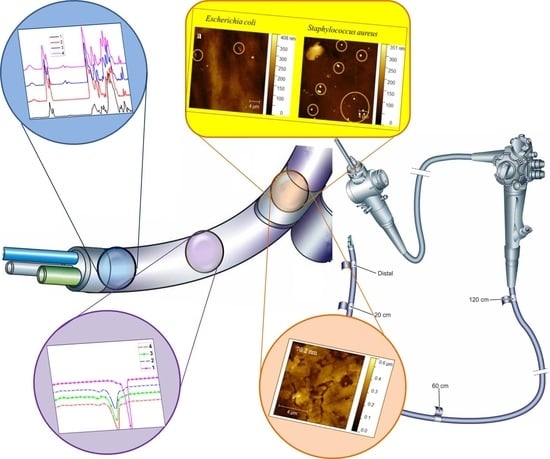
Duodenoscope-Associated Infections beyond the Elevator Channel: Alternative Causes for Difficult Reprocessing
Abstract
1. Introduction
2. Results and Discussion
2.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2. Thermal Behavior
2.3. Antimicrobial Activity
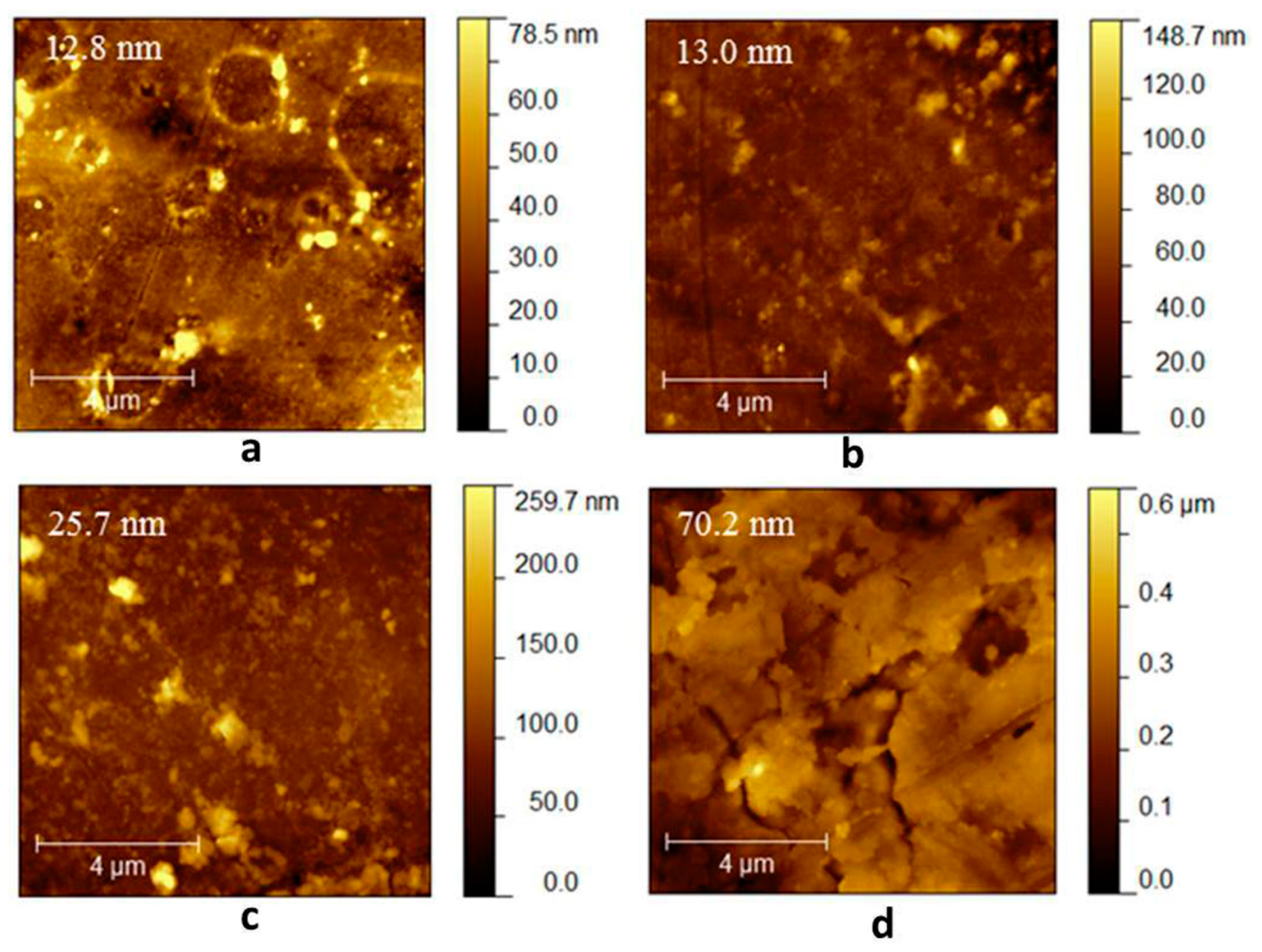
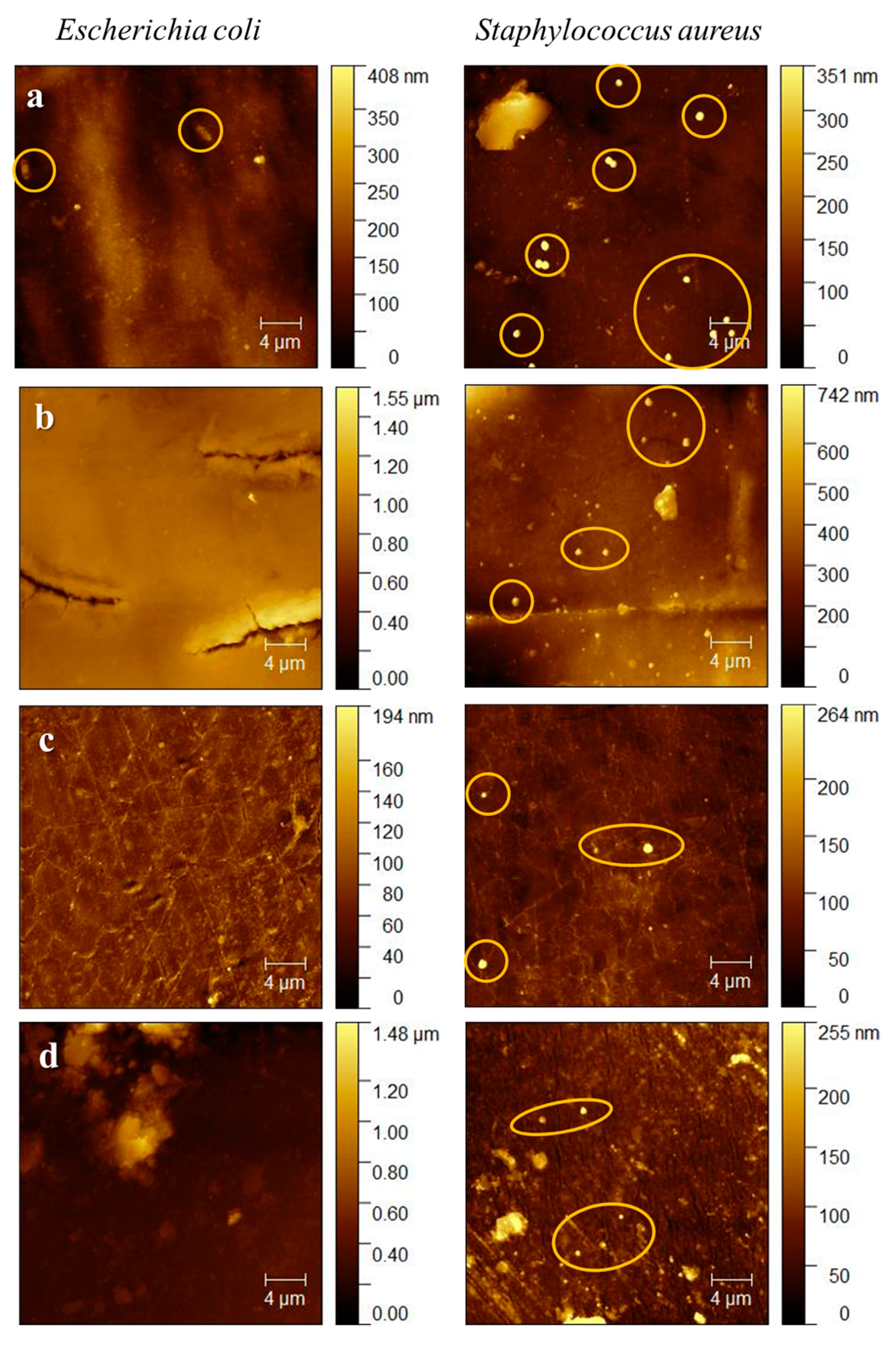
2.4. Atomic Force Microscopy (AFM)
2.4.1. Morphological Characterization
2.4.2. Evaluation of Biofilm Formation on Duodenoscope Samples
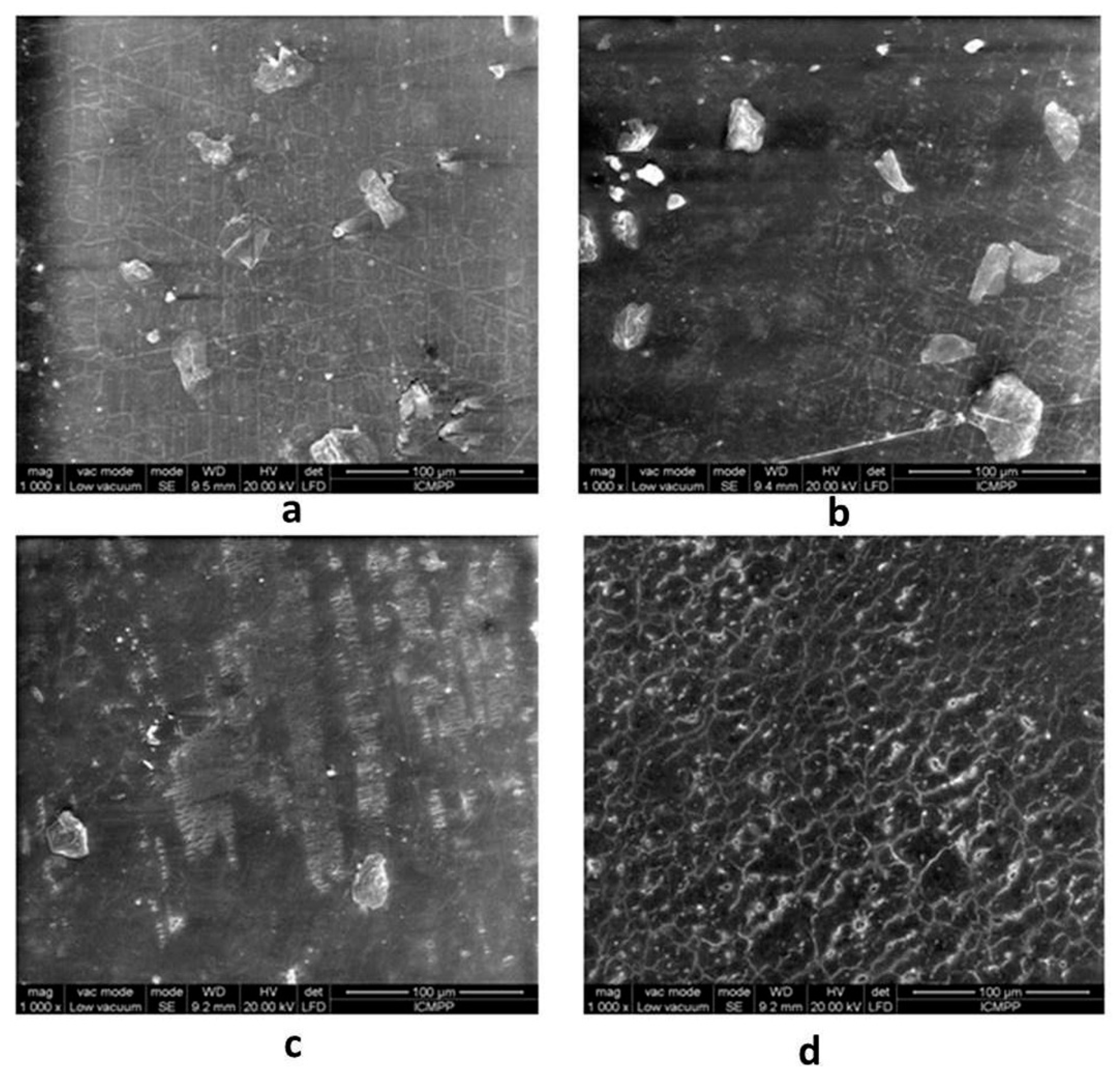
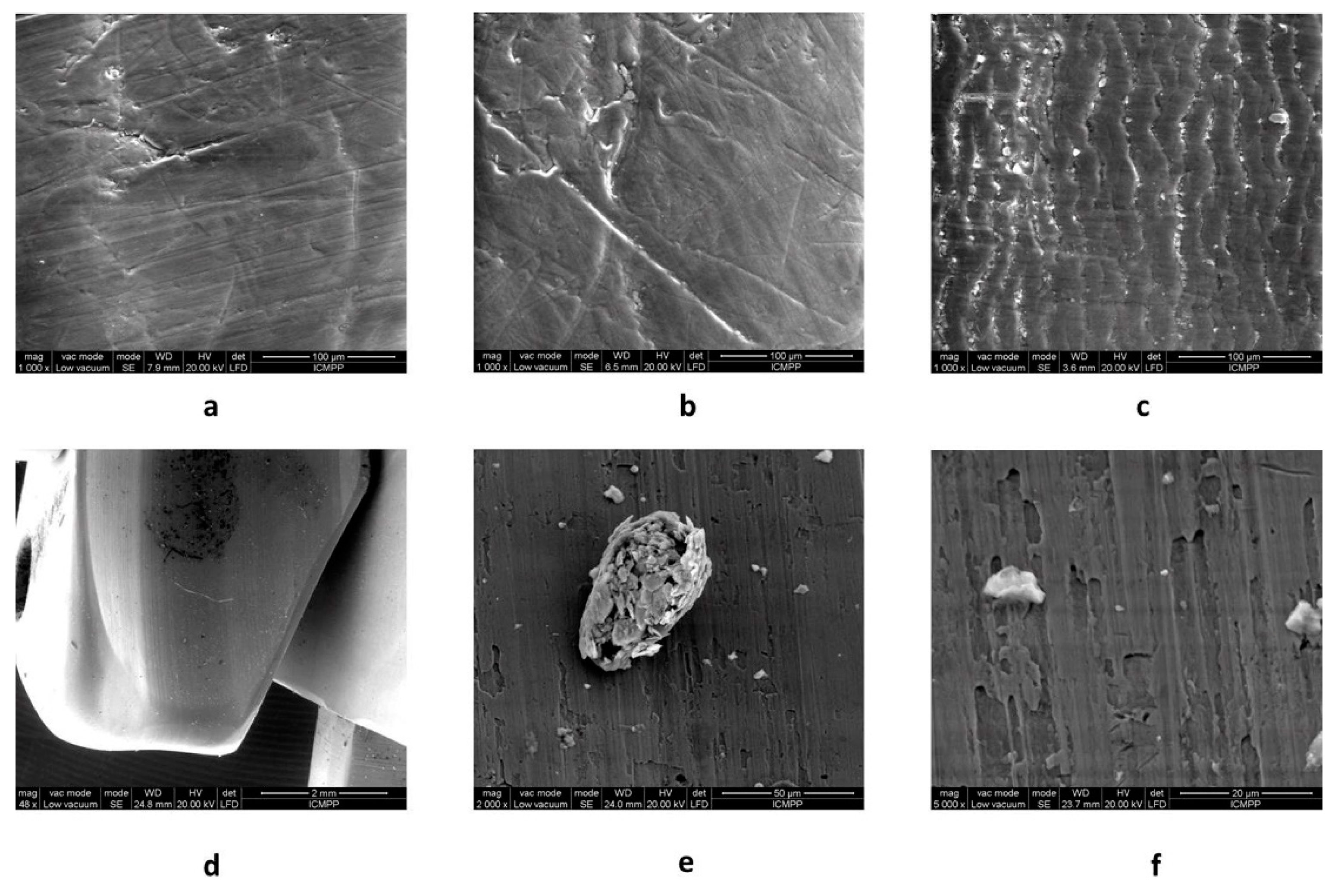
2.5. Scanning Electron Microscopy (SEM)
2.6. Impact on Public Health
3. Materials and Methods
3.1. Duodenoscope Samples
3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3. Differential Scanning Calorimetry (DSC)
3.4. Thermogravimetric Analysis (TGA)
3.5. Antibacterial Activity
3.6. Atomic Force Microscopy (AFM)
3.7. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allen, J.I.; Allen, M.O.; Olson, M.M.; Gerding, D.N.; Shanholtzer, C.J.; Meier, P.B.; Vennes, J.A.; Silvis, S.E. Pseudomonas infection of the biliary system resulting from use of a contaminated endoscope. Gastroenterology 1987, 92, 759–763. [Google Scholar] [CrossRef]
- Calderwood, A.H.; Day, L.W.; Muthusamy, V.R.; Collins, J.; Hambrick, R.D., 3rd; Brock, A.S.; Guda, N.M.; Buscaglia, J.M.; Petersen, B.T.; Buttar, N.S.; et al. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018, 87, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. ERCP scopes: What can we do to prevent infections? Infect. Control. Hosp. Epidemiol. 