Synthesis, Self-Assembly and Characterization of Tandem Triblock BPOSS-PDI-X Shape Amphiphiles
Abstract
1. Introduction
2. Results and Discussion
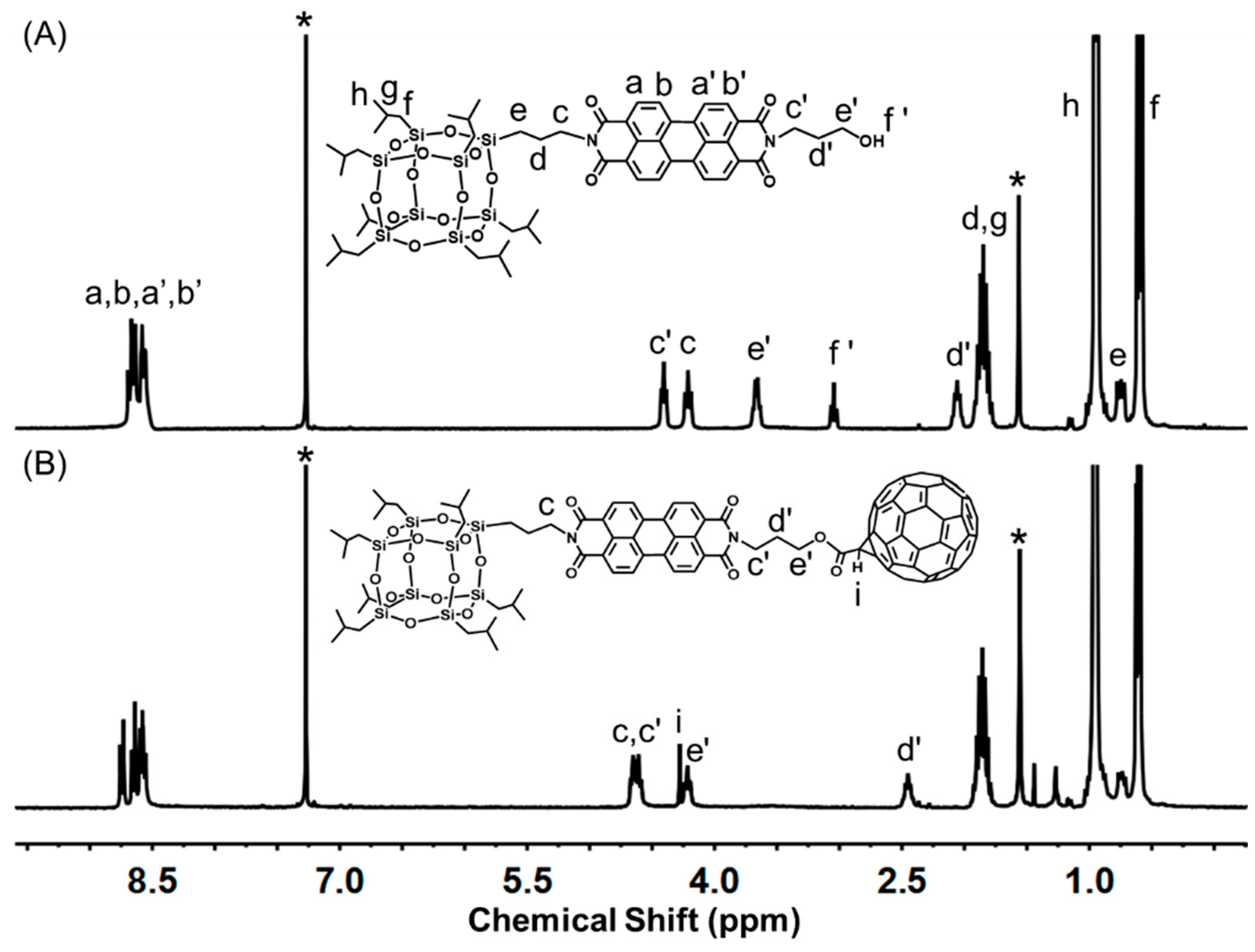
2.1. Molecular Design and Synthesis
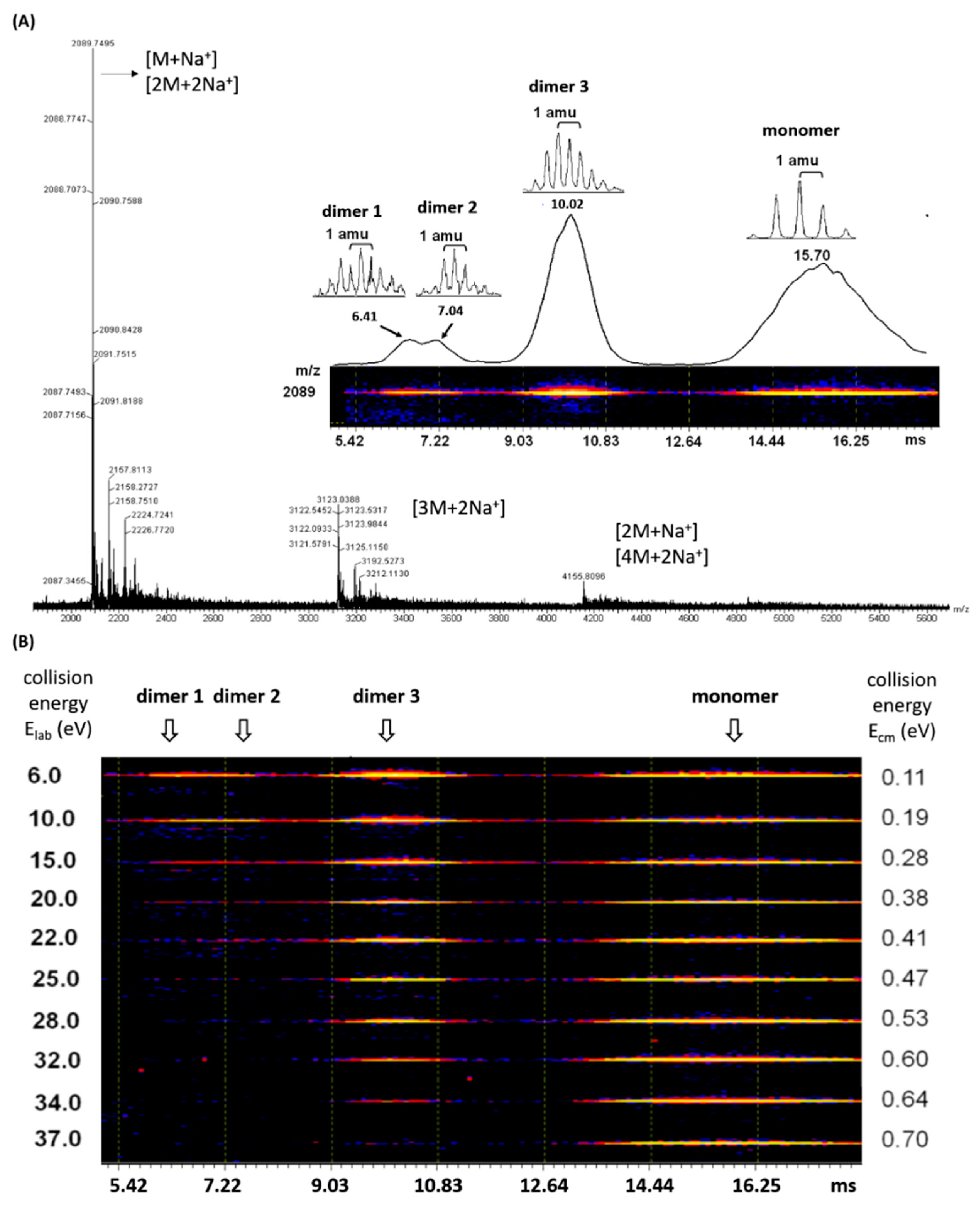
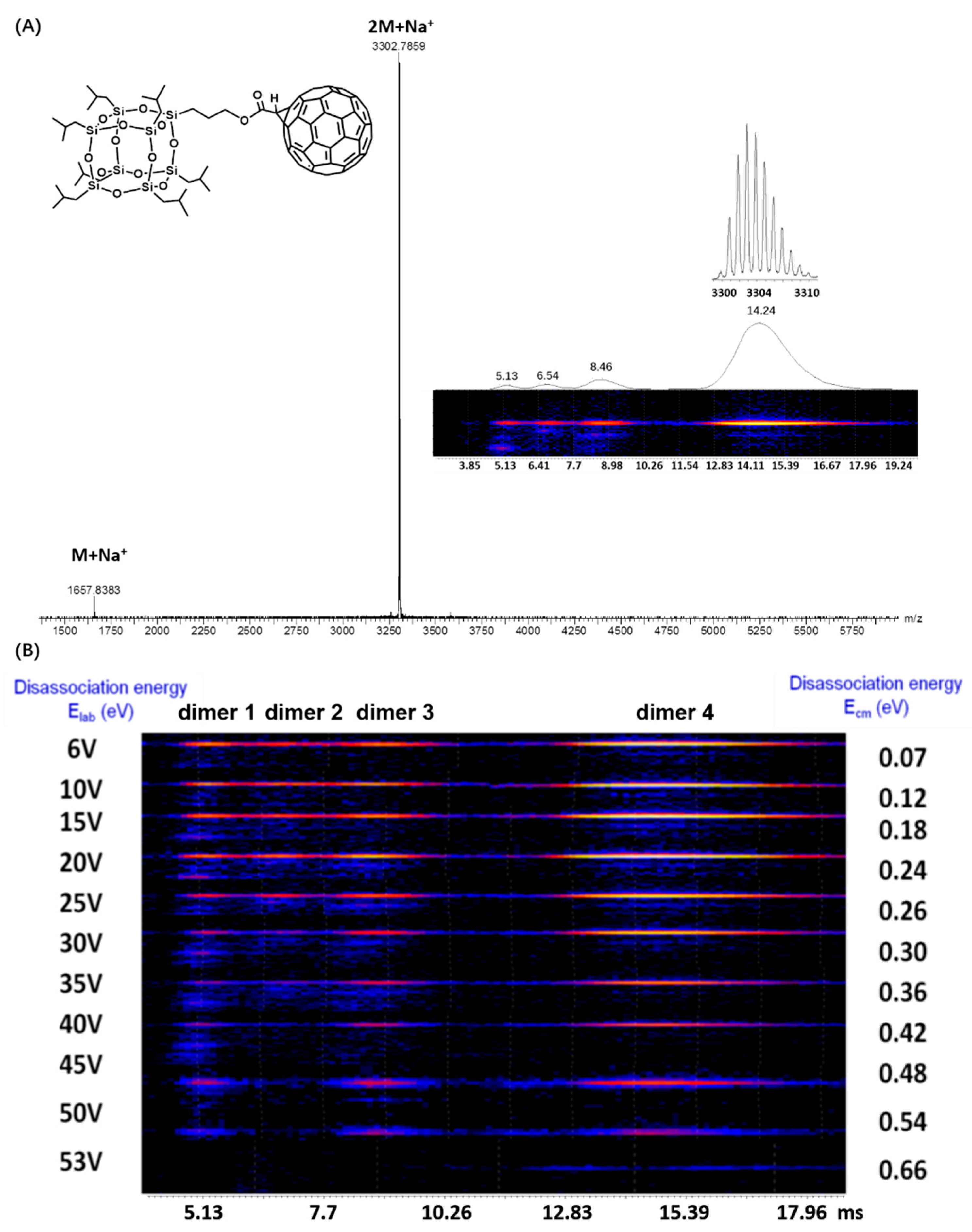
2.2. Self-Assembly in Gas Phase as Revealed by ESI-TWIM-MS Spectrometry
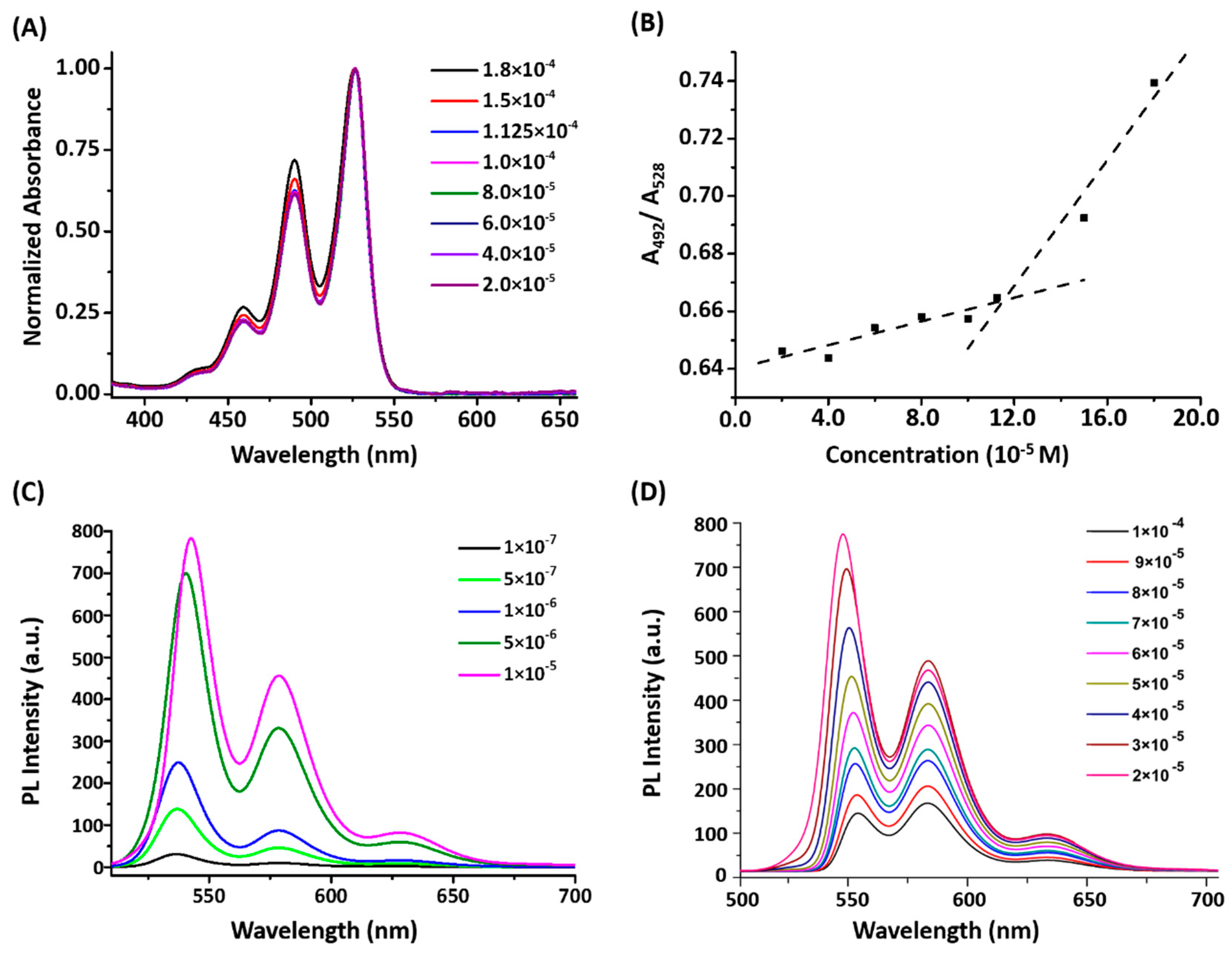
2.3. Solution Assembly as Revealed by UV/Vis and Fluorescence Spectrometry
2.4. Self-Assembly of BPOSS-PDI-OH into Single Crystalline Nanobelts
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis
3.2.1. Synthesis of BPOSS-PDI-OH
3.2.2. Synthesis of BPOSS-PDI-C60
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Materials Genome Initiative for Global Competitiveness; National Science and Technology Council: Washington, DC, USA, 2011.
- Damasceno, P.F.; Engel, M.; Glotzer, S.C. Predictive Self-Assembly of Polyhedra into Complex Structures. Science 2012, 337, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Glotzer, S.C.; Solomon, M.J. Anisotropy of building blocks and their assembly into complex structures. Nat. Mater. 2007, 6, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-B.; Cheng, S.Z.D. Toward rational and modular molecular design in soft matter engineering. Chin. J. Sci. 2015, 33, 797–814. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Zhang, W.-B.; Cheng, S.Z.D. Giant molecules: Where chemistry, physics, and bio-science meet. Sci. China Chem. 2017, 60, 338–352. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Wang, X.-M.; Wang, X.-W.; Liu, D.; Han, S.-Y.; Cheng, S.Z.D. Giant Molecules Based on Nano-Atoms. Prog. Chem. 2015, 27, 1333–1342. [Google Scholar]
- Zhang, W.-B.; Yu, X.; Wang, C.-L.; Sun, H.-J.; Hsieh, I.-F.; Li, Y.; Dong, X.-H.; Yue, K.; Van Horn, R.; Cheng, S.Z.D. Molecular Nanoparticles Are Unique Elements for Macromolecular Science: From “Nanoatoms” to Giant Molecules. Macromolecules 2014, 47, 1221–1239. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Wu, X.-L.; Yin, G.-Z.; Shao, Y.; Cheng, S.Z.D. From protein domains to molecular nanoparticles: What can giant molecules learn from proteins? Mater. Horiz. 2017, 4, 117–132. [Google Scholar] [CrossRef]
