Synthesis and Properties of New Dithienosilole Derivatives as Luminescent Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
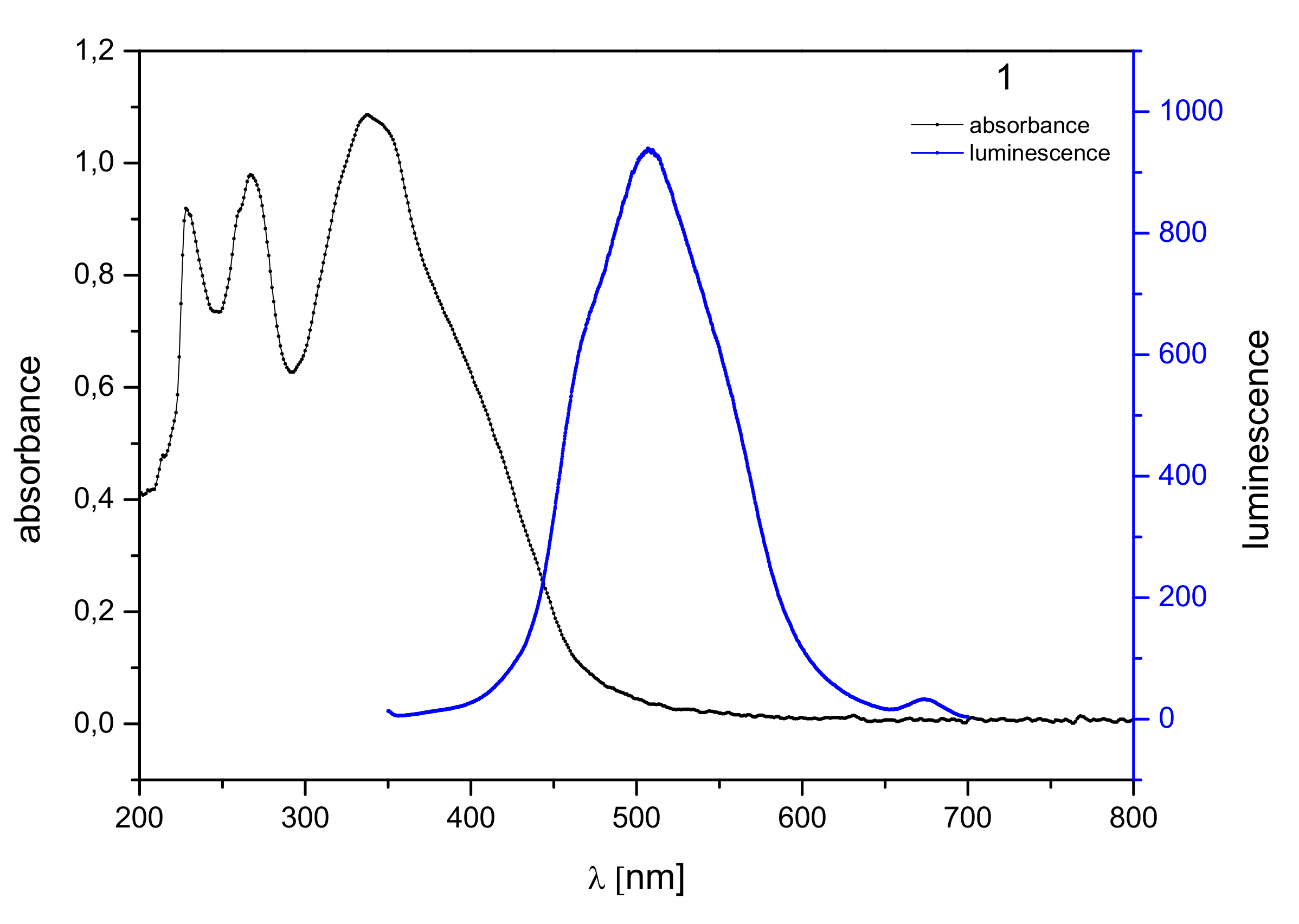
2.2. Optical Properties
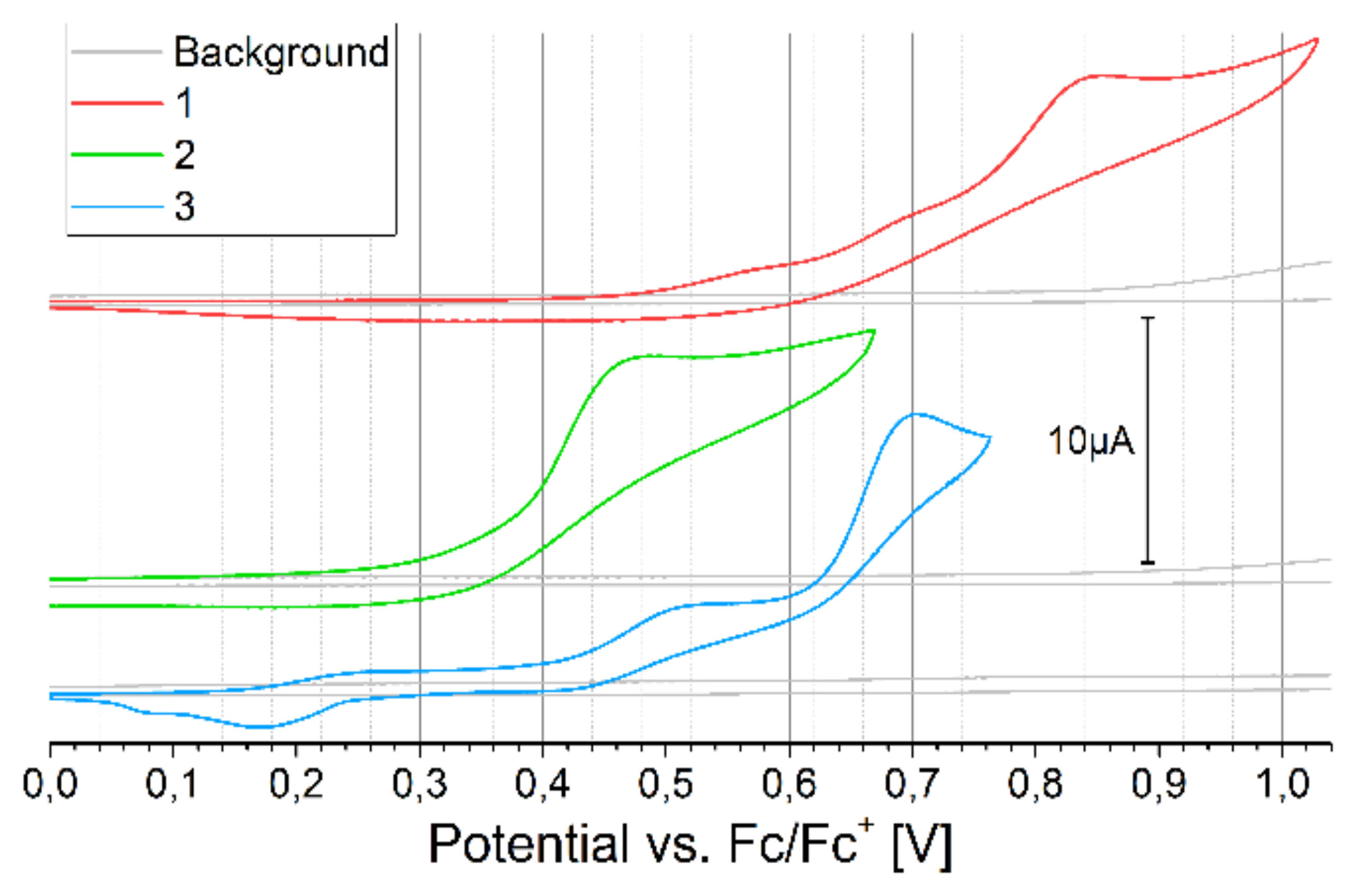
