Recent Advances in Biotransformation of Saponins
Abstract
1. Introduction
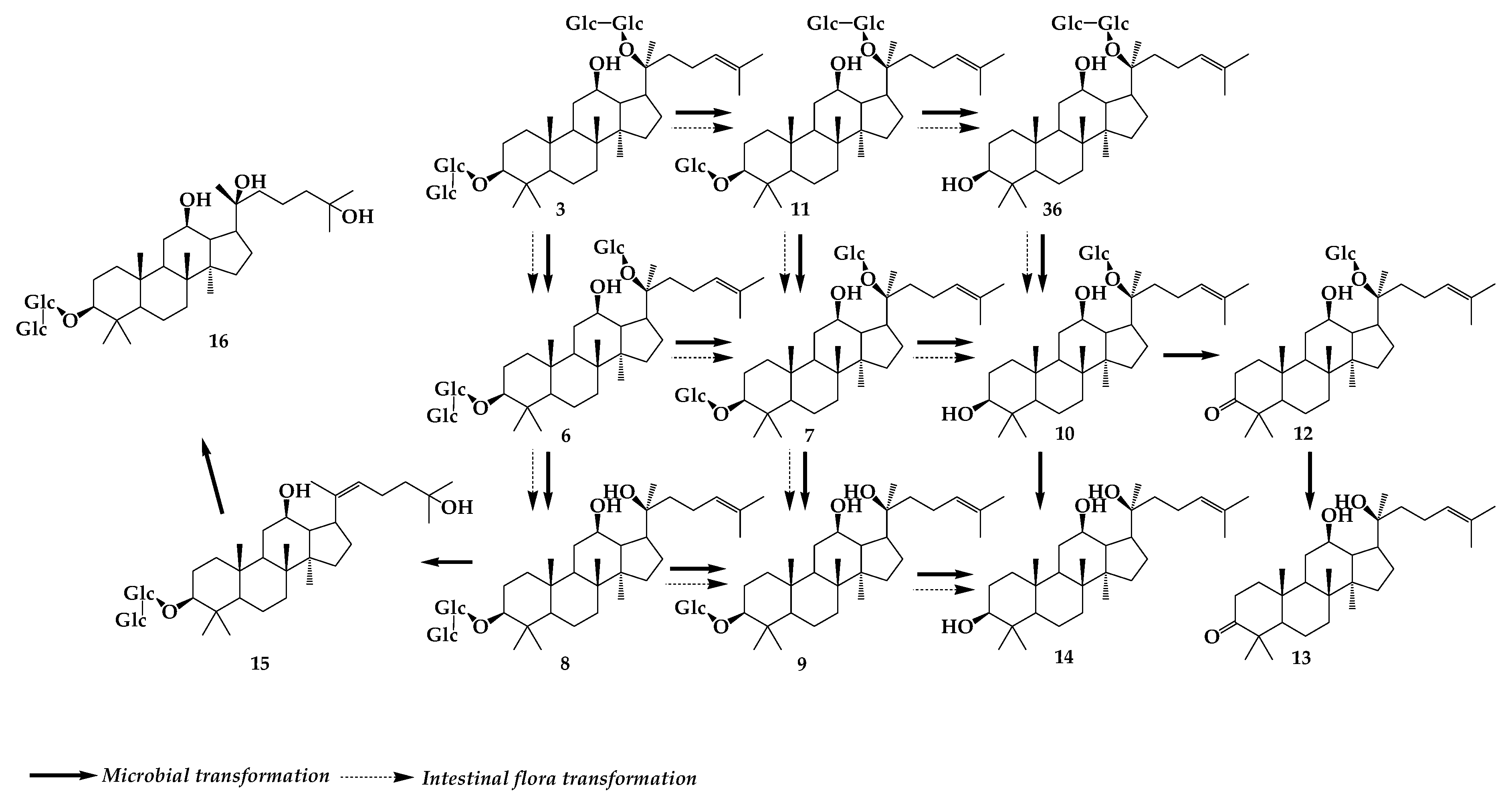
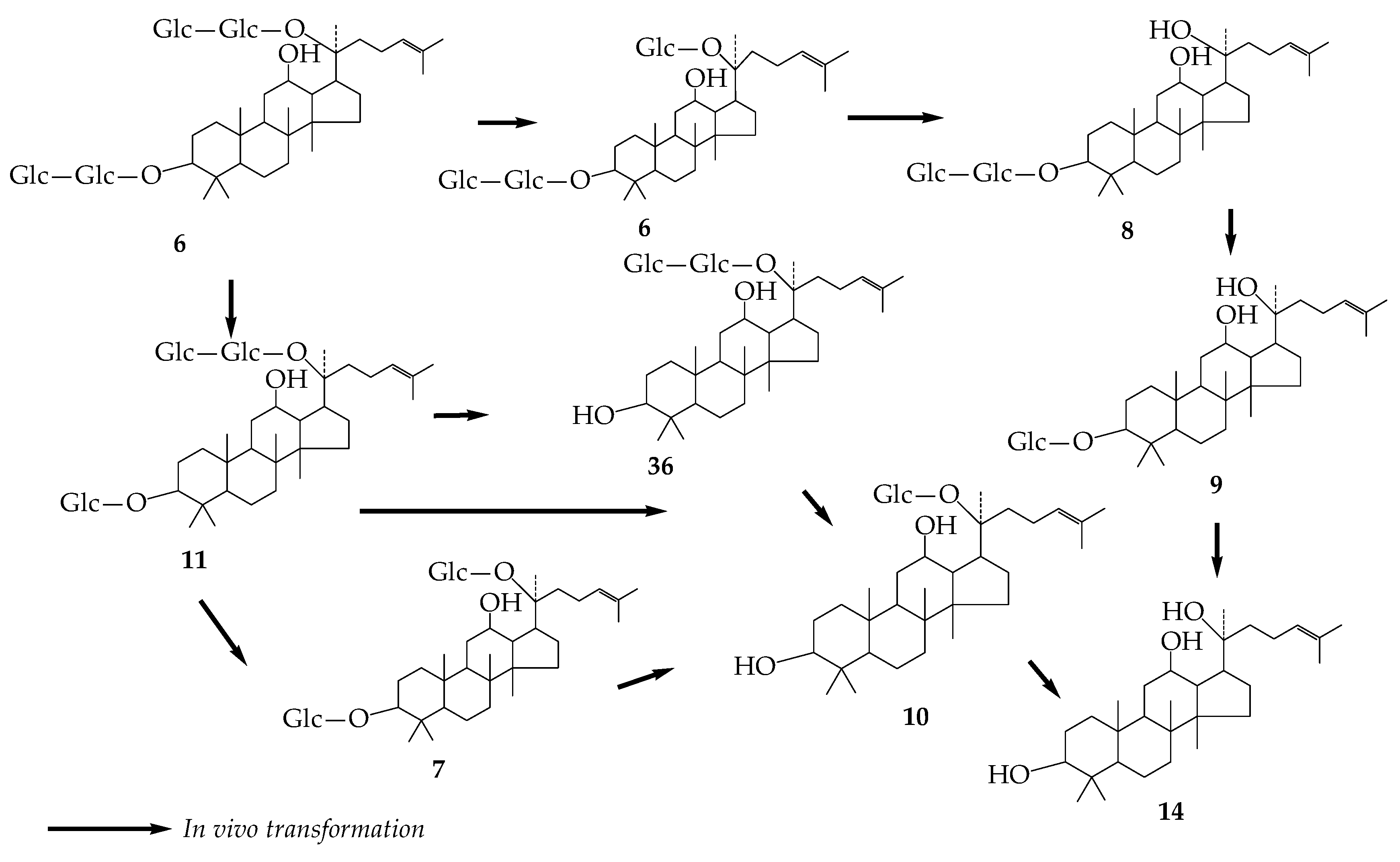
2. Ginsenoside
3. Gypenoside
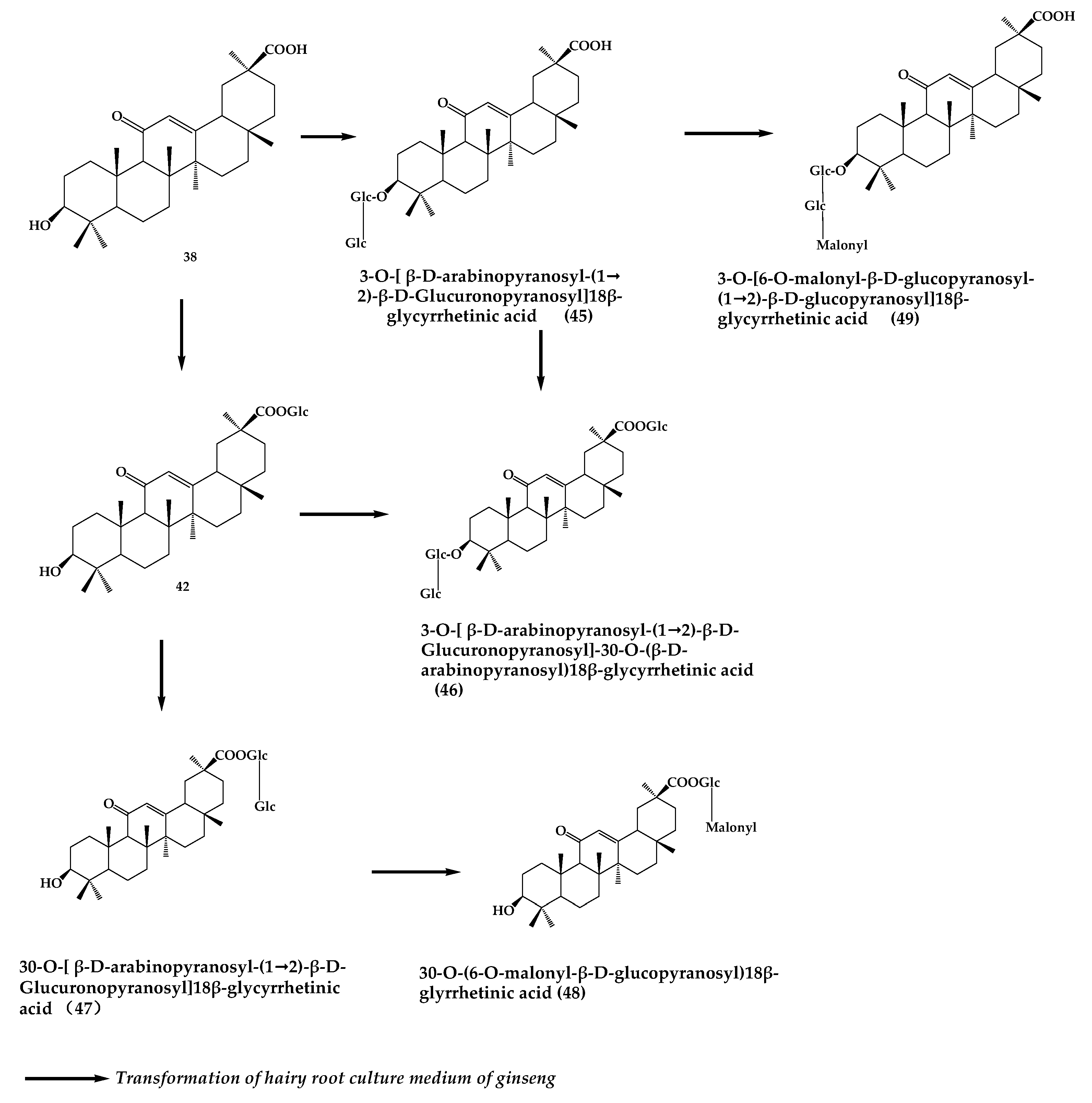
4. Glycyrrhizin
5. Saikosaponin
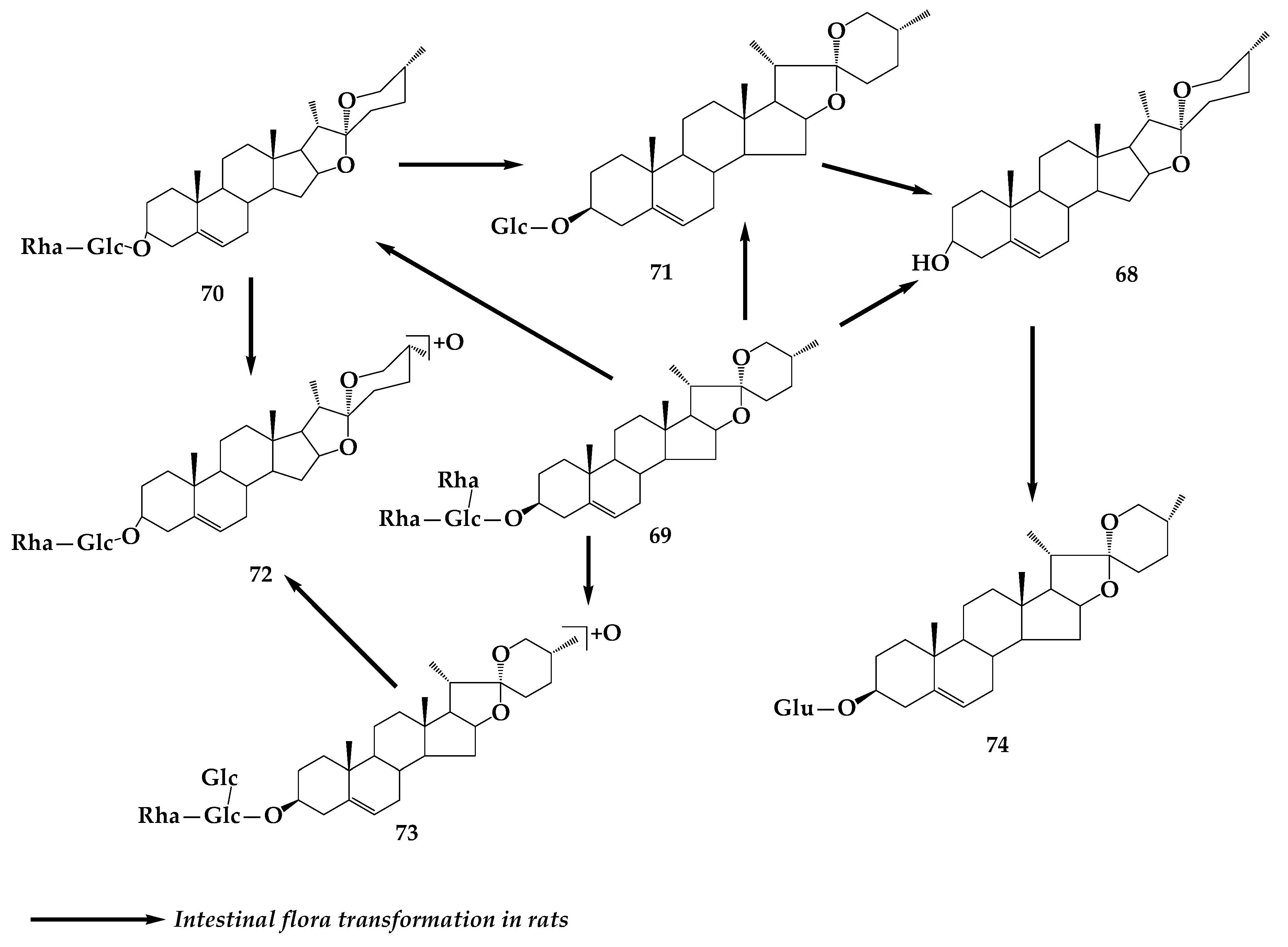
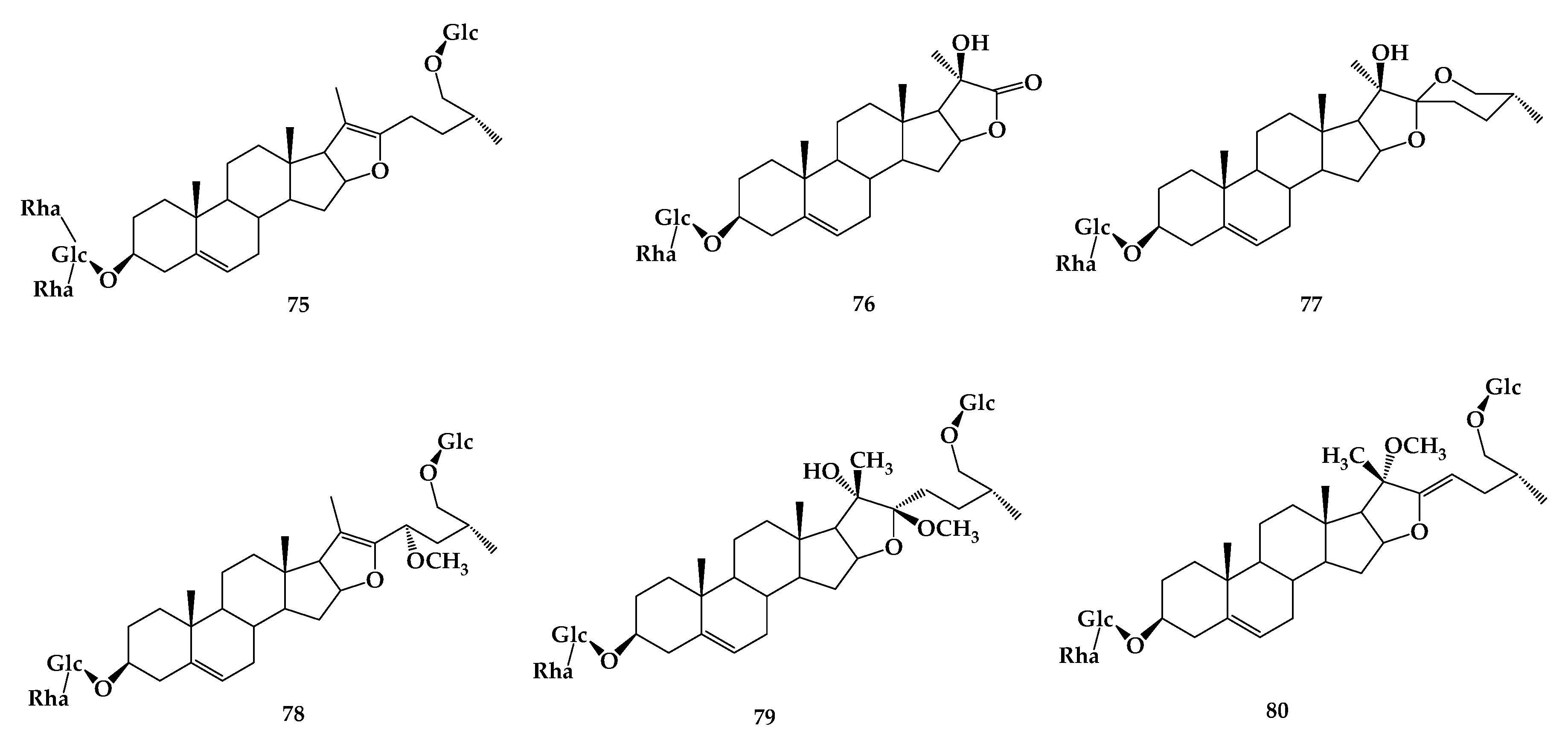
6. Dioscin
7. Timosaponin
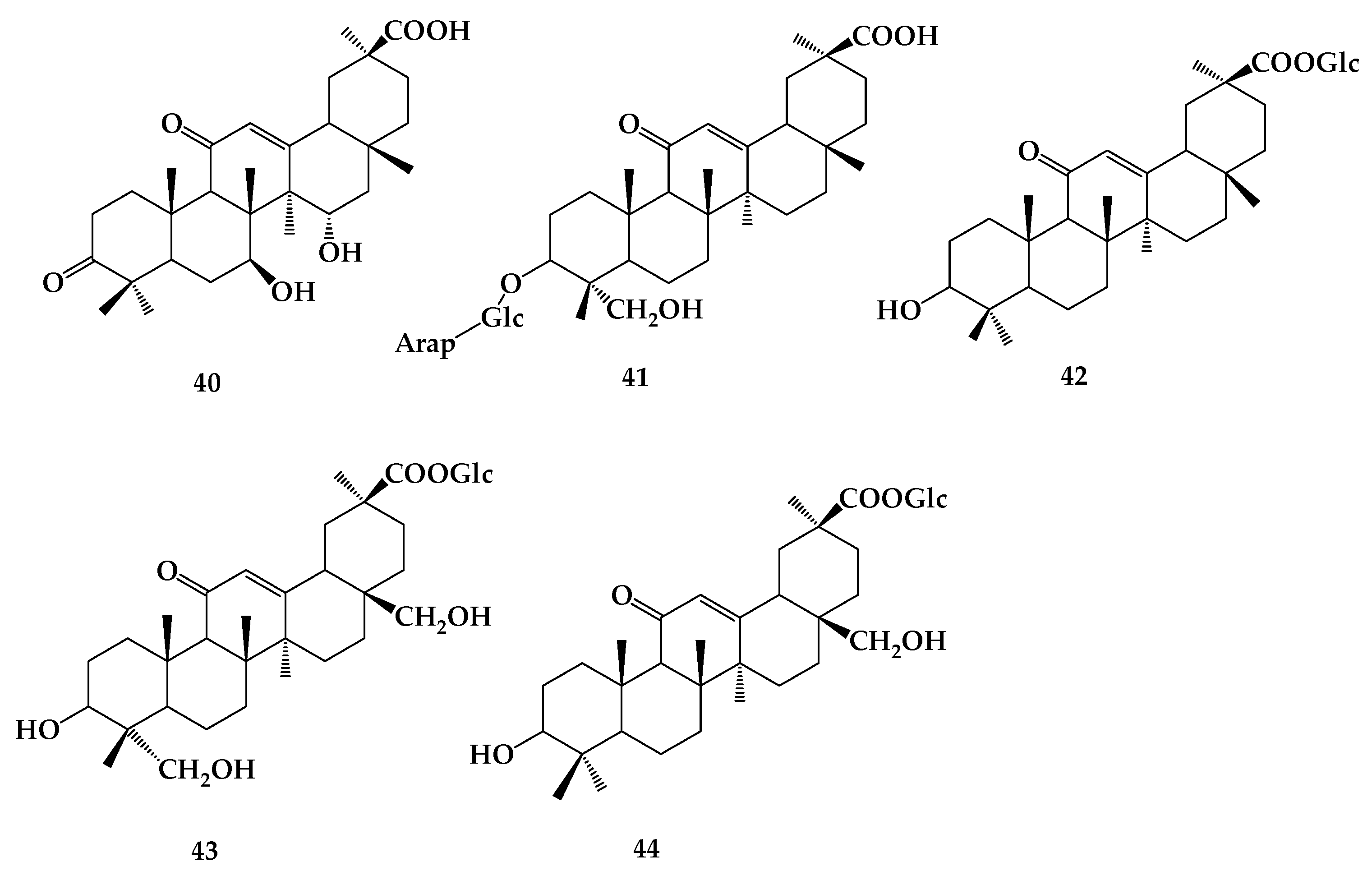
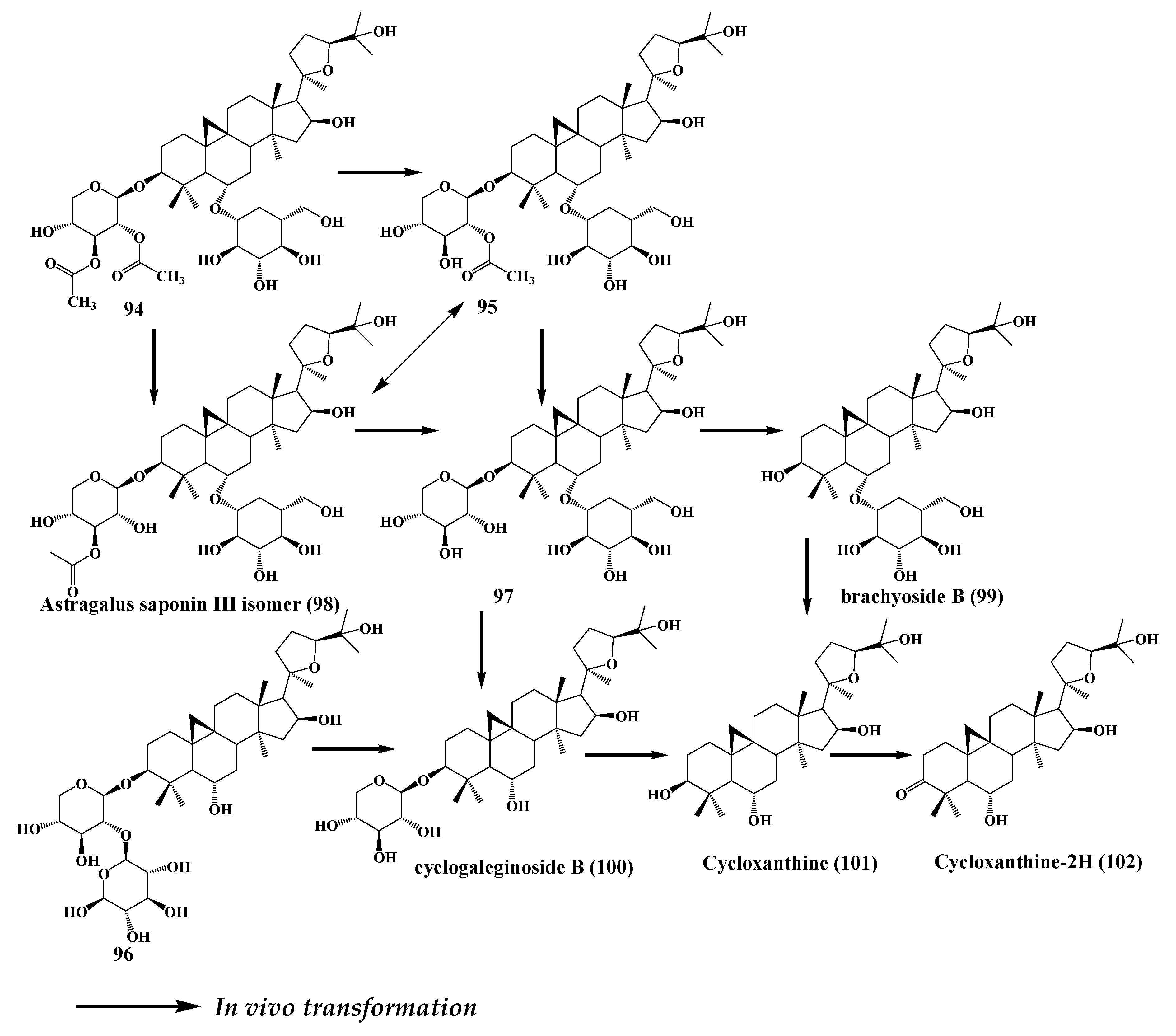
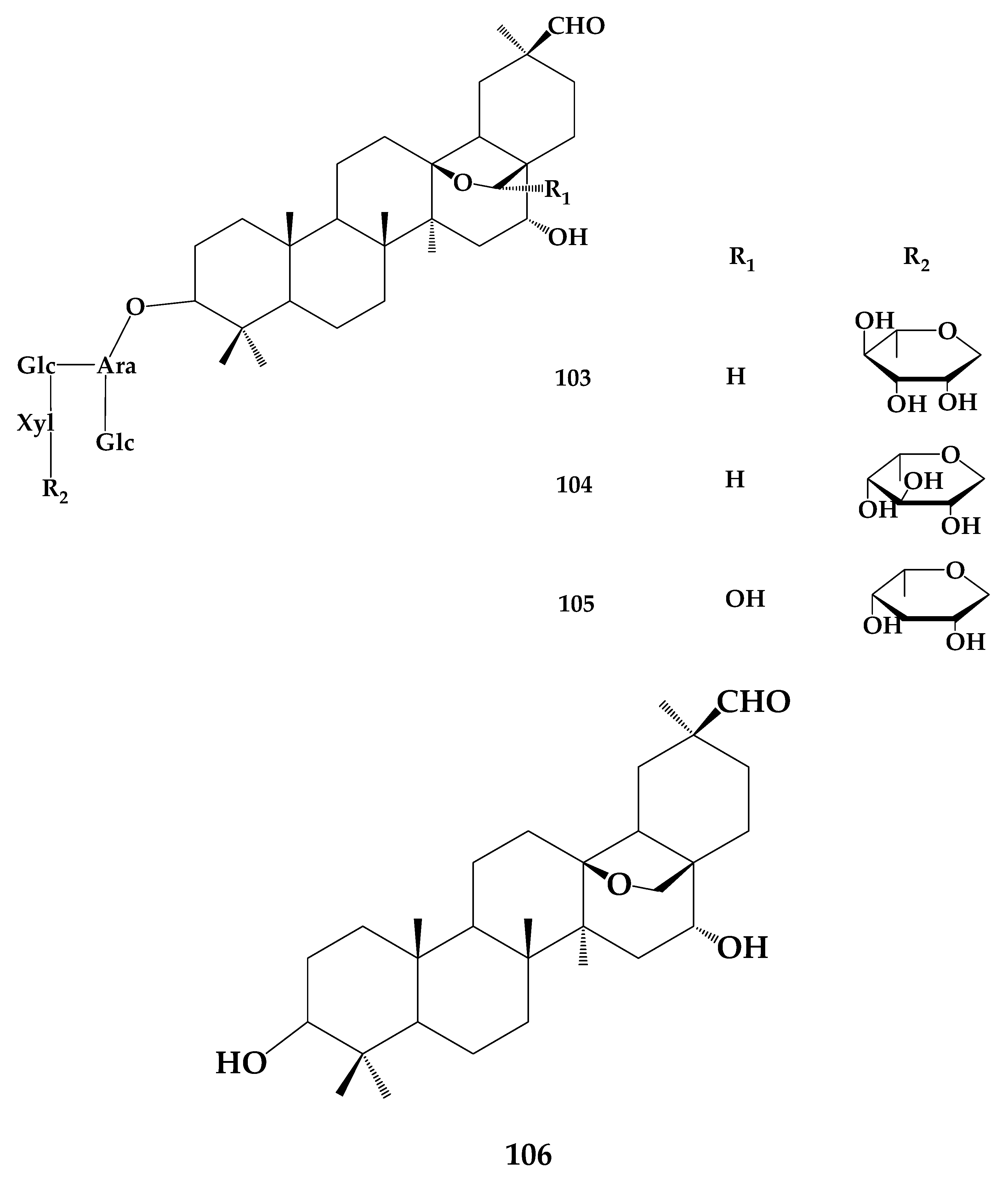
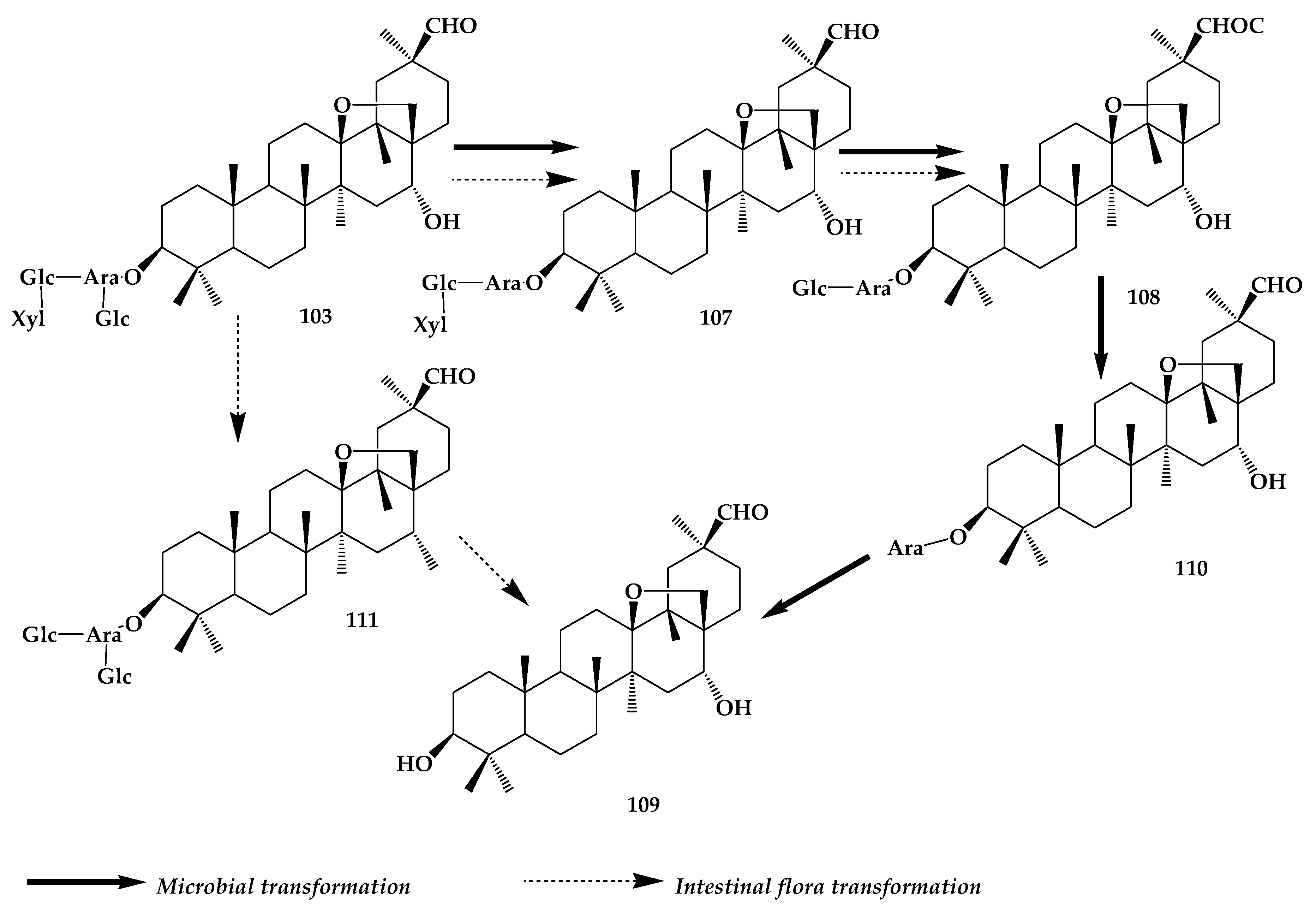
8. Astragaloside
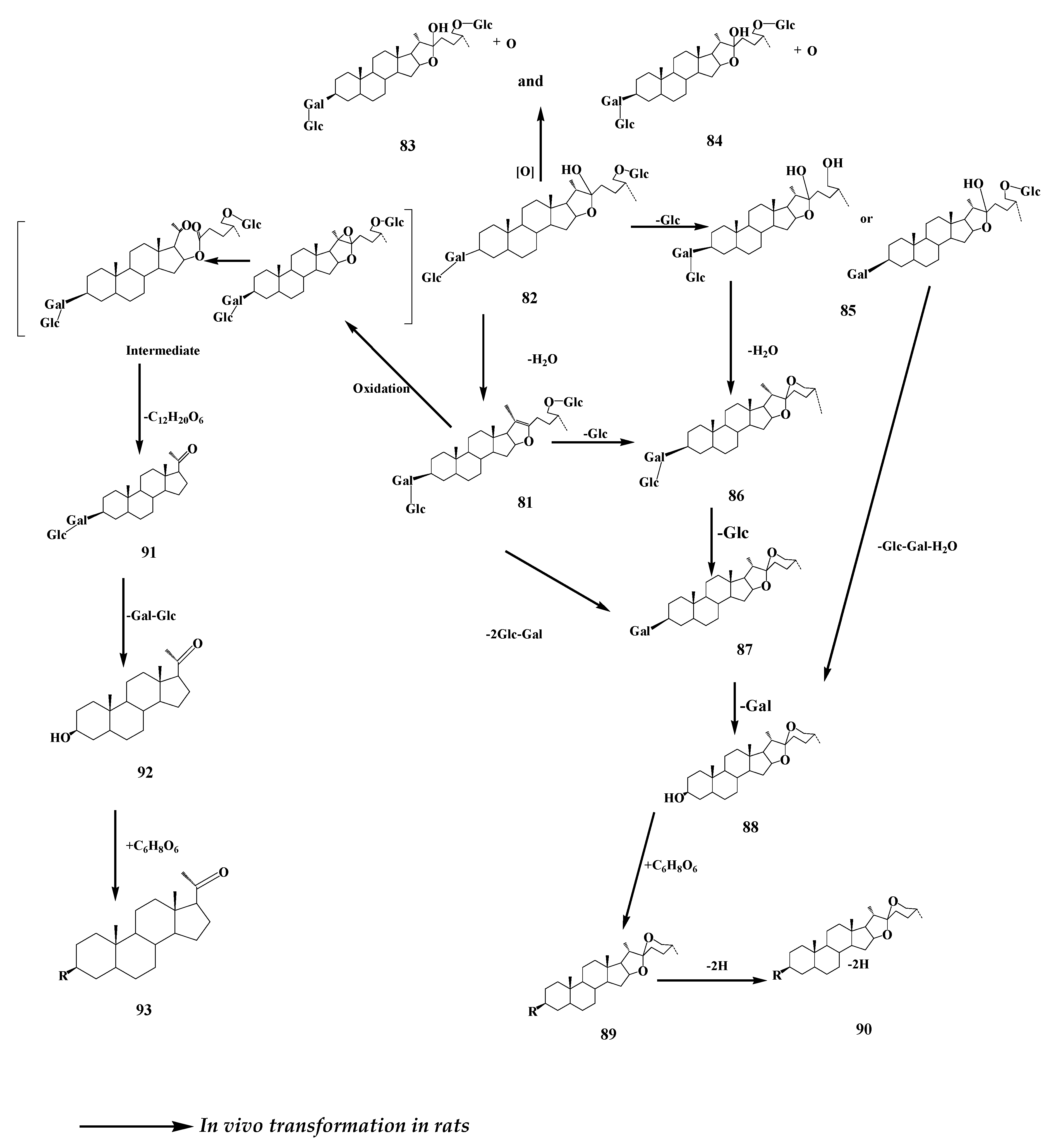
9. Ardipusilloside
10. Outlook
Funding
Conflicts of Interest
References
- Haralampidis, K.; Trojanowska, M.; Osbourn, A. Biosynthesis of triterpenoid saponins in plants. Adv. Biochem. Eng. Biotechnol. 2002, 75, 31–49. [Google Scholar] [PubMed]
- Estrada, A.; Katselis, G.S.; Laarveld, B.; Barl, B.; Diseases, I. Isolation and evaluation of immunological adjuvant activities of saponins from Polygala senega L. Comp. Immunol. Microbiol. Infect. Dis. 2000, 23, 27–43. [Google Scholar] [CrossRef]
- Cheeke, P.R. Actual and Potential Applications of Yucca Schidigera and Quillaja Saponaria Saponins in Human and Animal Nutrition. Sapon. Food Feedstuffs Med. Plants 2000, 45, 241–254. [Google Scholar]
- Matsuura, H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J. Nutr. 2001, 131, 1000S–1005S. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Park, S.K.; Kang, S.L.; Kang, H.C.; Oh, H.J.; Bae, C.Y.; Bae, D.H. Hypocholesterolemic property of Yucca schidigera and Quillaja saponaria extracts in human body. Arch. Pharm. Res. 2003, 26, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Gabriella, C.; Ernesto, F.; Virginia, L.; Raffaele, C.; Izzo, A.A. Antispasmodic saponins from bulbs of red onion, Allium cepa L. var. Tropea. J. Agric. Food Chem. 2005, 53, 935–940. [Google Scholar]
- Liu, M.; Wang, Z.; Ju, Y.; Wong, R.N.; Wu, Q.Y. Diosgenin induces cell cycle arrest and apoptosis in human leukemia K562 cells with the disruption of Ca2+ homeostasis. Cancer Chemother. Pharmacol. 2005, 55, 79–90. [Google Scholar] [CrossRef]
- Del Hierro, J.N.; Herrera, T.; Fornari, T.; Reglero, G.; Martin, D. The gastrointestinal behavior of saponins and its significance for their bioavailability and bioactivities. J. Funct. Foods 2018, 40, 484–497. [Google Scholar] [CrossRef]