- Teng, F.-A.; Liu, F.-L.; Han, L.; Zhu, Z.-J.; Zhang, Y.-F.; Wu, Z.-J.; Han, Z.-W.; Zhang, W.-B.; Li, H. Design, synthesis, and optical/electronic properties of a series of sphere-rod shape amphiphiles based on the C60-oligofluorene conjugates. Chin. J. Sci. 2017, 35, 503–514. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Ni, H.; Pittman, C.U., Jr. Polyhedral Oligomeric Silsesquioxane (POSS) Polymers and Copolymers: A Review. J. Inorg. Organomet. Polym. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C. Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: Berlin, Germany, 2011. [Google Scholar]
- Lin, Z.; Yang, X.; Xu, H.; Sakurai, T.; Matsuda, W.; Seki, S.; Zhou, Y.; Sun, J.; Wu, K.-Y.; Yan, X.-Y.; et al. Topologically Directed Assemblies of Semiconducting Sphere–Rod Conjugates. J. Am. Chem. Soc. 2017, 139, 18616–18622. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Liang, W.-W.; Huang, C.-F.; Wu, K.-Y.; Wu, S.-L.; Chang, S.-T.; Cheng, Y.-J.; Wang, C.-L. Flat-on ambipolar triphenylamine/C60nano-stacks formed from the self-organization of a pyramid-sphere-shaped amphiphile. Chem. Sci. 2016, 7, 2768–2774. [Google Scholar] [CrossRef] [PubMed]
- Balch, A.L.; Winkler, K. Two-Component Polymeric Materials of Fullerenes and the Transition Metal Complexes: A Bridge between Metal–Organic Frameworks and Conducting Polymers. Chem. Rev. 2016, 116, 3812–3882. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, F.; Martín, N. Fullerene Polymers: Synthesis and Properties. Chem. Rev. 2006, 106, 5136–5190. [Google Scholar] [CrossRef]
- Georgakilas, V.; Pellarini, F.; Prato, M.; Guldi, D.M.; Melle-Franco, M.; Zerbetto, F. Supramolecular self-assembled fullerene nanostructures. Proc. Natl. Acad. Sci. USA 2002, 99, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ding, M.; Xu, Y.; Zhao, F.; Dai, H.; Miao, X.-R.; Yang, S.; Li, H.; Elemans, J. Synthesis and Self-Assembly of Shape Amphiphiles Based on POSS-Dendron Conjugates. Molecules 2017, 22, 622. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Graham, M.J.; Sun, B.; Cheng, S.Z.D.; Tu, Y.; Wang, C.-L.; Van Horn, R.M.; Tsai, C.-C.; Lotz, B.; Zhang, W.-B. Hierarchical structure and polymorphism of a sphere-cubic shape amphiphile based on a polyhedral oligomeric silsesquioxane–[60]fullerene conjugate. J. Mater. Chem. 2011, 21, 14240. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Tu, Y.; Sun, H.-J.; Yue, K.; Gong, X.; Cheng, S.Z.D. Polymer solar cells with an inverted device configuration using polyhedral oligomeric silsesquioxane-[60]fullerene dyad as a novel electron acceptor. Sci. China Chem. 2012, 55, 749–754. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, K.; Hou, P.; Lyu, X.; Pan, H.-B.; Shen, Z.; Fan, X.H. Hierarchically ordered structures of disk-cube triads containing hexa-peri-hexabenzocoronene and polyhedral oligomeric silsesquioxane. Soft Matter 2018, 14, 6774–6782. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, X.; Mao, J.; Hsu, C.H.; Mu, G.; Huang, M.; Guo, Q.; Liu, H.; Wesdemiotis, C.; Li, T.; Zhang, W.-B.; Li, Y.; Cheng, S.Z.D. Sequence-Mandated, Distinct Assembly of Giant Molecules. Angew. Chem. Int. Ed. 2017, 56, 15014–15019. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, R.; Li, Y.; Hong, Y.L.; Guo, D.; Lang, K.; Wu, K.Y.; Huang, M.; Mao, J.; Wesdemiotis, C.; Nishiyama, Y.; Zhang, W.; Zhang, W.; Miyoshi, T.; Li, T.; Cheng, S.Z.D. Hierarchical Self-Organization of AB n Dendron-like Molecules into a Supramolecular Lattice Sequence. ACS Cent. Sci. 2017, 3, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Huang, M.; Marson, R.L.; He, J.; Huang, J.; Zhou, Z.; Wang, J.; Liu, C.; Yan, X.; Wu, K.; et al. Geometry induced sequence of nanoscale Frank–Kasper and quasicrystal mesophases in giant surfactants. Proc. Natl. Acad. Sci. USA 2016, 113, 14195–14200. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Hsu, C.-H.; Wang, J.; Mei, S.; Dong, X.; Li, Y.; Li, M.; Liu, H.; Zhang, W.; Aida, T.; et al. Selective assemblies of giant tetrahedra via precisely controlled positional interactions. Science 2015, 348, 424–428. [Google Scholar] [CrossRef]
- Bates, F.S.; Hillmyer, M.A.; Lodge, T.P.; Bates, C.M.; Delaney, K.T.; Fredrickson, G.H. Multiblock Polymers: Panacea or Pandora’s Box? Science 2012, 336, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Yin, H.; Jin, P.-F.; Jiang, Y.-S.; Yang, S.; Zhang, W.-B. Regioisomeric Tandem Triblock Shape Amphiphiles Based on Polyhedral Oligomeric Silsesquioxanes. Chem. Eur. J. 2018, 24, 12389–12396. [Google Scholar] [CrossRef] [PubMed]