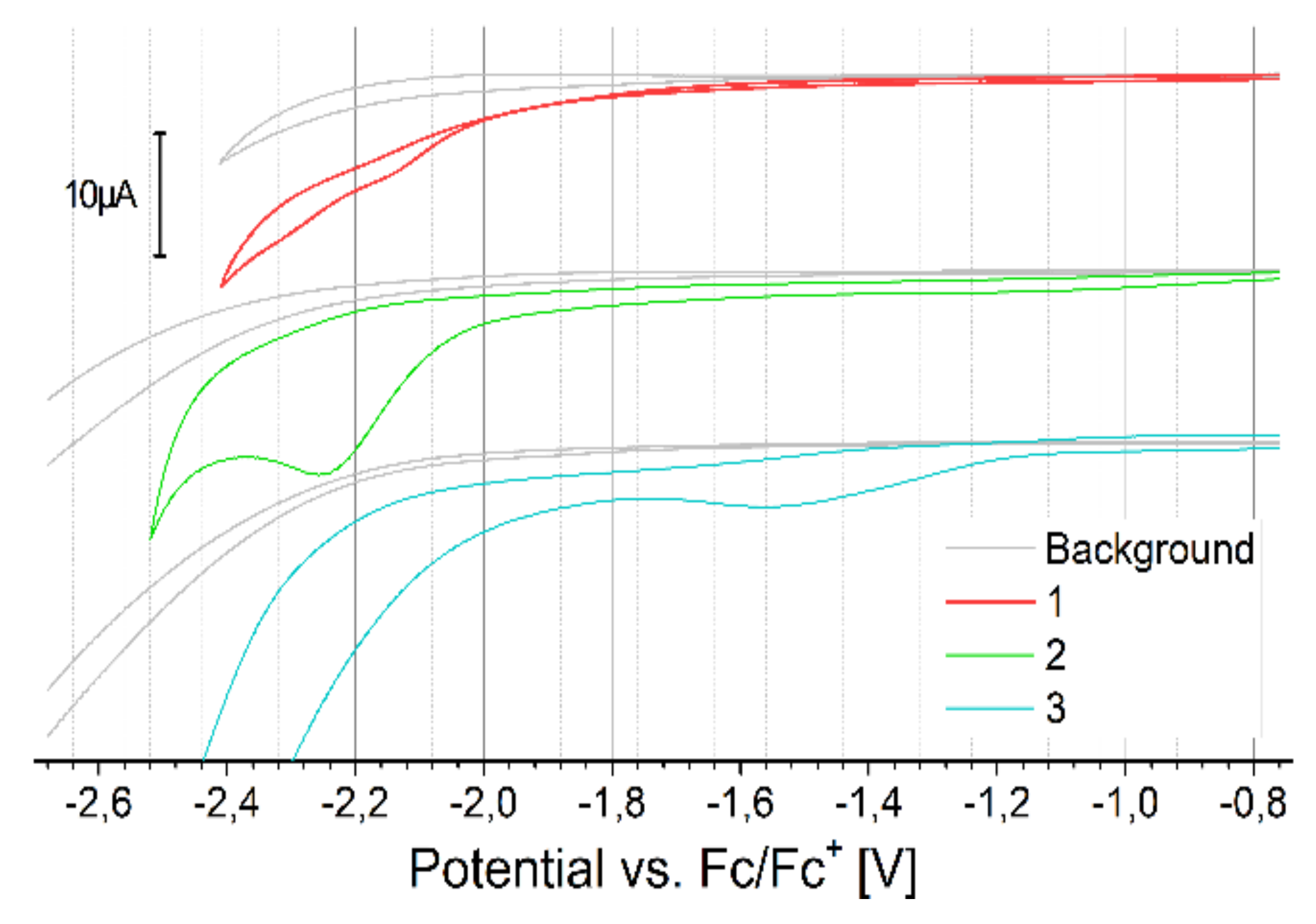
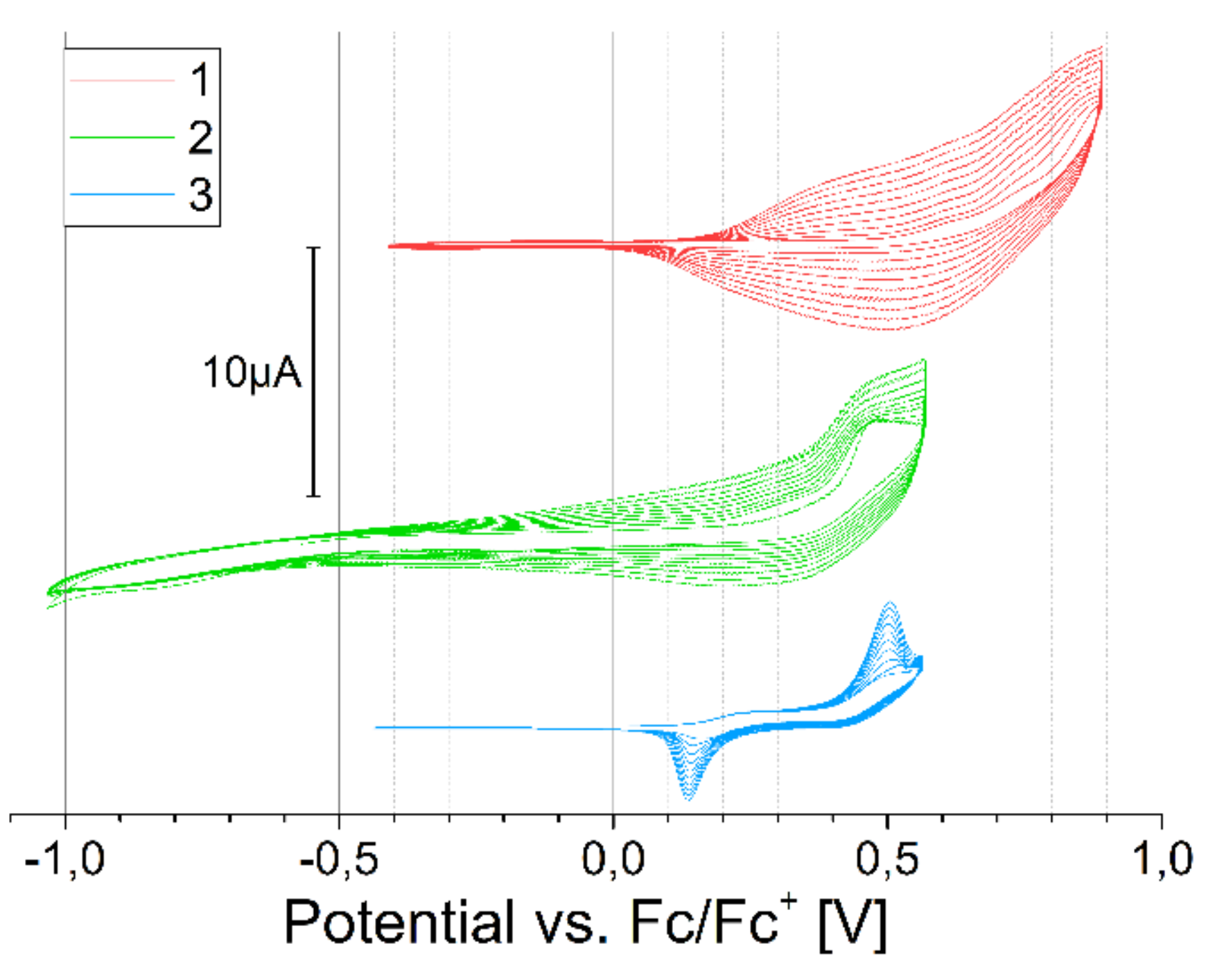
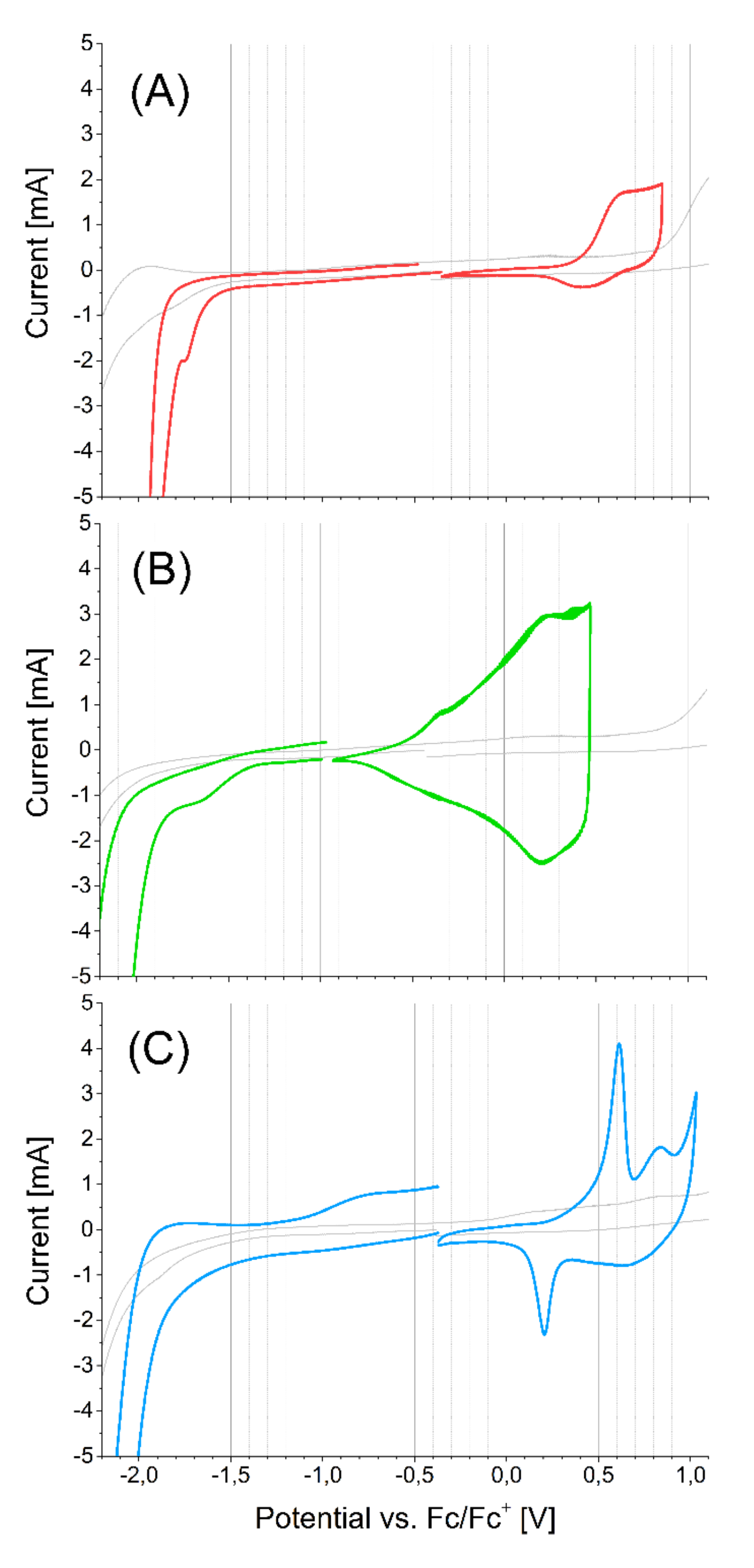
2.3. Electrochemical Properties
3. Materials and Methods
3.1. Chemistry
3.1.1. Preparation of 2,6-Di(selenophen-2-yl)-4-Methyl-4-Octyl-Dithienosilole (1)
