Methanol Extract of Aerial Parts of Pavetta indica L. Enhances the Cytotoxic Effect of Doxorubicin and Induces Radiation Sensitization in MDA-MB-231 Triple-Negative Breast Cancer Cells
Abstract
1. Introduction
2. Results
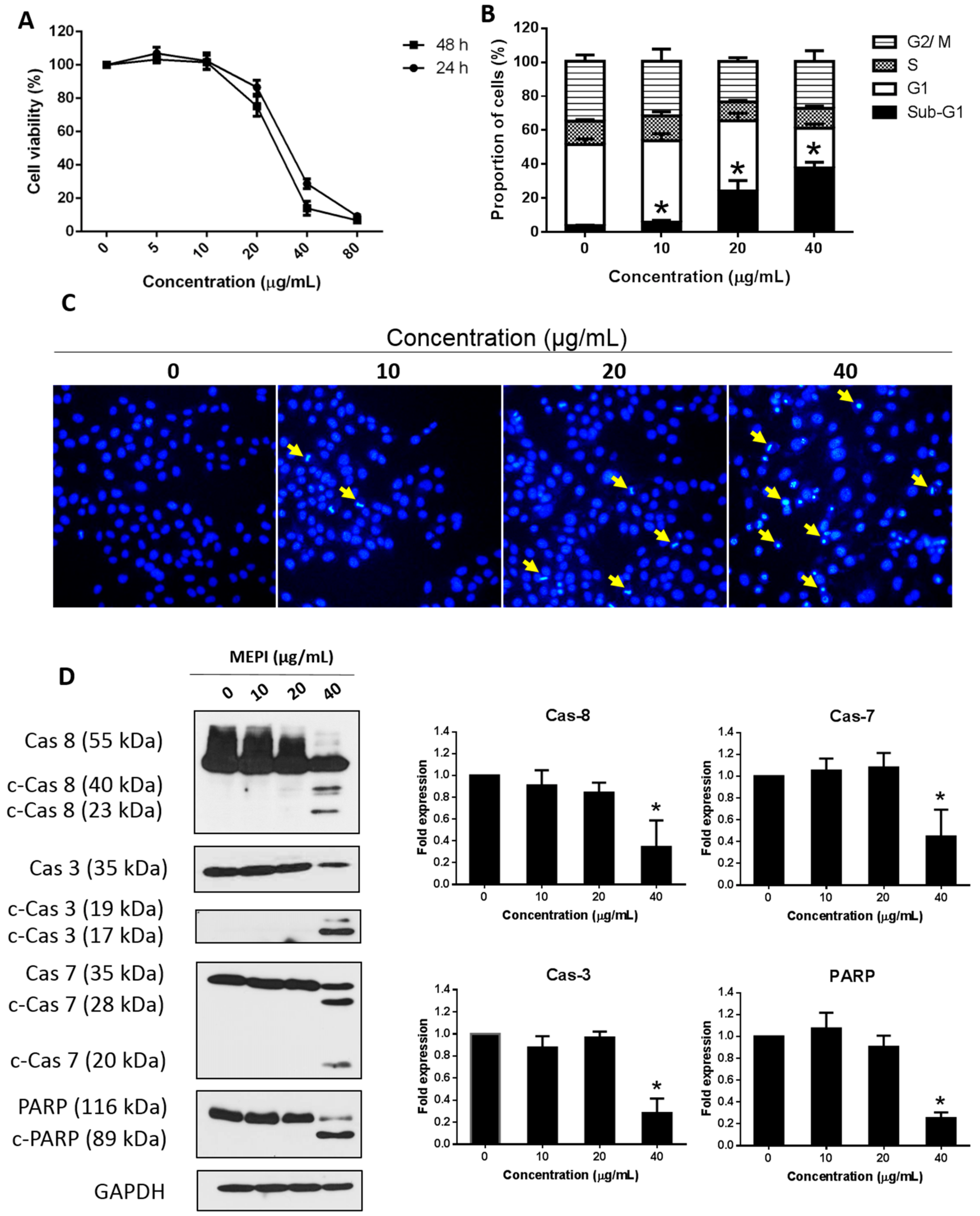
2.1. MEPI Induced Apoptosis of MDA-MB-231 Cells
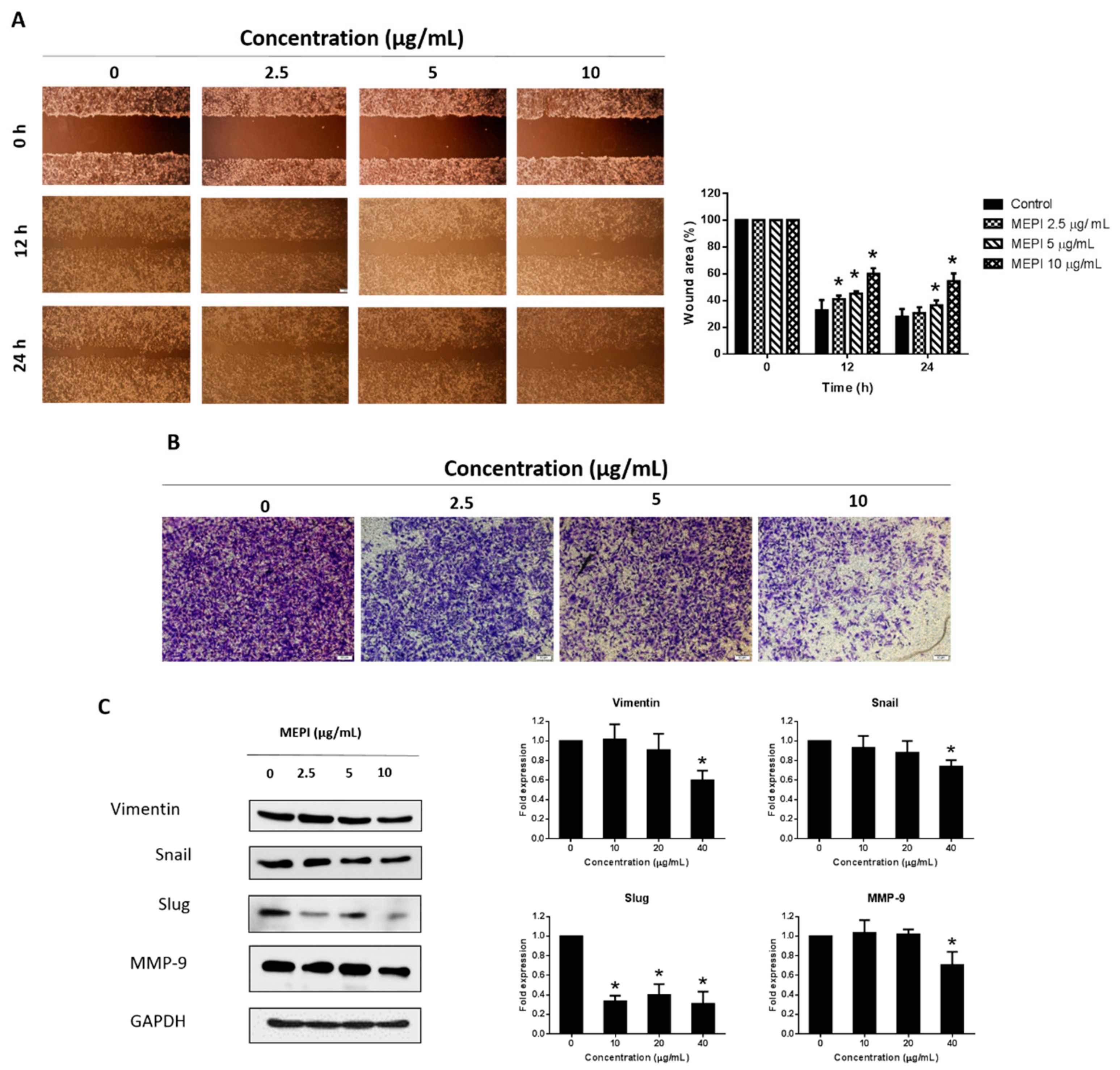
2.2. MEPI Inhibited the EMT in MDA-MB-231 Cells
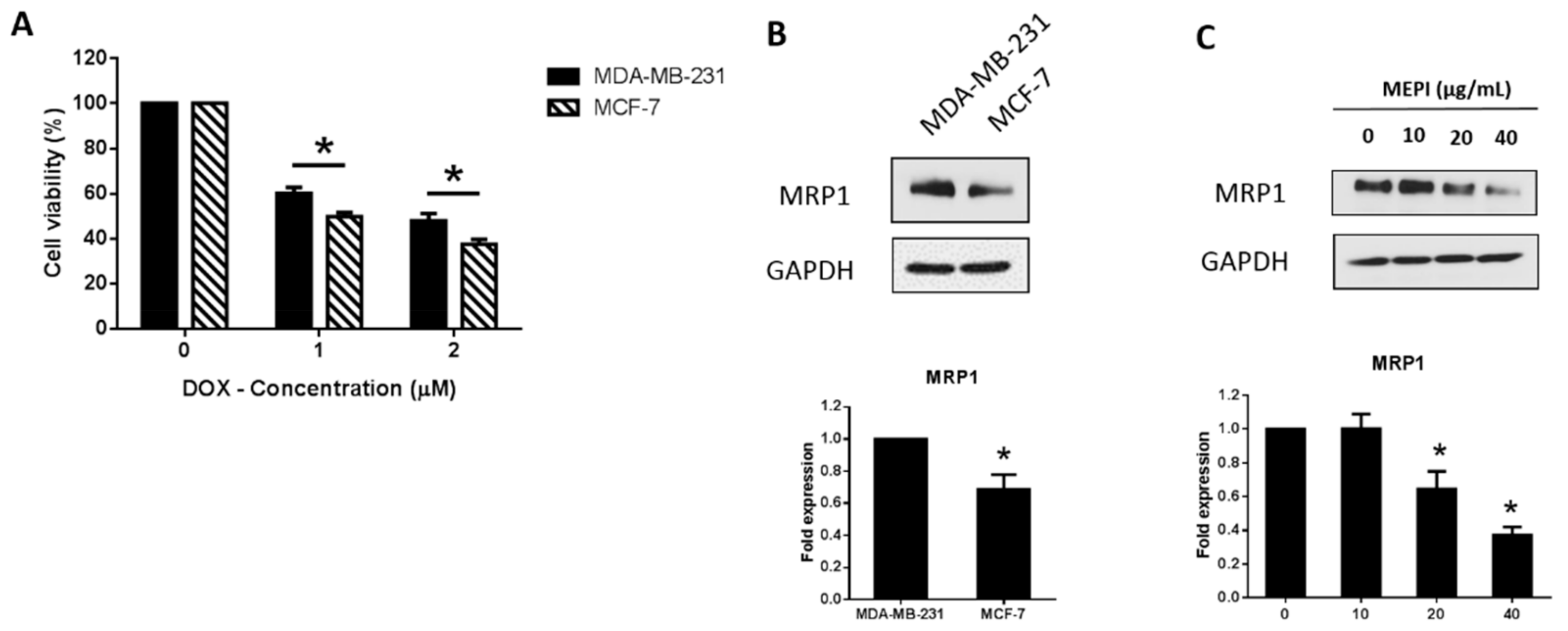
2.3. MEPI and Doxorubicin Synergistically Suppressed the Proliferation of MDA-MB-231 Cells
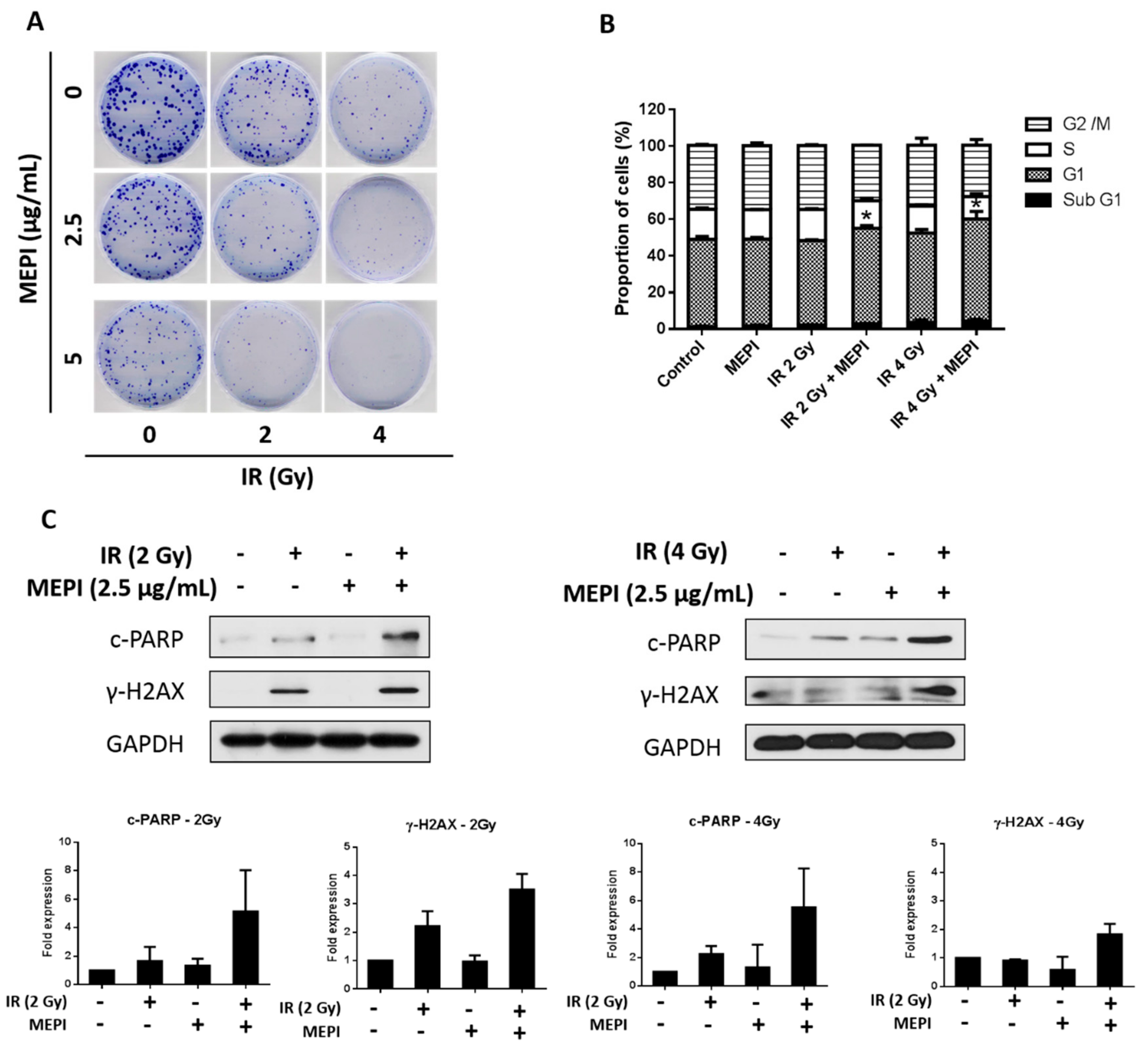
2.4. MEPI Induced Radiation Sensitization of MDA-MB-231 Cells
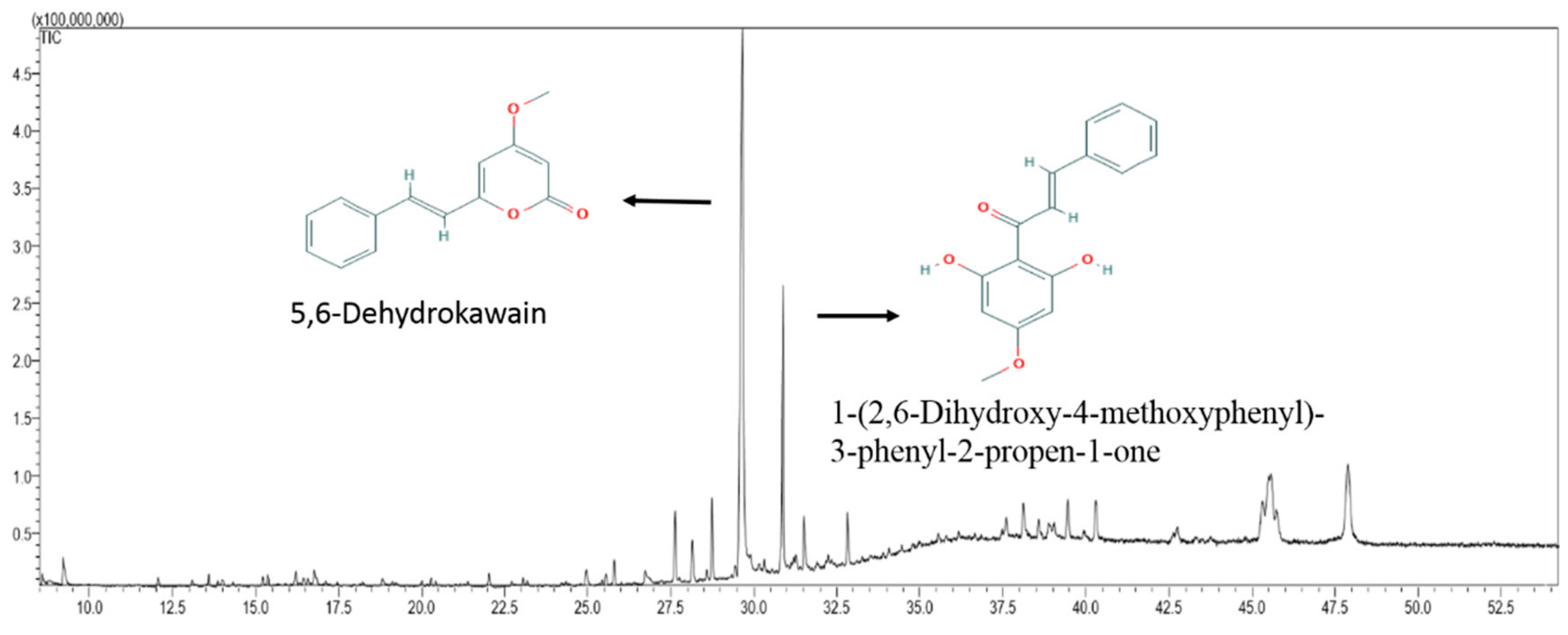
2.5. Chemical Composition of MEPI
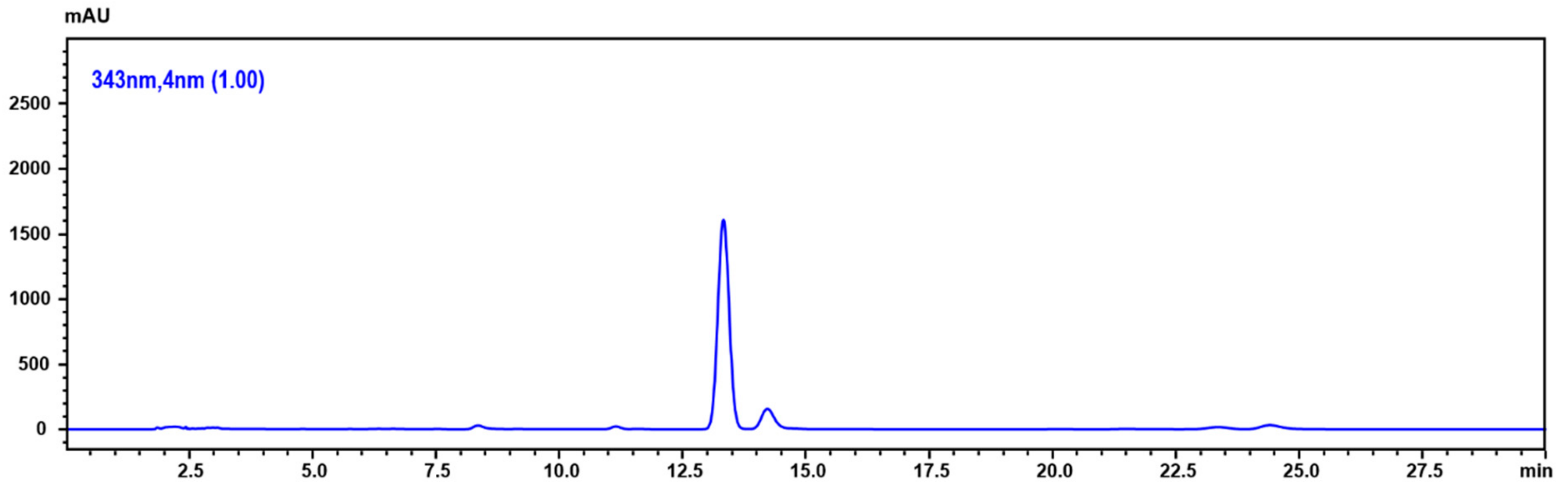
2.6. Determination of 5,6-Dehydrokawain in Pavetta Indica Methanol Extract
2.7. 5,6-Dehydrokawain Inhibited the EMT in MDA-MB-231 Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of the P. indica Methanol Extracts
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Flow Cytometric Analysis of the Cell-Cycle Distribution
4.5. Cell Migration Assay
4.6. Cell Invasion Assay
4.7. Western Blot Analysis
4.8. Analysis the Effects of Drug Combinations
4.9. Gas Chromatography–Mass Spectrometry
4.10. Determination of 5,6-Dehydrokawain
4.11. Irradiation
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mandal, S.C.; Mohana Lakshmi, S.; Ashok Kumar, C.K.; Sur, T.K.; Boominathan, R. Evaluation of anti-inflammatory potential of Pavetta indica Linn. leaf extract (family: Rubiaceae) in rats. Phytother. Res. 2003, 17, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Sastri, B. The Wealth of India: A Dictionary of Indian Raw Materials and Industrial Products. Raw Mater. 1962, 6, 483. [Google Scholar]