2015, 36, 643–648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Epstein, L.; Hunter, J.C.; Arwady, M.A.; Tsai, V.; Stein, L.; Gribogiannis, M.; Frias, M.; Guh, A.Y.; Laufer, A.S.; Black, S.; et al. New Delhi metallo-β-lactamase–producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 2014, 312, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Verfaillie, C.J.; Bruno, M.J.; Voor in ’t Holt, A.F.; Buijs, J.G.; Poley, J.W.; Loeve, A.J.; Severin, J.A.; Abel, L.F.; Smit, B.J.; de Goeij, I.; et al. Withdrawal of a novel-design duodenoscope ends outbreak of a VIM-2-producing Pseudomonas aeruginosa. Endoscopy 2015, 47, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Bălan, G.G.; Roşca, I.; Ursu, E.L.; Doroftei, F.; Bostănaru, A.C.; Hnatiuc, E.; Năstasă, V.; Şandru, V.; Ştefănescu, G.; Trifan, A.; et al. Plasma activated water—A new and effective alternative for duodenoscope reprocessing. Infect. Drug. Resist. 2018, 11, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Polivkova, M.; Hubacek, T.; Staszek, M.; Svorcik, V.; Siegel, J. Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology. Int. J. Mol. Sci. 2017, 18, 419. [Google Scholar] [CrossRef] [PubMed]
- Boumitri, C.; Kumta, N.A.; Kahaleh, M. Endoscopic retrograde cholangiopancreatography. In Endoscopic retrograde cholangiopancreatography; Wallace, M.B., Fockens, P., Sung, I.I.J., Eds.; Theime: Stuttgart, Germany, 2018; p. 115. [Google Scholar]
- Tokar, J.L.; Allen, J.I.; Kochman, M.L. Getting to zero: Reducing the risk for duodenoscope-related infections. Ann. Intern. Med. 2015, 163, 873–874. [Google Scholar] [CrossRef]