- 1H NMR (600 MHz, CDCl3) δ (ppm): δ 7.84 (d, J = 5.6 Hz, 2H), 7.59 (d, J = 7.6 Hz, 2H), 7.28 (d, J = 3.6 Hz, 2H), 7.08 (s, 2H), 1.24–1.23 (m, 12H), 0.93–0.89 (m, 2H), 0.85 (t, J = 6.8 Hz, 3H), 0.41 (s, 3H).
- 13C NMR (600 MHz, CDCl3) δ (ppm): δ 143.5, 140.8, 140.6, 138.7, 135.1, 132.5, 131.0, 130.8, 130.4, 129.1, 129.0, 128.6, 127.3, 127.1, 126.7, 125.7, 125.4, 33.2, 31.9, 29.8, 29.3, 24.2, 22.7, 14.3, 13.3.
3.1.2. Preparation of 2,6-Bis(3,4-Ethylenedioxythiophen-2-yl)-4-Methyl-4-Octyl-Dithienosilole (2)
- 1H NMR (400 MHz, CDCl3)δ (ppm): δ 7.16 (s, 2H), 6.27 (s, 2H), 4.25–4.33 (m, 4H), 4.25–4.23 (m, 4H), 1.24–1.17 (m, 12H), 0.96–0.93 (m, 2H), 0.84 (t, J = 10.2 Hz, 3H), 0.63 (s, 3H).