- Christensen, L.P.; John, L. Ginsenosides: Chemistry, Biosynthesis, Analysis, and Potential Health Effects. Adv. Food Nutr. Res. 2009, 55, 1–99. [Google Scholar]
- Kim, S.H.; Min, J.W.; Quan, L.H.; Lee, S.; Yang, D.U.; Yang, D. Enzymatic Transformation of Ginsenoside Rb1 by Lactobacillus pentosus Strain 6105 from Kimchi. J. Ginseng Res. 2012, 36, 291–297. [Google Scholar] [CrossRef]
- Quan, L.H.; Kim, Y.J.; Li, G.H.; Choi, K.T.; Yang, D.C. Microbial transformation of ginsenoside Rb1 to compound K by Lactobacillus paralimentarius. World J. Microbiol. Biotechnol. 2013, 29, 1001–1007. [Google Scholar] [CrossRef]
- Quan, L.H.; Min, J.W.; Yang, D.U.; Kim, Y.J.; Yang, D. Enzymatic biotransformation of ginsenoside Rb1 to 20(S)-Rg3 by recombinant β-glucosidase from Microbacterium esteraromaticum. Appl. Microbiol. Biotechnol. 2012, 94, 377–384. [Google Scholar] [CrossRef]
- Jin, Y.; Jung, S.Y.; Kim, Y.J.; Lee, D.Y.; Aceituno, V.C.; Wang, C.; Yang, D.C. Microbial deglycosylation and ketonization of ginsenoside by Cladosporium cladosporioide and their anticancer activity. Antonie Van Leeuwenhoek 2016, 109, 179–185. [Google Scholar] [CrossRef]
- Feng, L.; Xu, C.; Li, Z.; Li, J.; Dai, Y.; Han, H.; Yu, S.; Liu, S. Microbial conversion of ginsenoside Rd from Rb1 by the fungus mutant Aspergillus niger strain TH-10a. Prep. Biochem. Biotechnol. 2016, 46, 336–341. [Google Scholar] [CrossRef]
- Chen, G.T.; Yang, M.; Song, Y.; Lu, Z.Q.; Zhang, J.Q.; Huang, H.L.; Wu, L.J.; Guo, D.A. Microbial transformation of ginsenoside Rb1 by Acremonium strictum. Appl. Microbiol. Biotechnol. 2008, 77, 1345–1350. [Google Scholar] [CrossRef]
- Chen, G.; Yang, M.; Song, Y.; Lu, Z.; Zhang, J.; Huang, H.; Guan, S.; Wu, L.; Guo, D.A. Comparative analysis on microbial and rat metabolism of ginsenoside Rb1 by high-performance liquid chromatography coupled with tandem mass spectrometry. Biomed. Chromatogr. 2008, 22, 779–785. [Google Scholar] [CrossRef]
- Park, S.E.; Na, C.S.; Yoo, S.A.; Seo, S.H.; Son, H.S. Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria. J. Ginseng Res. 2017, 41, 36–42. [Google Scholar] [CrossRef]
- Hou, J.; Xue, J.; Wang, C.; Liu, L.; Zhang, D.; Wang, Z.; Li, W.; Zheng, Y.; Sung, C. Microbial transformation of ginsenoside Rg3 to ginsenoside Rh2 by Esteya vermicola CNU 120806. World J. Microbiol. Biotechnol. 2012, 28, 1807–1811. [Google Scholar] [CrossRef]
- Liu, L.; Gu, L.J.; Zhang, D.L.; Wang, Z.; Wang, C.Y.; Li, Z.; Sung, C.K. Microbial conversion of rare ginsenoside Rf to 20(S)-protopanaxatriol by Aspergillus niger. Biosci. Biotechnol. Biochem. 2010, 74, 96–100. [Google Scholar] [CrossRef][Green Version]
