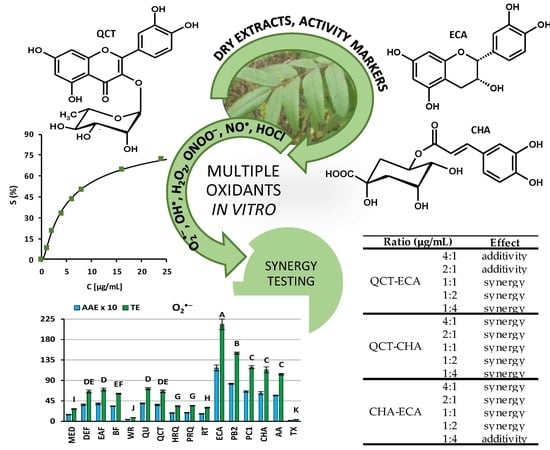
Sorbus domestica Leaf Extracts and Their Activity Markers: Antioxidant Potential and Synergy Effects in Scavenging Assays of Multiple Oxidants
Abstract
1. Introduction
2. Results and Discussion
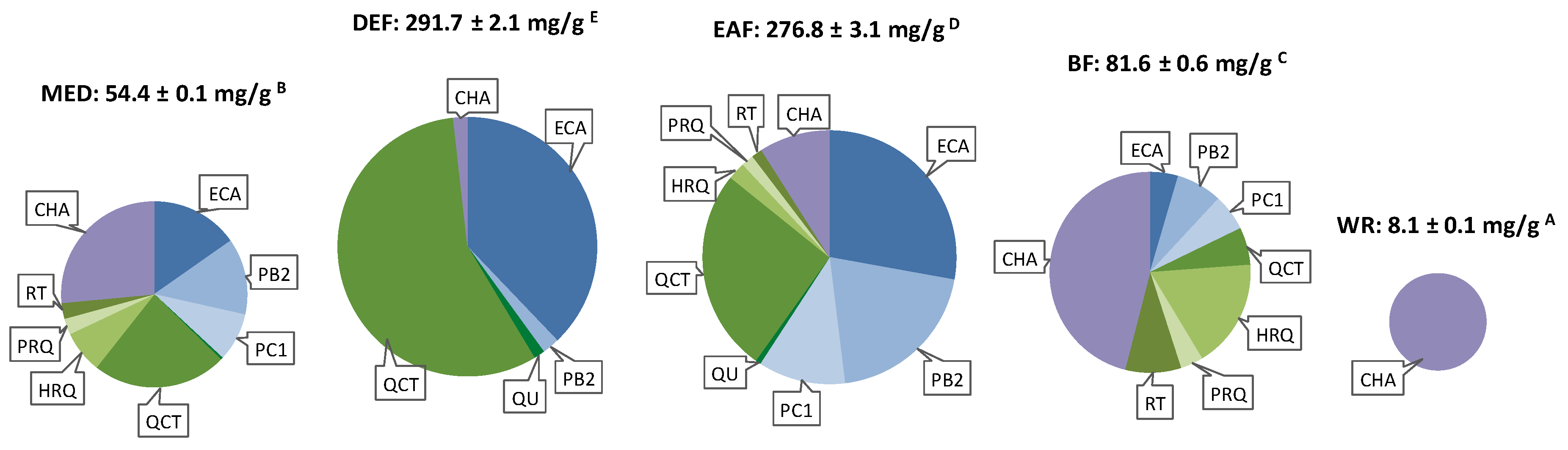
2.1. Scavenging of Multiple Oxidants
2.2. Synergistic Effects in O2•− Scavenging
3. Materials and Methods
3.1. General
3.2. Plant Material and Extracts Preparation
3.3. Antioxidant Activity Measurements
3.4. PEA Index Calculation
3.5. Synergistic Effect Measurement
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kültür, S. Medicinal plants used in Kirklareli province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants, 1st ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 4, pp. 590–593. [Google Scholar]
- Matczak, M.; Marchelak, A.; Michel, P.; Owczarek, A.; Piszczan, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Sorbus domestica L. leaf extracts as functional products: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. J. Funct. Foods 2018, 40, 207–218. [Google Scholar] [CrossRef]
- Rutkowska, M.; Owczarek, A.; Kolodziejczyk-Czepas, J.; Michel, P.; Piotrowska, D.G.; Kapusta, P.; Nowak, P.; Olszewska, M.A. Identification of bioactivity markers of Sorbus domestica leaves in chromatographic, spectroscopic and biological capacity tests: Application for the quality control. Phytochem. Lett. 2019, 30, 278–287. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the phisiological control of cell functions. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Antioxidant capacity of foods for scavenging reactive oxidants and inhibition of plasma lipid oxidation induced by multiple oxidants. Food Funct. 2016, 7, 2156–2168. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.B. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 5698931. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Sonam, K.S.; Guleria, S. Synergistic antioxidant activity of natural products. Annal. Pharmacol. Pharm. 2017, 2, 1–6. [Google Scholar]