- 13C NMR (600 MHz, CDCl3)δ (ppm): δ 141.90, 138.76, 136.72, 126.41, 125.65, 112.38, 110.65, 98.40, 65.19, 64.62, 33.56, 32.03, 29.78, 29.39, 26.97, 26.65, 26.38, 22.78, 13.58.
3.1.3. Preparation of 2,6-Di([2,2’-Bithiophen]-5-yl)-4-Methyl-4-Octyl-Dithienosilole (3)
- 1H NMR (600 MHz, CDCl3)δ (ppm): δ 7.64 (d, J = 8.4 Hz, 2H), 7.42 (t, J = 7.8 Hz, 2H), 7.27–7.21 (m, 4H), 7.18 (d, J = 3.6 Hz, 1H), 7.11 (d, J = 3.0 Hz, 1H), 7.07–7.04 (m, 2H), 1.39–1.27 (m, 12H), 1.08 (t, J = 7.8 Hz 2H), 0.92 (t, J = 7.2 Hz, 3H), 0.47 (s, 3H).
- 13C NMR (600 MHz, CDCl3) δ (ppm): δ 143.76, 142.14, 138.16, 137.30, 136.60, 136.44, 135.88, 134.16, 129.04, 128.01, 127.68, 126.32, 125.71, 124.70, 124.50, 124.47, 123.96, 123.81, 123.72, 123.69, 33.23, 32.00, 29.30, 29.20, 27.78, 27.50, 24.23, 22.80, 14.24, 13.80, 13.30.
3.2. Cyclic Voltammetry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ni, W.; Li, M.; Liu, F.; Wan, X.; Feng, H.; Kan, B.; Zhang, Q.; Zhang, H.; Chen, Y. Dithienosilole-Based Small-Molecule Organic Solar Cells with an Efficiency over 8%: Investigation of the Relationship between the Molecular Structure and Photovoltaic Performance. Chem. Mater. 2015, 27, 6077–6084. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Li, M.; Guo, Y.; Song, J.; Wang, H. Random dithienosilole-based terpolymers: Synthesis and application in polymer solar cells. Dyes Pigment. 2016, 130, 63–69. [Google Scholar] [CrossRef]
- Qi, F.; Zhang, Y.; Wan, M.; Liu, J.; Huo, L. Enhanced photovoltaic performance of polymer solar cells through design of a fused dithienosilolodithiophene structure with an enlarged p-conjugated system. J. Mater. Chem. C 2018, 6, 4208–4216. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.; Zhao, C.; Zhou, Y.; Guo, Y.; Song, J.; Wang, H. Side chain engineering of dithienosilole-based polymers for application in polymer solar cells. Dyes Pigment. 2016, 134, 480–486. [Google Scholar] [CrossRef]
- Chena, X.; Sunb, Y.; Wanga, Z.; Gaob, H.; Lina, Z.; Keb, X.; Hea, T.; Yina, S.; Chenb, Y.; Zhanga, Q.; et al. Dithienosilole-based small molecule donors for efficient all-small-moleculeorganic solar cells. Dyes Pigment. 2018, 158, 445–450. [Google Scholar] [CrossRef]
- Heo, H.; Kim, H.; Nam, G.; Lee, D.; Lee, Y. Multi-Donor Random Terpolymers Based on Benzodithiophene and Dithienosilole Segments with Different Monomer Compositions for High-Performance Polymer Solar Cells. Macromol. Res. 2018, 26, 238–245. [Google Scholar] [CrossRef]
- Huang, J.; Ie, Y.; Karakawa, M.; Saito, M.; Osaka, I.; Aso, Y. Enhanced Photovoltaic Performance of Amorphous Copolymers Based on Dithienosilole and Dioxocycloalkene-annelated Thiophene. Chem. Mater. 2014, 26, 6971–6978. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Liu, S.; Yang, C.; Yi, J.; Yang, C. Thieno [3,2-b]thiophene-Bridged Conjugated Polymers Based on Dithieno [3,2-b:2′,3′-d] silole and Thieno m[3¨C-c]pyrrole-4,6-dione for Polymer Solar Cells: Influence of Side Chains on Optoelectronic Properties. Macromol. Chem. Phys. 2018, 219, 1800297. [Google Scholar] [CrossRef]