- Han, Y.; Sun, B.; Jiang, B.; Hu, X.; Spranger, M.I.; Zhang, Y.; Zhao, Y. Microbial transformation of ginsenosides Rb1, Rb3 and Rc by Fusarium sacchari. J. Appl. Microbiol. 2010, 109, 792–798. [Google Scholar] [CrossRef]
- Chi, H.; Kim, D.H.; Ji, G.E. Transformation of ginsenosides Rb2 and Rc from Panax ginseng by food microorganisms. Biol. Pharm. Bull. 2005, 28, 2102–2105. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wu, S.Q.; Zhao, C.A.; Yin, C.R. Microbial conversion of major ginsenosides in ginseng total saponins by Platycodon grandiflorum endophytes. J. Ginseng Res. 2016, 40, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yang, X.B.; Yang, X.W.; Liu, J.X. Biotransformation ofprim-O-glucosylcimifugin by human intestinal flora and its inhibition on NO production and DPPH free radical. J. Asian Nat. Prod. Res. 2012, 14, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Eunah, B.; Han, M.K.; Sunyoung, P.; Donghyun, K.; Bulletin, P. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol. Pharm. Bull. 2002, 25, 58–63. [Google Scholar]
- Liu, X.; Qiao, L.; Xie, D.; Zhang, Y.; Zou, J.; Chen, X.; Dai, J. Microbial transformation of ginsenoside-Rg(1) by Absidia coerulea and the reversal activity of the metabolites towards multi-drug resistant tumor cells. Fitoterapia 2011, 82, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, M.; Nong, S.; Yang, X.; Ling, Y.; Wang, D.; Wang, X.; Zhang, W. Microbial transformation of 20(S)-protopanaxadiol by Absidia corymbifera. Cytotoxic activity of the metabolites against human prostate cancer cells. Fitoterapia 2013, 84, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Huang, H.; Zhu, H.; Zhou, P.; Shi, X. New metabolites from the biotransformation of ginsenoside Rb1 by Paecilomyces bainier sp.229 and activities in inducing osteogenic differentiation by Wnt/β-catenin signaling activation. J. Ginseng Res. 2018, 42, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyam, S.; Mathiyalagan, R.; Gyo, I.J.; Bum-Soo, L.; Sungyoung, L.; Yang, D. Transcriptome profiling and insilico analysis of Gynostemma pentaphyllum using a next generation sequencer. Plant Cell Rep. 2011, 30, 2075–2083. [Google Scholar] [CrossRef]
- Shi, L.; Song, D.P.; Pan, M. Research advances on the saponins of Gynostemma pentaphyllum. Drug Eval. Res. 2011, 34, 456–464. [Google Scholar]
