Mechanism Investigation of Tagetes patula L. against Chronic Nonbacterial Prostatitis by Metabolomics and Network Pharmacology
Abstract
1. Introduction
2. Results
2.1. The Levels of Physiological Indexes
2.2. The Activity of Na+/K+-ATPase
2.3. Results of Energy Metabolic Parameters
2.4. LC-MS Analysis of Metabolic Profiling
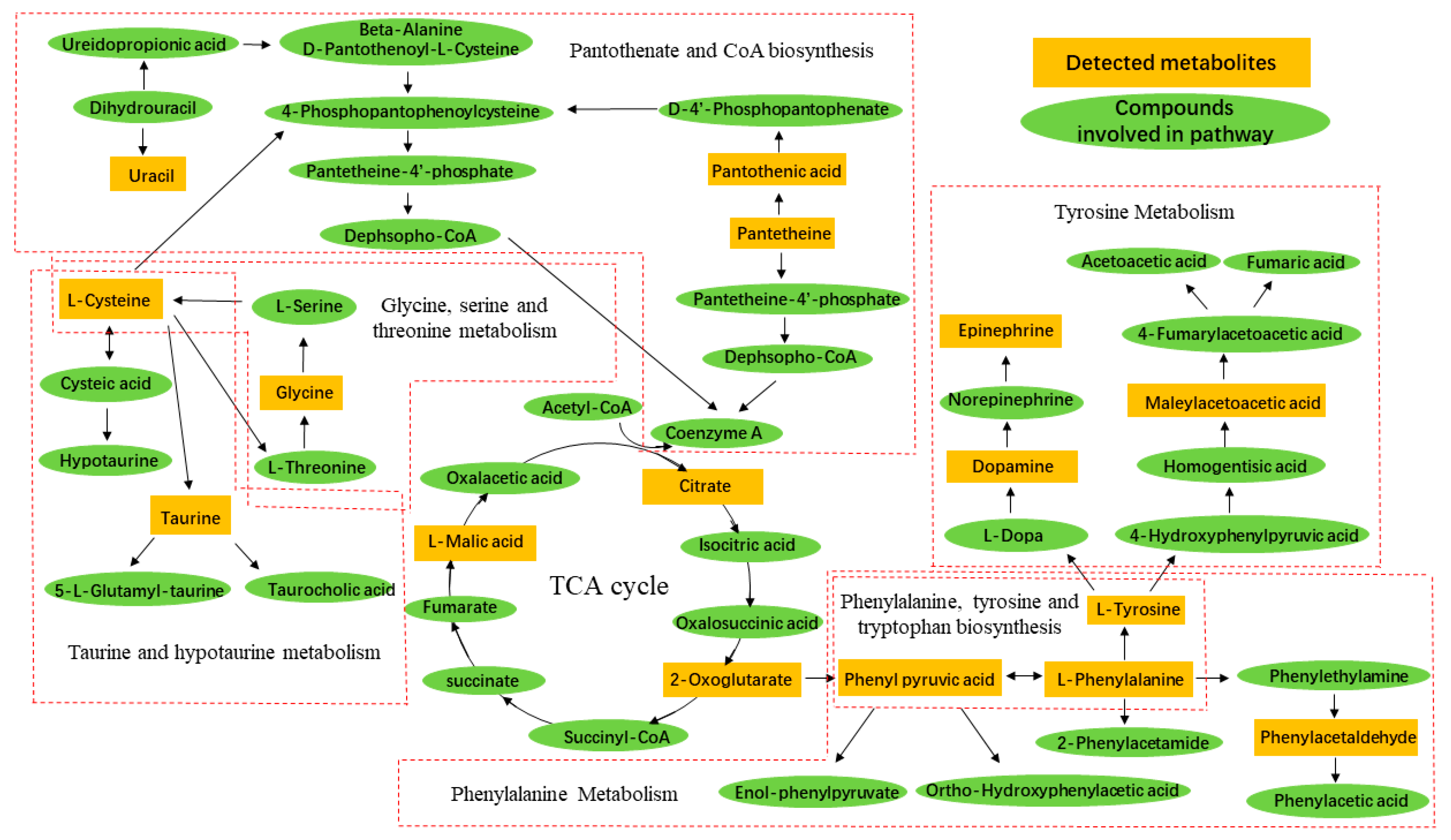
2.5. Identification of Metabolite Candidates
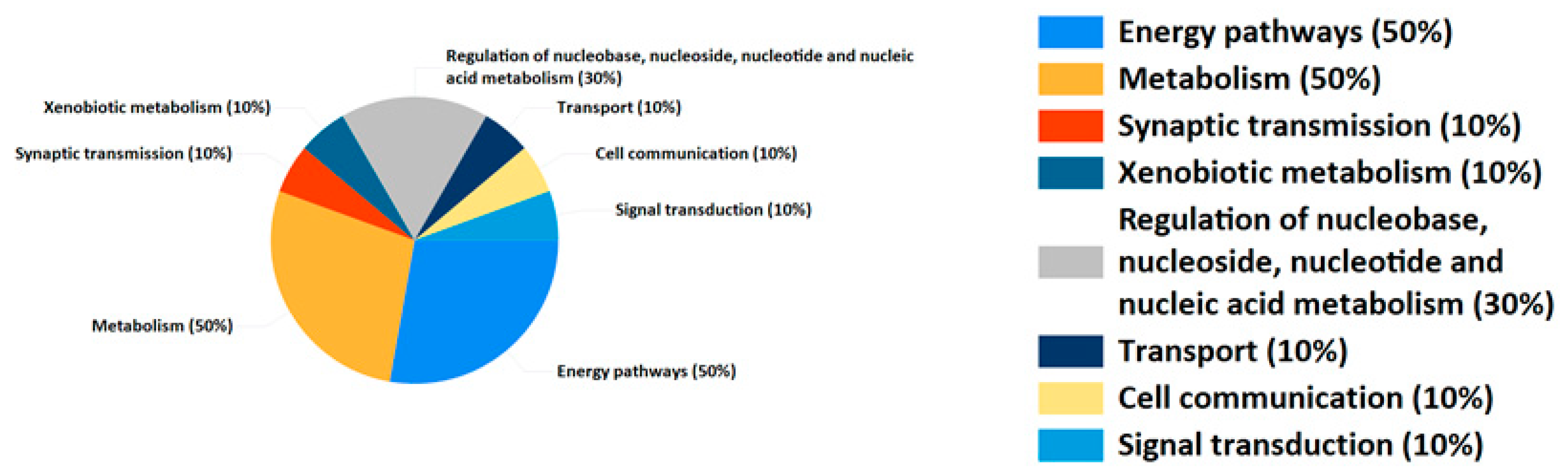
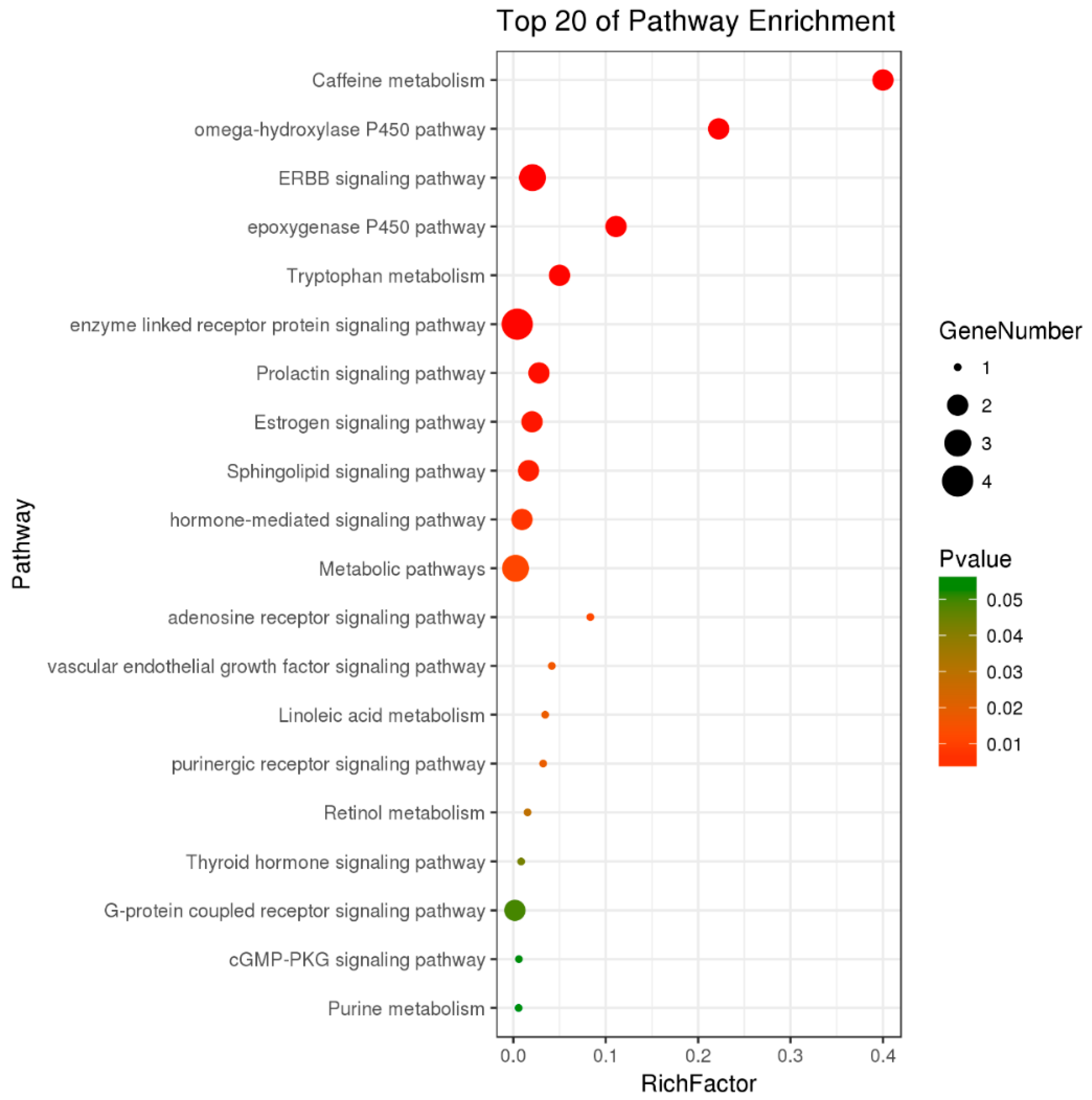
2.6. Pathway Analysis
2.7. Network Pharmacology
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animals
4.3. Determination Eenergy Metabolic Parameters
4.4. Collection and Preparation of Biosamples
4.5. Physiological Indexes
4.6. Metabolic Profiling Chromatography
4.7. Mass Spectrometry
4.8. Data Processing
4.9. Multivariate Data Analysis
4.10. Biomarkers Identification
4.11. Metabolic Pathway Analysis
4.12. Statistical Analysis
4.13. Network Pharmacology Predict Pathway
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CNP | Chronic nonbacterial prostatitis |
| PSA | prostate-specific antigen |
| TNF-α | tumor necrosis factor-α |
| DHT | dihydrotestosterone |
| SOG | Sham-operated group |
| MOG | Chronic nonbacterial prostatitis model group |
| WAD | Water decoction treatment group |
| EOC | Essential oil treatment group |
| POL | Polysaccharide treatment group |
| FLA | Flavonoids treatment group |
| TCM | Pule’an tablet treatment group |