- Lubos, E.; Handy, D.E.; Loscalzo, J. Role of oxidative stress and nitric oxide in atherothrombosis. Front. Biosci. 2008, 13, 5323–5344. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.A. Lipid oxidation by hypochlorous acid: Chlorinated lipids in atherosclerosis and myocardial ischemia. Clin. Lipidol. 2010, 5, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Vissers, M.C.M.; Winterbourn, C.C. Living with a killer: The effects of hypochlorous acid on mammalian cells. IUBMB Life 2000, 50, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Bartesaghia, S.; Radia, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Muselík, J.; García-Alonso, M.; Martín-López, M.P.; Žemlička, M.; Rivas-Gonzalo, J.C. Measurement of antioxidant activity of wine catechins, procyanidins, anthocyanins and pyranoanthocyanins. Int. J. Mol. Sci. 2007, 8, 797–809. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Hu, J.P.; Calomme, M.; Lasure, A.; De Bruyne, T.; Pieters, L.; Vlietinck, A.; Venden Berghe, D.A. Structure-activity relationship of flavonoids with superoxide scavenging activity. Biol. Trace Elem. Res. 1995, 47, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, T.; Guo, J.; Sun, M.; Wong, M.W.; Huang, D. Dietary flavonoids scavenge hypochlorous acid via chlorination on A- and C-rings as primary reaction sites: Structure and reactivity relationship. J. Agric. Food Chem. 2019, 67, 4346–4354. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. A supposed mechanism of synergistic action of catechol-containing natural polyphenols. Int. J. Phytomed. 2017, 9, 207–212. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharmacogn. (RJP) 2014, 1, 35–40. [Google Scholar]
- Tallarida, R.J. Quantitative methods for assessing drug synergism. Genes Cancer 2011, 2, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.A.; Curran, T.P.; Gowen, A.; Vazquez, J.A. An efficient methodology for quantification of synergy and antagonism in single electron transfer antioxidant assays. Food Res. Int. 2015, 67, 284–298. [Google Scholar] [CrossRef]
- Zhao, L.; Au, J.L.S.; Wientjes, M.G. Comparison of methods for evaluating drug-drug interaction. Front. Biosci. 2010, 2, 241–249. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Rutkowska, M.; Magiera, A.; Michel, P.; Rejman, M.W.; Nowak, P.; Owczarek, A. The effect of standardised flower extracts of Sorbus aucuparia L. on proinflammatory enzymes, multiple oxidants, and oxidative/nitrative damage of human plasma components in vitro. Oxid. Med. Cell. Longev. 2019, 9746358. [Google Scholar] [CrossRef]