- Yu, H.; Liu, H.; Zhang, C.; Tan, D.; Lu, M.; Jin, F. Purification and characterization of gypenoside-α-rhamnosidase hydrolyzing gypenoside-5 into ginsenoside Rd. Process Biochem. 2004, 39, 861–867. [Google Scholar] [CrossRef]
- Ma, S.L.; Wang, F.X.; Yang, X.; Wan, S.N.; Wei, X.Y. Diversity of Endogeny Eumycetes in Gynostemma pentaphyllum and Its Correlation with Gypenoside XLIX. J. Chin. Med. Mater. 2015, 38, 476–480. (In Chinese) [Google Scholar]
- Chen, D.J.; Hu, H.G.; Xing, S.F.; Liu, H.M.; Piao, X. Metabolite profiling of gypenoside LVI in rat after oral and intravenous administration. Arch. Pharm. Res. 2015, 38, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Leung, W.I.; Ruan, J.Q.; Li, S.L.; Lei, J.P.; Wang, Y.T.; Yan, R.J. Biotransformation of ginsenoside Rb1 via the gypenoside pathway by human gut bacteria. Chin. Med. 2013, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.H.; Piao, J.Y.; Min, J.W.; Kim, H.B.; Kim, S.R.; Yang, D.U.; Yang, D. Biotransformation of Ginsenoside Rb1 to Prosapogenins, Gypenoside XVII, Ginsenoside Rd, Ginsenoside F2, and Compound K by Leuconostoc mesenteroides DC102. J. Ginseng Res. 2011, 35, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, L.; Jeger, O.; Ingold, W. TriterpenesLXXXIV. New proof for the difference in position of the carboxylic acid group in oleanolic and glycyrrhetinic acids. Helvetica Chimica Acta 1943, 26, 2278–2282. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, M.; Bulletin, P. Biotransformation of glycyrrhizin to 18β-glycyrrhetinic acid-3-O-β-D-glucuronide by Streptococcus LJ-22, a human intestinal bacterium. Biol. Pharm. Bull. 1999, 22, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, N.; Kido, A.; Kubota, K.; Tsuchiya, N.; Morita, T.; Kase, Y.; Takeda, S. A possible involvement of 3-monoglucuronyl-glycyrrhetinic acid, a metabolite of glycyrrhizin (GL), in GL-induced pseudoaldosteronism. Sci. China-Life Sci. 2007, 80, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Dhingra, V.; Giri, C.C.; Singh, A.; Ward, O.P.; Narasu, M. Biotransformations using plant cells, organ cultures and enzyme systems: Current trends and future prospects. Biotechnol. Adv. 2001, 19, 175–199. [Google Scholar] [CrossRef]
- Akao, T.; Hattori, M.; Kanaoka, M.; Yamamoto, K.; Namba, T.; Kobashi, K. Hydrolysis of glycyrrhizin to 18β-glycyrrhetyl monoglucuronide by lysosomal β-D-glucuronidase of animal livers. Biochem. Pharmacol. 1991, 41, 1025. [Google Scholar] [CrossRef]