- Chen, X.; Feng, H.; Lin, Z.; Jiang, Z.; He, T.; Yin, S.; Wan, X.; Chen, Y.; Zhang, Q.; Qiu, H. Impact of end-capped groups on the properties of dithienosilole-based small molecules for solution-processed organic solar cells. Dyes Pigment. 2017, 147, 183–189. [Google Scholar] [CrossRef]
- Tamao, K.; Uchida, M.; Izumizawa, T.; Furukawa, K.; Yamaguchi, S. Silole Derivatives as Efficient Electron Transporting Materials. J. Am. Chem. Soc. 1996, 118, 11974–11975. [Google Scholar] [CrossRef]
- Chan, K.L.; McKiernan, M.J.; Towns, C.R.; Holmes, A.B. Poly (2,7-dibenzosilole): A Blue Light Emitting Polymer. J. Am. Chem. Soc. 2005, 127, 7662–7663. [Google Scholar] [CrossRef]
- Park, H.; Rao, Y.; Varlan, M.; Kim, J.; Ko, S.B.; Wang, S.; Kang, Y. Synthesis and characterization of fluorene and carbazole dithienosilole derivatives for potential applications in organic light-emitting diodes. Tetrahedron 2012, 68, 9278–9283. [Google Scholar] [CrossRef]
- Chen, H.Y.; Hou, J.; Hayden, A.E.; Yang, H.; Houk, K.N.; Yang, Y. Silicon Atom Substitution Enhances Interchain Packing in a Thiophene-Based Polymer System. Adv. Mater. 2010, 22, 371–375. [Google Scholar] [CrossRef]
- Jung, H.; Hwang, H.; Park, K.M.; Kim, J.; Kim, D.H.; Kang, Y. Palladium-Catalyzed Cross-Coupling Reactions of Dithienosilole with Indium Reagents: Synthesis and Characterization of Dithienosilole Derivatives and Their Application to Organic Light-Emitting Diodes. Organometallics 2010, 29, 2715–2723. [Google Scholar] [CrossRef]
- Ohshita, J.; Tominaga, Y.; Tanaka, D.; Ooyama, Y.; Mizumo, T.; Kobayashi, N.; Higashimura, H. Synthesis of dithienosilole-based highly photoluminescent donor–acceptor type compounds. Dalton Trans. 2013, 42, 3646–3652. [Google Scholar] [CrossRef]
- Sanchez, J.C.; Urbas, S.A.; Toal, S.J.; DiPasquale, A.G.; Rheingold, A.L.; Trogler, W.C. Catalytic Hydrosilylation Routes to Divinylbenzene Bridged Silole and Silafluorene Polymers. Applications to Surface Imaging of Explosive Particulates. Macromolecules 2008, 41, 1237–1245. [Google Scholar] [CrossRef]
- Chen, J.; Cao, Y. Silole-containing polymers: Chemistry and optoelectronic properties. Macromol. Rapid Commun. 2007, 28, 1714–1742. [Google Scholar] [CrossRef]
- Yam, V.W.W.; Cheng, E.C.C. Highlights on the recent advances in gold chemistry--a photophysical perspective. Chem. Soc. Rev. 2008, 37, 1806–1813. [Google Scholar] [CrossRef]
- Huang, H.; Youn, J.; Ortiz, R.P.; Zheng, Y.; Facchetti, A.; Marks, T. Very Large Silacylic Substituent Effects on Response in Silole-Based Polymer Transistors. Chem. Mater. 2011, 23, 2185–2200. [Google Scholar] [CrossRef]
- Kang, W.; Jung, M.; Cha, W.; Jang, S.; Yoon, Y.; Kim, H.; Son, H.J.; Lee, D.K.; Kim, B.S.; Cho, J.H. High Crystalline Dithienosilole-Cored Small Molecule Semiconductor for Ambipolar Transistor and Nonvolatile Memory. ACS Appl. Mater. Interfaces 2014, 6, 6589–6597. [Google Scholar] [CrossRef]
- Ohshita, J. Conjugated Oligomers and Polymers Containing Dithienosilole Units. Macromol. Chem. Phys. 2009, 210, 1360–1370. [Google Scholar] [CrossRef]