- Alfa, M.J.; Singh, H.; Duerksen, D.R.; Schultz, G.; Reidy, C.; DeGagne, P.; Olson, N. Improper positioning of the elevator lever of duodenoscopes may lead to sequestered bacteria that survive disinfection by automated endoscope reprocessors. Am. J. Infect. Control. 2018, 46, 73–75. [Google Scholar] [CrossRef]
- Singh, H.; Duerksen, D.R.; Schultz, G.; Reidy, C.; DeGagne, P.; Olson, N.; Nugent, Z.; Bernard, K.A.; Alfa, M.J. Impact of cleaning monitoring combined with channel purge storage on elimination of Escherichia coli and environmental bacteria from duodenoscopes. Gastrointest. Endosc. 2018, 88, 292–302. [Google Scholar] [CrossRef]
- Petersen, B.T.; Koch, J.; Ginsberg, G.G. Infection using ERCP endoscopes. Gastroenterology 2016, 151, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, D.B.; Kim, H.Y.; Baek, H.S.; Kwon, S.Y.; Lee, M.H.; Park, J.C. Increasing potential risks of contamination from repetitive use of endoscope. Am. J. Infect. Control. 2015, 43, e13–e17. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Vickery, K.; Walker, J.T.; deLancey Pulcini, E.; Stoodley, P.; Goldenberg, S.D.; Salkeld, J.A.; Chewins, J.; Yezli, S.; Edgeworth, J.D. Surface-attached cells, biofilms and biocide susceptibility: Implications for hospital cleaning and disinfection. J. Hosp. Infect. 2015, 89, 16–27. [Google Scholar] [CrossRef]
- Kovaleva, J.; Peters, F.T.; van der Mei, H.C.; Degener, J.E. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin. Microbiol. Rev. 2013, 26, 231–254. [Google Scholar] [CrossRef] [PubMed]
