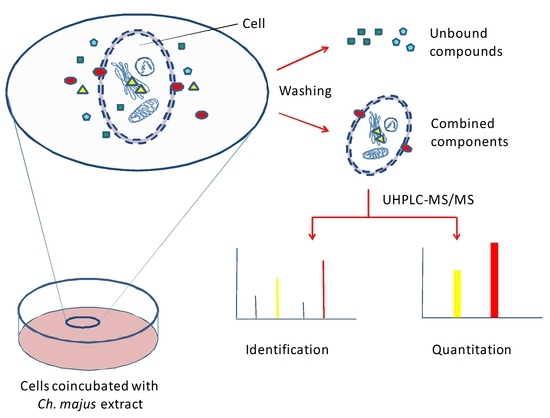
:1. Introduction
2. Results and Discussion
2.1. Selection of Hepatic Cell Line
2.2. Optimization of Cell Lysate Preparation
2.3. Optimization of LC–MS/MS Conditions
2.4. Method Validation
2.4.1. Specificity and Crosstalk Effect
2.4.2. Linearity, LLOQ, and ULOQ
2.4.3. Precision and Accuracy
2.4.4. Extraction Recovery and Matrix Effect
2.4.5. Stability
2.5. Identification of Ch. majus Components
2.6. Determination of Intracellular Accumulation
2.7. Cell Viability Assay
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Calibration Standards and QC Samples
3.3. Sample Preparation
3.4. Cell Culture
3.5. Cell Exposure to the Test Sample
3.6. Cell Lysate Preparation
3.7. LC–MS/MS Analysis
3.8. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jing, J.; Teschke, R. Traditional Chinese Medicine and Herb-induced Liver Injury: Comparison with Drug-induced Liver Injury. J. Clin. Transl. Hepatology 2018, 6, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Zhang, L.; Long, H.; Schwarzenboeck, A.; Schmidt-Taenzer, W.; Genthner, A.; Wolff, A.; Frenzel, C.; Schulze, J.; Eickhoff, A. Traditional Chinese Medicine and herbal hepatotoxicity: A tabular compilation of reported cases. Annuals hepatology 2015, 14, 7–19. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, A.; Fan, X.; Hu, L.; Hao, F.; Li, Y. Safety Research in Traditional Chinese Medicine: Methods, Applications, and Outlook. Engineering 2019, 5, 76–82. [Google Scholar] [CrossRef]
- Xue, L.M.; Zhang, Q.Y.; Han, P.; Jiang, Y.P.; Yan, R.D.; Wang, Y.; Rahman, K.; Jia, M.; Han, T.; Qin, L.P. Hepatotoxic constituents and toxicological mechanism of Xanthium strumarium L. fruits. J. Ethnopharmacol. 2014, 152, 272–282. [Google Scholar] [CrossRef]
- Song, H.; Lin, J.; Zhu, X.; Chen, Q. Developments in high-speed countercurrent chromatography and its applications in the separation of terpenoids and saponins. J. Sep. Sci. 2016, 39, 1574–1591. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Tung, C.W.; Fulop, F.; Li, J.H. Developing a QSAR model for hepatotoxicity screening of the active compounds in traditional Chinese medicines. Food Chem. Toxicol. 2015, 78, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, B.; Wang, C. Acute Liver Failure Study Team. Hepatotoxicity evaluation of traditional Chinese medicines using a computational molecular model. Clin. Toxicol. 2017, 55, 996–1000. [Google Scholar] [CrossRef]
- Lin, L.; Lin, H.; Zhang, M.; Ni, B.; Yin, X.; Qu, C.; Ni, J. A novel method to analyze hepatotoxic components in Polygonum multiflorum using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Hazard. Mater. 2015, 299, 249–259. [Google Scholar] [CrossRef]
- Liang, J.; Chen, Y.; Ren, G.; Dong, W.; Shi, M.; Xiong, L.; Li, J.; Dong, J.; Li, F.; Yuan, J. Screening Hepatotoxic Components in Euodia rutaecarpa by UHPLC-QTOF/MS Based on the Spectrum-Toxicity Relationship. Molecules 2017, 22, 1264. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, C.; Zhao, D.; Wang, L.; Li, P.; Li, H. Discovery of Hepatotoxic Equivalent Combinatorial Markers from Dioscorea bulbifera tuber by Fingerprint-Toxicity Relationship Modeling. Sci. Rep. 2018, 8, 462. [Google Scholar] [CrossRef]