- Kirtikar, K.; Basu, B. Indian Medicinal Plants Vol-3; Bishen Singh Mahendra Pal Singh and Periodical Experts: Dehra Dun, India, 1918. [Google Scholar]
- Thabrew, M.I.; Joice, P.D.; Rajatissa, W. A comparative study of the efficacy of Pavetta indica and Osbeckia octandra in the treatment of liver dysfunction. Planta Med. 1987, 53, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M. Molecular stratification of triple-negative breast cancers. Oncologist 2011, 16, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265. [Google Scholar] [CrossRef]
- Kim, B.; Fatayer, H.; Hanby, A.M.; Horgan, K.; Perry, S.L.; Valleley, E.M.; Verghese, E.T.; Williams, B.J.; Thorne, J.L.; Hughes, T.A. Neoadjuvant chemotherapy induces expression levels of breast cancer resistance protein that predict disease-free survival in breast cancer. PLoS ONE 2013, 8, e62766. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Li, Y.; Wang, Z.; Sarkar, F. Cancer stem cells and epithelial-to-mesenchymal transition (EMT)-phenotypic cells: Are they cousins or twins? Cancers 2011, 3, 716–729. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; He, X.; Zhang, P.; Sun, C.; Xu, X.; Lu, Y.; Li, F. TGF-beta plays a vital role in triple-negative breast cancer (TNBC) drug-resistance through regulating stemness, EMT and apoptosis. Biochem. Biophys. Res. Commun. 2018, 502, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Mahjoubi, F. MRP1 but not MDR1 is associated with response to neoadjuvant chemotherapy in breast cancer patients. Dis. Markers 2013, 34, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.; Foekens, J.A.; Look, M.P.; Meijer-van Gelder, M.E.; Klijn, J.G.; Wiemer, E.A.; Stoter, G.; Nooter, K. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: Correlation with chemotherapeutic response. Clin. Cancer Res. 2003, 9, 827–836. [Google Scholar] [PubMed]
