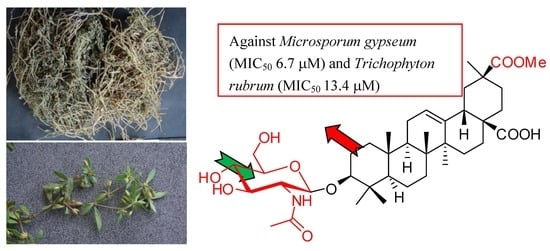
Triterpenoids and Their Glycosides from Glinus Oppositifolius with Antifungal Activities against Microsporum Gypseum and Trichophyton Rubrum
Abstract
1. Introduction
2. Results and Discussion
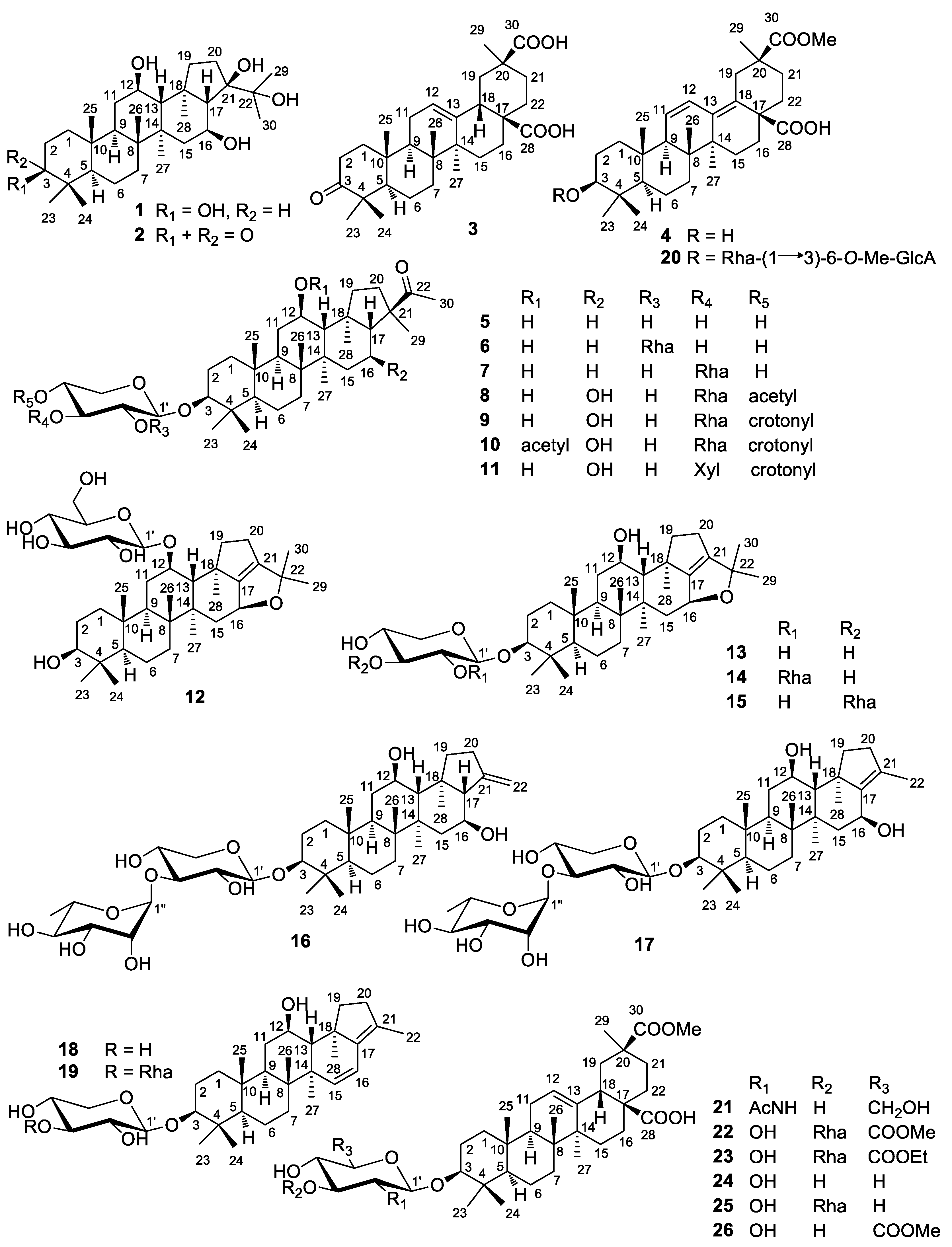
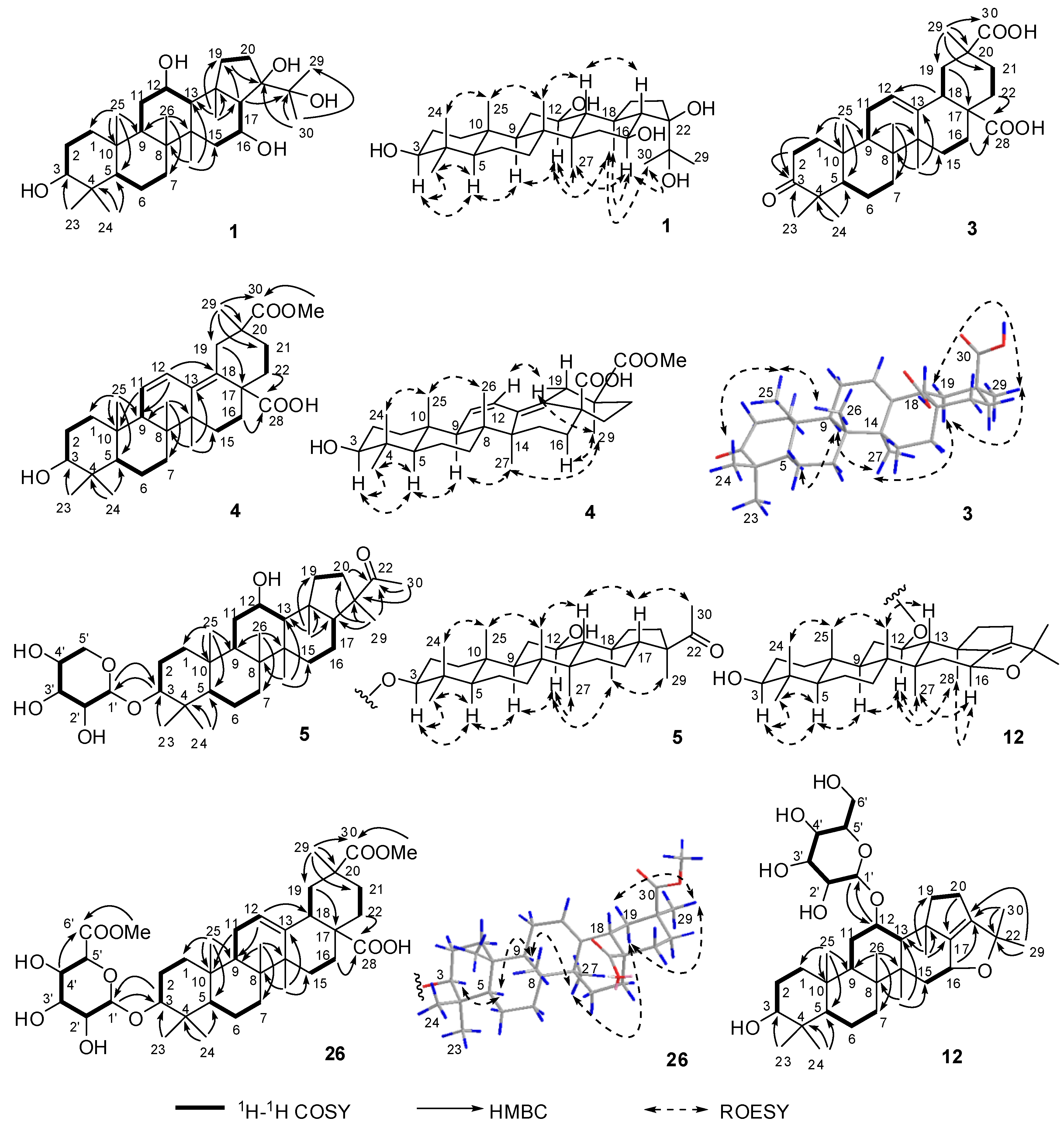
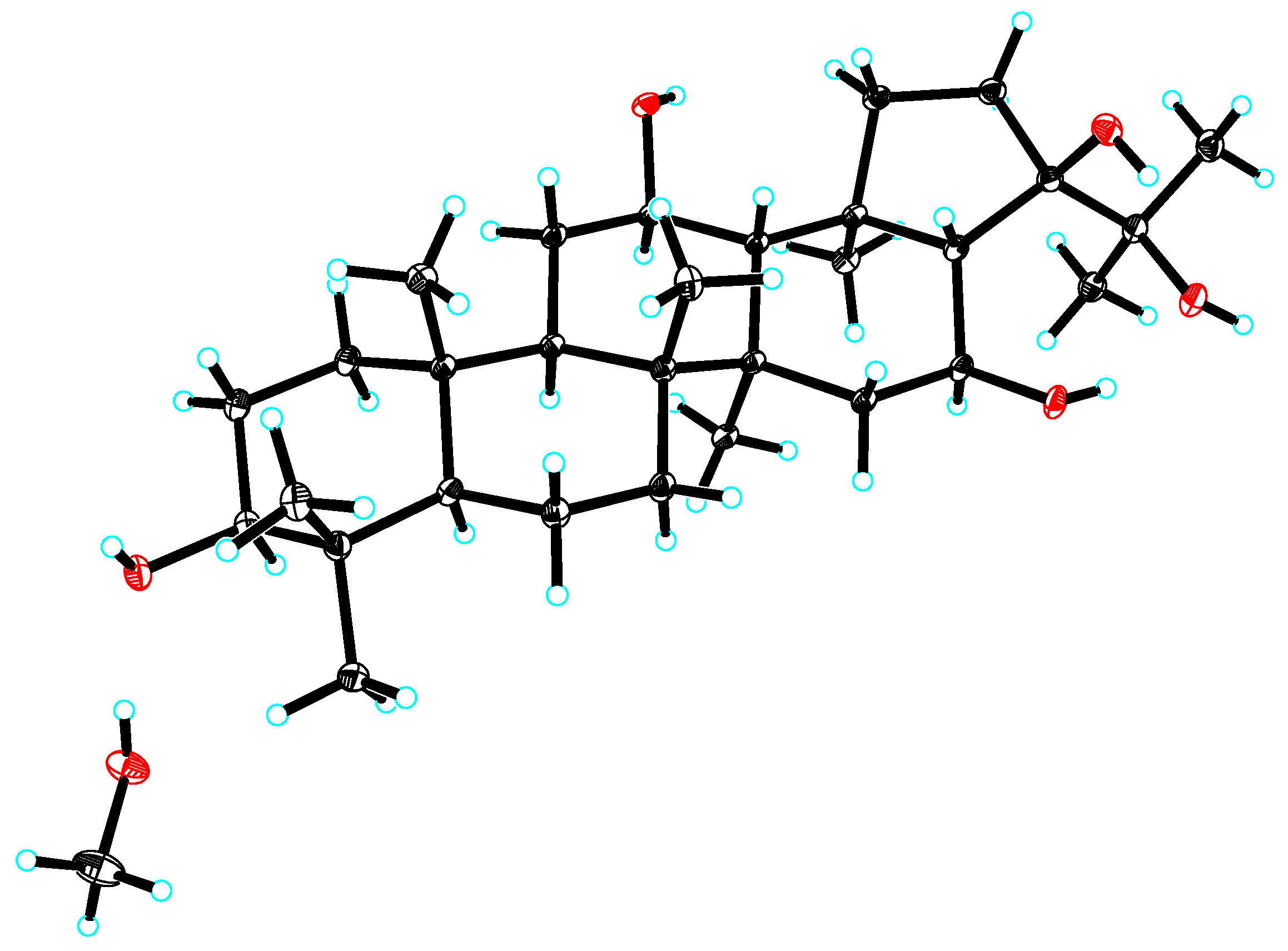
2.1. Structure Elucidation of the Compounds
2.2. Biological Evaluation
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic and Physical Data
3.5. Acid Hydrolysis and Sugar Analysis
3.5.1. Acid Hydrolysis of 31 and Acetylation of Xylose
3.5.2. Acid Hydrolysis of the Saponin Mixture and Acetylation of Rhamnose
3.6. Antimicrobial Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abd Elmegeed, A.S.M.; Ouf, S.A.; Moussa, T.A.A.; Eltahlawi, S.M.R. Dermatophytes and other associated fungi in patients attending to some hospitals in Egypt. Braz. J. Microbiol. 2015, 46, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.V.S.; Lima, M.I.O.; Cardoso, G.N.; Santos, A.S.; Silva, G.S.; Pereira, F.O. Inhibitory effects of linalool on fungal pathogenicity of clinical isolates of Microsporum canis and Microsporum gypseum. Mycoses 2017, 60, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Hartmann, H.E.K. Molluginaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2003; Volume 5, pp. 437–439. [Google Scholar]
- Sheu, S.Y.; Yao, C.H.; Lei, Y.C.; Kuo, T.F. Recent progress in Glinus oppositifolius research. Pharm. Biol. 2014, 52, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Ashin Nargathaynar Biwantha. Ashin Nargathein: Pohn-pya-say-abidan (Illustrated dictionary of traditional Medicinal plants) (Myanmar version); Mingalar Press: Yangon, Myanmar, 1971; Volume 5, pp. 57–58. [Google Scholar]
- Editorial Board of “Zhonghua Bencao”, State Administration of Traditional Chinese Medicine of the People’s Republic of China. Zhonghua Bencao; Shanghai Scientific and Technical Publishers: Shanghai, China, 1999; Volume 2, pp. 751–752. [Google Scholar]
- Hariharan, V.; Rangaswami, S. Steroids and triterpenoids from the roots of Mollugo spergula. Phytochemistry 1971, 10, 621–624. [Google Scholar] [CrossRef]
- Kumar, D.; Shah, V.; Ghosh, R.; Pal, B.C. A new triterpenoid saponin from Glinus oppositifolius with α-glucosidase inhibitory activity. Nat. Prod. Res. 2013, 27, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Cabrera, E.C.; Torres, O.B.; Buluran, A.I.; Espineli, D.L.; Raga, D.D.; Shen, C.C. Chemical constituents and bioactivities of Glinus oppositifolius. Pharmacognosy Res. 2015, 7, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Traore, F.; Faure, R.; Ollivier, E.; Gasquet, M.; Azas, N.; Debrauwer, L.; Keita, A.; Timon-David, P.; Balansard, G. Structure and antiprotozoal activity of triterpenoid saponins from Glinus oppositifolius. Planta Med. 2000, 66, 368–371. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Qian, L.W.; Zhang, J.; Liu, J.H.; Yu, B.Y. New approaches to the structural modification of olean-type pentacylic triterpenes via microbial oxidation and glycosylation. Tetrahedron 2011, 67, 4206–4211. [Google Scholar] [CrossRef]
- Mizui, F.; Kasai, R.; Ohtani, K.; Tanaka, O. Saponins from bran of quinoa, Chenopodium quinoa Willd. II. Chem. Pharm. Bull. 1990, 38, 375–377. [Google Scholar] [CrossRef]
- Sahu, N.P.; Koike, K.; Banerjee, S.; Achari, B.; Nikaido, T. Triterpenoid saponins from Mollugo spergula. Phytochemistry 2001, 58, 1177–1182. [Google Scholar] [CrossRef]