- Chhaya, R.; Bhatwadekar, K. Microbial bio-film an unpredictable trouble on medical devices. Int. J. Basic Appl. Med. Sci. 2015, 5, 83–93. [Google Scholar]
- Xue, Y.; Patel, A.; Sant, V.; Sant, S. Semiquantitative FTIR analysis of the crosslinking density of poly(ester amide)-based thermoset elastomers. Macromol. Mater. Eng. 2016, 301, 296–305. [Google Scholar] [CrossRef]
- Infrared Spectroscopy Absorbtion Table. Available online: https://chem.libretexts.org/Reference/Reference_Tables/Spectroscopic_Parameters/Infrared_Spectroscopy_Absorption_Table (accessed on 24 May 2018).
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Varganici, C.D.; Marangoci, N.; Rosu, L.; Barbu-Mic, C.; Rosu, D.; Pinteala, M.; Simionescu, B.C. TGA/DTA–FTIR–MS coupling as analytical tool for confirming inclusion complexes occurrence in supramolecular host–guest architectures. J. Anal. Appl. Pyrolysis 2015, 115, 132–142. [Google Scholar] [CrossRef]
- JIS Z 2801: 2000. Antimicrobial products—Test for antimicrobial activity and efficacy. 2001. Japanese Industrial Standard. Available online: http://lotusyapi.com.tr/Antibacterial/JIS%20Z%202801%202000.pdf (accessed on 24 May 2018).
- Balan, G.; Pavel, L.; Sandu, A.V.; Stefanescu, G.; Trifan, A.V. Preliminary study on erosion of polymer coatings of duodenoscopes. Materiale Plastice 2016, 53, 791–795. [Google Scholar]
- Polivkova, M.; Valova, M.; Siegel, J.; Rimpelova, S.; Hubacek, T.; Lyutakov, O.; Svorcik, V. Antibacterial properties of palladium nanostructures sputtered on polyethylene naphthalate. RSC Adv. 2015, 5, 73767–73774. [Google Scholar] [CrossRef]