- Lou, L.; Liu, Y.; Zhou, J.; Wei, Y.; Deng, J.; Dong, B.; Chai, L. Chlorogenic acid and luteolin synergistically inhibit the proliferation of interleukin-1β-induced fibroblast-like synoviocytes through regulating the activation of NF-κB and JAK/STAT-signaling pathways. Immunopharmacol. Immunotoxicol. 2015, 37, 499–507. [Google Scholar] [CrossRef]
- Freeman, B.L.; Eggett, D.L.; Parker, T.L. Synergistic and antagonistic interactions of phenolic compounds found in navel oranges. J. Food Sci. 2010, 75, 570–576. [Google Scholar] [CrossRef]
- Mao, S.; Wang, K.; Lei, Y.; Yao, S.; Lu, B.; Huang, W. Antioxidant synergistic effects of Osmanthus fragrans flowers with green tea and their major contributed antioxidant compounds. Sci. Rep. 2017, 7, 46501. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Gil-Chávez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; González-Aguilar, G.A. Antioxidant interactions between major phenolic compounds found in ‘Ataulfo’ Mango Pulp: Chlorogenic, gallic, protocatechuic and vanillic acids. Molecules 2012, 17, 12657–12664. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.P.; Pancholi, S.S.; Patel, R. Synergistic antioxidant activity of green tea with some herbs. J. Adv. Pharm. Technol. Res. 2011, 2, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Granica, S.; Czerwińska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical composition, antioxidative and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera paradoxa Hudziok obtained after seeds cultivation. J. Agric. Food Chem. 2013, 61, 801–810. [Google Scholar] [CrossRef]
- Marchelak, A.; Owczarek, A.; Rutkowska, M.; Michel, P.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. New insights into antioxidant activity of Prunus spinosa flowers: Extracts, model polyphenols and their phenolic metabolites in plasma towards multiple in vivo-relevant oxidants. Phytochem. Lett. 2019, 30, 288–295. [Google Scholar] [CrossRef]
- Czerwińska, M.; Kiss, A.K.; Naruszewicz, M. A comparison of antioxidant activities of oleuropein and its dialdehydic derivative from olive oil, oleacein. Food Chem. 2012, 131, 940–947. [Google Scholar] [CrossRef]