| LEH | Levofloxacin treatment group |
| VO2 | O2 consumption |
| VCO2 | CO2 production |
| H | Heat production |
| EE | Energy expenditure |
| RER | Respiratory exchange ratio |
| ESI | electrospray ion source |
| QC | The quality control |
| PCA | principal component analysis |
| PLS-DA | partial least square discriminant analysis |
References
- Bano, H.; Ahmed, S.W.; Azhar, I.; Ali, M.S.; Alam, N. Chemical constituents of Tagetes patula L. Pak. J. Pharm. Sci. 2002, 15, 1–12. [Google Scholar] [PubMed]
- Kasahara, Y.; Yasukawa, K.; Kitanaka, S.; Khan, M.T.; Evans, F.J. Effect of methanol extract from flower petals of Tagetes patula L. on acute and chronic inflammation model. Phytotherapy Res. Ptr. 2002, 16, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ran, X.; Dou, D.; Cai, D. Effectiveness of Tagetes patula against chronic nonbacterial prostatitis in rat model. Bangl. J. Pharmacol. 2017, 12, 376–383. [Google Scholar] [CrossRef]
- Malik, R.; Aneni, E.C.; Shahrayar, S.; Freitas, W.M.; Ali, S.S.; Veledar, E.; Latif, M.A.; Aziz, M.; Ahmed, R.; Khan, S.A. Elevated serum uric acid is associated with vascular inflammation but not coronary artery calcification in the healthy octogenarians: The Brazilian study on healthy aging. Aging Clin. Exper. Res. 2016, 28, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, R.; Kichler, J. Uric Acid-Induced Inflammation Is Mediated by the Parathyroid Hormone: 25-Hydroxyvitamin D Ratio in Obese Adolescents. Metab. Syndrome Related Dis. 2016, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Sumner, S.J.; Fennell, T.R. Review of the metabolic fate of styrene. Crit. Rev. Toxicol. 