- Tanaka, N.; Yamauchi, K.; Murakami, T.; Saiki, Y.; Chen, C.M. Chemical and chemotactic investigations of filicales. 38. Chemical investigations of the substances contained in Diplazium subsinuatum (Wall) Tagawa. Chem. Pharm. Bull. (Tokyo) 1982, 30, 3632–3639. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, I.K.; Choi, S.U.; Lee, J.H.; Moon, E.; Kim, S.Y.; Lee, K.R. New triterpenoids from the tubers of Corydalis ternata: Structural elucidation and bioactivity evaluation. Planta Med. 2011, 77, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Awanchiri, S.S.; Trinh-Van-Dufat, H.; Shirri, J.C.; Dongfack, M.D.J.; Nguenang, G.M.; Boutefnouchet, S.; Fomum, Z.T.; Seguin, E.; Verite, P.; Tillequin, F.; et al. Triterpenoids with antimicrobial activity from Drypetes inaequalis. Phytochemistry 2009, 70, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Melek, F.R.; Miyase, T.; El-Gindi, O.D.; Abdel-Khalik, S.M.; Haggag, M.Y. Saponins from Fagonia mollis. Phytochemistry 1996, 42, 1405–1407. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Espineli, D.L.; Mandia, E.H.; Don, M.J.; Shen, C.C. A new triterpene from Glinus oppositifolius. Chin. J. Nat. Medicines 2012, 10, 284–286. [Google Scholar] [CrossRef]
- Kuang, H.X.; Yang, B.Y.; Xia, Y.G.; Feng, W.S. Chemical constituents from the flower of Datura metel L. Arch. Pharm. Res. 2008, 31, 1094–1097. [Google Scholar] [CrossRef]
- Corbally, R.P.; Mehta, L.K.; Parrick, J.; Short, E.L. Experimental and calculated 13C chemical shifts for α-, β-, γ-and δ-carbolines. Magn. Reson. Chem. 2000, 38, 1034–1036. [Google Scholar] [CrossRef]
- Šardzík, R.; Noble, G.T.; Weissenborn, M.J.; Martin, A.; Webb, S.J.; Flitsch, S.L. Preparation of aminoethyl glycosides for glycoconjugation. Beilstein J. Org. Chem. 2010, 6, 699–703. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, J.H.; He, H.P.; Zhou, H.; Yang, X.W.; Li, C.S.; Hao, X.J. Norsesquiterpenoid glucosides and a rhamnoside of pyrrolizidine alkaloid from Tephroseris kirilowii. J. Asian Nat. Prod. Res. 2008, 10, 25–31. [Google Scholar] [CrossRef]
- Li, E.W.; Gao, X.; Gao, K.; Jia, Z.J. Labdane diterpenoid glycosides from Aster veitchianus. Chem. Biodivers. 2007, 4, 531–538. [Google Scholar] [CrossRef]
- National Committee on Clinical Laboratory Standards (NCCLS). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved standard NCCLS document M27-A2; National Committee on Clinical Laboratory Standards: Wayne, PA, USA, 2002. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved standard, M7-A7; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent end points. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
Sample Availability: Samples of the compounds 8, 31, 33, and 34 are available from the authors. |
| 1 | 2 | |||
|---|---|---|---|---|
| No. | δH | δC | δH | δC |
| 1 | 1.68 m 0.97 m | 39.1 | 1.79 m 1.31 m | 39.4 |
| 2 | 1.81 m | 28.3 | 2.48 m 2.42 m | 34.4 |
| 3 | 3.44 br t (8.3) | 78.0 | 216.4 | |
| 4 | 39.5 | 47.4 | ||
| 5 | 0.78 dd (12.0, 1.7) | 55.7 | 1.28 m | 54.8 |
| 6 | 1.51 m 1.33 m | 18.9 | 1.37 m 1.31 m | 20.0 |
| 7 | 1.43 m 1.21 m | 33.6 | 1.38 m 1.19 m | 32.7 |
| 8 | 45.1 | 45.2 | ||
| 9 | 1.39 m | 49.3 | 1.39 m | 48.4 |
| 10 | 37.3 | 36.8 | ||
| 11 | 2.11 m 1.64 m | 33.2 | 2.04 m 1.65 m | 33.4 |
| 12 | 4.23 m | 69.1 | 4.20 m | 69.0 |
| 13 | 1.84 d (10.7) | 56.4 | 1.84 d (10.8) | 56.5 |
| 14 | 41.8 | 41.7 | ||
| 15 | 1.93 dd (12.7, 4.2) 1.71 m | 46.0 | 1.92 dd (12.6, 4.3) 1.70 m | 45.9 |
| 16 | 4.48 m | 66.8 | 4.48 m | 66.7 |
| 17 | 2.41 d (11.7) | 73.8 | 2.41 d (11.6) | 73.8 |
| 18 | 47.5 | 47.5 | ||
| 19 | 2.60 m 2.14 m | 43.1 | 2.60 m 2.13 m | 43.1 |
| 20 | 2.05 m 1.95 m | 37.8 | 2.07 m 1.96 m | 37.8 |
| 21 | 85.7 | 85.7 | ||
| 22 | 75.5 | 75.5 | ||
| 23 | 1.21 s | 28.7 | 1.11 s | 26.6 |
| 24 | 1.01 s | 16.3 | 1.00 s | 21.3 |
| 25 | 0.82 s | 16.1 | 0.81 s | 15.6 |
| 26 | 0.97 s | 17.0 | 0.95 s | 16.6 |
| 27 | 1.13 s | 19.5 | 1.10 s | 19.4 |
| 28 | 1.19 s | 17.3 | 1.19 s | 17.3 |
| 29 | 1.56 s | 26.6 | 1.56 s | 26.7 |
| 30 | 1.61 s | 27.3 | 1.61 s | 27.4 |
| 3-OH | 5.78 br s | |||
| 12-OH | 5.32 d (6.0) | 5.38 d (6.9) |
| 3 (Pyridine-d5) | 4 (Methanol-d4) | 4 (Pyridine-d5) | ||||
|---|---|---|---|---|---|---|
| No. | δH (500 MHz) | δC (126 MHz) | δH (600 MHz) | δC (151 MHz) | δH (500 MHz) | δC (126 MHz) |
| 1 | 1.65 m 1.30 m | 39.1 | 1.92 m 1.05 m | 39.4 | 1.88 m 1.07 m | 38.5 |
| 2 | 2.51 m 2.37 m | 34.4 | 1.69 m 1.64 m | 27.9 | 1.94 m 1.89 m | 28.1 |
| 3 | 216.3 | 3.18 dd (11.7, 4.9) | 79.8 | 3.49 dd (10.6, 5.0) | 78.1 | |
