Ratiometric Fluorescent Nanoprobe for Highly Sensitive Determination of Mercury Ions
Abstract
1. Introduction
2. Results and Discussion
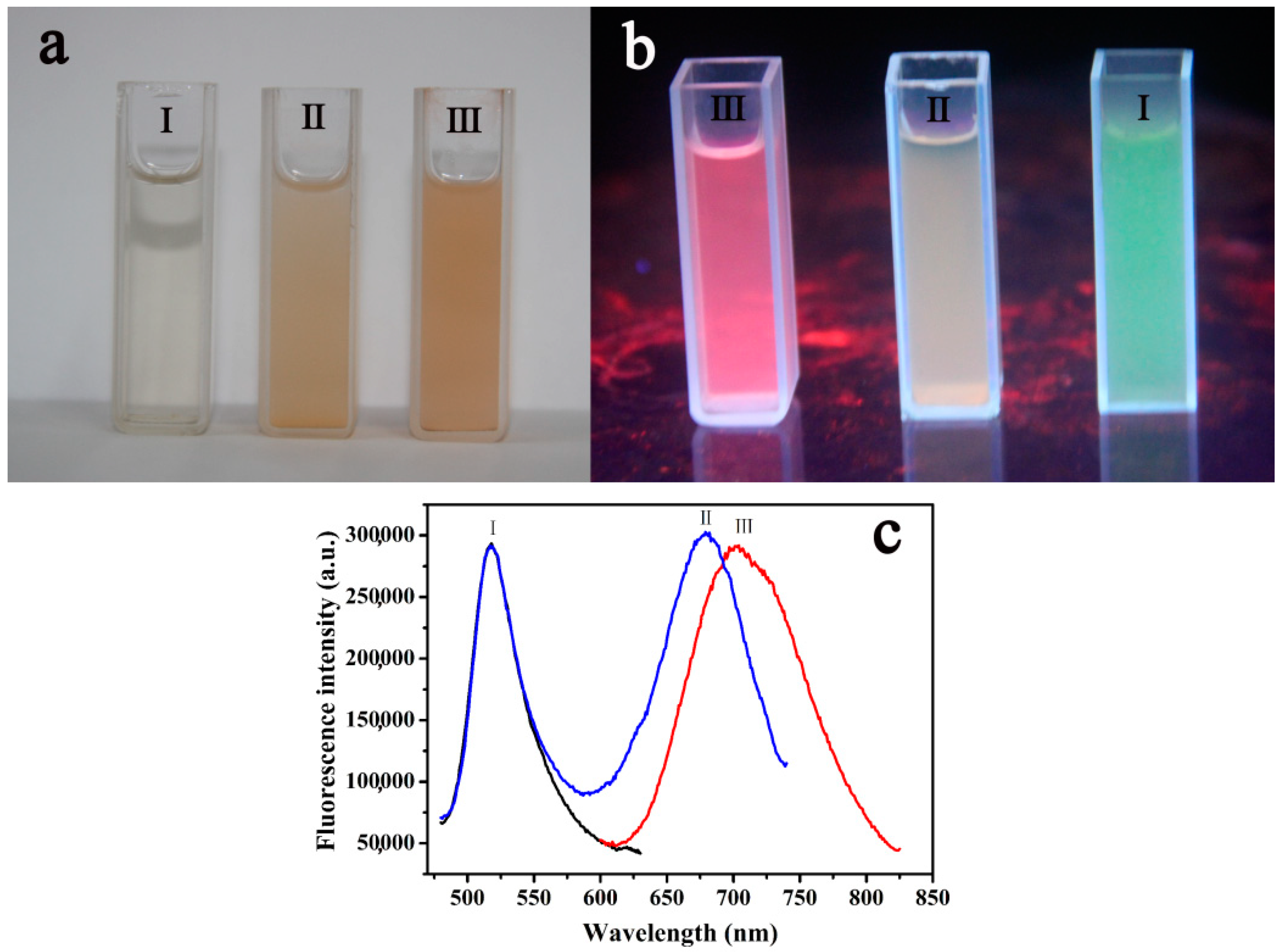
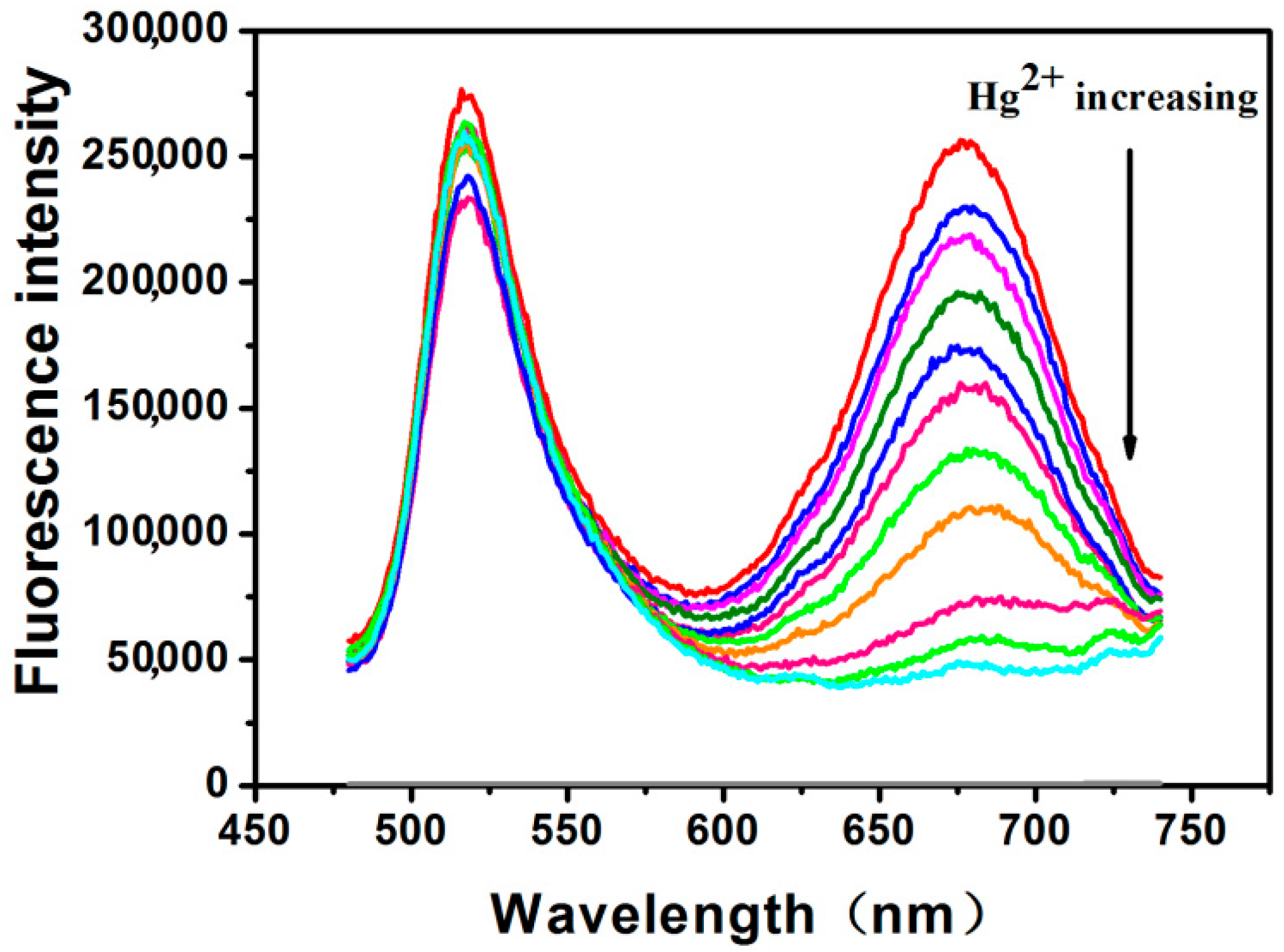
2.1. Fluorescence Spectra of RFN
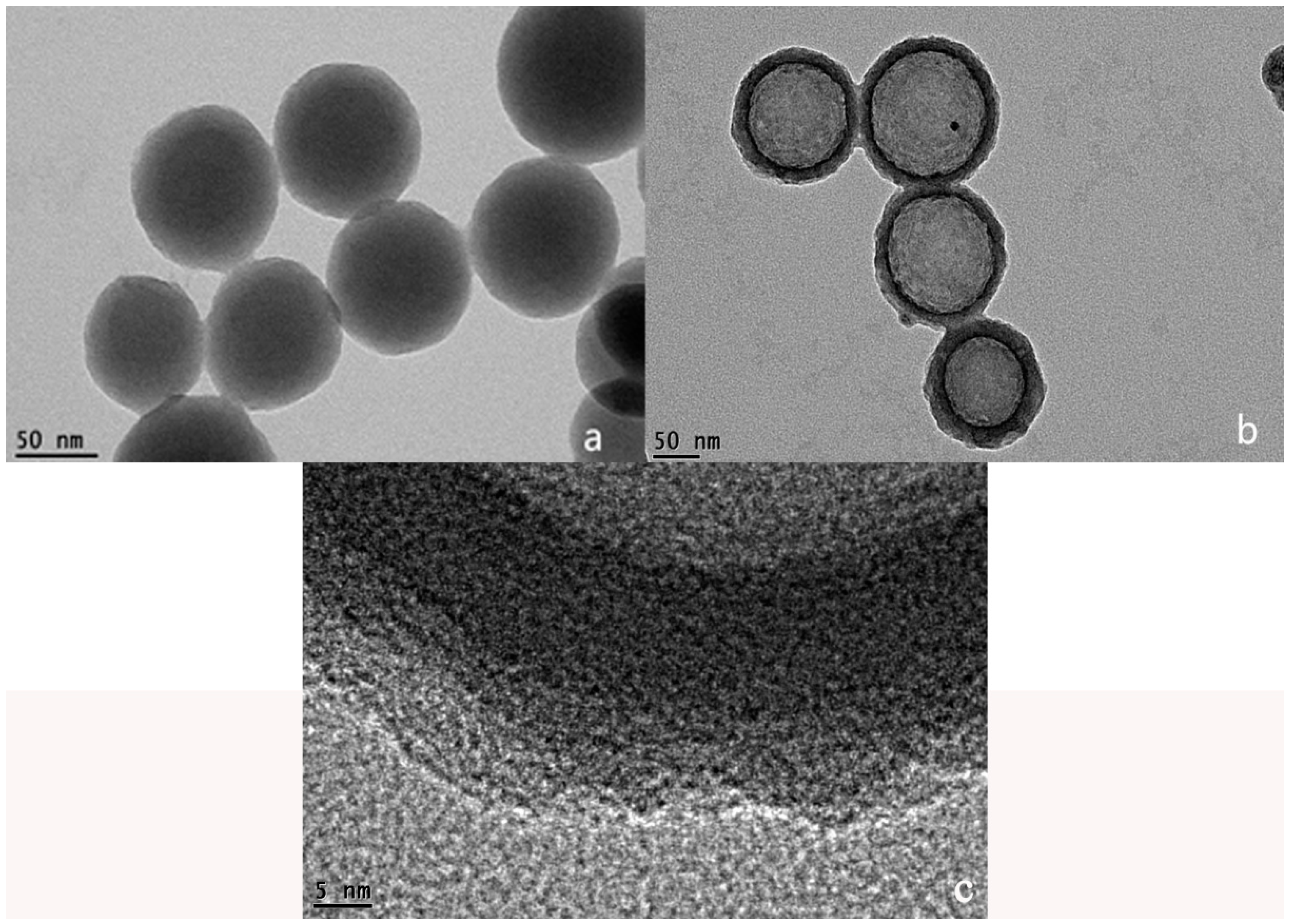
2.2. Transmission Electron Microscopy (TEM) of RFN
2.3. Energy Dispersive X-Ray Spectroscopy (EDS) of RFN
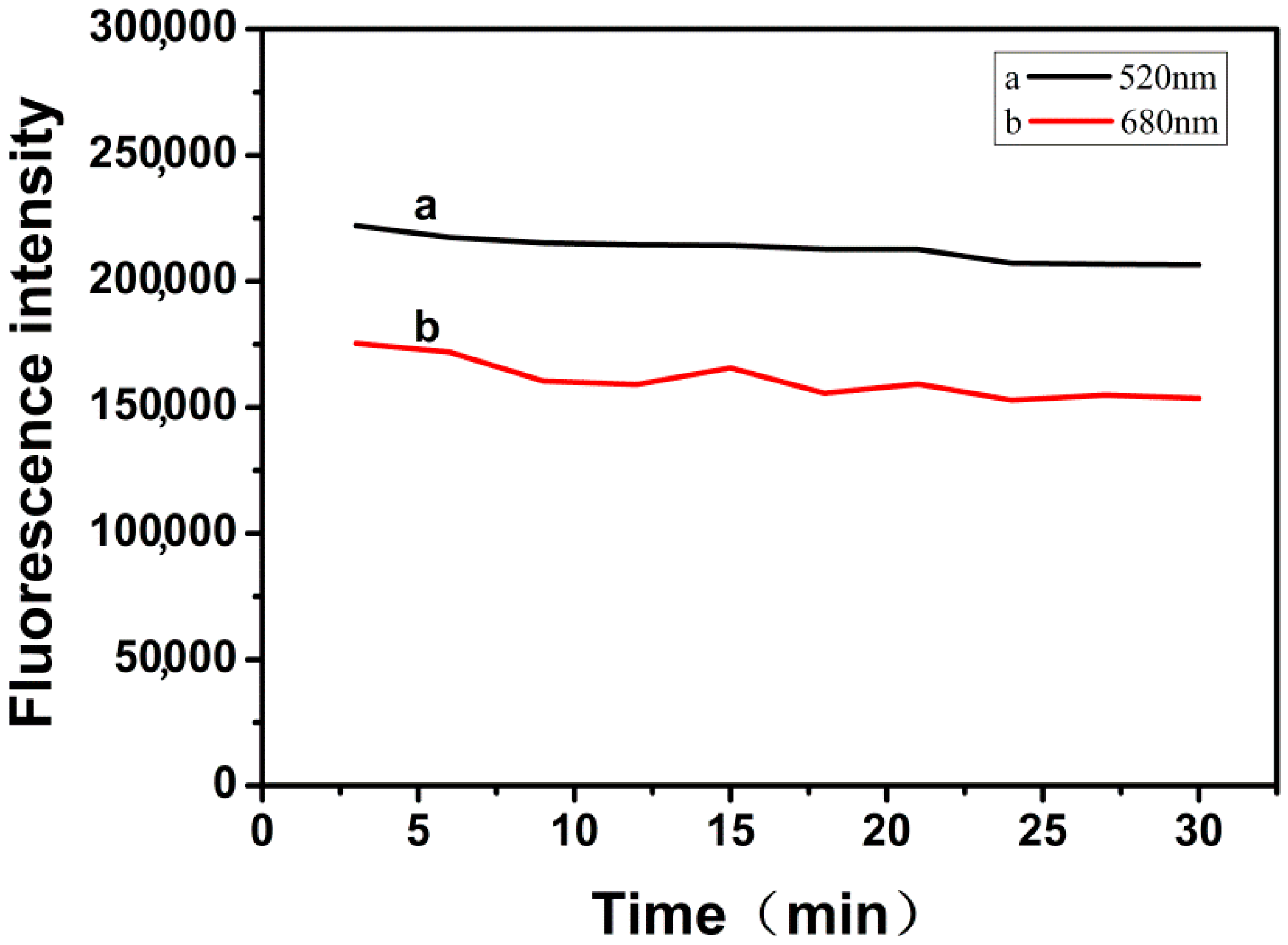
2.4. Fluorescence Stability of RFN
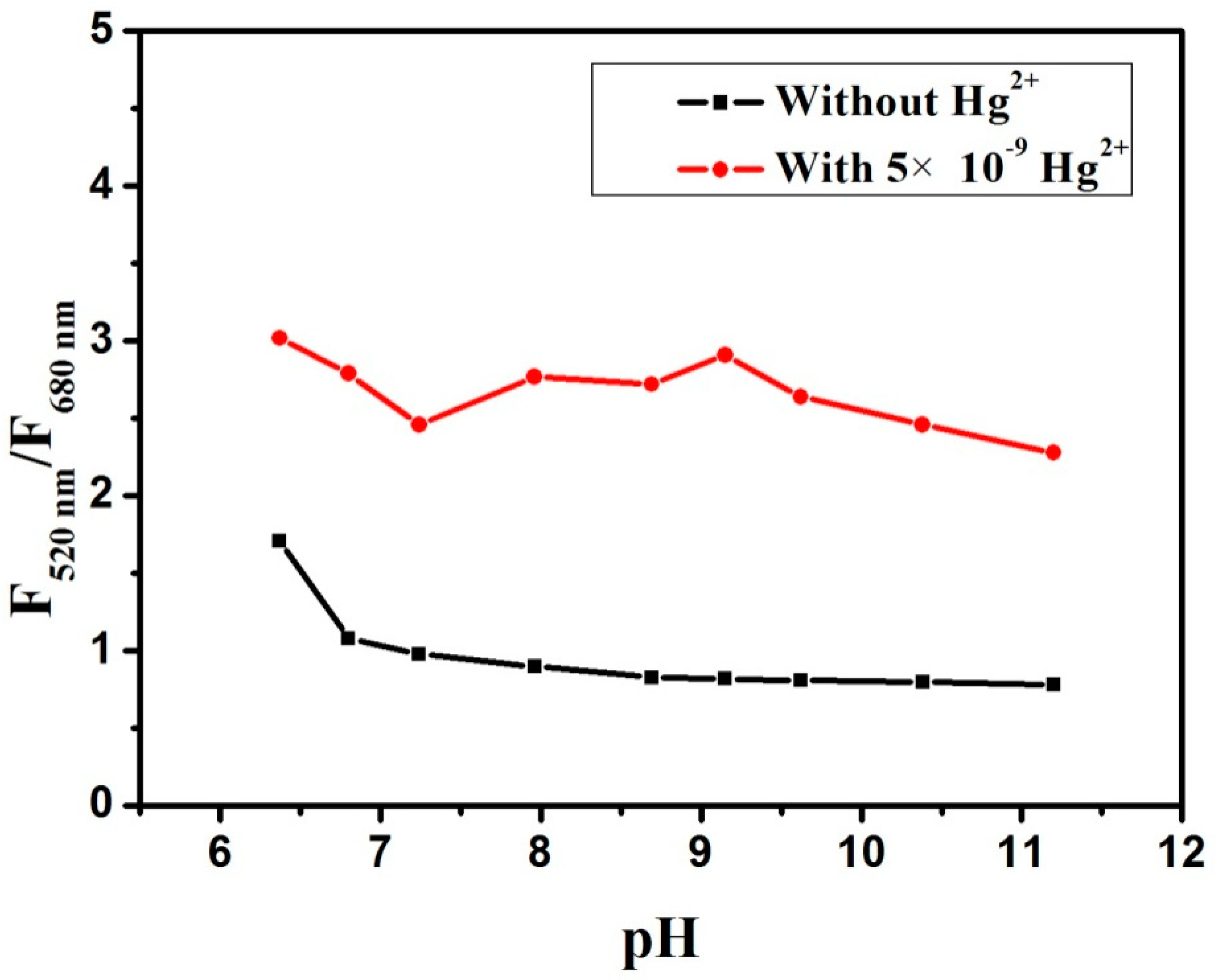
2.5. Effect of Buffer Solutions at Different pH Value on the RFN
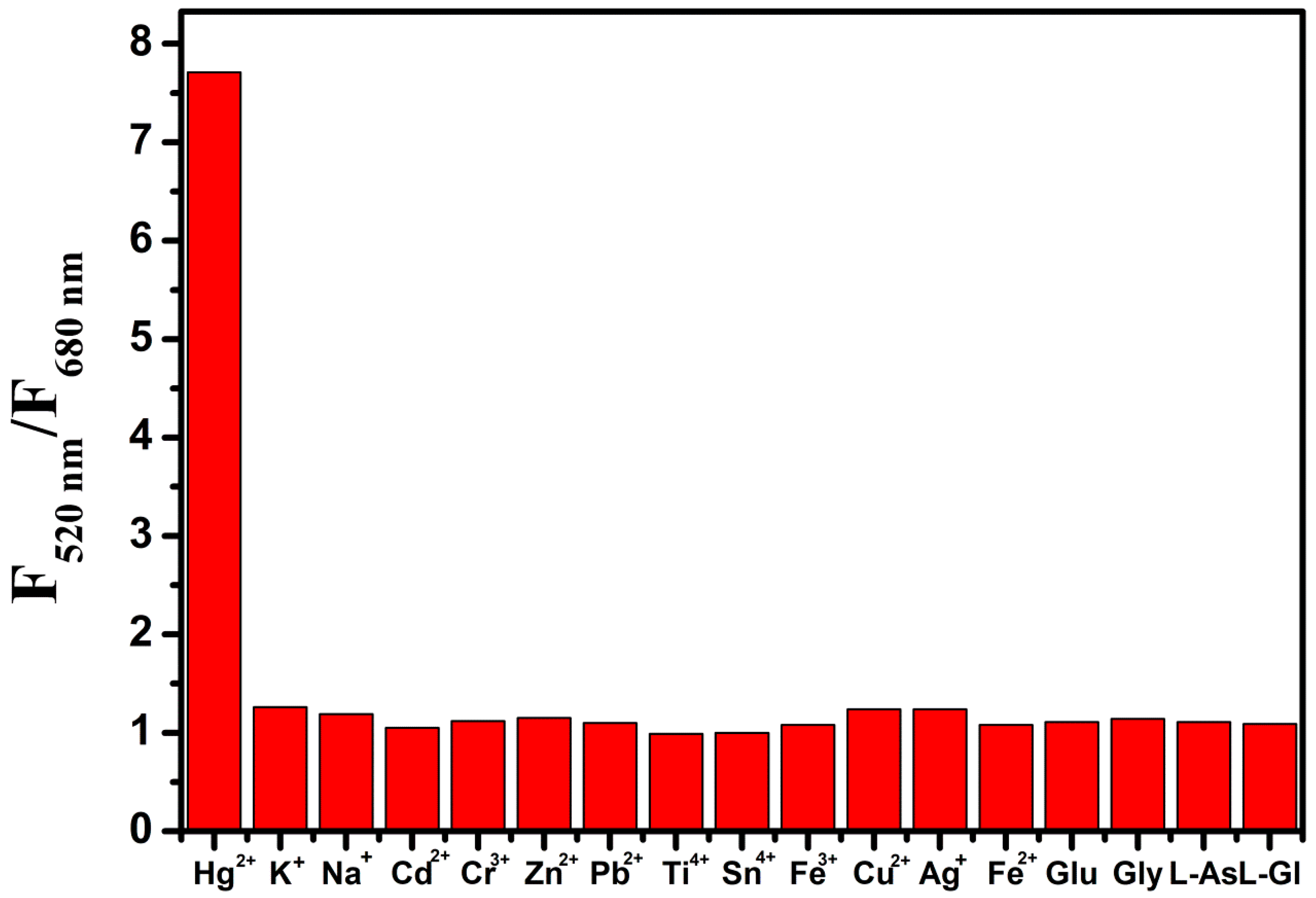
2.6. Selectivity of the RFN to Hg2+
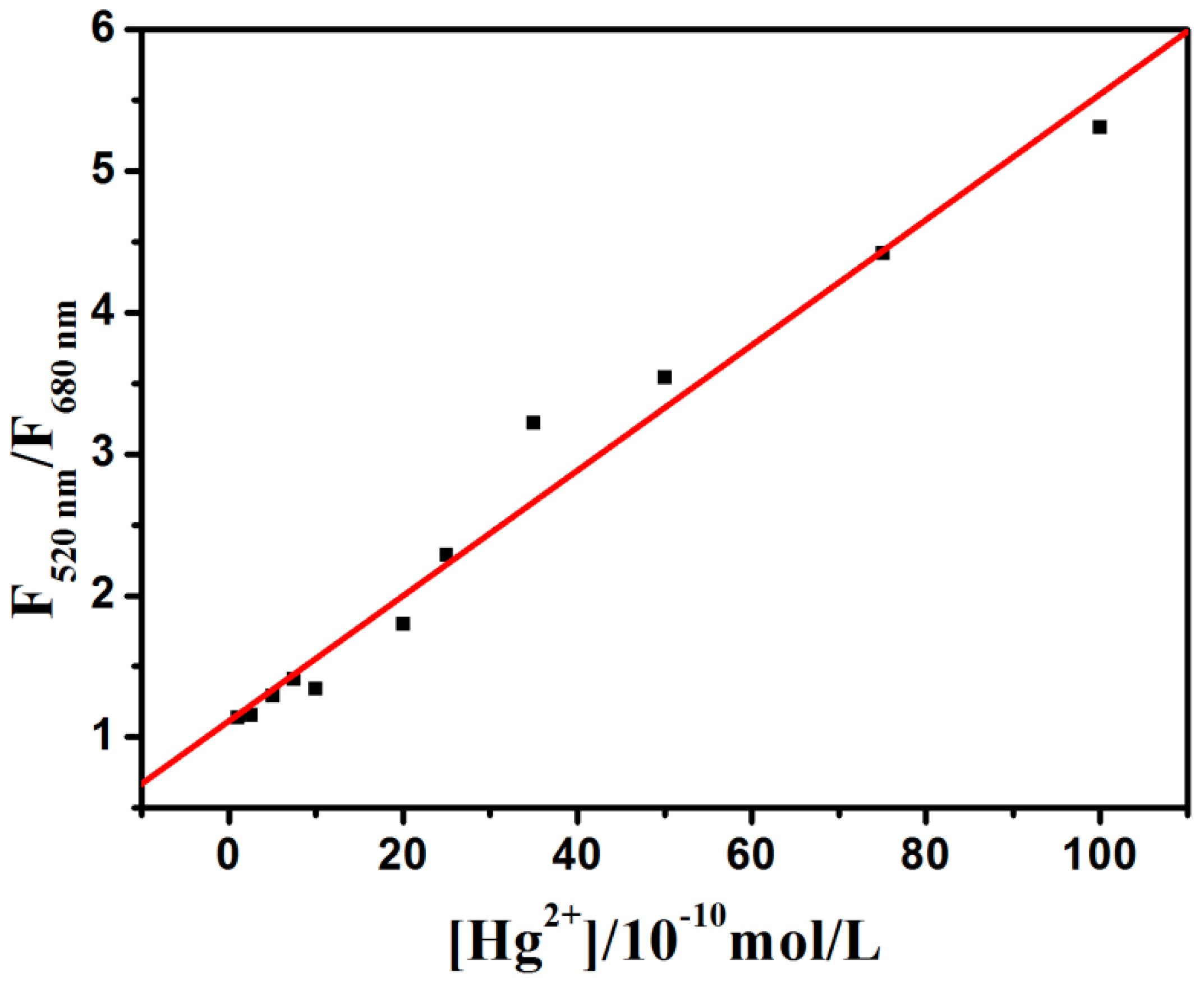
2.7. Determination of Mercury Ions
2.8. Detection of Water Samples
3. Materials and Methods
3.1. Reagents and Materials