1994, 24 (Suppl. 1), S11–S33. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Wang, Q. Phenylalanine metabolic disorders and diseases. J. Clin. Med. Pathol. 2001, 6, 451–453. [Google Scholar]
- Jin, G. Biochemistry; Shanghai Science and Technology Press: Shanghai, China, 2006; pp. 156–158. [Google Scholar]
- Liu, J.; Xu, C.; Ying, L.; Zang, S.; Zhuang, Z.; Lv, H.; Yang, W.; Luo, Y.; Ma, X.; Wang, L. Relationship of serum uric acid level with non-alcoholic fatty liver disease and its inflammation progression in nonobese adults. Hepatol. Res. Off. J. Japan Soc. Hepatol. 2016, 47, E104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, T. Research progress of tyrosine pharmacology. Chin. Pharmacol. Bull. 1993, 251–253. [Google Scholar]
- Yin, D.; Qi, Z. Biosynthesis and metabolism of catecholamines. Adv. Physiol. Sci. 1965, 58–67. [Google Scholar] [CrossRef]
- Boehm, G.; Cervantes, H.; Georgi, G.; Jelinek, J.; Sawatzki, G.; Wermuth, B.; Colombo, J.P. Effect of increasing dietary threonine intakes on amino acid metabolism of the central nervous system and peripheral tissues in growing rats. Pediatr. Res. 1998, 44, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, X.; Chen, Q. Protective effect of glycine in cardiovascular diseases. Adv. Biochem. Biophy. 2015, 810–816. [Google Scholar] [CrossRef]