- Struijk, C.W.; Sieval, A.B.; Dakhorst, J.E.J.; Van Dijk, M.; Kimkes, P.; Koehorst, R.B.M.; Donker, H.; Schaafsma, T.J.; Picken, S.J.; Van De Craats, A.M.; et al. Liquid Crystalline Perylene Diimides: Architecture and Charge Carrier Mobilities. J. Am. Chem. Soc. 2000, 122, 11057–11066. [Google Scholar] [CrossRef]
- Chesterfield, R.J.; McKeen, J.C.; Newman, C.R.; Ewbank, P.C.; Filho, D.A.D.S.; Brédas, J.-L.; Miller, L.L.; Mann, K.R.; Frisbie, C.D. Organic Thin Film Transistors Based onN-Alkyl Perylene Diimides: Charge Transport Kinetics as a Function of Gate Voltage and Temperature. J. Phys. Chem. B 2004, 108, 19281–19292. [Google Scholar] [CrossRef]
- Zeng, Y.; Lu, L.; Gao, T.; Feng, Y.; Zheng, J.-F.; Chen, E.-Q.; Ren, X.-K. Synthesis, helical columnar liquid crystalline structure, and charge transporting property of perylene diimide derivative bearing oligosiloxane chains. Dyes Pigments 2018, 152, 139–145. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, L.; Lv, F.; Wang, S. Design and Synthesis of Reactive Perylene Tetracarboxylic Diimide Derivatives for Rapid Cell Imaging. ACS Omega 2018, 3, 8691–8696. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, D. New Perylenediimide Polymer Acceptor Design and Their Applications to All-polymer Solar Cells. Acta Polym. Sin. 2018, 2, 174–185. [Google Scholar]
- Dai, S.; Zhang, S.; Ling, Q.; Zhan, X. Rylene Diimide and Dithienocyanovinylene Copolymers for Polymer Solar Cells. Chin. J. Polym. Sci. 2017, 35, 230–238. [Google Scholar] [CrossRef]
- Welsh, T.A.; LaVenture, A.; Welch, G.C. Direct (Hetero)Arylation for the Synthesis of Molecular Materials: Coupling Thieno[3,4-c]pyrrole-4,6-dione with Perylene Diimide to Yield Novel Non-Fullerene Acceptors for Organic Solar Cells. Molecules 2018, 23, 931. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.R.; Frisbie, C.D.; Filho, D.A.D.S.; Brédas, J.-L.; Ewbank, P.C.; Mann, K.R. Introduction to Organic Thin Film Transistors and Design of n-Channel Organic Semiconductors. Chem. Mater. 2004, 16, 4436–4451. [Google Scholar] [CrossRef]