| 4 | 47.5 | 40.1 | 39.6 | |||
| 5 | 1.32 m | 55.4 | 0.84 br d (12.1) | 56.4 | 0.91 dd (12.2, 1.8) | 55.3 |
| 6 | 1.35 m | 19.8 | 1.63 m 1.46 m | 19.6 | 1.61 m 1.44 m | 18.8 |
| 7 | 1.46 m 1.31 m | 32.7 | 1.35 m | 33.7 | 1.31 m | 32.9 |
| 8 | 39.7 | 42.2 | 41.2 | |||
| 9 | 1.70 m | 47.2 | 1.99 br s | 56.0 | 2.06 br s | 54.9 |
| 10 | 36.9 | 38.0 | 37.1 | |||
| 11 | 1.88 m | 23.8 | 5.72 br d (11.2) | 129.0 | 5.78 br d (10.5) | 127.8 |
| 12 | 5.72 br t (3.3) | 122.8 | 6.33 dd (11.2, 2.8) | 126.2 | 6.63 dd (10.5, 2.6) | 125.7 |
| 13 | 144.8 | 139.8 | 138.3 a | |||
| 14 | 42.2 | 43.6 | 42.7 | |||
| 15 | 2.18 m | 28.5 | 1.72 m 1.08 m | 26.2 | 1.97 m 1.08 m | 25.6 |
| 16α 16β | 2.19 m 2.08 m | 24.0 | 1.71 m 1.95 m | 33.8 | 1.78 m 2.25 m | 33.1 |
| 17 | 46.4 | 49.3 a | 48.8 | |||
| 18 | 3.63 dd (13.7, 4.0) | 43.5 | 130.8 | 131.2 a | ||
| 19α 19β | 1.92 dd (13.7, 13.7) 2.50 m | 43.1 | 2.85 dd (14.5, 1.7) 2.17 d (14.5) | 35.9 | 3.18 d (15.2) 2.78 d (15.2) | 35.6 |
| 20 | 44.1 | 44.8 | 43.9 | |||
| 21 | 2.41 m 1.47 m | 31.2 | 1.83 m 1.57 m | 33.0 | 2.28 m 1.78 m | 32.5 |
| 22 | 2.41 m 2.08 m | 34.8 | 2.29 m 1.41 m | 35.4 | 2.67 ddd (13.8, 3.5, 3.5) 1.52 m | 35.0 |
| 23 | 1.14 s | 26.6 | 0.98 s | 28.6 | 1.24 s | 28.6 |
| 24 | 0.99 s | 21.6 | 0.78 s | 15.9 | 1.03 s | 16.1 |
| 25 | 0.86 s | 14.9 | 0.94 s | 18.8 | 0.97 s | 18.4 |
| 26 | 1.00 s | 17.3 | 0.81 s | 17.3 | 1.07 s | 17.0 |
| 27 | 1.30 s | 26.1 | 1.00 s | 20.3 | 1.09 s | 20.1 |
| 28 | 180.1 | 179.9 | DAS b | |||
| 29 | 1.43 s | 29.1 | 1.11 s | 20.4 | 1.28 s | 20.2 |
| 30 | 179.5 | 180.4 | 178.5 | |||
| 30-OMe | 3.67 s | 52.6 | 3.60 s | 51.8 |
| 5 | 6 | 7 | ||||
|---|---|---|---|---|---|---|
| No. | δH (600 MHz) | δC (151 MHz) | δH (500 MHz) | δC (126 MHz) | δH (600 MHz) | δC (151 MHz) |
| 1 | 1.70 m 0.97 m | 39.3 | 1.63 m 0.90 m | 39.1 | 1.67 m 0.94 m | 39.3 |
| 2 | 2.21 m 1.91 m | 27.4 | 2.13 m 1.88 m | 27.0 | 2.14 m 1.87 m | 27.3 |
| 3 | 3.38 dd (11.7, 4.4) | 89.1 | 3.29 dd (11.8, 4.2) | 88.6 | 3.31 dd (11.9, 4.4) | 89.3 |
| 4 | 40.1 | 39.7 | 40.1 | |||
| 5 | 0.79 br d (11.9) | 56.2 | 0.71 br d (9.3) | 56.0 | 0.76 br d (12.0) | 56.2 |
| 6 | 1.51 m 1.33 m | 19.1 | 1.46 m 1.33 m | 18.6 | 1.50 m 1.32 m | 19.0 |
| 7 | 1.39 m 1.19 m | 34.0 | 1.33 m 1.15 m | 33.5 | 1.40 m 1.19 m | 34.0 |
| 8 | 43.8 | 43.4 | 43.8 | |||
| 9 | 1.40 m | 50.0 | 1.35 m | 49.6 | 1.40 m | 50.0 |
| 10 | 37.4 | 37.0 | 37.4 | |||
| 11 | 2.12 m 1.64 m | 33.5 | 2.07 m 1.60 m | 33.0 | 2.10 m 1.63 m | 33.5 |
| 12 | 4.20 m | 69.2 | 4.17 m | 68.7 | 4.20 m | 69.1 |
| 13 | 1.72 d (10.7) | 56.9 | 1.68 d (11.1) | 56.5 | 1.72 overlapped | 57.0 |
| 14 | 42.1 | 41.6 | 42.1 | |||
| 15 | 1.51 m 1.20 m | 34.8 | 1.48 m 1.17 m | 34.4 | 1.52 m 1.21 m | 34.8 |
| 16 | 1.44 m | 20.2 | 1.42 m | 19.8 | 1.46 m | 20.2 |
| 17 | 1.82 dd (12.1, 2.9) | 56.3 | 1.78 dd (11.9, 2.9) | 55.9 | 1.82 dd (12.0, 2.8) | 56.3 |
| 18 | 45.8 | 45.4 | 45.8 | |||
| 19 | 2.58 m 1.68 m | 45.2 | 2.55 m 1.65 m | 44.8 | 2.58 m 1.68 m | 45.2 |
| 20 | 2.17 m 1.68 m | 36.2 | 2.14 m 1.65 m | 35.7 | 2.17 m 1.68 m | 36.2 |
| 21 | 54.5 | 54.0 | 54.5 | |||
| 22 | 213.0 | 212.6 | 213.0 | |||
| 23 | 1.33 s | 28.6 | 1.25 s | 28.0 | 1.27 s | 28.5 |
| 24 | 1.01 s | 17.2 | 1.17 s | 16.9 | 0.97 s | 17.2 |
| 25 | 0.82 s | 16.5 | 0.79 s | 16.2 | 0.81 s | 16.5 |
| 26 | 0.99 s | 17.5 | 0.95 s | 17.0 | 0.99 s | 17.5 |
| 27 | 1.05 s | 18.4 | 1.01 s | 18.0 | 1.05 s | 18.4 |
| 28 | 1.12 s | 17.2 | 1.09 s | 16.8 | 1.12 s | 17.2 |
| 29 | 1.22 s | 21.5 | 1.19 s | 21.0 | 1.22 s | 21.5 |
| 30 | 2.19 s | 25.9 | 2.16 s | 25.4 | 2.19 s | 25.9 |
| 1′ | 4.87 d (7.6) | 108.2 | 4.83 d (7.3) | 106.2 | 4.77 d (7.5) | 107.8 |
| 2′ | 4.05 dd (8.6, 7.6) | 76.0 | 4.24 dd (8.2, 7.3) | 78.0 | 4.04 dd (8.6, 7.5) | 75.9 |
| 3′ | 4.20 m | 79.1 | 4.18 m | 79.7 | 4.32 dd (8.8, 8.8) | 83.5 |
| 4′ | 4.26 m | 71.7 | 4.15 m | 71.6 | 4.17 m | 70.2 |
| 5′ | 4.41 dd (11.3, 5.2) 3.80 dd (11.3, 10.5) | 67.6 | 4.33 m 3.71 dd (10.5, 9.9) | 67.0 | 4.36 m 3.74 dd (11.2, 10.3) | 67.4 |
| 1″ | 6.54 d (0.9) | 102.0 | 6.30 br s | 103.2 | ||