- Kang, L.P.; Zhang, J.; Yu, H.S.; Huang, H.Z.; Wang, Y.Z.; Ma, B. One new triterpenoid from biotransformation product of glycyrrhizic acid. J. Asian Nat. Prod. Res. 2008, 10, 463–466. [Google Scholar] [CrossRef]
- Hayashi, H.; Fukui, H.; Tabata, M. Biotransformation of 18β-glycyrrhetinic acid by cell suspension cultures of Glycyrrhiza glabra. Phytochemistry 1990, 29, 2149–2152. [Google Scholar] [CrossRef]
- Asada, Y.; Saito, H.; Yoshikawa, T.; Sakamoto, K.; Furuya, T. Biotransformation of 18β-glycyrrhetinic acid by ginseng hairy root culture. Phytochemistry 1993, 34, 1049–1052. [Google Scholar] [CrossRef]
- Orihara, Y.; Furuya, T. Biotransformation of 18β-glycyrrhetinic acid by cultured cells of Eucalyptus perriniana and Coffea arabica. Phytochemistry 1990, 29, 3123–3126. [Google Scholar] [CrossRef]
- Guo, Y.C.; Lin, Z.X.; Luo, W.H. Microbial Transformation of Aristolochic Acid I by Cunninghamella Blakesleana. Curr. Pharm. Biotechnol. 2015, 40, 451–455. [Google Scholar]
- Xin, X.; Liu, Y.; Ye, M.; Guo, H.; Guo, D. Microbial transformation of glycyrrhetinic acid by Mucor polymorphosporus. Planta Med. 2006, 72, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nakamura, A.; Yamamoto, K.; Kikuzaki, H. Biochemistry Transformation of Glycyrrhizic Acid by Aspergillus spp. B Chem. Soc. Jpn. 1994, 58, 436–437. [Google Scholar]
- Akao, T. Differences in the metabolism of glycyrrhizin, glycyrrhetic acid and glycyrrhetic acid monoglucuronide by human intestinal flora. Biol. Pharm. Bull. 2000, 23, 1418–1423. [Google Scholar] [CrossRef]
- Lin, S.P.; Tsai, S.Y.; Hou, Y.C.; Chao, P.D.L. Glycyrrhizin and licorice significantly affect the pharmacokinetics of methotrexate in rats. J. Agric. Food Chem. 2009, 57, 1854–1859. [Google Scholar] [CrossRef]
- Akao, T. Purification and characterization of glycyrrhetic acid mono-glucuronide β-D-glucuronidase in Eubacterium sp. GLH. Biol. Pharm. Bull. 1999, 22, 80–82. [Google Scholar] [CrossRef]
- Pan, S.L.; Pan, S.; Applications, C. Bupleurum Species: Scientific Evaluation and Clinical Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Li, X.Q.; Song, Y.N.; Wang, S.J.; Khalid, R.; Zhu, J.Y.; Zhang, H. Saikosaponins: A review of pharmacological effects. J. Asian Nat. Prod. Res. 2018, 20, 399–411. [Google Scholar] [CrossRef]