- Polivkova, M.; Strublova, V.; Hubacek, T.; Rimpelova, S.; Svorcik, V.; Siegel, J. Surface characterization and antibacterial response of silver nanowire arrays supported on laser-treated polyethylene naphthalate. Mater. Sci. Eng. C 2017, 72, 512–518. [Google Scholar] [CrossRef]
- Barakat, M.T.; Girotra, M.; Huang, R.J.; Banerjee, S. Scoping the scope: Endoscopic evaluation of endoscope working channels with a new high-resolution inspection endoscope. Gastroint. Endosc. 2018, 88, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Ofstead, C.L.; Wetzler, H.P.; Heymann, O.L.; Johnson, E.A.; Eiland, J.E.; Shaw, M.J. Longitudinal assessment of reprocessing effectiveness for colonoscopes and gastroscopes: Results of visual inspections, biochemical markers, and microbial cultures. Am. J. Infect. Control. 2017, 45, e26–e33. [Google Scholar] [CrossRef] [PubMed]
- Ofstead, C.L.; Wetzler, H.P.; Eiland, J.E.; Heymann, O.L.; Held, S.B.; Shaw, M.J. Assessing residual contamination and damage inside flexible endoscopes over time. Am. J. Infect. Control. 2016, 44, 1675–1677. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; McDonnell, G. Superbugs on duodenoscopes: The challenge of cleaning and disinfection of reusable devices. J. Clin. Microbiol. 2015, 53, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Duodenoscope Surveillance Sampling and Culturing Protocols developed by the FDA/CDC/ASM Working Group on Duodenoscope Culturing. Available online: https://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/ReprocessingofReusableMedicalDevices/UCM597949.pdf (accessed on 2 March 2018).