| 2″ | 4.88 m | 72.5 | 4.82 dd (3.4, 1.3) | 73.1 | ||
| 3″ | 4.69 dd (9.5, 3.1) | 72.6 | 4.62 dd (9.3, 3.4) | 73.2 | ||
| 4″ | 4.35 m | 74.2 | 4.37 dd (9.3, 9.3) | 74.6 | ||
| 5″ | 4.77 m | 69.8 | 5.01 m | 70.4 | ||
| 6″ | 1.70 d (6.2) | 18.8 | 1.71 d (6.2) | 19.1 | ||
| 12-OH | 5.23 d (6.2) | |||||
| 2″-OH | 6.69 br s | |||||
| 4″-OH | 6.72 br s |
| 8 | 9 | 10 | 11 | |||||
|---|---|---|---|---|---|---|---|---|
| No. | δH (500 MHz) | δC (126 MHz) | δH (500 MHz) | δC (126 MHz) | δH 800 MHz | δC (201 MHz) | δH (800 MHz) | δC (201 MHz) |
| 1 | 1.62 m 0.85 m | 38.8 | 1.63 m 0.86 m | 38.8 | 1.41 m 0.66 m | 38.6 | 1.64 m 0.87 m | 38.8 |
| 2 | 2.03 m 1.80 m | 26.8 | 2.04 m 1.81 m | 26.8 | 1.98 m 1.76 m | 26.6 | 2.05 m 1.82 m | 26.8 |
| 3 | 3.25 dd (11.8, 4.4) | 88.8 | 3.26 dd (11.8, 4.4) | 88.8 | 3.22 dd (11.9, 4.6) | 88.8 | 3.29 dd (11.9, 4.0) | 88.8 |
| 4 | 39.6 | 39.6 | 39.5 | 39.6 | ||||
| 5 | 0.69 d (11.7) | 55.7 | 0.69 br d (11.8) | 55.7 | 0.64 m | 55.5 | 0.70 br d (12.5) | 55.7 |
| 6 | 1.43 m 1.27 m | 18.6 | 1.44 m 1.27 m | 18.6 | 1.41 m 1.23 m | 18.4 | 1.41 m 1.31 m | 18.6 |
| 7 | 1.40 m 1.21 m | 33.6 | 1.41 m 1.22 m | 33.6 | 1.35 m 1.17 m | 33.3 | 1.42 m 1.23 m | 33.6 |
| 8 | 45.7 | 45.7 | 45.8 | 45.7 | ||||
| 9 | 1.33 m | 49.1 | 1.34 m | 49.1 | 1.28 m | 48.5 | 1.35 m | 49.1 |
| 10 | 36.9 | 36.9 | 36.8 | 36.9 | ||||
| 11 | 2.07 m 1.62 m | 33.0 | 2.09 m 1.63 m | 33.0 | 1.98 m 1.37 m | 28.1 | 2.09 m 1.63 m | 33.0 |
| 12 | 4.19 m | 68.6 | 4.21 m | 68.6 | 5.47 m | 72.2 | 4.20 m | 68.6 |
| 13 | 1.79 d (10.7) | 55.8 | 1.79 d (10.5) | 55.8 | 1.90 d (11.5) | 52.4 | 1.79 d (10.6) | 55.8 |
| 14 | 41.8 | 41.8 | 41.6 | 41.7 | ||||
| 15 | 1.87 m 1.69 m | 45.7 | 1.88 m 1.70 m | 45.7 | 1.83 m 1.64 m | 45.2 | 1.88 m 1.70 m | 45.6 |
| 16 | 4.12 m | 65.5 | 4.11 m | 65.5 | 4.06 m | 65.0 | 4.12 m | 65.5 |
| 17 | 2.28 d (11.3) | 63.7 | 2.28 d (11.4) | 63.7 | 2.23 d (11.5) | 63.3 | 2.28 d (11.7) | 63.7 |
| 18 | 47.1 | 47.1 | 46.4 | 47.1 | ||||
| 19 | 2.61 m 1.87 m | 45.8 | 2.63 m 1.90 m | 45.8 | 1.83 m 1.68 m | 44.8 | 2.62 m 1.89 m | 45.7 |
| 20 | 2.05 m 1.74 m | 37.6 | 2.06 m 1.74 m | 37.6 | 2.04 m 1.66 m | 37.5 | 2.06 m 1.73 m | 37.6 |
| 21 | 53.6 | 53.6 | 53.4 | 53.6 | ||||
| 22 | 214.9 | 215.0 | 214.6 | 214.9 | ||||
| 23 | 1.22 s | 28.0 | 1.22 s | 28.0 | 1.21 s | 27.9 | 1.25 s | 28.0 |
| 24 | 0.89 s | 16.6 | 0.90 s | 16.6 | 0.88 s | 16.6 | 0.94 s | 16.7 |
| 25 | 0.76 s | 16.0 | 0.76 s | 16.0 | 0.72 s | 15.9 | 0.77 s | 16.0 |
| 26 | 0.98 s | 17.0 | 0.99 s | 17.0 | 0.93 s | 16.8 | 0.99 s | 17.0 |
| 27 | 1.11 s | 19.2 | 1.11 s | 19.2 | 1.08 s | 18.9 | 1.12 s | 19.1 |
| 28 | 1.21 s | 17.8 | 1.21 s | 17.8 | 1.03 s | 17.7 | 1.21 s | 17.8 |
| 29 | 1.66 s | 21.0 | 1.66 s | 21.0 | 1.61 s | 20.9 | 1.66 s | 21.0 |
| 30 | 2.34 s | 26.3 | 2.34 s | 26.3 | 2.37 s | 26.3 | 2.35 s | 26.3 |
| 1′ | 4.74 d (7.7) | 107.2 | 4.76 d (7.5) | 107.2 | 4.75 d (7.5) | 107.2 | 4.83 d (7.3) | 106.9 |
| 2′ | 4.00 m | 75.9 | 4.00 dd (8.3, 7.5) | 75.8 | 4.01 dd (8.4, 7.5) | 75.8 | 4.08 m | 75.0 |
| 3′ | 4.40 m | 78.3 | 4.43 m | 79.0 | 4.43 m | 79.0 | 4.35 m | 83.9 |
| 4′ | 5.28 ddd (9.7, 9.7, 5.5) | 71.3 | 5.38 overlapped | 71.0 | 5.39 m | 70.9 | 5.44 m | 70.7 |
| 5′ | 4.30 m 3.58 dd (11.3, 11.0) | 63.1 | 4.35 m 3.63 dd (11.3 10.0) | 63.2 | 4.38 m 3.65 m | 63.2 | 4.35 m 3.68 m | 63.3 |
| 1″ | 6.26 d (1.2) | 102.6 | 6.24 br s | 102.8 | 6.25 br s | 102.9 | 5.25 d (7.8) | 106.9 |
| 2″ | 4.69 br s | 72.4 | 4.74 dd (3.2, 1.4) | 72.5 | 4.72 m | 72.5 | 3.99 t (7.8) | 75.8 |
| 3″ | 4.45 m | 72.7 | 4.45 m | 72.6 | 4.44 m | 72.6 | 4.14 m | 78.4 |
| 4″ | 4.30 t (9.3) | 73.9 | 4.30 t (9.4) | 73.9 | 4.29 dd (9.5, 9.5) | 74.0 | 4.15 m | 71.0 |
| 5″ | 4.43 m | 70.0 | 4.40 m | 70.0 | 4.40 m | 70.1 | 4.31 m 3.70 m | 67.6 |
| 6″ | 1.70 d (6.2) | 18.8 | 1.68 d (6.3) | 18.9 | 1.69 d (6.2) | 18.9 | ||
| 1′′′ | 170.4 | 165.9 | 165.9 | 165.9 | ||||
| 2′′′ | 2.15 s | 21.1 | 6.02 dq (15.6, 1.6) | 122.7 | 6.04 br d (14.6) | 122.8 | 5.99 br d (15.5) | 123.1 |