- Maciejczyk, A.; Jagoda, E.; Wysocka, T.; Matkowski, R.; Györffy, B.; Lage, H.; Surowiak, P. ABCC2 (MRP2, cMOAT) localized in the nuclear envelope of breast carcinoma cells correlates with poor clinical outcome. Pathol. Oncol. Res. 2012, 18, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Langlands, F.; Horgan, K.; Dodwell, D.; Smith, L. Breast cancer subtypes: Response to radiotherapy and potential radiosensitisation. Br. J. Radiol. 2013, 86, 20120601. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Jiang, M.; Yu, L.; Han, Q.; Shen, Z. p53 independent G1 arrest and apoptosis induced by adriamycin. Chin. Med Sci. J. Chung-Kuo I Hsueh K’o Hsueh Tsa Chih 1997, 12, 71–75. [Google Scholar] [PubMed]
- You, R.; Dai, J.; Zhang, P.; Barding, G.A., Jr.; Raftery, D. Dynamic Metabolic Response to Adriamycin-Induced Senescence in Breast Cancer Cells. Metabolites 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wang, L.; Wang, J.; Chen, T.; Li, H.; Zhang, K.; Chen, J.; Zhen, S.; Tuluhong, D.; Li, J.; et al. microRNA-129-5p suppresses Adriamycin resistance in breast cancer by targeting SOX2. Arch. Biochem. Biophys. 2018, 651, 52–60. [Google Scholar] [CrossRef]
- Ta, H.Q.; Thomas, K.S.; Schrecengost, R.S.; Bouton, A.H. A novel association between p130Cas and resistance to the chemotherapeutic drug adriamycin in human breast cancer cells. Cancer Res. 2008, 68, 8796–8804. [Google Scholar] [CrossRef]