- Liu, Q.X.; Tan, L.; Bai, Y.J.; Liang, H.; Zhao, Y. A survey of the studies on saponins from Bupleurum in past 10 years. China J. Chin. Mater. Med. 2002, 27, 7–11. (In Chinese) [Google Scholar]
- Shimizu, K.; Amagaya, S.; Ogihara, Y. Structural transformation of saikosaponins by gastric juice and intestinal flora. J. Pharm.-Dyn. 1985, 8, 718. [Google Scholar] [CrossRef]
- Yu, K.U.; Jang, I.S.; Kang, K.H.; Sung, C.K.; Kim, D. Metabolism of saikosaponin c and naringin by human intestinal bacteria. Arch. Pharm. Res. 1997, 20, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Kida, H.; Akao, T.; Meselhy, M.R.; Hattori, M. Metabolism and pharmacokinetics of orally administered saikosaponin b1 in conventional, germ-free and Eubacterium sp. A-44-infected gnotobiote rats. Biol. Pharm. Bull. 1998, 21, 588. [Google Scholar] [CrossRef] [PubMed]
- Meselhy, M. Human intestinal bacteria responsible for the metabolism of saikosaponins. Ho Han Med. Sch. Eternal Rec. 2000, 17, 1–11. [Google Scholar]
- Gao, R.R.; Gao, S.H.; Dong, X.R.; Hu, X.H.; Qiao, Y.; Sun, D.A. Microbial Transformation of Gracillin by Penicillium Lilacinum ACCC 31890. China Pharm. 2017, 20, 988–999. (In Chinese) [Google Scholar]
- Zhu, H.; Xu, J.D.; Mao, Q.; Shen, H.; Kong, M.; Chen, J.P.; Li, S. Metabolic profiles of dioscin in rats revealed by ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr. 2015, 29, 1415–1421. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Huang, W.; Ni, J.R.; Liu, W.; Li, H. Biotechnology Production of diosgenin from Dioscorea zingiberensis tubers through enzymatic saccharification and microbial transformation. Appl. Microbiol. Biotechnol. 2010, 85, 1409–1416. [Google Scholar] [CrossRef]
- Zheng, T.; Yu, L.; Zhu, Y.; Zhao, B.; Equipment, B. Evaluation of different pretreatments on microbial transformation of saponins in Dioscorea zingiberensis for diosgenin production. Biotechnol. Biotecnol. Equip. 2014, 28, 740–746. [Google Scholar] [CrossRef]
- Dong, X.; Gao, Z.; Hu, H.; Gao, R.; Sun, D. Microbial transformation of Pseudoprotodioscin by Chaetomium olivaceum. J. Mol. Catal. B-Enzym. 2016, 130, 88–95. [Google Scholar] [CrossRef]
- Takeda, Y.; Togashi, H.; Matsuo, T.; Shinzawa, H.; Takeda, Y.; Takahashi, T. Growth inhibition and apoptosis of gastric cancer cell lines by Anemarrhena asphodeloides Bunge. J. Gastroenterol. 2001, 36, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jiang, T.; Han, B.; Li, L.; Bing, F.; Kang, L.; Jie, Z.; Yan, F.; Xiong, C.; Jia, D.; et al. Preparation of some metabolites of timosaponin B-II by biotransformation in vitro. Process Biochem. 2015, 50, 2182–2187. [Google Scholar]