| 3′′′ | 7.06 m | 146.0 | 7.07 m | 145.9 | 7.09 m | 145.4 | ||
| 4′′′ | 1.66 d (6.8) | 17.8 | 1.61 dd (7.0, 1.5) | 17.8 | 1.61 br d (6.9) | 17.8 | ||
| 1′′′′ | 170.4 | |||||||
| 2′′′′ | 2.15 s | 21.9 | ||||||
| 12-OH | 5.34 d (6.1) | |||||||
| 16-OH | 5.49 d (5.0) | |||||||
| 2′-OH | 7.68 d (6.1) | |||||||
| 2″-OH | 6.80 br s | |||||||
| 4″-OH | 6.80 br s |
| 12 | 13 | 14 | ||||
|---|---|---|---|---|---|---|
| No. | δH (500 MHz) | δC (126 MHz) | δH (600 MHz) | δC (151 MHz) | δH (600 MHz) | δC (151 MHz) |
| 1 | 1.61 m 0.84 m | 39.0 | 1.72 m 0.98 m | 39.5 | 1.68 m 0.95 m | 39.7 |
| 2 | 1.78 m | 28.3 | 2.21 m 1.90 m | 27.4 | 2.17 m 1.92 m | 27.5 |
| 3 | 3.41 m | 78.1 | 3.37 dd (11.8, 4.5) | 89.0 | 3.31 dd (11.9, 4.2) | 88.9 |
| 4 | 39.5 | 40.1 | 40.1 | |||
| 5 | 0.69 m | 56.0 | 0.75 br d (10.8) | 56.3 | 0.71 br d (11.5) | 56.6 |
| 6 | 1.49 m 1.28 m | 18.8 | 1.51 m 1.33 m | 19.0 | 1.47 m 1.31 m | 19.0 |
| 7 | 1.27 m | 34.2 | 1.32 m | 34.6 | 1.29 m | 34.6 |
| 8 | 46.8 | 47.2 | 47.2 | |||
| 9 | 1.22 m | 48.4 | 1.40 m | 49.5 | 1.37 m | 49.5 |
| 10 | 37.6 | 37.6 | 37.5 | |||
| 11 | 2.37 m 1.48 m | 27.2 | 2.09 m 1.66 m | 33.5 | 2.07 m 1.65 m | 33.5 |
| 12 | 4.37 m | 74.5 | 4.04 m | 69.7 | 4.04 m | 69.7 |
| 13 | 1.87 d (11.2) | 54.0 | 1.92 d (11.1) | 56.2 | 1.91 d (11.1) | 56.2 |
| 14 | 41.5 | 41.9 | 41.9 | |||
| 15 | 1.92 m 1.29 m | 43.0 | 1.99 dd (11.6. 6.2) 1.38 m | 43.7 | 1.97 dd (11.7, 6.1) 1.36 m | 43.7 |
| 16 | 4.84 m | 76.0 | 4.93 m | 76.6 | 4.92 m | 76.6 |
| 17 | 152.5 | 153.0 | 153.0 | |||
| 18 | 46.0 | 46.4 | 46.4 | |||
| 19 | 2.88 m 2.54 m | 53.7 | 3.15 m 2.70 m | 54.6 | 3.15 m 2.70 ddd (13.6, 8.2, 2.6) | 54.5 |
| 20 | 2.37 m 2.04 m | 25.6 | 2.50 m 2.20 m | 26.1 | 2.50 m 2.21 m | 26.1 |
| 21 | 146.8 | 147.5 | 147.5 | |||
| 22 | 84.2 | 84.5 | 84.5 | |||
| 23 | 1.20 s | 28.7 | 1.31 s | 28.5 | 1.25 s | 28.3 |
| 24 | 0.99 s | 16.4 | 0.99 s | 17.2 | 1.19 s | 17.4 |
| 25 | 0.79 s | 16.6 | 0.85 s | 17.0 | 0.85 s | 17.1 |
| 26 | 0.88 s | 16.5 | 1.02 s | 16.9 | 1.01 s | 16.9 |
| 27 | 0.94 s | 15.9 | 1.07 s | 16.2 | 1.06 s | 16.2 |
| 28 | 1.67 s | 22.2 | 1.59 s | 23.0 | 1.59 s | 23.0 |
| 29 | 1.44 s | 29.2 | 1.49 s | 29.2 | 1.49 s | 29.2 |
| 30 | 1.44 s | 28.6 | 1.47 s | 29.5 | 1.47 s | 29.5 |
| 1′ | 5.19 d (7.5) | 100.1 | 4.86 d (7.5) | 108.2 | 4.85 d (7.4) | 106.6 |
| 2′ | 4.07 m | 75.9 | 4.05 m | 76.0 | 4.27 dd (8.3, 7.4) | 78.4 |
| 3′ | 4.37 m | 78.8 | 4.20 dd (8.7, 8.7) | 79.1 | 4.20 dd (8.7, 8.3) | 80.1 |
| 4′ | 4.26 dd (9.5, 9.5) | 72.4 | 4.26 m | 71.7 | 4.17 m | 72.0 |
| 5′ | 4.05 m | 78.4 | 4.39 dd (11.3, 5.2) 3.79 dd (11.3, 10.7) | 67.6 | 4.34 dd (11.4, 4.8) 3.73 dd (11.4, 9.9) | 67.4 |
| 6′ | 4.57 dd (11.7, 2.2) 4.36 m | 63.4 | ||||
| 1″ | 6.58 br s | 102.4 | ||||
| 2″ | 4.90 dd (3.4, 1.4) | 72.9 | ||||
| 3″ | 4.71 dd (9.4, 3.4) | 73.0 | ||||
| 4″ | 4.38 dd (9.3, 9.3) | 74.6 | ||||
| 5″ | 4.80 m | 70.2 | ||||
| 6″ | 1.73 d (6.2) | 19.2 | ||||
| 12-OH | 5.46 br s |
| 15 | 16 | 17 | ||||
|---|---|---|---|---|---|---|
| No. | δH (600 MHz) | δC (151 MHz) | δH (500 MHz) | δC (126 MHz) | δH (800 MHz) | δC (201 MHz) |
| 1 | 1.69 m 0.95 m | 39.5 | 1.64 m 0.90 m | 38.8 | 1.63 m 0.91 m | 39.0 |
| 2 | 2.14 m 1.87 m | 27.3 | 2.11 m 1.83 m | 26.8 | 2.10 m 1.83 m | 26.9 |
| 3 | 3.30 dd (11.9, 4.3) | 89.2 | 3.27 dd (11.8, 4.4) | 88.8 | 3.26 dd (12.0, 4.3) | 88.8 |
| 4 | 40.1 | 39.6 | 39.6 | |||
| 5 | 0.73 br d (11.3) | 56.3 | 0.72 br d (11.9) | 55.7 | 0.73 br d (11.7) | 55.9 |
| 6 | 1.48 m 1.31 m | 19.0 | 1.47 m 1.30 m | 18.6 | 1.44 m 1.30 m | 18.6 |
| 7 | 1.31 m | 34.6 | 1.45 m 1.27 m | 33.5 | 1.40 m 1.31 m | 33.6 |
| 8 | 47.2 | 45.4 | 44.8 | |||
| 9 | 1.39 m | 49.5 | 1.38 m | 49.2 | 1.47 m | 49.6 |
| 10 | 37.5 | 36.9 | 37.0 | |||
| 11 | 2.09 m 1.65 m | 33.5 | 2.07 m 1.61 m | 33.1 | 2.08 m 1.62 m | 33.4 |
| 12 | 4.04 m | 69.7 | 4.19 m | 69.3 | 4.08 m | 69.6 |
| 13 | 1.92 d (11.1) | 56.2 | 1.77 d (10.9) | 53.9 | 1.91 m | 55.1 |
| 14 | 47.2 | 41.8 | 41.7 | |||
| 15 | 2.00 m 1.38 m | 43.7 | 1.87 dd (12.5, 4.2) 1.73 m | 45.1 | 1.92 m 1.87 m | 44.1 |