- Jie, N. Vitamin B5. Chinese Health Food 2013, 12–14. [Google Scholar]
- Costello, L.C.; Franklin, R.B. Testosterone and Prolactin Regulation of Metabolic Genes and Citrate Metabolism of Prostate Epithelial Cells. Horm. Metab. Res. 2002, 34, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, C.; Ying, L.; Zang, S.; Zhuang, Z.; Lv, H.; Yang, W.; Luo, Y.; Ma, X.; Wang, L. Research and Application on l-Malic Acid. China Food Addict. 2003, 52–56. [Google Scholar]
- Veronika, F.; Janez, K.; Urban, B. Inverse Molecular Docking as a Novel Approach to Study Anticarcinogenic and Anti-Neuroinflammatory Effects of Curcumin. Molecules 2018, 23, 3351. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, X.; Liu, J.; Cheng, C.; Jiang, T. Study on structure-antioxidation relationship of plant flavonoids. Food Sci. 2006, 26, 69–73. [Google Scholar] [CrossRef]
- Robinette, C.L. Sex-hormone-induced inflammation and fibromuscular proliferation in the rat lateral prostate. Prostate 1988, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2013, 11, 2498–2504. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Chisanga, D.; Alessandro, R.; Ang, C.S.; Askenase, P.; Batagov, A.O.; Benito-Martin, A.; Camussi, G.; Clayton, A.; et al. A novel community driven software for functional enrichment analysis of extracellular vesicles data. J. Extracell. Vesicles 2017, 1, 1321455. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Ang, C.S.; Gangoda, L.; Quek, C.M.J.; Williamson, N.J.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: a standalone tool for functional enrichment analysis. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
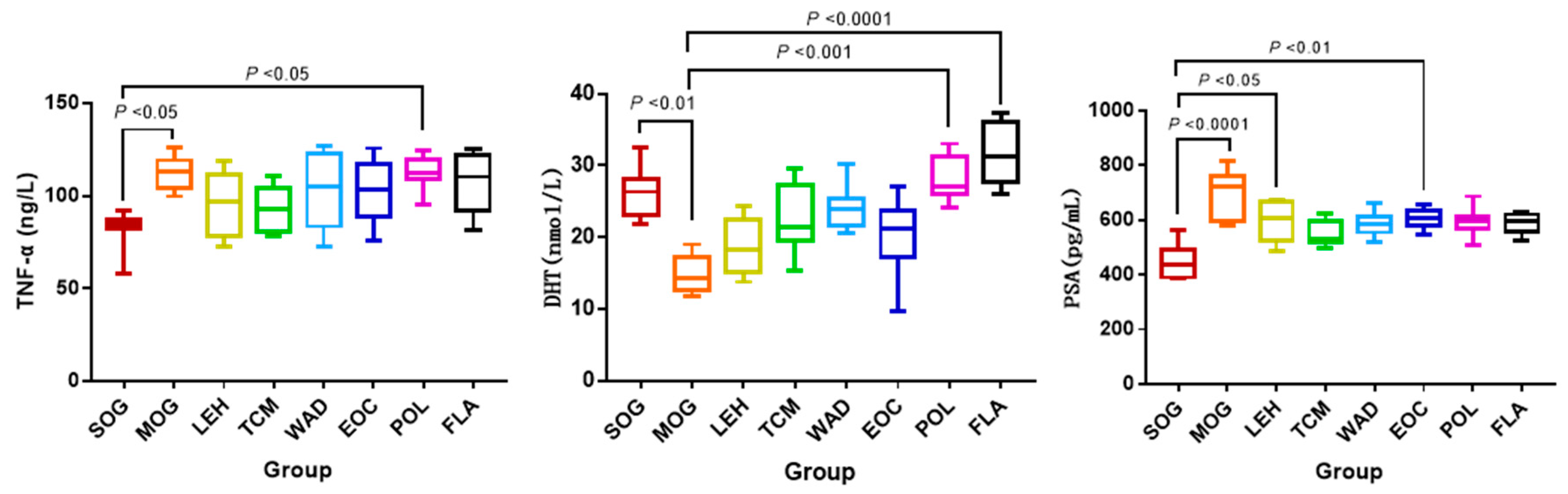
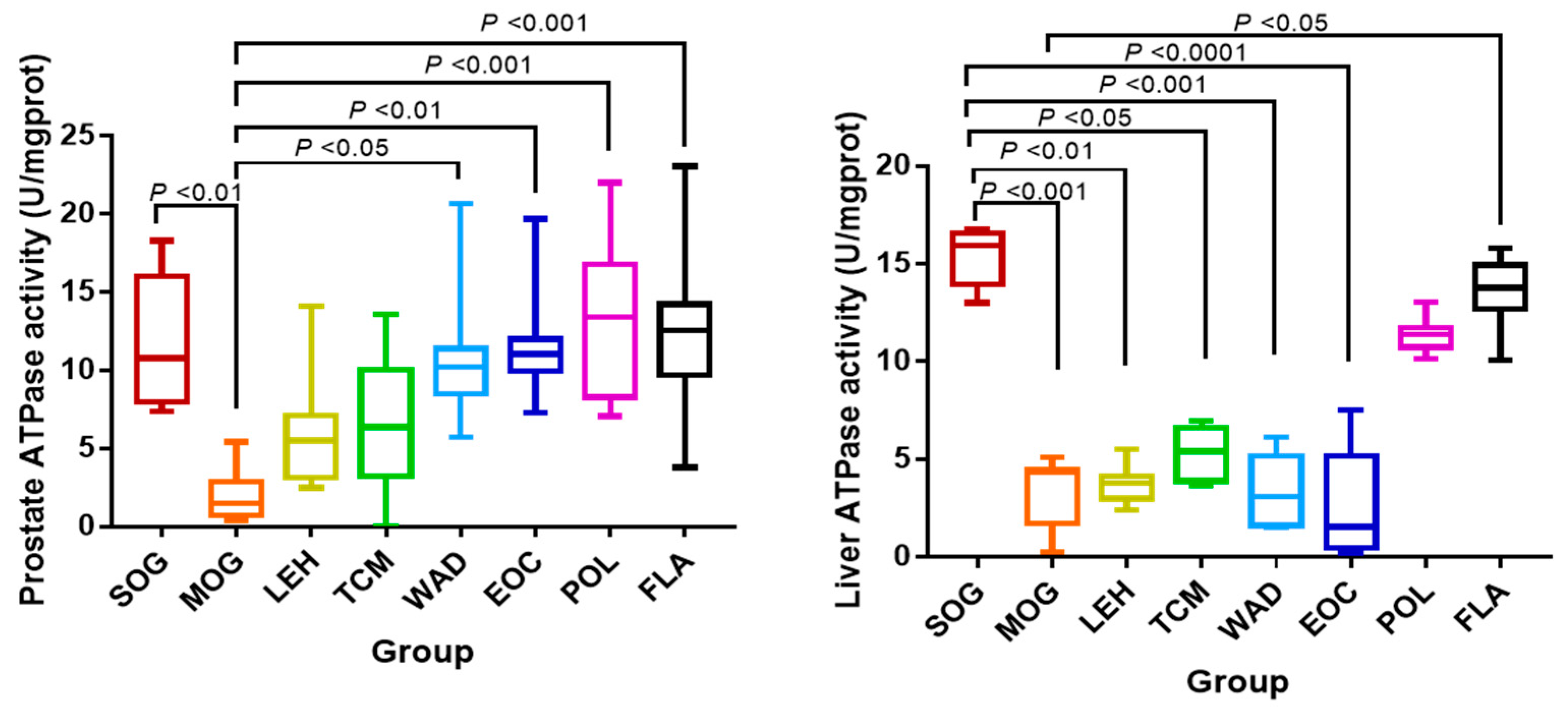
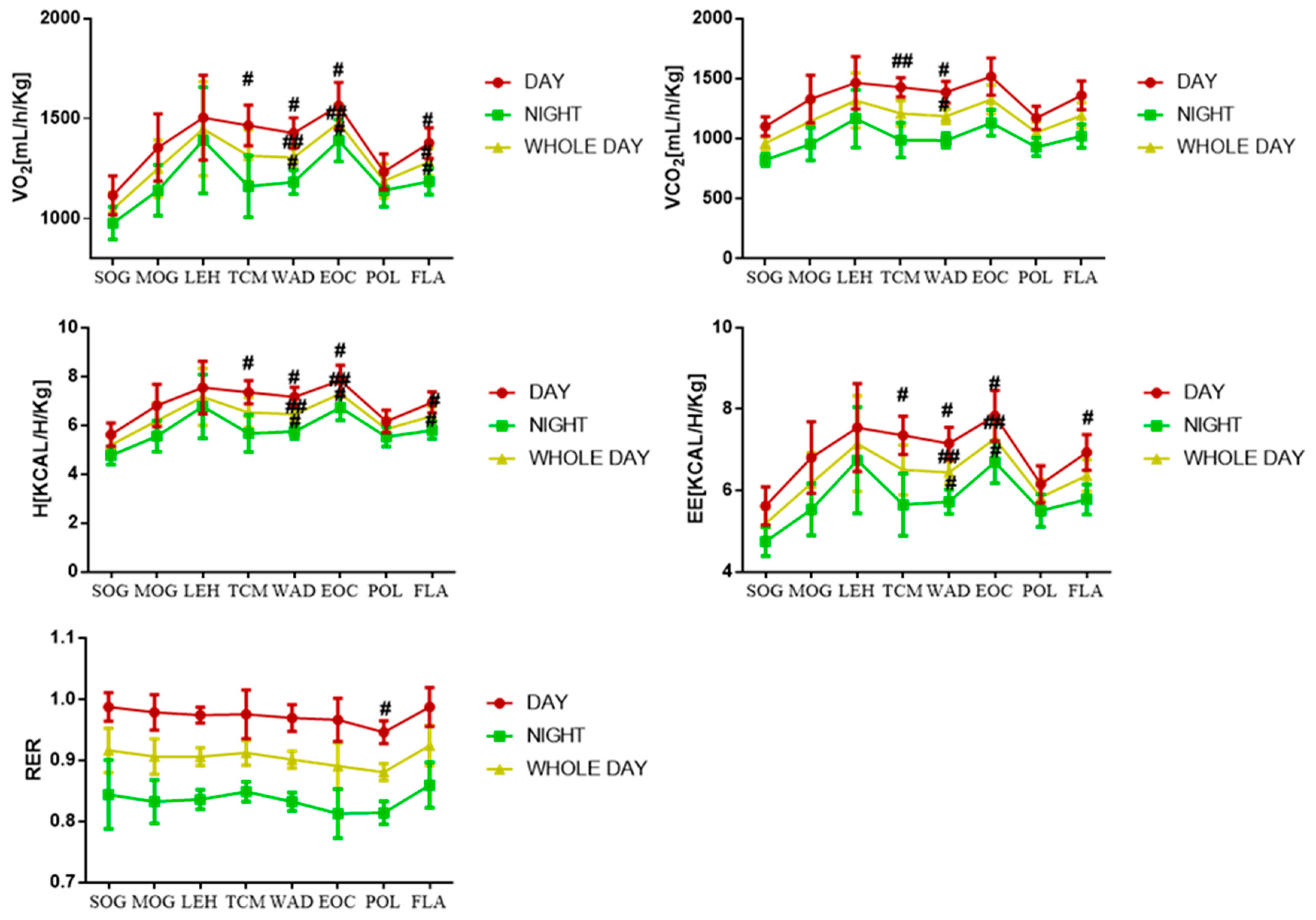


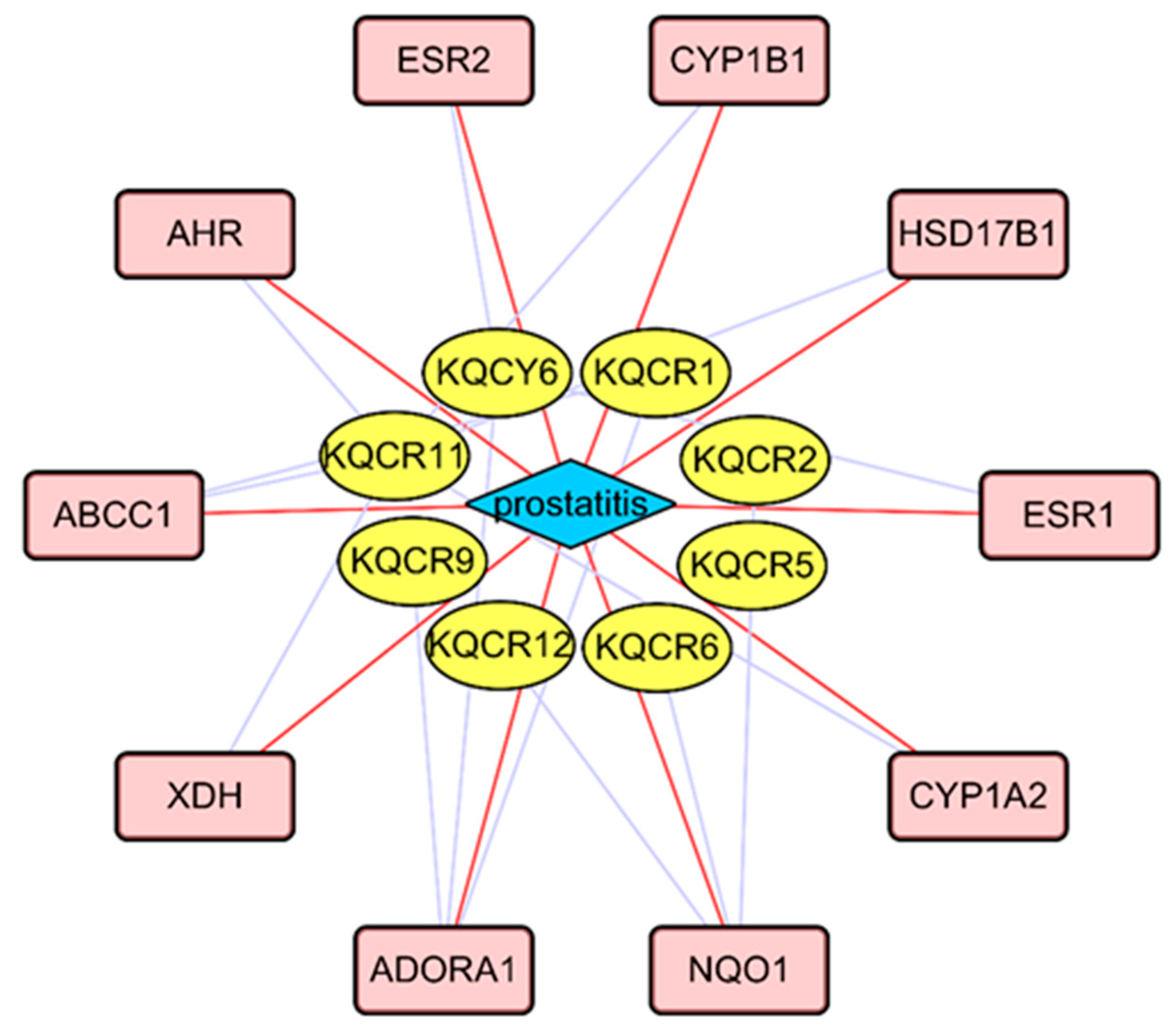
| Compound ID | Metabolite | Molecular Formula | Mode | Ret. Time | M/Z | Trend |
|---|---|---|---|---|---|---|
| HMDB0000158 | Δl-tyrosine | C9H11NO3 | ESI - | 1.3987 | 180.0632 | - |
| HMDB0006236 | Phenylacetaldehyde | C8H8O | ESI + | 1.5029 | 121.0663 | - |
| HMDB0000205 | Phenylpyruvic acid | C9H8O3 | ESI - | 3.5141 | 163.0386 | - |
| HMDB0000159 | Δl-phenylalanine | C9H11NO2 | ESI + | 2.1193 | 120.0800 | - |
| HMDB0003426 | Pantetheine | C11H22N2O4S | ESI + | 2.5712 | 279.1378 | - |
| HMDB0000574 | l-Cysteine | C3H7NO2S | ESI + | 4.8468 | 122.0283 | - |
| HMDB0000210 | Pantothenic Acid | C9H17NO5 | ESI - | 2.3662 | 218.1016 | - |
| HMDB0000300 | Uracil | C7H10N2OS | ESI + | 1.5955 | 113.0465 | + |
| HMDB0000929 | Tryptophan | C11H12N2O2 | ESI + | 2.9052 | 205.1002 | + |
| HMDB0000259 | Serotonin | C10H12N2O | ESI + | 1.8983 | 177.1051 | - |
| HMDB0000684 | l-Kynurenine | C10H12N2O3 | ESI + | 2.3458 | 191.0838 | - |
| HMDB0000734 | Indoleacrylic acid | C11H9NO2 | ESI - | 6.86 | 186.0561 | + |