- Yao, J.; Fu, Z.; Li, Z.; Pei, H.; Rui, X.; Chen, M.; Xiang, T.; Huang, C. In-vivo and In-vitro Metabolism Study of Timosaponin B-II Using HPLC-ESI-MS n. Chromatographia 2015, 78, 1175–1184. [Google Scholar]
- Hu, Y.M.; Yu, Z.L.; Fong, W. Biotechnology Stereoselective Biotransformation of Timosaponin A-III by Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2011, 21, 582–589. [Google Scholar]
- Lu, L.; Liu, Y.P.; Ding, Y.; Hou, J.W.; Zhang, Y.; Xue, H.P.; Zhang, T. Preparation of highly purified timosaponin AIII from rhizoma anemarrhenae through an enzymatic method combined with preparative liquid chromatography. Nat. Prod. Res. 2016, 30, 2364–2367. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xue, R.; Li, Z.; Chen, M.; Jiang, W.; Huang, C. In Vivo Metabolism Study of Timosaponin B-III in Rat Using HPLC-QTOF-MS/MS. Chromatographia 2014, 77, 853–858. [Google Scholar] [CrossRef]
- Liu, Z.R.; Zhu, D.L.; Lv, L.; Li, Y.Y.; Dong, X.; Zhu, Z.Y.; Cai, Y.F. Metabolism profile of timosaponin B-II in urine after oral administration to rats by ultrahigh-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.H.; Fan, M.S.; Tang, Y.H.; Li, Z.X.; Sun, Z.L.; Ye, G.; Huang, C.G. Identification of major alkaloids and steroidal saponins in rat serum by HPLC-diode array detection-MS/MS following oral administration of Huangbai-Zhimu herb-pair Extract. Biomed. Chromatogr. 2010, 22, 835–850. [Google Scholar] [CrossRef]
- Li, X.X.; Qu, L.; Dong, Y.Z.; Han, L.F.; Liu, E.W.; Fang, S.M.; Zhang, Y.; Wang, T. A review of recent research progress on the astragalus genus. Molecules 2014, 19, 18850–18880. [Google Scholar] [CrossRef]
- Meng, X.T.; Yue, S.J.; Yang, Z.R.; Liu, J.; Feng, W.W.; Su, J.; Zhang, Y. Investigation on Biotransformation Characteristics of Astragalosides in Human Intestinal Microbiota. Food Drug 2018, 20, 161–167. (In Chinese) [Google Scholar]
- Zhai, Y.; Li, P.; Wang, M.; Gong, M.; Qiu, F. Determination of astragaloside III in rat plasma by liquid chromatography-tandem mass spectrometry and its application to a rat pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y. Efficient Biotransformation of Astragaloside IV to Cycloastragenol Bacillus sp. LG-502. Appl. Biochem. Biotechnol. 2017, 183, 1488–1502. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Li, Y.; Feng, M.; Pei, Z.; Shi, X. The biotransformation of astragalosides by a novel acetyl esterase from Absidia corymbifera AS2. Process Biochem. 2014, 49, 1464–1471. [Google Scholar]