- Hong, M.; Ma, H.Y.; Wu, X.R.; Hua, Y.Q.; Zhu, Q.; Fan, H.W. A method of hepatocyte extraction conjugated with HPLC is established for screening potential active components in Chinese medicines—probing Herba Artemisiae Scopariae as an exemplifying approach. Molecules 2012, 17, 1468–1482. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tan, Z.; Li, P.; Gao, X.; Zeng, Y.; Wang, S. HepG2 cells biospecific extraction and HPLC-ESI-MS analysis for screening potential antiatherosclerotic active components in Bupeuri radix. J. Pharm. Biomed. Anal. 2016, 121, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, P.; Xiao, W.; Zhao, L.; Wang, Z.; Yu, L. Screening and analyzing the potential bioactive components from reduning injection, using macrophage cell extraction and ultra-high performance liquid chromatography coupled with mass spectrometry. Am. J. Chin. Med. 2013, 41, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, R.; Piotrowski, M.; Zhang, H.; Leach, K.L. Intracellular concentrations determine the cytotoxicity of adefovir, cidofovir and tenofovir. Toxicol. In Vitro 2015, 29, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.L.; Bosisio, E. Pharmacological activities of Chelidonium majus L. (papaveraceae). Pharmacol. Res. 1996, 33, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Capistrano, I.R.; Wouters, A.; Lardon, F.; Gravekamp, C.; Apers, S.; Pieters, L. In vitro and in vivo investigations on the antitumour activity of Chelidonium majus. Phytomedicine 2015, 22, 1279–1287. [Google Scholar] [CrossRef]
- Orland, A.; Knapp, K.; Konig, G.M.; Ulrich-Merzenich, G.; Knoss, W. Combining metabolomic analysis and microarray gene expression analysis in the characterization of the medicinal plant Chelidonium majus L. Phytomedicine 2014, 21, 1587–1596. [Google Scholar] [CrossRef]
- Gao, L.; Schmitz, H.J.; Merz, K.H.; Schrenk, D. Characterization of the cytotoxicity of selected Chelidonium alkaloids in rat hepatocytes. Toxicol. Lett. 2019, 311, 91–97. [Google Scholar] [CrossRef]
- Teschke, R.; Glass, X.; Schulze, J. Herbal hepatotoxicity by Greater Celandine (Chelidonium majus): causality assessment of 22 spontaneous reports. Regul. Toxicol. Pharmacol. 2011, 61, 282–291. [Google Scholar] [CrossRef]
- Van den Hof, W.F.; Coonen, M.L.J.; van Herwijnen, M.; Brauers, K.; Wodzig, W.K.W.H.; van Delft, J.H.M.; Kleinjans, J.C.S. Classification of hepatotoxicants using HepG2 cells: A proof of principle study. Chem. Res. Toxicol. 2014, 27, 433–442. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, Y.; Wang, X.; Di, X. A sensitive and selective liquid chromatography-tandem mass spectrometry method for simultaneous determination of five isoquinoline alkaloids from Chelidonium majus L. in rat plasma and its application to a pharmacokinetic study. J. Mass Spectrom. 2013, 48, 111–118. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/media/70858/download (accessed on 10 June 2019).