| HMDB0000978 | 4-(2-Aminophenyl)-2,4-dioxobutanoic acid | C10H9NO4 | ESI + | 3.434 | 208.0615 | - |
| HMDB0000197 | 3-Indoleacetic Acid | C10H9NO2 | ESI - | 6.1042 | 174.0572 | - |
| HMDB0001644 | d-Xylulose | C5H10O5 | ESI - | 1.6288 | 151.0607 | - |
| HMDB0000127 | d-Glucuronic acid | C6H10O7 | ESI - | 3.6827 | 193.0474 | + |
| HMDB0000177 | Δl-histidine | C6H9N3O2 | ESI + | 0.6353 | 156.0459 | - |
| HMDB0062781 | 2-oxoglutarate | C5H6O5 | ESI - | 0.8023 | 145.0105 | + |
| HMDB0000156 | Δl-Malic acid | C4H6O5 | ESI - | 0.7309 | 133.0072 | + |
| HMDB0000094 | Δ Citrate | C6H8O7 | ESI + | 0.9318 | 193.0375 | + |
| HMDB0000068 | Epinephrine | C6H7N5O | ESI + | 2.3582 | 184.0994 | - |
| HMDB0000073 | Δ Dopamine | C8H11NO2 | ESI + | 0.8783 | 154.0985 | + |
| HMDB0002052 | Maleylacetoacetic acid | C8H8O6 | ESI + | 2.8867 | 201.042 | - |
| HMDB0000251 | Δ Taurine | C2H7NO3S | ESI - | 0.6159 | 124.0016 | - |
| HMDB0000167 | Δl-threonine | C4H9NO3 | ESI + | 0.7366 | 120.0690 | + |
| HMDB0000123 | Δ Glycine | C2H5NO2 | ESI + | 4.2937 | 76.0406 | - |
| HMDB0000289 | Uric acid | C5H4N4O3 | ESI + | 1.0763 | 169.0381 | - |
| HMDB0000034 | Adenine | C6H7N5 | ESI + | 0.9096 | 136.0654 | - |
| No. | Pathway Name | Match Status | P | Impact |
|---|---|---|---|---|
| 1 | Δ Phenylalanine, tyrosine and tryptophan biosynthesis | 3/4 | 2.52 × 10−5 | 1 |
| 2 | Δ Phenylalanine metabolism | 4/9 | 1.29 × 10−5 | 0.77778 |
| 3 | Δ Taurine and hypotaurine metabolism | 2/8 | 0.0093149 | 0.42857 |