3.2. Measurements
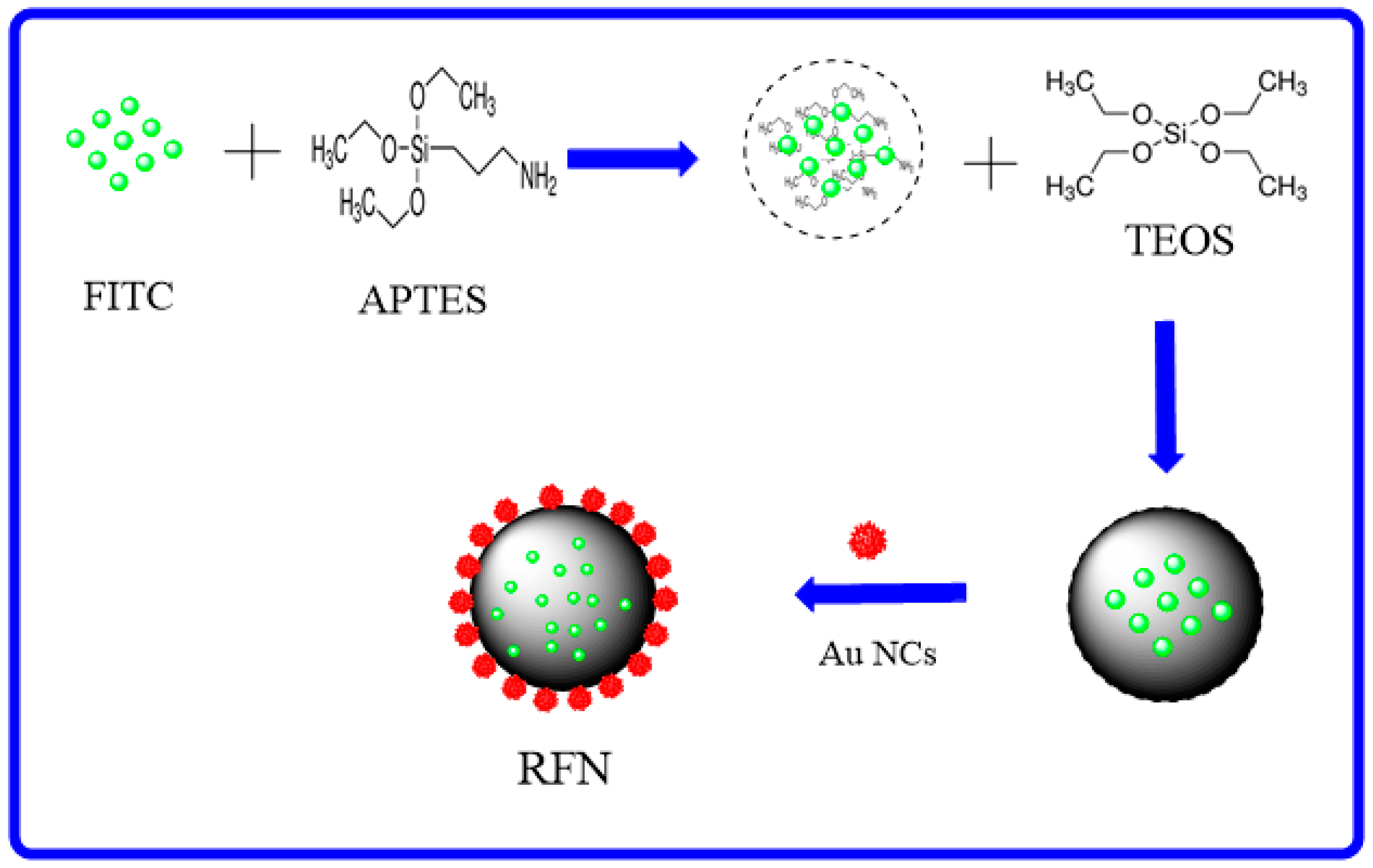
3.3. Synthesis of FITC–SiO2 Microspheres
3.4. Synthesis of AuNCs
3.5. Synthesis of Amino-Modified FITC–SiO2 Microspheres
3.6. Synthesis of RFN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gustin, S.M.; Coolbaugh, M.; Engle, M.; Fitzgerald, B.; Keislar, R.; Lindberg, S.; Nacht, D.; Quashnick, J.; Rytuba, J.; Sladek, C.; et al. Atmospheric mercury emissions frommine wastes and surrounding geologically enriched terranes. Env. Geol. 2003, 43, 339–351. [Google Scholar] [CrossRef]
- Clarkson, L.; Ma, T.W. Overview of the clinical toxicity of mercury. Ann Clin Biochem. 2006, 43, 257–268. [Google Scholar]
- Lippard, E.M.; Na, S.J. Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectro. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Adlnasab, L.; Ebrahimzadeh, H.; Asgharinezhad, A.A.; Aghdam, M.N.; Dehghani, A.; Esmaeilpour, S. A Preconcentration Procedure for Determination of Ultra-Trace Mercury (II) in Environmental Samples Employing Continuous-Flow Cold Vapor Atomic Absorption Spectrometry. Food Anal. Meth. 2014, 7, 616–628. [Google Scholar] [CrossRef]
- Noël, M.; Christensen, J.R.; Spence, J.; Robbins, C.T. Using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to characterize copper, zinc and mercury along grizzly bear hair providing estimate of diet. Sci. Total. Env. 2015, 529, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Amiri, N.; Rofouei, M.K.; Ghasemi, J.B. Multivariate optimization, preconcentration and determination of mercury ions with (1-(p-acetyl phenyl)-3-(o-methyl benzoate)) triazene in aqueous samples using ICP-AES. Anal. Meth. 2016, 8, 1111–1119. [Google Scholar] [CrossRef]
- Drummen, G. Fluorescent probes and fluorescence (microscopy) techniques—illuminating biological and biomedical research. Molecules 2012, 17, 14067–14090. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, K.; Fang, D.; Ma, L. Highly Sensitive and Selective Fluorescent Detection of Gossypol Based on BSA-Stabilized Copper Nanoclusters. Molecules 2019, 24, 95. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-K.; Yook, K.-J.; Tae, J. A Rhodamine-Based Fluorescent and Colorimetric Chemodosimeter for the Rapid Detection of Hg2+ Ions in Aqueous Media. J. Am. Chem. Soc. 2005, 127, 16760–16761. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Ye, Z.; Zhang, R.; Wang, G.; Yuan, J. Design and synthesis of a terbium (III) complex-based luminescence probe for time-gated luminescence detection of mercury (II) Ions. J. Fluoresc. 