- Schneider, S.L.; Fuqua, S.A.; Speeg, K.V.; Tandon, A.K.; McGuire, W.L. Isolation and characterization of an adriamycin-resistant breast tumor cell line. In Vitro Cell. Dev. Biol. 1990, 26, 621–628. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Yang, T.-Y.; Chen, K.-C.; Wu, C.-L.; Hsu, S.-L.; Hsueh, C.-M. Hypoxia can impair doxorubicin resistance of non-small cell lung cancer cells by inhibiting MRP1 and P-gp expression and boosting the chemosensitizing effects of MRP1 and P-gp blockers. Cell. Oncol. 2016, 39, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Herceg, Z.; Wang, Z.-Q. Functions of poly (ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2001, 477, 97–110. [Google Scholar] [CrossRef]
- Mallini, P.; Lennard, T.; Kirby, J.; Meeson, A. Epithelial-to-mesenchymal transition: What is the impact on breast cancer stem cells and drug resistance. Cancer Treat. Rev. 2014, 40, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tong, D.; Liu, G.; Xu, J.; Do, K.; Geary, K.; Zhang, D.; Zhang, J.; Zhang, Y.; Li, Y. Metformin reverses prostate cancer resistance to enzalutamide by targeting TGF-β1/STAT3 axis-regulated EMT. Cell Death Dis. 2017, 8, e3007. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, W.; Li, Y.; Zhu, J.Y.; Fang, D.; Ding, H.F.; Dong, Z.; Jing, Q.; Su, S.B.; Huang, S. Triple negative breast cancer development can be selectively suppressed by sustaining an elevated level of cellular cyclic AMP through simultaneously blocking its efflux and decomposition. Oncotarget 2016, 7, 87232–87245. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xiang, Y.; Liu, X.; Zhang, T.; Yang, R.; Chen, S.; Xu, L.; Yu, Q.; Zhao, H.; Zhang, L. Cucurbitacin B Induces the Lysosomal Degradation of EGFR and Suppresses the CIP2A/PP2A/Akt Signaling Axis in Gefitinib-Resistant Non-Small Cell Lung Cancer. Molecules 2019, 24, 647. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Cao, W.; Shen, W.; Zhang, L.; Gu, X.; Guo, Y.; Tsai, H.