- Liang, L.; Liming, D. Photovoltaic-Active Dithienosilole-Containing Polymers. Macromolecules 2007, 40, 9406–9412. [Google Scholar]
- Grisorio, R.; Suranna, G.P.; Mastrorilli, P.; Allegretta, G.; Loiudice, A.; Rizzo, A.; Gigli, G.; Manoli, K.; Magliulo, M.; Torsi, L. All-Donor Poly(arylene-ethynylene)s Containing Anthracene and Silole-Based Units: Synthesis, Electronic, and Photovoltaic properties. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4860–4872. [Google Scholar] [CrossRef]
- Zhan, X.; Haldi, A.; Risko, C.; Chan, C.K.; Zhao, W.; Timofeeva, T.V.; Korlyukov, A.; Antipin, M.Y.; Montgomery, S.; Thompson, E.; et al. Fluorenyl-substituted silole molecules: Geometric, electronic, optical, and device properties. J. Mater. Chem. 2008, 18, 3157–3166. [Google Scholar] [CrossRef]
- Zając, D.; Sołoducho, J.; Jarosz, T.; Roszak, S.; Łapkowski, M. Push-pull structures of symmetric silane derivatives as a novel hosting materials. Indian J. Appl. Res. 2017, 7, 58–66. [Google Scholar]
- Vollmer, F.; Rettig, W.; Birckner, E. Photochemical Mechanisms Producing Large Fluorescence Stokes Shifts. J. Fluoresc. 1994, 4, 65–69. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- Eken, S.; Ergun, E.G.C.; Onal, A.M. Synthesis and electrochemical polymerization of dithienosilole-based monomers bearing different donor units. J. Electroch. Soc. 2016, 163, G69–G74. [Google Scholar] [CrossRef]
- Waltman, R.J.; Bargon, J. Reactivity/Structure Correlations for the Electropolymerization of Pyrrole: An INDO/CNDO Study of the Reactive Sites of Oligomeric Radical Cations. Tetrahedron 1984, 40, 3963–3970. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |




| Compound | Eox onset (V) | Eredonset (V) | IP (eV) | EA (eV) | ΔEgel (eV) |
|---|---|---|---|---|---|
| 1 | 0.48 | −1.81 | 5.58 | 3.29 | 2.29 |
| 2 | 0.39 | −1.87 | 5.49 | 3.23 | 2.26 |
| 3 | 0.42 | −1.81 | 5.52 | 3.29 | 2.23 |
| 3a | 0.15 | −1.18 | 5.25 | 3.92 | 1.33 |
| Compound | Eox onset (V) | Eredonset (V) | IP (eV) | EA (eV) | ΔEgel (eV) |
|---|---|---|---|---|---|
| P1 | 0.27 | −1.48 | 5.37 | 3.62 | 1.75 |
| P2 | −0.63 | −1.34 | 4.47 | 3.76 | 0.71 |
| P3 | 0.21 | −1.31 | 5.31 | 3.79 | 1.52 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, D.; Honisz, D.; Łapkowski, M.; Sołoducho, J. Synthesis and Properties of New Dithienosilole Derivatives as Luminescent Materials. Molecules 2019, 24, 2259. https://doi.org/10.3390/molecules24122259
Zając D, Honisz D, Łapkowski M, Sołoducho J. Synthesis and Properties of New Dithienosilole Derivatives as Luminescent Materials. Molecules. 2019; 24(12):2259. https://doi.org/10.3390/molecules24122259
Chicago/Turabian StyleZając, Dorota, Damian Honisz, Mieczysław Łapkowski, and Jadwiga Sołoducho. 2019. "Synthesis and Properties of New Dithienosilole Derivatives as Luminescent Materials" Molecules 24, no. 12: 2259. https://doi.org/10.3390/molecules24122259
APA StyleZając, D., Honisz, D., Łapkowski, M., & Sołoducho, J. (2019). Synthesis and Properties of New Dithienosilole Derivatives as Luminescent Materials. Molecules, 24(12), 2259. https://doi.org/10.3390/molecules24122259