| 16 | 4.93 m | 76.6 | 4.32 m | 67.2 | 4.99 overlapped | 68.1 |
| 17 | 153.0 | 2.14 d (10.8) | 62.2 | 143.9 | ||
| 18 | 46.4 | 45.4 | 52.6 | |||
| 19 | 3.15 m 2.70 m | 54.6 | 2.53 m 1.78 m | 43.2 | 2.58 m 2.20 m | 46.5 |
| 20 | 2.50 m 2.21 m | 26.1 | 2.42 m | 29.9 | 2.54 m 2.18 m | 38.1 |
| 21 | 147.5 | 152.2 | 128.5 | |||
| 22 | 84.5 | 6.06 t (2.0) 5.14 t (2.0) | 106.1 | 2.33 s | 16.0 | |
| 23 | 1.25 s | 28.4 | 1.23 s | 28.0 | 1.21 s | 28.0 |
| 24 | 0.95 s | 17.2 | 0.93 s | 16.7 | 0.92 s | 16.7 |
| 25 | 0.83 s | 17.0 | 0.78 s | 16.0 | 0.79 s | 16.4 |
| 26 | 1.02 s | 16.9 | 1.01 s | 17.0 | 0.98 s | 16.5 |
| 27 | 1.07 s | 16.2 | 1.16 s | 19.1 | 1.25 s | 17.5 |
| 28 | 1.59 s | 23.0 | 1.06 s | 16.0 | 1.39 s | 20.6 |
| 29 | 1.49 s | 29.2 | ||||
| 30 | 1.47 s | 29.5 | ||||
| 1′ | 4.76 d (7.5) | 107.8 | 4.74 d (7.4) | 107.4 | 4.73 d (7.5) | 107.4 |
| 2′ | 4.03 m | 75.9 | 4.01 m | 75.4 | 4.00 m | 75.4 |
| 3′ | 4.32 dd (8.9, 8.9) | 83.5 | 4.30 t (8.9) | 83.0 | 4.29 t (8.8) | 83.0 |
| 4′ | 4.16 m | 70.2 | 4.14 m | 69.7 | 4.13 m | 69.7 |
| 5′ | 4.35 m 3.73 dd (11.2, 10.4) | 67.4 | 4.32 m 3.72 dd (11.3, 10.2) | 67.0 | 4.33 m 3.71 dd (11.2, 10.3) | 66.9 |
| 1″ | 6.30 br s | 103.3 | 6.26 d (1.2) | 102.9 | 6.26 br s | 102.8 |
| 2″ | 4.82 dd (3.3, 1.5) | 73.1 | 4.78 br s | 72.6 | 4.78 br s | 72.6 |
| 3″ | 4.62 dd (9.4, 3.3) | 73.2 | 4.60 br d (9.1) | 72.8 | 4.59 br d (8.7) | 72.8 |
| 4″ | 4.37 dd (9.4, 9.4) | 74.6 | 4.34 m | 74.2 | 4.33 m | 74.2 |
| 5″ | 5.00 m | 70.4 | 4.98 overlapped | 70.0 | 4.97 overlapped | 70.0 |
| 6″ | 1.71 d (6.2) | 19.2 | 1.68 d (6.2) | 18.7 | 1.68 d (6.1) | 18.7 |
| 12-OH | 5.27 d (6.5) | 5.20 d (6.2) | ||||
| 16-OH | 5.72 d (5.9) | 6.03 d (5.5) | ||||
| 2′-OH | 7.29 d (6.0) | 7.26 d (6.0) | ||||
| 4′-OH | 6.79 d (5.9) | 6.76 d (5.6) | ||||
| 2″-OH | 6.74 br s | 6.71 br s | ||||
| 3″-OH | 6.47 br s | 6.43 br s | ||||
| 4″-OH | 6.74 br s | 6.71 br s |
| 18 | 19 | 20 | ||||
|---|---|---|---|---|---|---|
| No. | δH (600 MHz) | δC (151 MHz) | δH (500 MHz) | δC (126 MHz) | δH (J in Hz) (600 MHz) | δC (151 MHz) |
| 1 | 1.69 m 0.98 m | 39.2 | 1.61 m 0.90 m | 38.8 | 1.70 m 0.89 m | 38.5 |
| 2 | 2.21 m 1.91 m | 27.4 | 2.10 m 1.82 m | 26.9 | 2.14 m 1.87 m | 27.0 |
| 3 | 3.39 dd (11.9, 4.3) | 89.1 | 3.27 dd (11.9, 4.4) | 88.8 | 3.32 dd (11.9, 4.5) | 89.8 |
| 4 | 40.2 | 39.7 | 40.1 | |||
| 5 | 0.84 m | 56.5 | 0.77 overlapped | 56.0 | 0.79 br d (12.1) | 55.6 |
| 6 | 1.57 m 1.42 m | 19.1 | 1.54 m 1.37 m | 18.6 | 1.52 m 1.33 m | 18.8 |
| 7 | 1.52 m | 33.9 | 1.48 m | 33.5 | 1.28 m | 33.2 |
| 8 | 41.8 | 41.3 | 41.6 | |||
| 9 | 1.50 m | 50.2 | 1.45 m | 49.7 | 1.98 br s | 55.2 |
| 10 | 37.7 | 37.3 | 37.1 | |||
| 11 | 2.18 m 1.66 m | 34.3 | 2.14 m 1.61 m | 33.9 | 5.69 br d (10.1) | 128.2 |
| 12 | 4.33 m | 69.4 | 4.28 m | 69.0 | 6.60 dd (10.1, 2.9) | 126.2 |
| 13 | 2.17 d (11.1) | 53.3 | 2.13 d (11.1) | 52.8 | 138.7 | |
| 14 | 47.5 | 47.0 | 43.1 | |||
| 15 | 5.76 d (10.3) | 135.5 | 5.72 d (10.3) | 135.1 | 1.96 m 1.09 m | 26.0 |
| 16 | 6.42 d (10.3) | 120.4 | 6.39 d (10.3) | 120.0 | 2.26 m 1.78 m | 33.5 |
| 17 | 141.9 | 141.5 | 49.2 | |||
| 18 | 48.6 | 48.2 | 131.4 | |||
| 19 | 2.59 m 2.24 m | 45.3 | 2.55 m 2.21 m | 44.9 | 3.17 d (14.5) 2.77 d (14.5) | 36.0 |
| 20 | 2.60 m 2.14 m | 36.8 | 2.55 m 2.09 m | 36.3 | 44.3 | |
| 21 | 131.8 | 131.4 | 2.31 m 1.79 m | 32.9 | ||
| 22 | 1.73 s | 14.4 | 1.70 s | 14.0 | 2.66 m 1.52 m | 35.3 |
| 23 | 1.35 s | 28.6 | 1.25 s | 28.0 | 1.25 s | 28.2 |
| 24 | 1.02 s | 17.2 | 0.94 s | 16.7 | 0.89 s | 16.8 |
| 25 | 0.81 s | 16.3 | 0.76 s | 15.9 | 0.84 s | 18.7 |
| 26 | 1.03 s | 19.7 | 0.99 s | 19.3 | 1.02 s | 17.4 |
| 27 | 1.34 s | 19.5 | 1.30 s | 19.0 | 1.11 s | 20.5 |
| 28 | 1.32 s | 20.4 | 1.29 s | 20.0 | 179.1 | |
| 29 | 1.27 s | 20.7 | ||||
| 30 | 178.9 | |||||
| 30-Me | 3.60 s | 52.2 | ||||
| 1′ | 4.88 d (7.7) | 108.2 | 4.74 d (7.5) | 107.4 | 4.92 d (7.9) | 107.6 |
| 2′ | 4.05 dd (8.4, 7.7) | 76.0 | 4.00 dd (8.4, 7.5) | 75.4 | 4.07 dd (8.7, 7.9) | 76.2 |
| 3′ | 4.20 dd (8.8, 8.4) | 79.1 | 4.28 m | 83.0 | 4.45 dd (9.3, 8.7) | 82.3 |