- Krzyzanowska-Kowalczyk, J.; Kolodziejczyk-Czepas, J.; Kowalczyk, M.; Pecio, Ł.; Nowak, P.; Stochmal, A. Yunnaneic Acid B, a component of Pulmonaria officinalis extract, prevents peroxinitrite-induced oxidative stress in vitro. J. Agric. Food Chem. 2017, 65, 3827–3834. [Google Scholar] [CrossRef]
- Weibull, W. A statistical distribution function of wide applicability. J. Appl. Mech. 1951, 18, 293–297. [Google Scholar]
Sample Availability: Plant samples are available from the authors. |
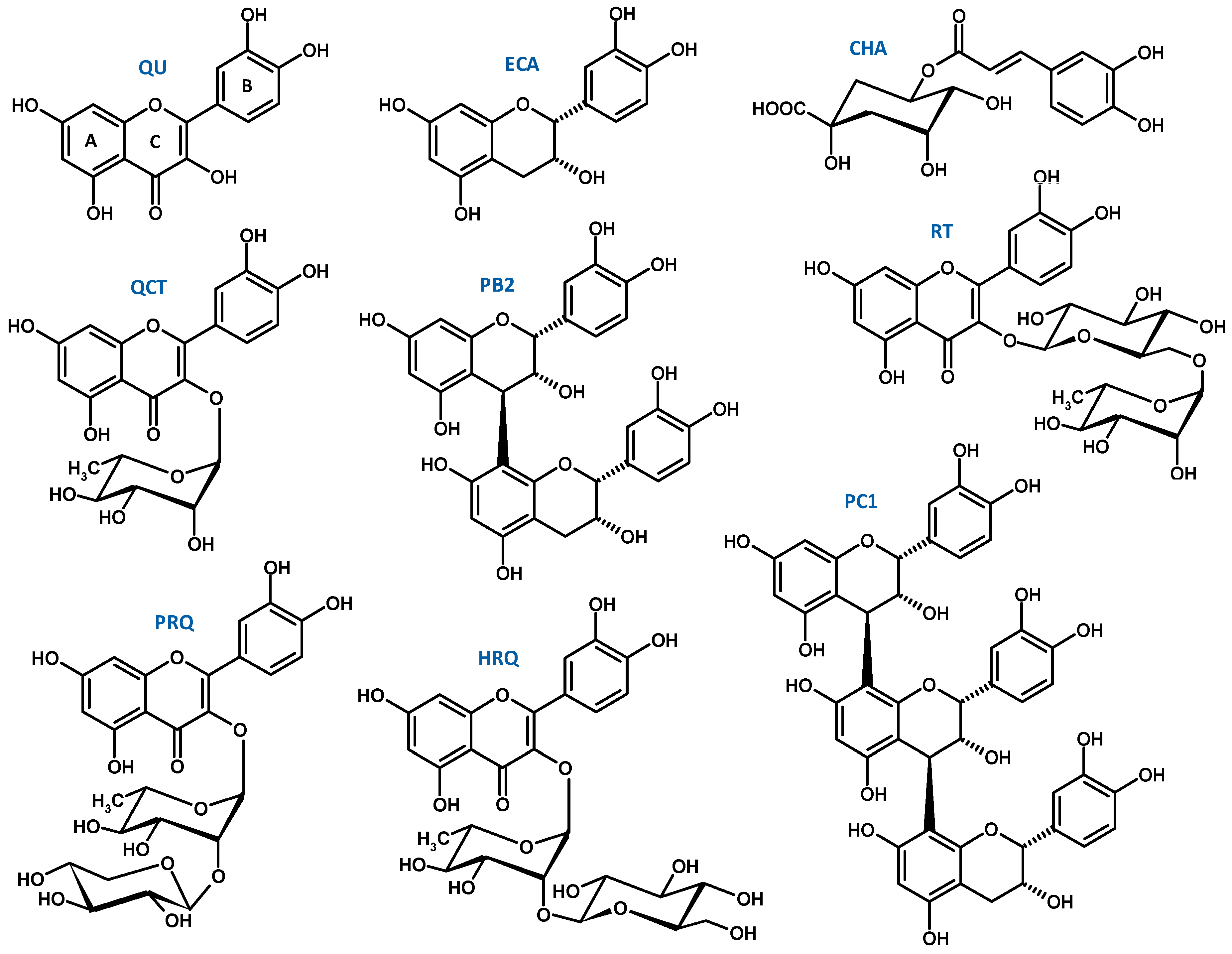


| AAEMR6 | TEMR6 | AAEMR6 | TEMR6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| μmol/mg | mol/mol | μmol/mg | mol/mol | μmol/mg | mol/mol | μmol/mg | mol/mol | ||
| Extracts | Markers | ||||||||
| MED | 14.31 | - | 38.79 | - | QU | 72.24 | 21.82 | 145.33 | 43.89 |
| DEF | 42.37 | - | 102.16 | - | QCT | 49.06 | 21.98 | 116.09 | 52.01 |
| EAF | 47.36 | - | 106.71 | - | HRQ | 29.77 | 18.16 | 62.45 | 38.09 |
| BF | 31.20 | - | 84.82 | - | PRQ | 29.62 | 17.18 | 62.40 | 36.19 |
| WR | 4.21 | - | 11.62 | - | RT | 25.39 | 15.49 | 57.23 | 34.91 |
| Standards | ECA | 59.27 | 17.19 | 253.26 | 73.45 | ||||
| PB2 | 55.68 | 32.18 | 189.75 | 109.68 | |||||
| AA | 34.07 | 6.00 | 130.06 | 22.89 | PC1 | 55.07 | 47.69 | 156.56 | 135.58 |
| TX | 97.10 | 24.28 | 23.97 | 6.0 | CHA | 45.69 | 16.17 | 154.12 | 54.56 |
| PEA | ||||||
|---|---|---|---|---|---|---|
| Extracts | O2•− | OH• | H2O2 | HClO | NO• | ONOO– |
| MED | 0.35 | 0.68 | 0.26 | 0.48 | 0.19 | 0.75 |
| DEF | 1.99 | 3.97 | 1.72 | 3.46 | 0.95 | 3.54 |
| EAF | 2.02 | 3.74 | 1.52 | 2.90 | 0.82 | 4.27 |
| BF | 0.45 | 0.93 | 0.32 | 0.52 | 0.31 | 0.99 |
| WR | 0.06 | 0.13 | 0.04 | 0.02 | 0.04 | 0.10 |
| r (p) | ||||||
| AAE | 0.8072 (0.099) | 0.8794 (0.049) * | 0.9043 (0.035) * | 0.9227 (0.026) * | 0.9779 (0.004) * | 0.9035 (0.035) * |
| Compound | Parameters | Model Significance | ||||
|---|---|---|---|---|---|---|
| m | k | SSE | R2 | F-Test | p | |
| ECA | 2.622 | −0.552 | 14.581 | 0.9964 | 2793.66 | 1.23 × 10−9 |
| QCT | 7.795 | −0.638 | 5.580 | 0.9988 | 8296.13 | 4.72 × 10−11 |
| CHA | 4.192 | −0.611 | 1.626 | 0.9997 | 41,930.01 | 3.66 × 10−13 |
| Compound Combination | Concentration Ratio (µg/mL) | Theoretical Scavenging Efficacy (%) | Experimental Scavenging Efficacy (%) | CI ± 95% Conf. | Effect |