- Choy, C.-S.; Cheah, K.-P.; Chiou, H.-Y.; Li, J.-S.; Liu, Y.-H.; Yong, S.-F.; Chiu, W.-T.; Liao, J.-W.; Hu, C.-M. Induction of hepatotoxicity by sanguinarine is associated with oxidation of protein thiols and disturbance of mitochondrial respiration. J. Appl. Toxicol. 2008, 28, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Ulrichová, J.; Walterová, D.; Vavrečková, C.; Kamarád, V.; Šimánek, V. Cytotoxicity of benzo[c]phenanthridinium alkaloids in isolated rat hepatocytes. Phytother. Res. 1996, 10, 220–223. [Google Scholar] [CrossRef]
- Zdařilová, A.; Vrzal, R.; Rypka, M.; Ulrichová, J.; Dvořák, Z. Investigation of sanguinarine and chelerythrine effects on CYP1A1 expression and activity in human hepatoma cells. Food Chem. Toxicol. 2006, 44, 242–249. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |





| Compound | Regression Equation | r | Linear Range (ng/mL) | LLOQ | ULOQ | ||
|---|---|---|---|---|---|---|---|
| Found Concentration (ng/mL) | RE (%) | Found Concentration (ng/mL) | RE (%) | ||||
| Protopine | y = 1.6558x + 0.0078 | 0.9992 | 0.75–30 | 0.728 | −2.9 | 28.62 | −4.6 |
| Chelidonine | y = 4.2789x + 0.0087 | 0.9987 | 0.5–20 | 0.495 | −1.0 | 20.48 | 2.4 |
| Coptisine | y = 1.1156x + 0.0399 | 0.9992 | 12.5–500 | 13.35 | 6.8 | 533.5 | 6.7 |
| Sanguinarine | y = 0.1732x + 0.0784 | 0.9989 | 20.0–800 | 20.72 | 3.6 | 770.4 | −3.7 |
| Chelerythrine | y = 0.2818x − 0.0068 | 0.9949 | 6.0–240 | 5.508 | −8.2 | 252.7 | 5.3 |
| Compound | Nominal Concentration (ng/mL) | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|---|
| Found Concentration (ng/mL) | RE (%) | RSD (%) | Found Concentration (ng/mL) | RE (%) | RSD (%) | ||
| Protopine | 0.75 | 0.776 | 3.5 | 4.7 | 0.772 | 2.9 | 6.5 |
| 1.875 | 1.978 | 5.5 | 3.4 | 1.954 | 4.2 | 4.1 | |
| 6.0 | 5.91 | −1.5 | 4.2 | 5.934 | −1.1 | 2.4 | |
| 22.5 | 21.02 | −6.6 | 3.0 | 21.22 | −5.7 | 3.8 | |
| Chelidonine | 0.5 | 0.526 | 5.2 | 3.7 | 0.523 | 4.6 | 7.1 |
| 1.25 | 1.295 | 3.6 | 4.1 | 1.319 | 5.5 | 2.2 | |
| 4.0 | 3.860 | −3.5 | 2.9 | 3.916 | −2.1 | 3.5 | |
| 15 | 13.74 | −8.4 | 3.0 | 13.85 | −7.7 | 2.8 | |
| Coptisine | 12.5 | 12.74 | 1.9 | 4.8 | 12.96 | 3.7 | 5.9 |
| 31.25 | 32.75 | 4.8 | 5.0 | 31.97 | 2.3 | 7.5 | |
| 100 | 97.9 | −2.1 | 3.4 | 99.70 | −0.3 | 4.1 | |
| 375 | 363.4 | −3.1 | 3.7 | 367.1 | −2.1 | 2.1 | |
| Sanguinarine | 20 | 20.54 | 2.7 | 3.8 | 20.72 | 3.6 | 4.2 |
| 50 | 52.45 | 4.9 | 2.6 | 51.85 | 3.7 | 3.9 | |
| 160 | 155.2 | −3.0 | 4.3 | 156.2 | −2.4 | 1.5 | |
| 600 | 567.6 | −5.4 | 3.7 | 583.8 | −2.7 | 6.0 | |
| Chelerythrine | 6.0 | 5.604 | −6.6 | 7.3 | 5.766 | −3.9 | 6.7 |
| 15 | 13.54 | −9.7 | 3.2 | 13.43 | −10.5 | 3.9 | |
| 48 | 43.78 | −8.8 | 2.8 | 44.06 | −8.2 | 1.3 | |
| 180 | 187.6 | 4.2 | 3.8 | 181.6 | 0.9 | 4.6 | |
| Compound | Nominal Concentration | Extraction Recovery | Matrix Effect | ||
|---|---|---|---|---|---|
| (ng/mL) | Mean (%) | RSD (%) | Mean (%) | RSD (%) | |
| Protopine | 1.875 | 108.7 | 13.4 | 93.5 | 12.1 |
| 6.0 | 90.2 | 8.0 | 99.9 | 3.1 | |
| 22.5 | 96.3 | 10.1 | 107.2 | 3.0 | |
| Chelidonine | 1.25 | 98.3 | 7.7 | 89.4 | 1.6 |
| 4.0 | 90.8 | 9.4 | 101.7 | 4.4 | |
| 15.0 | 101.1 | 9.4 | 106.7 | 2.6 | |
| Coptisine | 31.25 | 88.7 | 5.2 | 91.8 | 2.1 |
| 100 | 81.3 | 8.3 | 96.4 | 10.3 | |
| 375 | 96.1 | 8.5 | 107.1 | 7.0 | |
| Sanguinarine | 50 | 88.2 | 10.1 | 87.5 | 1.5 |