-i.; Liu, X.; Li, J.; Zhang, J. Arctigenin inhibits STAT3 and exhibits anticancer potential in human triple-negative breast cancer therapy. Oncotarget 2017, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Sak, A.; Stuschke, M. Use of γH2AX and other biomarkers of double-strand breaks during radiotherapy. Semin. Radiat. Oncol. 2010, 20, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit. Rev. ™Eukaryot. Gene Expr. 2014, 24, 15–28. [Google Scholar] [CrossRef]
- Gherman, C.; Culea, M.; Cozar, O. Comparative analysis of some active principles of herb plants by GC/MS. Talanta 2000, 53, 253–262. [Google Scholar] [CrossRef]
- Prasad, K.; Moulekhi, K.; Bisht, G. Chemical composition of the essential oil of Pavetta indica L. leaves. Res. J. Phytochem. 2011, 5, 66–69. [Google Scholar] [CrossRef]
- Teng, C.-M.; Hsu, S.-Y.; Lin, C.-H.; Yu, S.-M.; Wang, K.-J.; Lin, M.-H.; Chen, C.-F. Antiplatelet action of dehydrokawain derivatives isolated from Alpinia speciosa rhizoma. Chin. J. Physiol. 1990, 33, 41–48. [Google Scholar] [PubMed]
- Malami, I.; Muhammad, A.; Abubakar, I.B.; Etti, I.C.; Waziri, P.M.; Abubakar, R.M.; Mshelia, H.E. 5,6-dehydrokawain from the rhizome of Alpinia mutica Roxb. induced proangiogenic tumour-derived VEGF of HT-29 colorectal cancer. Nat. Prod. Res. 2018, 32, 2964–2967. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Rao Ravu, R.; Tekwani, B.L.; Li, W.; Liu, W.B.; Jacob, M.R.; Khan, S.I.; Cai, X.; Peng, C.Y.; Khan, I.A.; et al. Biological evaluation of phytoconstituents from Polygonum hydropiper. Nat. Prod. Res. 2017, 31, 2053–2057. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, M.; Mishima, T.; Watanabe, A.; Harada, T.; Yoshida, I.; Fujita, K.; Watai, M.; Tawata, S.; Nishikawa, K.; Morimoto, Y. 5,6-Dehydrokawain from Alpinia zerumbet promotes osteoblastic MC3T3-E1 cell differentiation. Biosci. Biotechnol. Biochem. 2016, 80, 1425–1432. [Google Scholar] [CrossRef]