- Zeng, H.; Huang, Q.; Liu, J.; Huang, Y.; Zhou, J.; Zhao, S.; Lu, Z. A Red-Emissive Sextuple Hydrogen-Bonding Self-Assembly Molecular Duplex Bearing Perylene Diimide Fluorophores for Warm-White Organic Light-Emitting Diode Application. Chin. J. Chem. 2016, 34, 387–396. [Google Scholar] [CrossRef]
- Wurthner, F.; Saha-Moller, C.R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. [Google Scholar] [CrossRef] [PubMed]
- Bialas, D.; Brüning, C.; Schlosser, F.; Fimmel, B.; Thein, J.; Engel, V.; Würthner, F. Exciton-Vibrational Couplings in Homo- and Heterodimer Stacks of Perylene Bisimide Dyes within Cyclophanes: Studies on Absorption Properties and Theoretical Analysis. Chem. Eur. J. 2016, 22, 15011–15018. [Google Scholar] [CrossRef]
- Bhavsar, G.A.; Asha, S.K. Pentadecyl Phenol- and Cardanol-Functionalized Fluorescent, Room-Temperature Liquid-Crystalline Perylene Bisimides: Effect of Pendant Chain Unsaturation on Self-Assembly. Chem. Eur. J. 2011, 17, 12646–12658. [Google Scholar] [CrossRef]
- Zang, L.; Che, Y.; Moore, J.S. One-dimensional self-assembly of planar pi-conjugated molecules: Adaptable building blocks for organic nanodevices. Acc. Chem. Res. 2008, 41, 1596–1608. [Google Scholar] [CrossRef]
- Fron, E.; Schweitzer, G.; Osswald, P.; Würthner, F.; Marsal, P.; Beljonne, D.; Müllen, K.; De Schryver, F.C.; Van Der Auweraer, M. Photophysical study of bay substituted perylenediimides. Photochem. Photobiol. Sci. 2008, 7, 1509. [Google Scholar] [CrossRef]
- Song, W.; You, Y.; Li, T.-J.; Li, J.; Ding, L. Perylene Bisimide In-chain Polyethylene with Unique Self-assembly Nanostructure through Acyclic Diene Metathesis (ADMET) Polymerization. Chin. J. Sci. 2018, 36, 703–711. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.-R.; Zeng, Y.; Ben, H.-J.; Yao, F.-L.; Yang, S.; Chen, E.-Q.; Ren, X.-K. Ionic self-assembled derivatives of perylene diimide: Synthesis, aggregated structure and molecular packing behavior. Dyes Pigments 2017, 139, 79–86. [Google Scholar] [CrossRef]
- Baffreau, J.; Anh, N.V.; Williams, R.M.; Hudhomme, P.; Leroy-Lhez, S.; Leroy-Lhez, S. Fullerene C60–Perylene-3,4:9,10-bis(dicarboximide) Light-Harvesting Dyads: Spacer-Length and Bay-Substituent Effects on Intramolecular Singlet and Triplet Energy Transfer. Chem. Eur. J. 2008, 14, 4974–4992. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin, Germany, 2006. [Google Scholar]