- Pajkos, A.; Vickery, K.; Cossart, Y. Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J. Hosp. Infect. 2004, 58, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Higa, J.T.; Choe, J.; Tombs, D.; Gluck, M.; Ross, A.S. Optimizing duodenoscope reprocessing: Rigorous assessment of a culture and quarantine protocol. Gastrointest. Endosc. 2018, 88, 223–229. [Google Scholar] [CrossRef]
- Wang, P.; Xu, T.; Ngamruengphong, S.; Makary, M.A.; Kalloo, A.; Hutfless, S. Rates of infection after colonoscopy and esophagogastroduodenoscopy in ambulatory surgery centres in the USA. Gut 2018, 67, 1626–1636. [Google Scholar] [CrossRef]
- Center for devices and radiological health. Division of epidemiology. Protecting & promoting public health through device surveillance and research. 522 Postmarket Surveillance (PS) Studies Program. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfm (accessed on 30 June 2018).
Sample Availability: Not available. |
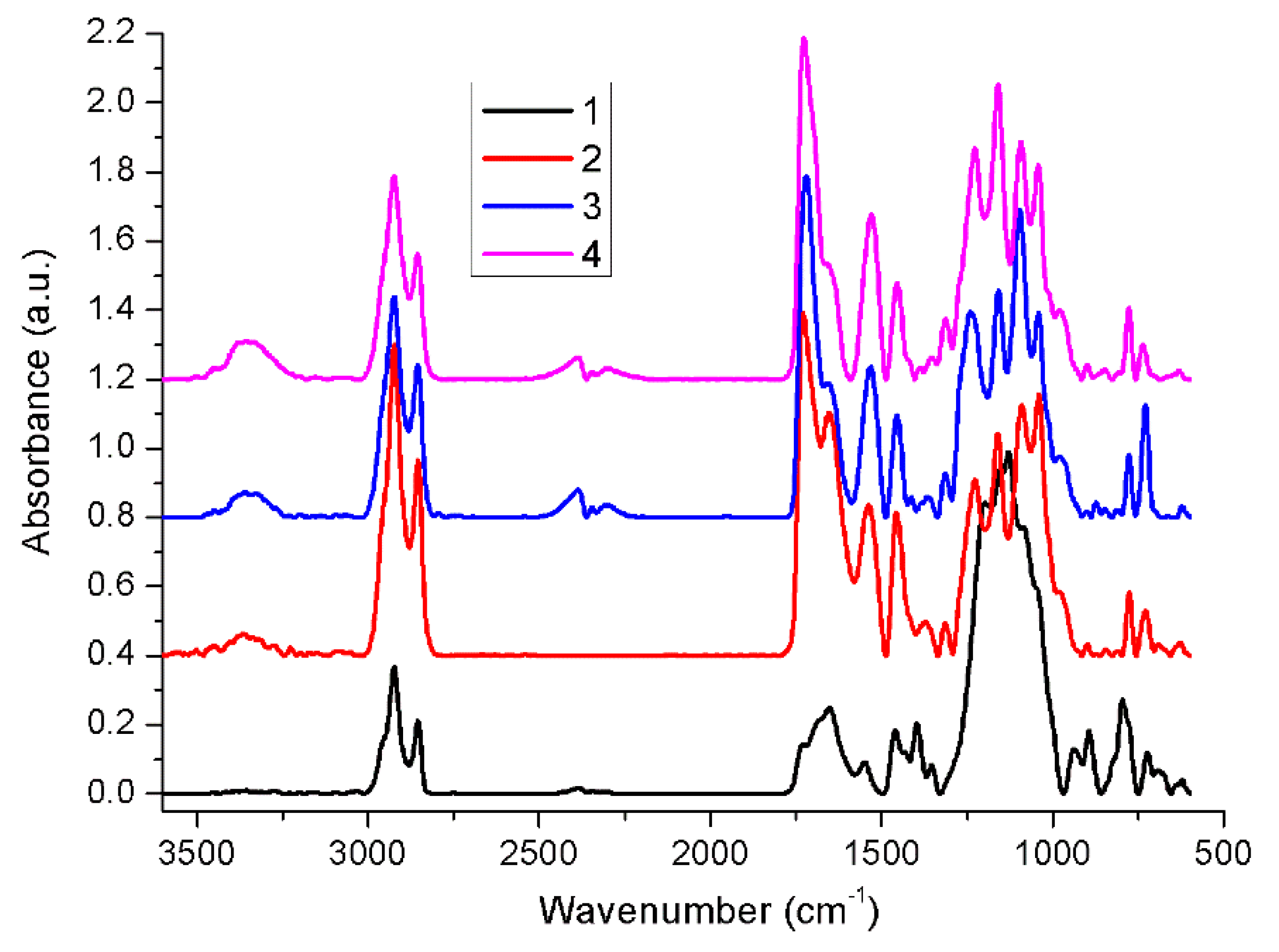
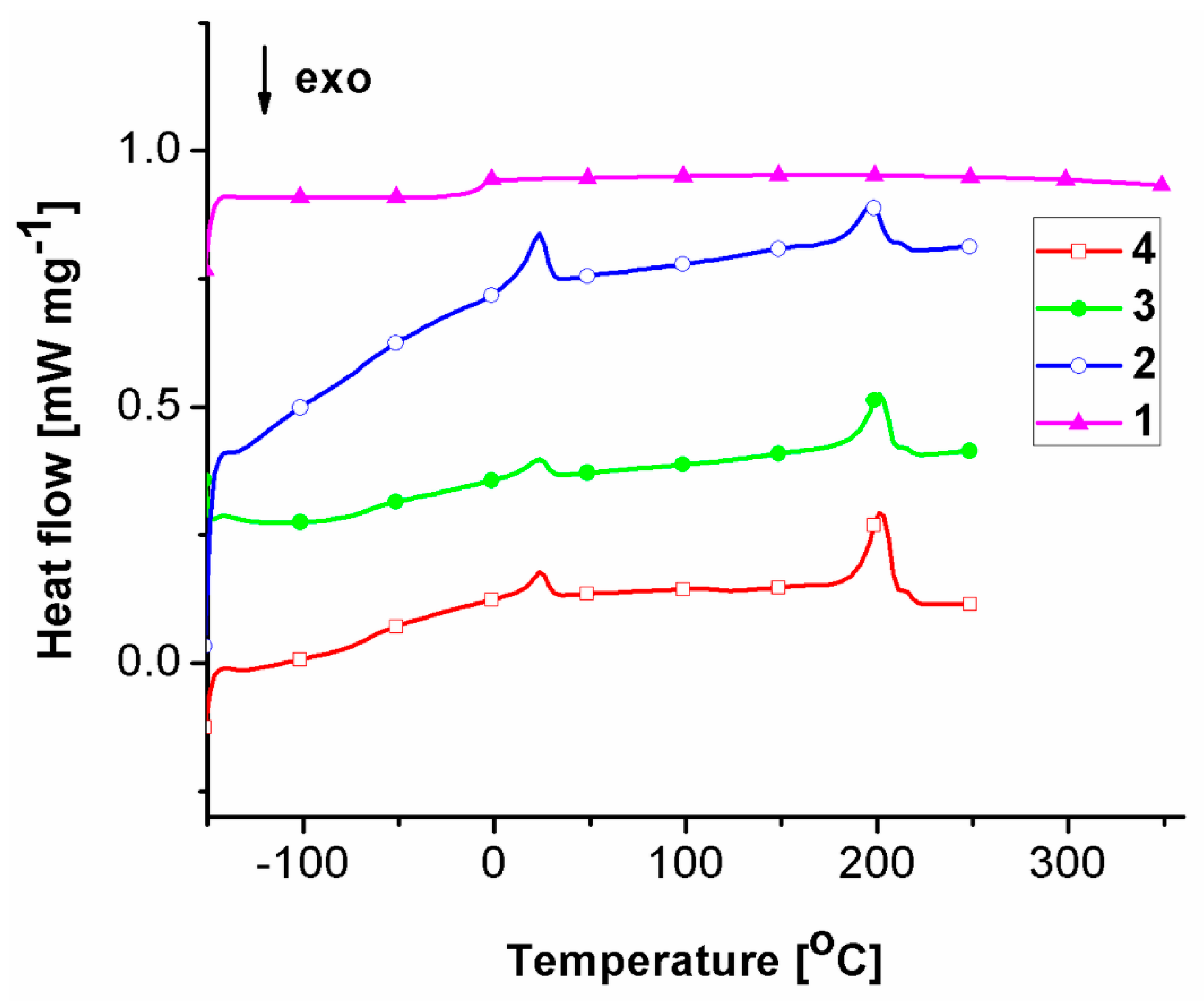
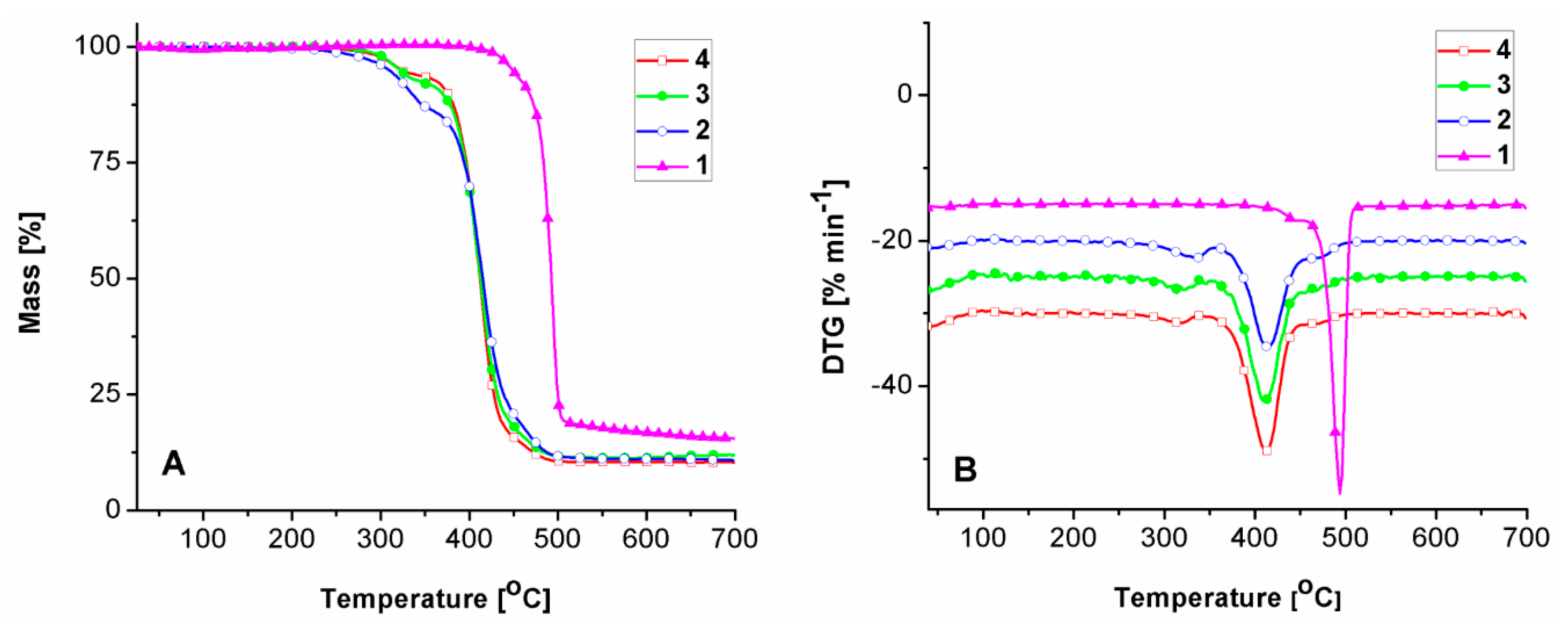
| Sample | Tg (°C) | Tm (°C) | ΔHm (J g−1) | ||
|---|---|---|---|---|---|