- Penumala, M.; Zinka, R.B.; Shaik, J.B.; Amooru Gangaiah, D. In Vitro Screening of Three Indian Medicinal Plants for Their Phytochemicals, Anticholinesterase, Antiglucosidase, Antioxidant, and Neuroprotective Effects. Biomed. Res. Int. 2017, 2017, 5140506. [Google Scholar] [CrossRef]
- Malek, S.N.; Phang, C.W.; Ibrahim, H.; Norhanom, A.W.; Sim, K.S. Phytochemical and cytotoxic investigations of Alpinia mutica rhizomes. Molecules 2011, 16, 583–589. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Ann, Y.S.; Ko, C.I.; Lee, S.H.; Lee, W.W.; Jeon, Y.J. Apoptotic and antiproliferative effects of Stigmast-5-en-3-ol from Dendronephthya gigantea on human leukemia HL-60 and human breast cancer MCF-7 cells. Toxicol. In Vitro 2018, 52, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kapur, A.; Felder, M.; Fass, L.; Kaur, J.; Czarnecki, A.; Rathi, K.; Zeng, S.; Osowski, K.K.; Howell, C.; Xiong, M.P.; et al. Modulation of oxidative stress and subsequent induction of apoptosis and endoplasmic reticulum stress allows citral to decrease cancer cell proliferation. Sci. Rep. 2016, 6, 27530. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, S.R.; Ramani, S.; Natarajan, S.; Kim, Y.J.; Perumalsamy, H. Integrated transcriptome and in vitro analysis revealed anti-proliferative effect of citral in human stomach cancer through apoptosis. Sci. Rep. 2019, 9, 4883. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.B.; Thakkar, V.R.; Patel, J.S. Cellular Effect of Curcumin and Citral Combination on Breast Cancer Cells: Induction of Apoptosis and Cell Cycle Arrest. J. Breast Cancer 2015, 18, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Lee, H.J.; Jung, J.H.; Kim, Y.H.; Jung, D.B.; Sohn, E.J.; Lee, J.H.; Woo, H.J.; Baek, N.I.; Kim, Y.C.; et al. Activation of Caspase-9/3 and Inhibition of Epithelial Mesenchymal Transition are Critically Involved in Antitumor Effect of Phytol in Hepatocellular Carcinoma Cells. Phytother. Res. 2015, 29, 1026–1031. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. Rev. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef]
- Hyun, H.; Moon, J.; Cho, S. Quercetin suppresses cyr61-mediated multidrug resistance in human gastric adenocarcinoma ags cells. Molecules 2018, 23, 209. [Google Scholar] [CrossRef]
- Miller, L. Analyzing Gels and Western Blots with ImageJ. Available online: https://lukemiller.org/index.php/2010/11/analyzing-gels-and-western-blots-with-image-j/ (accessed on 13 June 2019).
Sample Availability: Not available. |
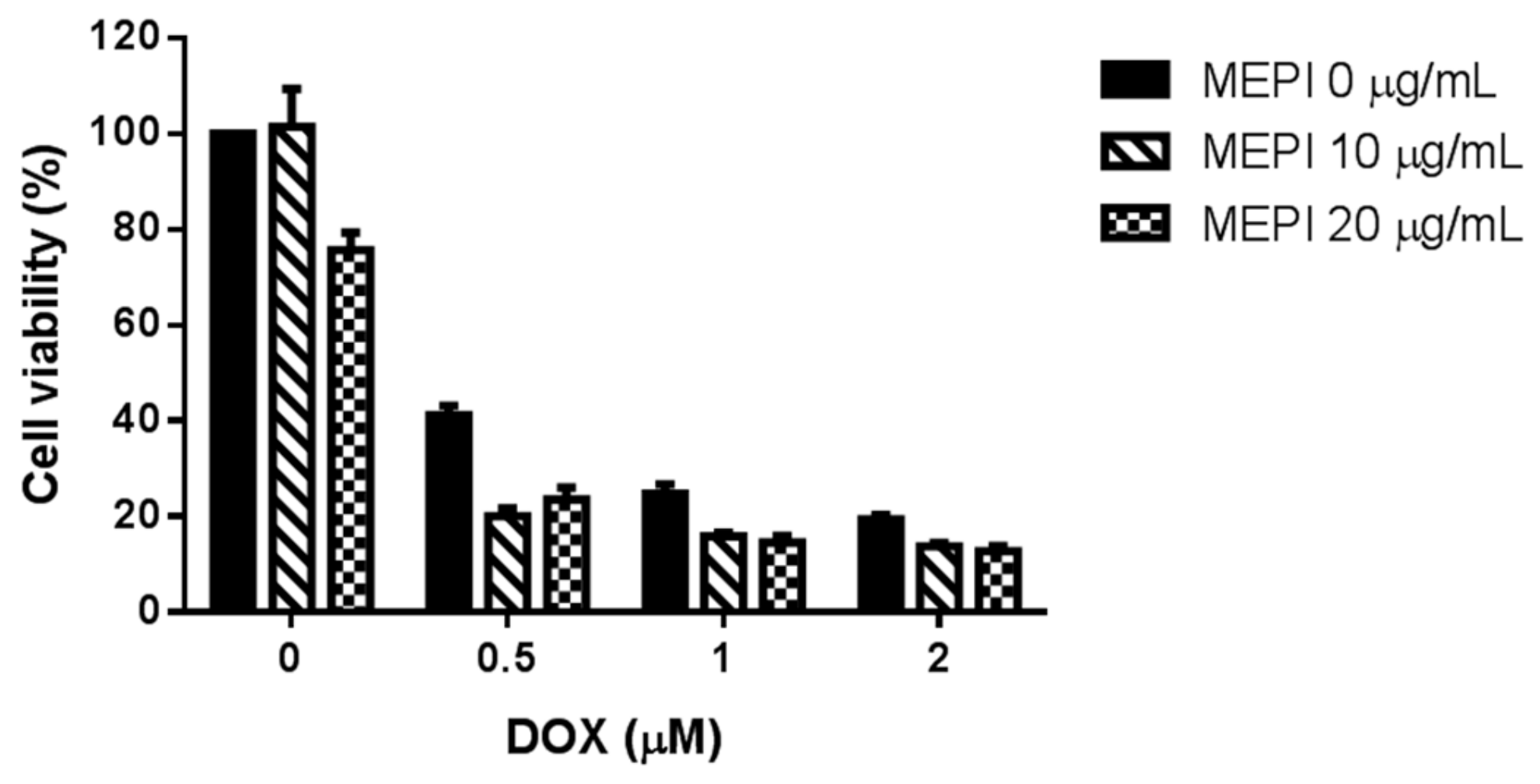

| MEPI (μg/mL) | DOX (μM) | CI Value |
|---|---|---|
| 10 | 0.5 | 0.564 |
| 10 | 1 | 0.658 |
| 10 | 2 | 0.889 |
| 20 | 0.5 | 0.957 |
| 20 | 1 | 0.85 |
| 20 | 2 | 1.055 |
| Peak No. | RT (min) | Compound Name | % Total | Mol. Formula | Mol. Wt (g/mol) |
|---|---|---|---|---|---|
| 1 | 9.206 | Citral | 2.25 | C10H16O | 152.237 |
| 2 | 12.064 | beta-Caryophyllene | 0.68 | C15H24 | 204.357 |
| 3 | 13.594 | 2,4-Di-tert-butylphenol | 0.62 | C14H22O | 206.329 |
| 4 | 15.218 | (-)-Spathulenol | 0.53 | C15H24O | 220.356 |
| 5 | 15.362 | Caryophyllene Oxide | 0.73 | C15H24O | 220.356 |
| 6 | 16.208 | Isospathulenol | 0.97 | C15H24O | 220.356 |
| 7 | 22.024 | Methyl palmitate | 0.75 | C17H34O2 | 270.457 |
| 8 | 25.795 | Phytol | 1.43 | C20H40O | 296.539 |