| 4 | Ascorbate and aldarate metabolism | 1/9 | 0.16098 | 0.4 |
| 5 | Δ Tryptophan metabolism | 5/41 | 8.37 × 10−4 | 0.35955 |
| 6 | Δ Tyrosine metabolism | 4/42 | 0.0074067 | 0.32959 |
| 7 | Δ Glyoxylate and dicarboxylate metabolism | 2/16 | 0.036307 | 0.2963 |
| 8 | Δ Glycine, serine and threonine metabolism | 3/32 | 0.021783 | 0.29197 |
| 9 | Histidine metabolism | 1/15 | 0.25411 | 0.24194 |
| 10 | Δ Citrate cycle (TCA cycle) | 3/20 | 0.0058413 | 0.16675 |
| 11 | Cysteine and methionine metabolism | 1/28 | 0.42297 | 0.12829 |
| 12 | Pantothenate and CoA biosynthesis | 4/15 | 1.29 × 10−4 | 0.08163 |
| 13 | Alanine, aspartate and glutamate metabolism | 1/24 | 0.37538 | 0.06329 |
| 14 | Primary bile acid biosynthesis | 2/46 | 0.22107 | 0.05952 |
| 15 | Pyrimidine metabolism | 1/41 | 0.5547 | 0.04182 |
| 16 | Purine metabolism | 2/68 | 0.38017 | 0.02549 |
| 17 | Glutathione metabolism | 2/26 | 0.087435 | 0.00955 |
| 18 | Ubiquinone and other terpenoid-quinone biosynthesis | 1/3 | 0.056709 | 0 |
| 19 | Thiamine metabolism | 1/7 | 0.12752 | 0 |
| 20 | Starch and sucrose metabolism | 1/23 | 0.36291 | 0 |
| 21 | Pyruvate metabolism | 1/22 | 0.35019 | 0 |
| 22 | Porphyrin and chlorophyll metabolism | 1/27 | 0.41141 | 0 |
| 23 | Pentose and glucuronate interconversions | 2/14 | 0.028192 | 0 |
| 24 | Nitrogen metabolism | 2/9 | 0.011835 | 0 |
| 25 | Methane metabolism | 1/9 | 0.16098 | 0 |