- Ren, X.; Sun, B.; Tsai, C.-C.; Tu, Y.; Leng, S.; Li, K.; Kang, Z.; Van Horn, R.M.; Li, X.; Zhu, M.; et al. Synthesis, Self-assembly, and Crystal Structure of a Shape-Persistent Polyhedral-Oligosilsesquioxane-Nanoparticle-Tethered Perylene Diimide. J. Phys. Chem. B 2010, 114, 4802–4810. [Google Scholar] [CrossRef]
- Ben, H.-J.; Ren, X.-K.; Song, B.; Li, X.; Feng, Y.; Jiang, W.; Chen, E.-Q.; Wang, Z.; Jiang, S. Synthesis, crystal structure, enhanced photoluminescence properties and fluoride detection ability of S-heterocyclic annulated perylene diimide-polyhedral oligosilsesquioxane dye. J. Mater. Chem. C 2017, 5, 2566–2576. [Google Scholar] [CrossRef]
- Lucenti, E.; Botta, C.; Cariati, E.; Righetto, S.; Scarpellini, M.; Tordin, E.; Ugo, R. New organic–inorganic hybrid materials based on perylene diimide–polyhedral oligomeric silsesquioxane dyes with reduced quenching of the emission in the solid state. Dyes Pigments 2013, 96, 748–755. [Google Scholar] [CrossRef]
- Du, F.; Jin, B.; Tian, J.; Wang, H.; Liu, B.; Bai, R. Synthesis and Luminescence of POSS-Containing Perylene Bisimide-Bridged Amphiphilic Polymers. Macromolecules 2012, 45, 3086–3093. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.-Z.; Ren, X.; Zhang, X.; Wang, J.; Guo, K.; Li, X.; Wesdemiotis, C.; Zhang, W.-B.; Yang, S.; et al. Engineering π–π interactions for enhanced photoluminescent properties: Unique discrete dimeric packing of perylene diimides. RSC Adv. 2017, 7, 6530–6537. [Google Scholar] [CrossRef]
- López, A.M.; Mateo-Alonso, A.; Prato, M. Materials chemistry of fullerene C 60 derivatives. J. Mater. Chem. 2011, 21, 1305–1318. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, X.; Wei, Y.; Yuan, Q. Applications of DNA Nanotechnology in Synthesis and Assembly of Inorganic Nanomaterials. Chin. J. Chem. 2016, 34, 291–298. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef]
- Zheng, Y.-Q.; Wang, J.-Y.; Pei, J. One-dimensional (1D) micro/nanostructures of organic semiconductors for field-effect transistors. Sci. China Chem. 2015, 58, 937–946. [Google Scholar] [CrossRef]
- Dou, J.-H.; Zheng, Y.-Q.; Yao, Z.-F.; Lei, T.; Shen, X.; Luo, X.-Y.; Yu, Z.-A.; Zhang, S.-D.; Han, G.; Wang, Z.; et al. A Cofacially Stacked Electron-Deficient Small Molecule with a High Electron Mobility of over 10 cm2V−1s−1in Air. Adv. Mater. 2015, 27, 8051–8055. [Google Scholar] [CrossRef] [PubMed]