| Lower Profile | Upper Profile | Lower Profile | Upper Profile | ||
| 4 | −67 | 24 | 202 | 2.968 | 14.88 |
| 3 | −65 | 24 | 201 | 2.218 | 10.55 |
| 2 | −63 | 24 | 197 | 7.5 | 9.169 |
| 1 | −5 | – | – | – | – |
| Sample | Stage | T5% (°C) | Tmax (°C) | m (%) | Tendset (°C) | Wrez (%) |
|---|---|---|---|---|---|---|
| 4 | I II III | 328 – – | 318 413 471 | 6.08 74.49 9.08 | 326 437 487 | 10.33 |
| 3 | I II III | 323 – – | 322 414 473 | 7.88 70.84 9.51 | 335 437 488 | 11.43 |
| 2 | I II III | 309 – – | 336 416 476 | 13.58 61.32 14.46 | 345 439 489 | 10.84 |
| 1 | I II | 445 – | 441 494 | 8.25 76.46 | 463 503 | 15.19 |
| Sample | R factor | |
|---|---|---|
| S. aureus | E. coli | |
| 1 | 4.3 | 4.7 |
| 2 | 5.8 | 4.9 |
| 3 | 5.6 | 5.8 |
| 4 | 5.7 | 5.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balan, G.G.; Rosca, I.; Ursu, E.-L.; Fifere, A.; Varganici, C.-D.; Doroftei, F.; Turin-Moleavin, I.-A.; Sandru, V.; Constantinescu, G.; Timofte, D.; et al. Duodenoscope-Associated Infections beyond the Elevator Channel: Alternative Causes for Difficult Reprocessing. Molecules 2019, 24, 2343. https://doi.org/10.3390/molecules24122343