2012, 22, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Chen, Y.; Li, Y.; Shahzad, S.A.; Wang, Y.; Yang, M.; Yu, C. Fluorescence turn-on detection of mercury ions based on the controlled adsorption of a perylene probe onto the gold nanoparticles. Analyst 2016, 141, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Finder, T.; Willner, I. Multiplexed Analysis of Hg2+ and Ag+ Ions by Nucleic Acid Functionalized CdSe/ZnS Quantum Dots and Their Use for Logic Gate Operations. Angew. Chem. 2009, 121, 7958–7961. [Google Scholar] [CrossRef]
- Joshi, B.P.; Park, J.; Lee, W.I.; Lee, K.-H. Ratiometric and turn-on monitoring for heavy and transition metal ions in aqueous solution with a fluorescent peptide sensor. Talanta 2009, 78, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Zhao, T.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. A novel core-satellite CdTe/Silica/Au NCs hybrid sphere as dual-emission ratiometric fluorescent probe for Cu2+. Biosen. Bioelectro. 2014, 51, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, N.; Bing, T.; Shangguan, D. Carbon Dots Based Dual-Emission Silica Nanoparticles as a Ratiometric Nanosensor for Cu2+. Anal. Chem. 2014, 86, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, C.; Wang, X.; Wang, L.; Chen, A.; Hu, J.; Luo, Z. Fabrication of an “ion-imprinting” dual-emission quantum dot nanohybrid for selective fluorescence turn-on and ratiometric detection of cadmium ions. Analyst 2016, 141, 5886–5892. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J.Y. Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of Au nanoclusters by Hg2+-Au+ interactions. Chem. Comm. 2010, 46, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Zhang, B.; Lu, L. Europium-Based Fluorescence Nanoparticle Sensor for Rapid and Ultrasensitive Detection of an Anthrax Biomarker. Angew. Chem. 2009, 121, 310–314. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Sample | Hg2+ Concentration (10−10 mol/L) | |||
|---|---|---|---|---|
| Present Method | Added | Total | Recovery | |
| Tiannan lake | 4.88 | 5 | 9.84 | 99.2% |
| 20 | 24.51 | 98.15% | ||
| Tap water | 1.49 | 5 | 6.46 | 99.4% |
| 20 | 21.58 | 100.45% | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Xu, H.; Ning, B.; Guo, Z.; Li, N.; Chen, L.; Huang, G.; Li, C.; Zheng, B. Ratiometric Fluorescent Nanoprobe for Highly Sensitive Determination of Mercury Ions. Molecules 2019, 24, 2278. https://doi.org/10.3390/molecules24122278
Luo Z, Xu H, Ning B, Guo Z, Li N, Chen L, Huang G, Li C, Zheng B. Ratiometric Fluorescent Nanoprobe for Highly Sensitive Determination of Mercury Ions. Molecules. 2019; 24(12):2278. https://doi.org/10.3390/molecules24122278
Chicago/Turabian StyleLuo, Zhihui, Hui Xu, Baogui Ning, ZeBin Guo, Na Li, Lina Chen, Guobao Huang, Charlie Li, and Baodong Zheng. 2019. "Ratiometric Fluorescent Nanoprobe for Highly Sensitive Determination of Mercury Ions" Molecules 24, no. 12: 2278. https://doi.org/10.3390/molecules24122278
APA StyleLuo, Z., Xu, H., Ning, B., Guo, Z., Li, N., Chen, L., Huang, G., Li, C., & Zheng, B. (2019). Ratiometric Fluorescent Nanoprobe for Highly Sensitive Determination of Mercury Ions. Molecules, 24(12), 2278. https://doi.org/10.3390/molecules24122278