- Robb, M.J.; Newton, B.; Fors, B.P.; Hawker, C.J. One-Step Synthesis of Unsymmetrical N -Alkyl- N ′-aryl Perylene Diimides. J. Org. Chem. 2014, 79, 6360–6365. [Google Scholar] [CrossRef] [PubMed]
- Thalassinos, K.; Scrivens, J. Applications of Traveling Wave Ion Mobility-Mass Spectrometry. In Practical Aspects of Trapped Ion Mass Spectrometry; CRC Press: Boca Raton, FL, USA, 2009; pp. 205–236. [Google Scholar]
- Hohenstein, E.G.; Sherrill, C.D. Effects of heteroatoms on aromatic pi-pi interactions: Benzene-pyridine and pyridine dimer. J. Phys. Chem. A 2009, 113, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Sinnokrot, M.O.; Sherrill, C.D. Substituent effects in pi-pi interactions: Sandwich and T-shaped configurations. J. Am. Chem. Soc. 2004, 126, 7690–7697. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-T.; Li, X.; Soler, M.; Wang, J.-L.; Wesdemiotis, C.; Newkome, G.R. Self-Assembly and Traveling Wave Ion Mobility Mass Spectrometry Analysis of Hexacadmium Macrocycles. J. Am. Chem. Soc. 2009, 131, 16395–16397. [Google Scholar] [CrossRef] [PubMed]
- Wesdemiotis, C. Multidimensional Mass Spectrometry of Synthetic Polymers and Advanced Materials. Angew. Chem. Int. Ed. 2017, 56, 1452–1464. [Google Scholar] [CrossRef]
- Perez, E.M.; Martin, N. pi-pi interactions in carbon nanostructures. Chem. Soc. Rev. 2015, 44, 6425–6433. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Chowdhury, A.; Holman, M.W.; Adams, D.M. Self-Organized Perylene Diimide Nanofibers. J. Phys. Chem. B 2005, 109, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liang, B.; Wang, D.; Wu, H.; Xue, L.; Li, X. Synthesis and Aggregation Behavior of Perylenetetracarboxylic Diimide Trimers with Different Substituents at Bay Positions. Langmuir 2008, 24, 11209–11215. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, L.S.; Helms, G.; Zhou, H.H.; Li, A.D. To fold or to assemble? J. Am. Chem. Soc. 2003, 125, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Shibano, Y.; Umeyama, T.; Matano, Y.; Tkachenko, N.V.; Lemmetyinen, H.; Imahori, H. Synthesis and Photophysical Properties of Electron-Rich Perylenediimide-Fullerene Dyad. Org. Lett. 2006, 8, 4425–4428. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhang, X.; Liang, K.; Wang, J.; Lin, Y.; Yang, S.; Zhang, W.-B.; Zhu, M.; Sun, B. How does the interplay between bromine substitution at bay area and bulky substituents at imide position influence the photophysical properties of perylene diimides? RSC Adv. 2017, 7, 16155–16162. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. An Overview of Strengths and Directionalities of Noncovalent Interactions: σ-Holes and π-Holes. Crystals 2019, 9, 165. [Google Scholar] [CrossRef]
- Thalacker, C.; Röger, C.; Würthner, F. Synthesis and Optical and Redox Properties of Core-Substituted Naphthalene Diimide Dyes. J. Org. Chem. 2006, 71, 8098–8105. [Google Scholar] [CrossRef] [PubMed]
- Langhals, H. Dyes with high dielectric constants. Chem. Phys. Lett. 1988, 150, 321–324. [Google Scholar] [CrossRef][Green Version]
Sample Availability: Samples of the compounds BPOSS-PDI-OH and BPOSS-PDI-C60 are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Chen, J.; Ren, X.-K.; Zhang, X.; Yin, G.-Z.; Li, X.; Wang, J.; Wesdemiotis, C.; Zhang, W.-B.; Yang, S.; et al. Synthesis, Self-Assembly and Characterization of Tandem Triblock BPOSS-PDI-X Shape Amphiphiles. Molecules 2019, 24, 2114. https://doi.org/10.3390/molecules24112114
Shao Y, Chen J, Ren X-K, Zhang X, Yin G-Z, Li X, Wang J, Wesdemiotis C, Zhang W-B, Yang S, et al. Synthesis, Self-Assembly and Characterization of Tandem Triblock BPOSS-PDI-X Shape Amphiphiles. Molecules. 2019; 24(11):2114. https://doi.org/10.3390/molecules24112114
Chicago/Turabian StyleShao, Yu, Jia Chen, Xiang-Kui Ren, Xinlin Zhang, Guang-Zhong Yin, Xiaopeng Li, Jing Wang, Chrys Wesdemiotis, Wen-Bin Zhang, Shuguang Yang, and et al. 2019. "Synthesis, Self-Assembly and Characterization of Tandem Triblock BPOSS-PDI-X Shape Amphiphiles" Molecules 24, no. 11: 2114. https://doi.org/10.3390/molecules24112114
APA StyleShao, Y., Chen, J., Ren, X.-K., Zhang, X., Yin, G.-Z., Li, X., Wang, J., Wesdemiotis, C., Zhang, W.-B., Yang, S., Sun, B., & Zhu, M. (2019). Synthesis, Self-Assembly and Characterization of Tandem Triblock BPOSS-PDI-X Shape Amphiphiles. Molecules, 24(11), 2114. https://doi.org/10.3390/molecules24112114