Balan GG, Rosca I, Ursu E-L, Fifere A, Varganici C-D, Doroftei F, Turin-Moleavin I-A, Sandru V, Constantinescu G, Timofte D, et al. Duodenoscope-Associated Infections beyond the Elevator Channel: Alternative Causes for Difficult Reprocessing. Molecules. 2019; 24(12):2343. https://doi.org/10.3390/molecules24122343
Chicago/Turabian StyleBalan, Gheorghe G., Irina Rosca, Elena-Laura Ursu, Adrian Fifere, Cristian-Dragos Varganici, Florica Doroftei, Ioana-Andreea Turin-Moleavin, Vasile Sandru, Gabriel Constantinescu, Daniel Timofte, and et al. 2019. "Duodenoscope-Associated Infections beyond the Elevator Channel: Alternative Causes for Difficult Reprocessing" Molecules 24, no. 12: 2343. https://doi.org/10.3390/molecules24122343
APA StyleBalan, G. G., Rosca, I., Ursu, E.-L., Fifere, A., Varganici, C.-D., Doroftei, F., Turin-Moleavin, I.-A., Sandru, V., Constantinescu, G., Timofte, D., Stefanescu, G., Trifan, A., & Sfarti, C. V. (2019). Duodenoscope-Associated Infections beyond the Elevator Channel: Alternative Causes for Difficult Reprocessing. Molecules, 24(12), 2343. https://doi.org/10.3390/molecules24122343