| 26 | Inositol phosphate metabolism | 1/26 | 0.39963 | 0 |
| 27 | d-glutamine and d-glutamate metabolism | 1/5 | 0.09278 | 0 |
| 28 | Cyanoamino acid metabolism | 1/6 | 0.11031 | 0 |
| 29 | Butanoate metabolism | 1/20 | 0.32403 | 0 |
| 30 | beta-Alanine metabolism | 1/19 | 0.31057 | 0 |
| 31 | Aminoacyl-tRNA biosynthesis | 7/67 | 1.72 × 10−4 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ran, X.; Riaz, M.; Kuang, H.; Dou, D.; Cai, D. Mechanism Investigation of Tagetes patula L. against Chronic Nonbacterial Prostatitis by Metabolomics and Network Pharmacology. Molecules 2019, 24, 2266. https://doi.org/10.3390/molecules24122266
Liu X, Ran X, Riaz M, Kuang H, Dou D, Cai D. Mechanism Investigation of Tagetes patula L. against Chronic Nonbacterial Prostatitis by Metabolomics and Network Pharmacology. Molecules. 2019; 24(12):2266. https://doi.org/10.3390/molecules24122266
Chicago/Turabian StyleLiu, Xueying, Xiaoku Ran, Muhammad Riaz, Haixue Kuang, Deqiang Dou, and Decheng Cai. 2019. "Mechanism Investigation of Tagetes patula L. against Chronic Nonbacterial Prostatitis by Metabolomics and Network Pharmacology" Molecules 24, no. 12: 2266. https://doi.org/10.3390/molecules24122266
APA StyleLiu, X., Ran, X., Riaz, M., Kuang, H., Dou, D., & Cai, D. (2019). Mechanism Investigation of Tagetes patula L. against Chronic Nonbacterial Prostatitis by Metabolomics and Network Pharmacology. Molecules, 24(12), 2266. https://doi.org/10.3390/molecules24122266