| 9 | 28.144 | 6,11-Dimethyl-2,6,10-dodecatrien-1-ol | 2.85 | C14H24O | 208.34l |
| 10 | 29.653 | 5,6-Dehydrokawain | 66.65 | C14H12O3 | 228.247 |
| 11 | 30.871 | 1-(2,6-Dihydroxy-4-methoxyphenyl)-3-phenyl-2-propen-1-one | 18.76 | C16H14O4 | 270.284 |
| 12 | 40.286 | Stigmast-5-en-3-ol | 3.78 | C29H50O | 414.718 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thi-Kim Nguyen, Y.; Moon, J.Y.; Ryu, J.-y.; Eum, S.; Bach, T.T.; Cho, S.K. Methanol Extract of Aerial Parts of Pavetta indica L. Enhances the Cytotoxic Effect of Doxorubicin and Induces Radiation Sensitization in MDA-MB-231 Triple-Negative Breast Cancer Cells. Molecules 2019, 24, 2273. https://doi.org/10.3390/molecules24122273
Thi-Kim Nguyen Y, Moon JY, Ryu J-y, Eum S, Bach TT, Cho SK. Methanol Extract of Aerial Parts of Pavetta indica L. Enhances the Cytotoxic Effect of Doxorubicin and Induces Radiation Sensitization in MDA-MB-231 Triple-Negative Breast Cancer Cells. Molecules. 2019; 24(12):2273. https://doi.org/10.3390/molecules24122273
Chicago/Turabian StyleThi-Kim Nguyen, Yen, Jeong Yong Moon, Ji-yeon Ryu, Sangmi Eum, Tran The Bach, and Somi Kim Cho. 2019. "Methanol Extract of Aerial Parts of Pavetta indica L. Enhances the Cytotoxic Effect of Doxorubicin and Induces Radiation Sensitization in MDA-MB-231 Triple-Negative Breast Cancer Cells" Molecules 24, no. 12: 2273. https://doi.org/10.3390/molecules24122273
APA StyleThi-Kim Nguyen, Y., Moon, J. Y., Ryu, J.-y., Eum, S., Bach, T. T., & Cho, S. K. (2019). Methanol Extract of Aerial Parts of Pavetta indica L. Enhances the Cytotoxic Effect of Doxorubicin and Induces Radiation Sensitization in MDA-MB-231 Triple-Negative Breast Cancer Cells. Molecules, 24(12), 2273. https://doi.org/10.3390/molecules24122273