| 160 | 90.0 | 9.1 | 98.1 | 2.8 | |
| 600 | 99.6 | 7.4 | 105.9 | 2.7 | |
| Chelerythrine | 15 | 98.6 | 12.1 | 90.7 | 1.8 |
| 48 | 92.9 | 3.4 | 104.2 | 2.9 | |
| 180 | 92.1 | 10.9 | 106.7 | 3.3 | |
| Palmatine (IS) | 32 | 92.6 | 8.9 | 100.5 | 5.5 |
| Compound | Nominal Concentration (ng/mL) | Freeze–Thaw Stability | Long-Term Stability | Bench-Top Stability | Post-Preparative Stability | ||||
|---|---|---|---|---|---|---|---|---|---|
| RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | ||
| Protopine | 1.875 | 12.6 | 1.9 | 12.6 | 0.9 | 12.1 | 2.7 | 11.9 | 2.0 |
| 22.5 | −3.9 | 1.7 | −4.9 | 1.9 | −5.4 | 1.7 | −2.5 | 2.2 | |
| Chelidonine | 1.250 | 5.2 | 2.4 | 8.2 | 2.1 | 7.8 | 2.5 | 8.2 | 2.4 |
| 15.0 | −8.9 | 3.4 | −7.1 | 1.6 | −9.6 | 2.3 | −6.3 | 2.2 | |
| Coptisine | 31.25 | 5.2 | 1.9 | 12.3 | 3.5 | 9.5 | 0.8 | 8.0 | 1.9 |
| 375 | −11.7 | 3.2 | −11.5 | 0.9 | −10.4 | 3.9 | −9.3 | 2.5 | |
| Sanguinarine | 50.0 | −4.8 | 1.7 | −3.8 | 2.6 | 2.2 | 0.5 | −4.1 | 1.6 |
| 600 | −6.3 | 1.5 | −6.0 | 1.5 | −5.3 | 2.3 | −4.4 | 1.7 | |
| Chelerythrine | 15.0 | −13.3 | 3.7 | −10.2 | 3.5 | −12.7 | 4.2 | −12.0 | 1.0 |
| 180 | 8.2 | 3.6 | 6.5 | 2.9 | 4.5 | 3.1 | 8.4 | 2.0 | |
| Compounds | Formular | tR (min) | [M + H]+ | [M]+ | MS2 |
|---|---|---|---|---|---|
| Magnoflorine | C20H24NO4 | 0.70 | - | 342 | 297,282,265,237,222 |
| Protopine | C20H20NO5 | 1.63 | 354 | - | 336,305,293,275,247,188 |
| Chelidonine | C20H20NO5 | 1.89 | 354 | - | 336,323,305,275,189 |
| Coptisine | C19H14NO4 | 2.00 | - | 320 | 292,277,262,249 |
| Allocryptopine | C21H24NO5 | 2.02 | 370 | - | 352,336,306,290,188 |
| Sanguinarine | C20H14NO4 | 2.70 | - | 332 | 317,304,289,274 |
| Berberine | C20H18NO4 | 3.07 | - | 336 | 321,320,206,292,278 |
| Chelerythrine | C21H18NO4 | 3.93 | - | 348 | 332,318,304,290 |
| Compound | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (eV) |
|---|---|---|---|
| Protopine | 354.0 | 188.0 | 33 |
| Chelidonine | 354.0 | 275.0 | 28 |
| Coptisine | 320.0 | 261.9 | 36 |
| Sanguinarine | 331.9 | 274.0 | 34 |
| Chelerythrine | 348.0 | 304.0 | 32 |
| Palmatine(IS) | 352.0 | 308.1 | 30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Wang, X.; Xu, M.; Liu, Y.; Di, X. Intracellular Accumulation as an Indicator of Cytotoxicity to Screen Hepatotoxic Components of Chelidonium majus L. by LC–MS/MS. Molecules 2019, 24, 2410. https://doi.org/10.3390/molecules24132410
Wu C, Wang X, Xu M, Liu Y, Di X. Intracellular Accumulation as an Indicator of Cytotoxicity to Screen Hepatotoxic Components of Chelidonium majus L. by LC–MS/MS. Molecules. 2019; 24(13):2410. https://doi.org/10.3390/molecules24132410
Chicago/Turabian StyleWu, Cuiting, Xin Wang, Ming Xu, Youping Liu, and Xin Di. 2019. "Intracellular Accumulation as an Indicator of Cytotoxicity to Screen Hepatotoxic Components of Chelidonium majus L. by LC–MS/MS" Molecules 24, no. 13: 2410. https://doi.org/10.3390/molecules24132410
APA StyleWu, C., Wang, X., Xu, M., Liu, Y., & Di, X. (2019). Intracellular Accumulation as an Indicator of Cytotoxicity to Screen Hepatotoxic Components of Chelidonium majus L. by LC–MS/MS. Molecules, 24(13), 2410. https://doi.org/10.3390/molecules24132410