| 4′ | 4.26 m | 71.7 | 4.12 m | 69.7 | 4.41 dd (9.3, 9.3) | 71.9 |
| 5′ | 4.40 dd (11.0, 5.1) 3.80 dd (11.0, 10.4) | 67.6 | 4.32 m 3.70 dd (11.0, 10.5) | 67.0 | 4.58 d (9.3) | 77.6 |
| 6′ | 171.3 | |||||
| 6′-OMe | 3.79 s | 52.7 | ||||
| 1″ | 6.27 br s | 102.8 | 6.33 br s | 103.4 | ||
| 2″ | 4.79 br s | 72.6 | 4.76 dd (3.3, 1.4) | 73.0 | ||
| 3″ | 4.59 dd (9.3, 3.0) | 72.8 | 4.57 dd (9.3, 3.3) | 73.2 | ||
| 4″ | 4.34 dd (9.3, 9.3) | 74.2 | 4.35 dd (9.3, 9.3) | 74.6 | ||
| 5″ | 4.97 m | 69.9 | 5.08 m | 70.3 | ||
| 6″ | 1.67 d (6.1) | 18.7 | 1.71 d (6.1) | 19.1 |
| 21 | 22 | 23 | ||||
|---|---|---|---|---|---|---|
| No. | δH (500 MHz) | δC (126 MHz) | δH (500 MHz) | δC (126 MHz) | δH (500 MHz) | δC (126 MHz) |
| 1 | 1.35 m 0.82 m | 38.6 | 1.36 m 0.82 m | 38.5 | 1.37 m 0.83 m | 38.5 |
| 2 | 2.19 m 1.76 m | 26.4 | 2.04 m 1.77 m | 26.6 | 2.07 m 1.78 m | 26.6 |
| 3 | 3.25 dd (11.9, 4.4) | 89.2 | 3.28 overlapped | 89.4 | 3.27 overlapped | 89.3 |
| 4 | 39.3 | 39.5 | 39.5 | |||
| 5 | 0.75 overlapped | 55.8 | 0.75 overlapped | 55.7 | 0.74 overlapped | 55.7 |
| 6 | 1.49 m 1.28 m | 18.6 | 1.46 m 1.25 m | 18.4 | 1.46 m 1.26 m | 18.4 |
| 7 | 1.46 m 1.27 m | 33.3 | 1.44 m 1.26 m | 33.2 | 1.45 m 1.26 m | 33.2 |
| 8 | 39.7 | 42.1 | 42.1 | |||
| 9 | 1.60 m | 48.1 | 1.61 m | 48.0 | 1.62 dd (8.6, 8.6) | 48.0 |
| 10 | 37.0 | 36.9 | 36.9 | |||
| 11 | 1.85 m | 23.8 | 1.85 m | 23.7 | 1.85 m | 23.7 |
| 12 | 5.58 br s | 122.5 | 5.59 br s | 123.1 | 5.59 dd (3.4, 3.4) | 123.1 |
| 13 | 145.5 | 144.5 | 144.5 | |||
| 14 | 42.2 | 39.7 | 39.7 | |||
| 15 | 2.21 m | 28.6 | 2.12 m 1.18 m | 28.4 | 2.13 m 1.27 m | 28.4 |
| 16 | 2.08 m | 24.2 | 2.12 m 2.02 m | 23.9 | 2.13 m 2.03 m | 23.9 |
| 17 | 46.4 | 46.2 | 46.2 | |||
| 18 | 3.36 dd (13.5, 3.6) | 43.6 | 3.29 overlapped | 43.4 | 3.29 overlapped | 43.4 |
| 19 | 2.26 m 1.82 m | 43.2 | 2.25 br d (13.2) 1.81 m | 42.7 | 2.25 br d (12.0) 1.82 m | 42.7 |
| 20 | 44.3 | 44.2 | 44.2 | |||
| 21 | 2.16 m 1.45 m | 31.2 | 2.19 m 1.46 m | 30.9 | 2.19 br d (13.5) 1.47 m | 30.9 |
| 22 | 2.06 m 1.96 m | 34.9 | 2.08 m 1.97 m | 34.6 | 2.09 m 1.98, m | 34.6 |
| 23 | 1.19 s | 28.2 | 1.24 s | 28.1 | 1.23 s | 28.1 |
| 24 | 0.98 s | 17.0 | 0.90 s | 16.9 | 0.90 s | 16.9 |
| 25 | 0.75 s | 15.4 | 0.75 s | 15.4 | 0.76 s | 15.4 |
| 26 | 0.89 br s | 17.6 | 0.95 s | 17.3 | 0.95 s | 17.3 |
| 27 | 1.31 s | 26.2 | 1.31 s | 26.2 | 1.31 s | 26.2 |
| 28 | DAP a | 179.9 | 179.9 | |||
| 29 | 1.22 s | 28.7 | 1.23 s | 28.5 | 1.23 s | 28.5 |
| 30 | 177.4 | 177.2 | 177.2 | |||
| 30-OMe | 3.63 s | 51.7 | 3.65 s | 51.8 | 3.65 s | 51.7 |
| 1′ | 5.05 d (8.2) | 105.0 | 4.88 d (7.8) | 107.1 | 4.88 d (7.9) | 107.2 |
| 2′ | 4.58 m | 58.1 | 4.34 m | 74.1 | 4.05 dd (8.4, 7.9) | 75.8 |
| 3′ | 4.41 dd (9.3, 8.7) | 76.3 | 4.42 dd (9.0, 9.0) | 81.9 | 4.44 dd (8.9, 8.4) | 81.9 |
| 4′ | 4.18 dd (9.3, 9.3) | 72.7 | 4.37 m | 71.4 | 4.41 dd (9.4, 8.9) | 71.4 |
| 5′ | 3.97 m | 78.4 | 4.56 d (9.4) | 77.2 | 4.55 d (9.4) | 77.2 |
| 6′ | 4.57 m 4.37 m | 63.0 | 170.8 | 170.3 | ||
| 6′-OMe, | 3.77 s | 52.2 | 4.29 m | 61.4 | ||
| OEt | 1.19 t (7.1) | 14.3 | ||||
| 1″ | 170.3 | 6.30 br s | 102.9 | 6.33 d (1.2) | 102.8 | |
| 2″ | 2.15 s | 23.8 | 4.75 m | 72.5 | 4.77 dd (3.4, 1.2) | 72.6 |
| 3″ | 4.55 m | 72.7 | 4.56 dd (9.2, 3.4) | 72.7 | ||
| 4″ | 4.34 m | 74.1 | 4.36 dd (9.2, 9.2) | 74.1 | ||
| 5″ | 5.05 m | 69.9 | 5.08 m | 69.8 | ||
| 6″ | 1.69 d (6.1) | 18.6 | 1.69 d (6.2) | 18.7 | ||
| NH | 8.94 d (9.0) |
| 24 | 25 | 26 | ||||
|---|---|---|---|---|---|---|
| No. | δH (500 MHz) | δC (126 MHz) | δH (500 MHz) | δC (126 MHz) | δH (500 MHz) | δC (126 MHz) |
| 1 | 1.51 m 0.97 m | 38.8 | 1.47 m 0.93 m | 38.7 | 1.39 m 0.85 m | 38.6 |
| 2 | 2.16 m 1.87 m | 26.8 | 2.09 m 1.81 m | 26.7 | 2.13 m 1.83 m | 26.6 |
| 3 | 3.35 dd (11.7, 4.4) | 88.7 | 3.28 overlapped | 88.8 | 3.36 dd (11.6, 4.3) | 89.2 |
| 4 | 39.6 | 39.5 | 39.6 | |||
| 5 | 0.82 overlapped | 55.9 | 0.79 overlapped | 55.8 | 0.79 overlapped | 55.8 |
| 6 | 1.49 m 1.28 m | 18.5 | 1.47 m 1.27 m | 18.5 | 1.47 m 1.26 m | 18.5 |