|---|---|---|---|---|---|
| QCT–ECA | 4:1 | 47.75 | 47.71 ± 2.43 | 1.00 ± 0.10 | additivity |
| 2:1 | 40.55 | 41.44 ± 2.22 | 0.97 ± 0.09 | additivity | |
| 1:1 | 35.95 | 39.82 ± 2.15 | 0.84 ± 0.08 | synergy | |
| 1:2 | 47.56 | 52.04 ± 2.86 | 0.81 ± 0.10 | synergy | |
| 1:4 | 59.12 | 61.57 ± 1.83 | 0.88 ± 0.08 | synergy | |
| QCT–CHA | 4:1 | 43.68 | 54.77 ± 3.14 | 0.61 ± 0.08 | synergy |
| 2:1 | 34.13 | 44.62 ± 3.02 | 0.64 ± 0.08 | synergy | |
| 1:1 | 27.46 | 37.44 ± 2.71 | 0.65 ± 0.07 | synergy | |
| 1:2 | 38.82 | 49.56 ± 3.86 | 0.62 ± 0.10 | synergy | |
| 1:4 | 51.61 | 61.04 ± 3.03 | 0.63 ± 0.09 | synergy | |
| CHA–ECA | 4:1 | 55.91 | 60.39 ± 1.84 | 0.78 ± 0.07 | synergy |
| 2:1 | 46.92 | 52.84 ± 2.66 | 0.75 ± 0.09 | synergy | |
| 1:1 | 40.12 | 51.82 ± 2.75 | 0.57 ± 0.07 | synergy | |
| 1:2 | 49.91 | 53.43 ± 2.63 | 0.84 ± 0.10 | synergy | |
| 1:4 | 60.26 | 60.33 ± 1.92 | 1.01 ± 0.10 | additivity |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowska, M.; Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Owczarek, A. Sorbus domestica Leaf Extracts and Their Activity Markers: Antioxidant Potential and Synergy Effects in Scavenging Assays of Multiple Oxidants. Molecules 2019, 24, 2289. https://doi.org/10.3390/molecules24122289
Rutkowska M, Olszewska MA, Kolodziejczyk-Czepas J, Nowak P, Owczarek A. Sorbus domestica Leaf Extracts and Their Activity Markers: Antioxidant Potential and Synergy Effects in Scavenging Assays of Multiple Oxidants. Molecules. 2019; 24(12):2289. https://doi.org/10.3390/molecules24122289
Chicago/Turabian StyleRutkowska, Magdalena, Monika Anna Olszewska, Joanna Kolodziejczyk-Czepas, Pawel Nowak, and Aleksandra Owczarek. 2019. "Sorbus domestica Leaf Extracts and Their Activity Markers: Antioxidant Potential and Synergy Effects in Scavenging Assays of Multiple Oxidants" Molecules 24, no. 12: 2289. https://doi.org/10.3390/molecules24122289
APA StyleRutkowska, M., Olszewska, M. A., Kolodziejczyk-Czepas, J., Nowak, P., & Owczarek, A. (2019). Sorbus domestica Leaf Extracts and Their Activity Markers: Antioxidant Potential and Synergy Effects in Scavenging Assays of Multiple Oxidants. Molecules, 24(12), 2289. https://doi.org/10.3390/molecules24122289