- Tian, Y.; Tang, H.F.; Qiu, F.; Wang, X.J.; Chen, X.L.; Wen, A.D. Triterpenoid saponins from Ardisia pusilla and their cytotoxic activity. Planta Med. 2008, 75, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.Y.; Wang, Y.N.; Wang, P.Y.; Lei, W.; Feng, B.; Wang, X.J. Ardipusilloside-I Metabolites from Human Intestinal Bacteria and Their Antitumor Activity. Molecules 2015, 20, 20569–20581. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.Y.; Wang, Y.N.; Wang, P.Y.; Lei, W.; Feng, B.; Wang, X.J. Study on biotransformation of Ardipusilloside-I by cultured human intestinal flora in vitro. Tianran Chanwu Yanjiu Yu Kaifa 2015, 27, 1357–1361. (In Chinese) [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
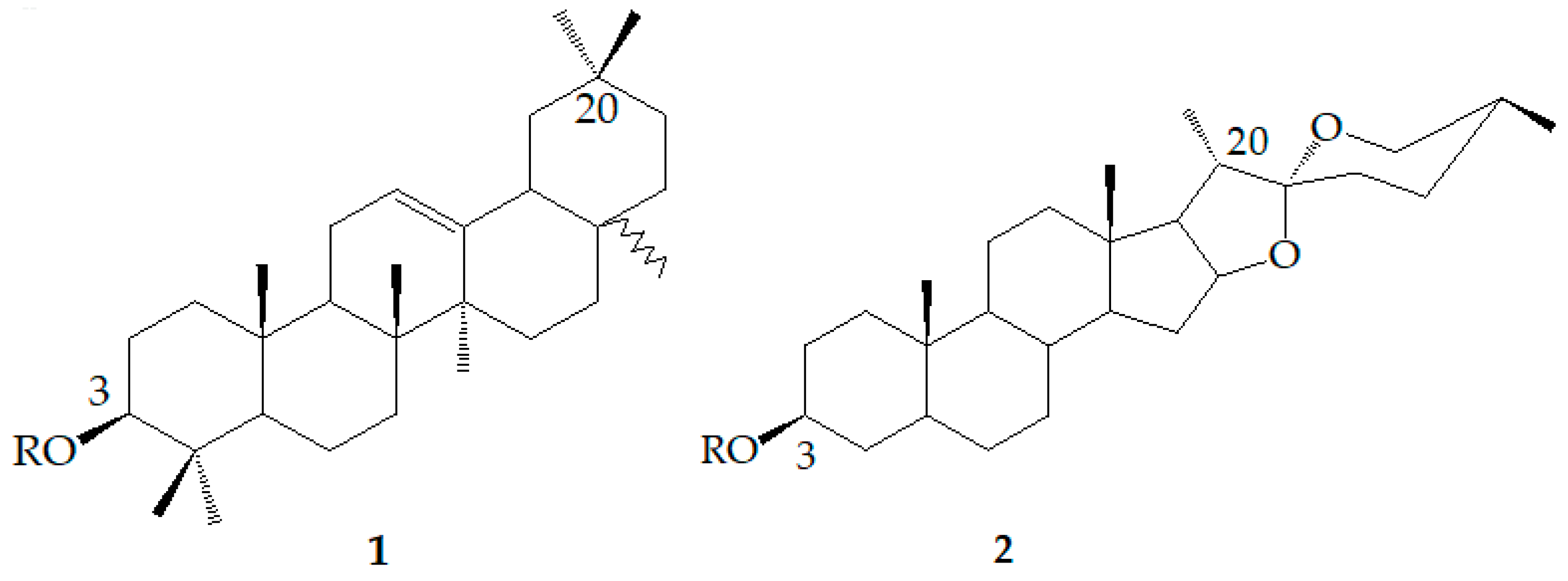
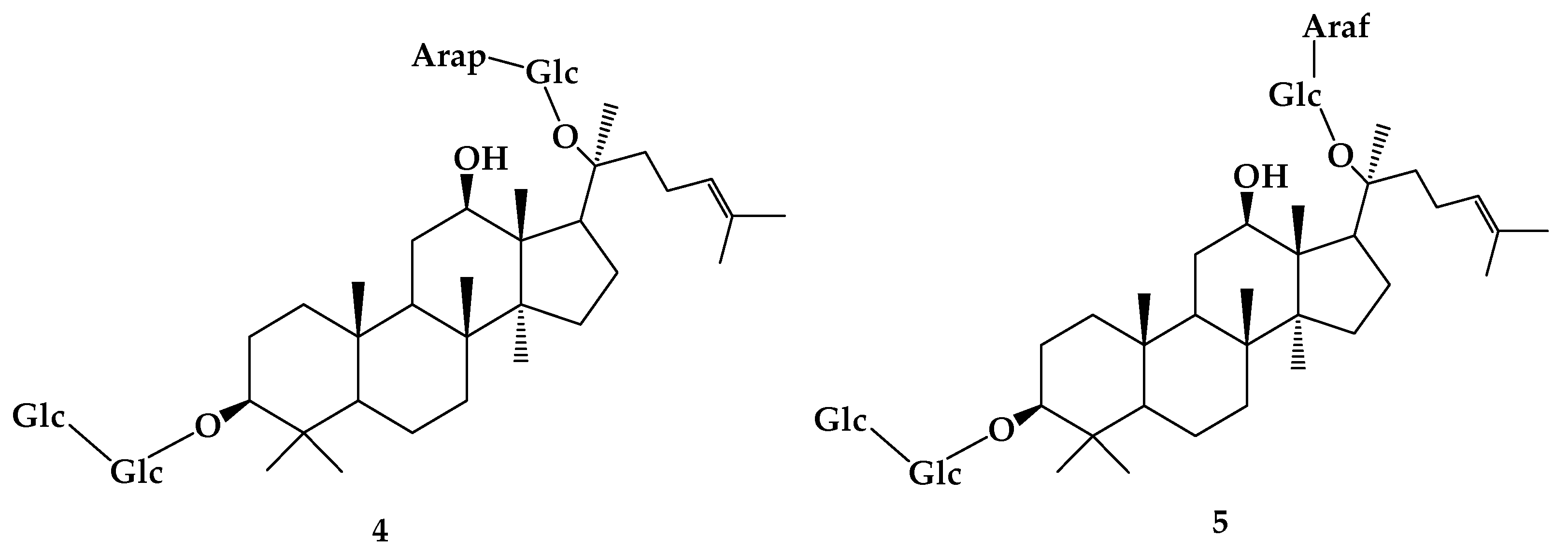
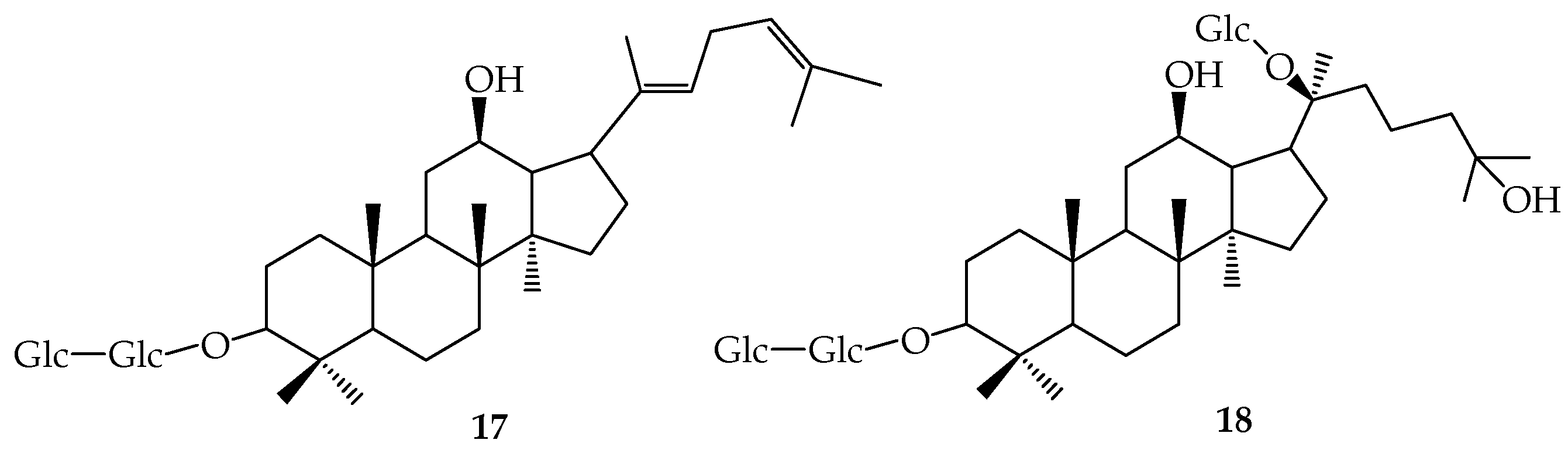
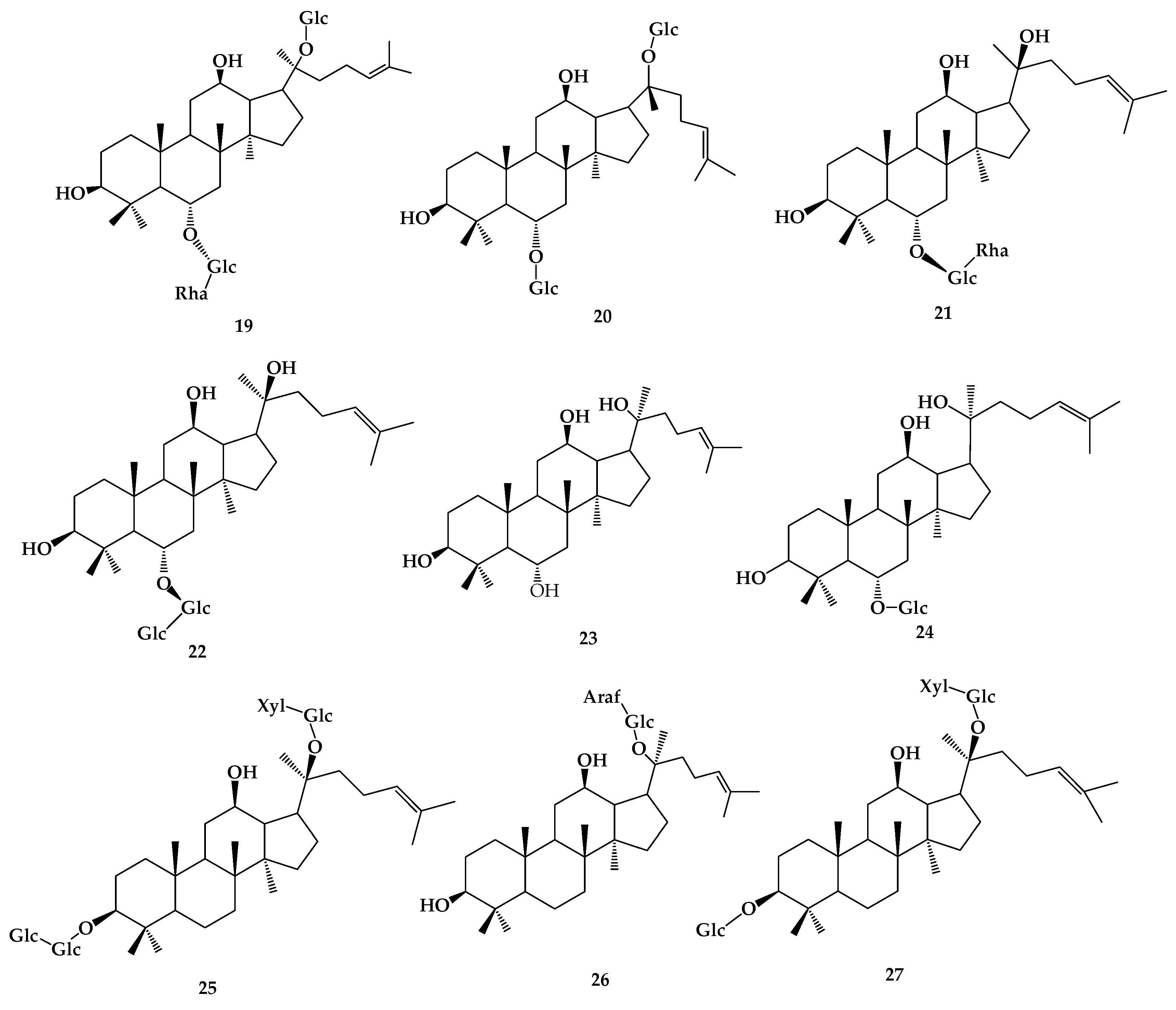
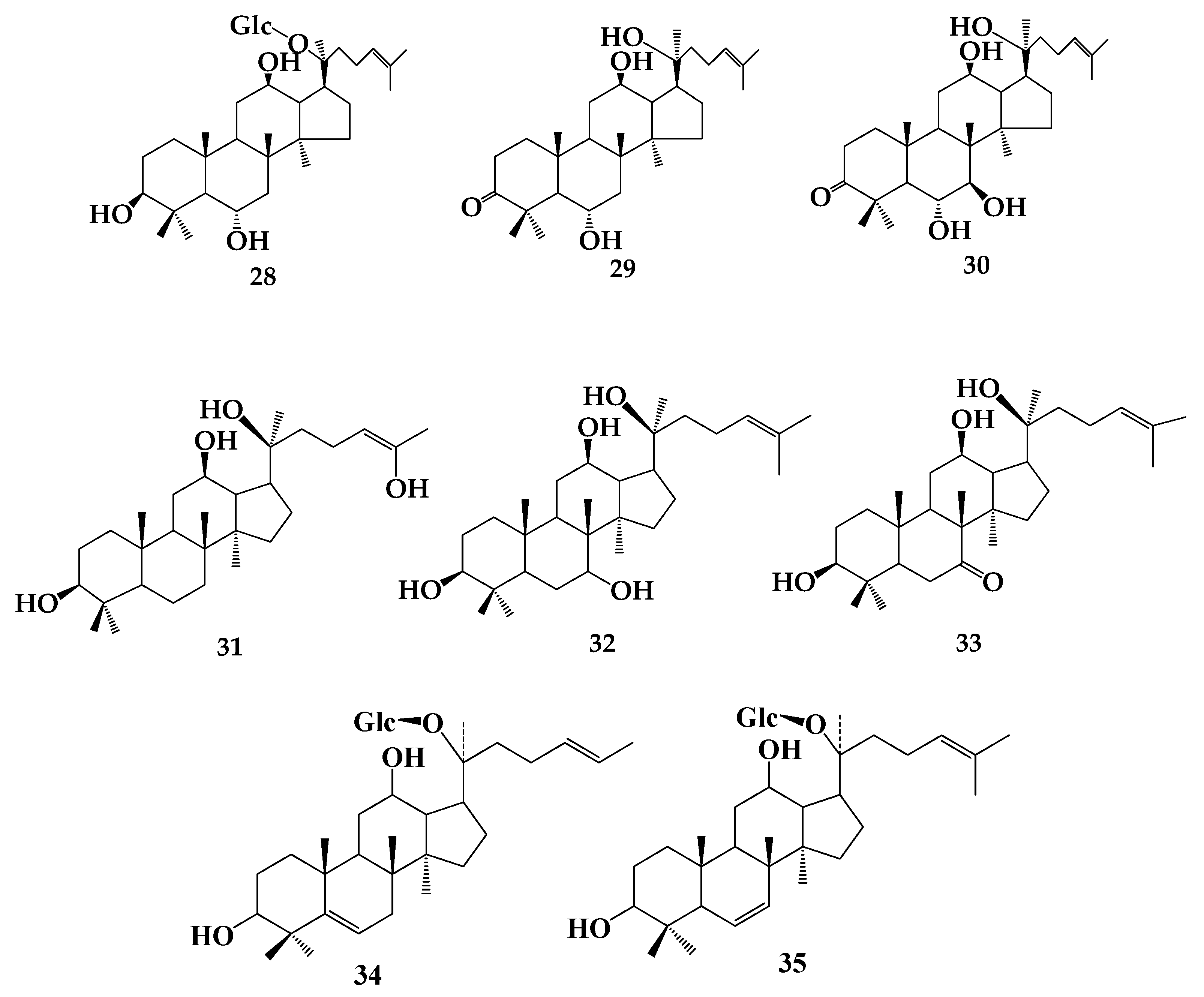




| R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | |
|---|---|---|---|---|---|---|---|---|---|
| 53 | OH | H | CH3 | H | H | H | OH | H | H |
| 54 | OH | H | CH3 | H | H | H | H | OH | H |
| 55 | OH | H | CH2OH | H | H | H | H | H | H |
| 56 | OH | H | CH3 | OH | H | H | H | H | H |
| 57 | OH | H | CH3 | H | H | OH | H | H | H |
| 58 | OAc | H | CH3 | H | H | H | OH | H | H |
| 59 | O | O | CH3 | H | H | H | OH | H | H |
| 60 | O | O | CH3 | H | H | H | H | OH | H |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent Advances in Biotransformation of Saponins. Molecules 2019, 24, 2365. https://doi.org/10.3390/molecules24132365
He Y, Hu Z, Li A, Zhu Z, Yang N, Ying Z, He J, Wang C, Yin S, Cheng S. Recent Advances in Biotransformation of Saponins. Molecules. 2019; 24(13):2365. https://doi.org/10.3390/molecules24132365
Chicago/Turabian StyleHe, Yi, Zhuoyu Hu, Aoran Li, Zhenzhou Zhu, Ning Yang, Zixuan Ying, Jingren He, Chengtao Wang, Sheng Yin, and Shuiyuan Cheng. 2019. "Recent Advances in Biotransformation of Saponins" Molecules 24, no. 13: 2365. https://doi.org/10.3390/molecules24132365
APA StyleHe, Y., Hu, Z., Li, A., Zhu, Z., Yang, N., Ying, Z., He, J., Wang, C., Yin, S., & Cheng, S. (2019). Recent Advances in Biotransformation of Saponins. Molecules, 24(13), 2365. https://doi.org/10.3390/molecules24132365