| 7 | 1.47 m 1.28 m | 33.2 | 1.46 m 1.27 m | 33.2 | 1.45 m 1.27 m | 33.2 |
| 8 | 39.7 | 42.0 | 39.7 | |||
| 9 | 1.67 dd (9.0, 8.7) | 48.1 | 1.65 m | 48.0 | 1.63 dd (9.0, 8.6) | 48.0 |
| 10 | 37.1 | 37.0 | 37.0 | |||
| 11 | 1.89 m | 23.8 | 1.88 m | 23.7 | 1.87 m | 23.7 |
| 12 | 5.60 br t (3.1) | 123.2 | 5.60 dd (3.4, 3.4) | 123.1 | 5.60 br t (3.3) | 123.1 |
| 13 | 144.5 | 144.4 | 144.5 | |||
| 14 | 42.1 | 39.7 | 42.1 | |||
| 15 | 2.13 m 1.19 m | 28.4 | 2.13 m 1.27 m | 28.4 | 2.13 m 1.20 m | 28.4 |
| 16 | 2.13 m 2.03 m | 23.9 | 2.12 m 2.02 m | 23.8 | 2.12 m 2.03 m | 23.9 |
| 17 | 46.2 | 46.2 | 46.2 | |||
| 18 | 3.30 dd (13.6, 3.6) | 43.4 | 3.29 overlapped | 43.4 | 3.29 dd (13.6, 3.9) | 43.4 |
| 19 | 2.26 m 1.82 dd (13.6, 13.6) | 42.7 | 2.26 br d (13.5) 1.82 dd (13.5, 13.5) | 42.7 | 2.26 m 1.82 m | 42.7 |
| 20 | 44.2 | 44.2 | 44.2 | |||
| 21 | 2.19 m 1.46 m | 30.9 | 2.19 br d (13.1) 1.46 m | 30.8 | 2.20 m 1.46 m | 30.9 |
| 22 | 2.10 m 1.98 m | 34.6 | 2.09 m 1.98 m | 34.5 | 2.09 m 1.98 m | 34.6 |
| 23 | 1.30 s | 28.2 | 1.24 s | 28.1 | 1.30 s | 28.2 |
| 24 | 0.97 s | 17.0 | 0.92 s | 16.9 | 0.95 s | 17.0 |
| 25 | 0.82 s | 15.5 | 0.80 s | 15.5 | 0.78 s | 15.5 |
| 26 | 0.97 s | 17.4 | 0.96 s | 17.3 | 0.95 s | 17.4 |
| 27 | 1.30 s | 26.2 | 1.30 s | 26.1 | 1.30 s | 26.2 |
| 28 | 179.9 | 179.9 | 179.9 | |||
| 29 | 1.23 s | 28.5 | 1.23 s | 28.5 | 1.23 s | 28.5 |
| 30 | 177.2 | 177.2 | 177.2 | |||
| 30-OMe | 3.65 s | 51.8 | 3.64 s | 51.7 | 3.65 s | 51.8 |
| 1′ | 4.83 d (7.6) | 107.8 | 4.73 d (7.5) | 107.4 | 5.00 d (7.8) | 107.3 |
| 2′ | 4.01 dd (8.7, 7.6) | 75.6 | 4.00 dd (8.8, 7.5) | 75.4 | 4.09 dd (9.0, 7.8) | 75.4 |
| 3′ | 4.17 dd (8.7, 8.7) | 78.7 | 4.29 dd (8.8, 8.8) | 82.9 | 4.28 dd (9.0, 9.0) | 77.9 |
| 4′ | 4.23 m | 71.3 | 4.13 m | 69.7 | 4.48 dd (9.0, 9.7) | 73.3 |
| 5′ | 4.38 dd (11.2, 5.1) 3.78 dd (11.2, 10.2) | 67.2 | 4.34 m 3.73 dd (10.6, 10.2) | 66.9 | 4.61 d (9.7) | 77.3 |
| 6′ | 170.9 | |||||
| 6′-OMe | 3.73 s | 52.1 | ||||
| 1″ | 6.27 d (1.2) | 102.7 | ||||
| 2″ | 4.79 dd (3.3, 1.2) | 72.6 | ||||
| 3″ | 4.60 dd (9.3, 3.3) | 72.7 | ||||
| 4″ | 4.34 dd (9.3, 9.3) | 74.1 | ||||
| 5″ | 4.98 m | 69.9 | ||||
| 6″ | 1.67 d (6.2) | 18.7 |
| Compound | MIC50 (μM) ± SD | |
|---|---|---|
| M. gypseum | T. rubrum | |
| 1 | 105.0 ± 0.6 | >300 |
| 3 | 128.1 ± 1.4 | >300 |
| 6 | 7.1 ± 1.2 | 14.3 ± 2.1 |
| 12 | 260.1 ± 2.3 | >300 |
| 15 | 46.8 ± 0.1 | >300 |
| 16 | 120.7 ± 1.4 | >300 |
| 18 | 29.3 ± 3.4 | >300 |
| 19 | 34.9 ± 1.2 | >300 |
| 21 | 6.7 ± 2.1 | 13.4 ± 1.1 |
| 22 | 40.3 ± 0.5 | >300 |
| 23 | 39.9 ± 1.2 | >300 |
| 24 | 6.8 ± 3.2 | 11.9 ± 0.3 |
| 25 | 11.1 ± 2.4 | 13.0 ± 1.3 |
| 26 | 22.0 ± 0.9 | >300 |
| terbinafine hydrochloride (positive control) | 0.008 ± 0.373 | 1.647 ± 0.101 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Fu, Y.; Yang, J.; Li, X.-N.; San, M.M.; Oo, T.N.; Wang, Y.; Yang, X. Triterpenoids and Their Glycosides from Glinus Oppositifolius with Antifungal Activities against Microsporum Gypseum and Trichophyton Rubrum. Molecules 2019, 24, 2206. https://doi.org/10.3390/molecules24122206
Zhang D, Fu Y, Yang J, Li X-N, San MM, Oo TN, Wang Y, Yang X. Triterpenoids and Their Glycosides from Glinus Oppositifolius with Antifungal Activities against Microsporum Gypseum and Trichophyton Rubrum. Molecules. 2019; 24(12):2206. https://doi.org/10.3390/molecules24122206
Chicago/Turabian StyleZhang, Dongdong, Yao Fu, Jun Yang, Xiao-Nian Li, Myint Myint San, Thaung Naing Oo, Yuehu Wang, and Xuefei Yang. 2019. "Triterpenoids and Their Glycosides from Glinus Oppositifolius with Antifungal Activities against Microsporum Gypseum and Trichophyton Rubrum" Molecules 24, no. 12: 2206. https://doi.org/10.3390/molecules24122206
APA StyleZhang, D., Fu, Y., Yang, J., Li, X.-N., San, M. M., Oo, T. N., Wang, Y., & Yang, X. (2019). Triterpenoids and Their Glycosides from Glinus Oppositifolius with Antifungal Activities against Microsporum Gypseum and Trichophyton Rubrum. Molecules, 24(12), 2206. https://doi.org/10.3390/molecules24122206