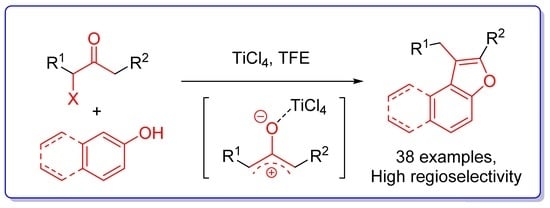
One-Step Regioselective Synthesis of Benzofurans from Phenols and α-Haloketones
Abstract
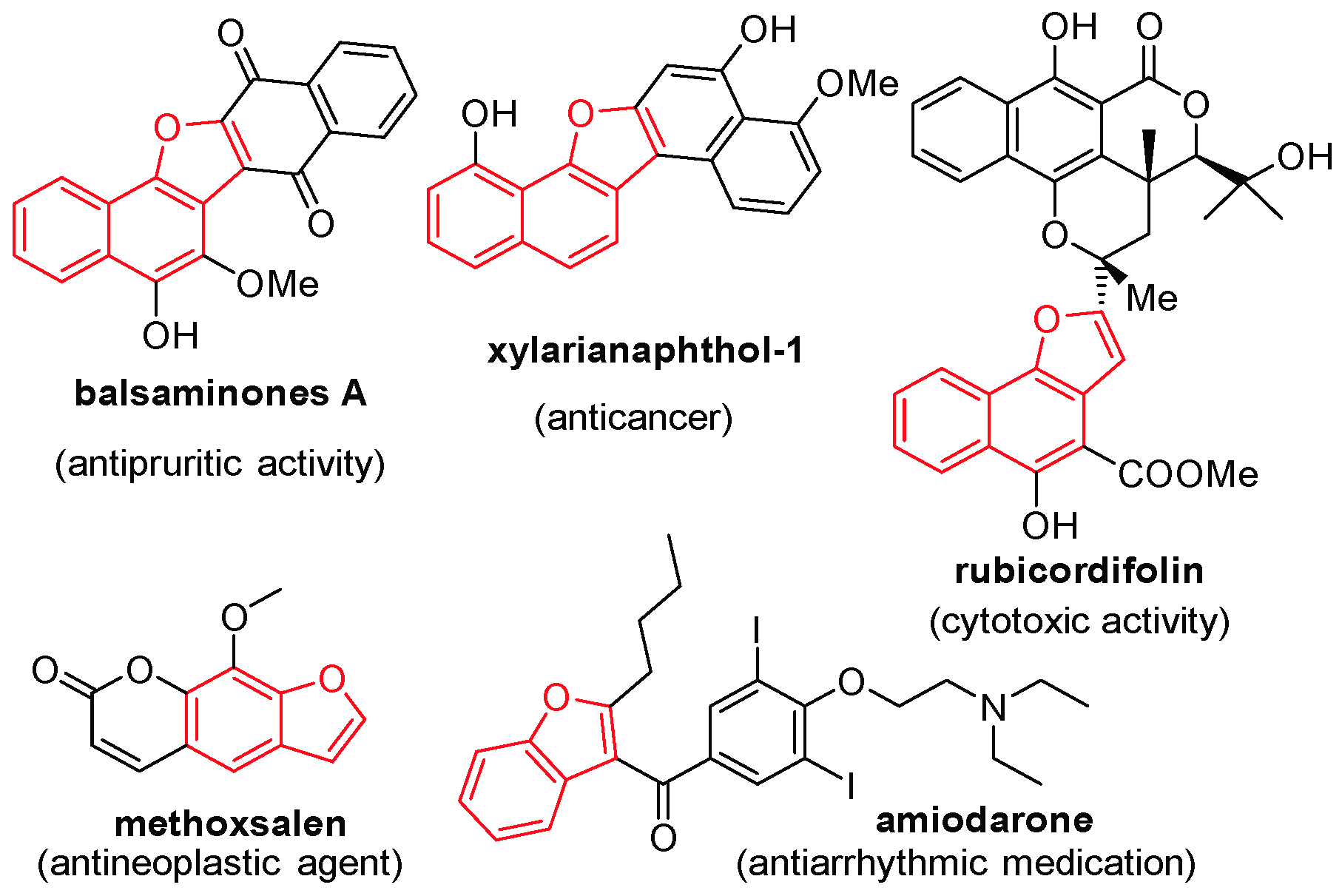
:1. Introduction
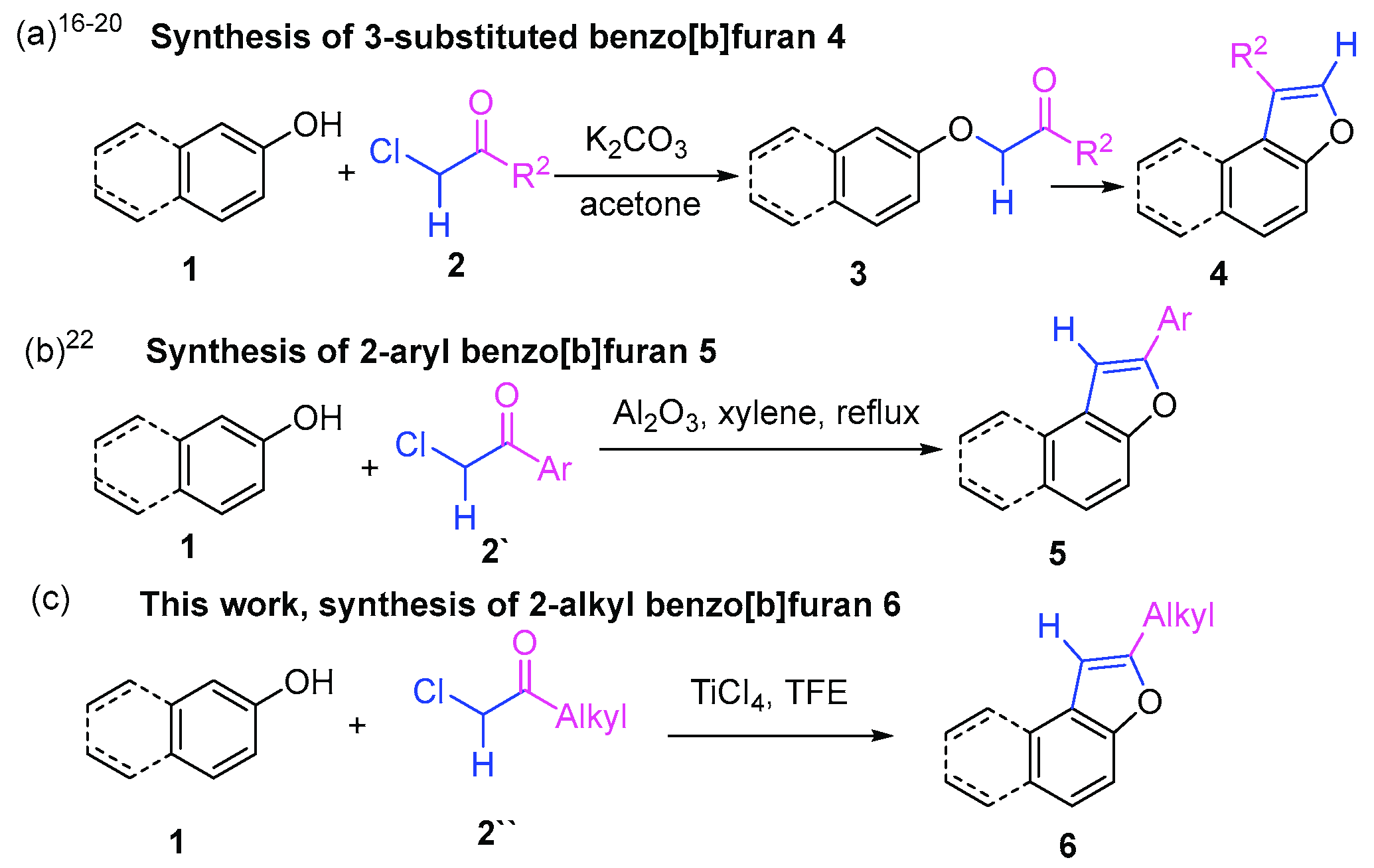
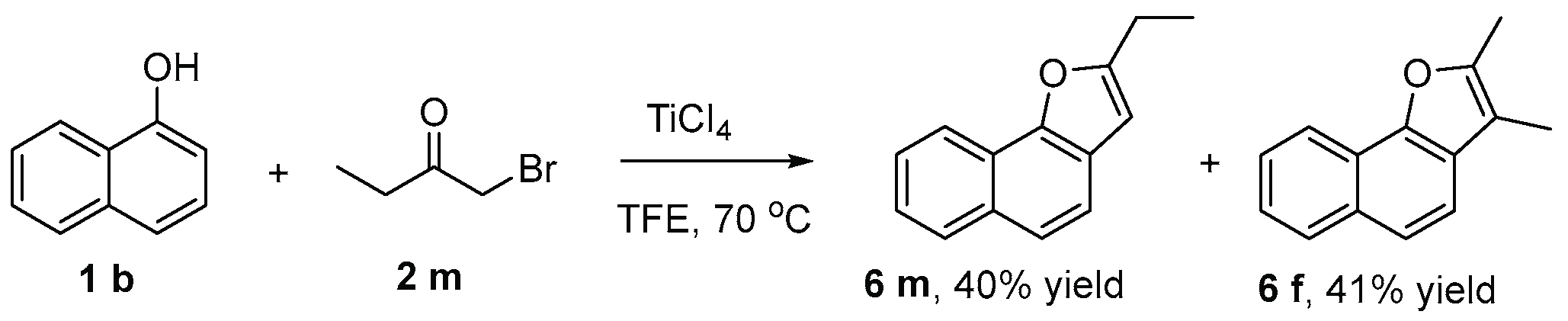
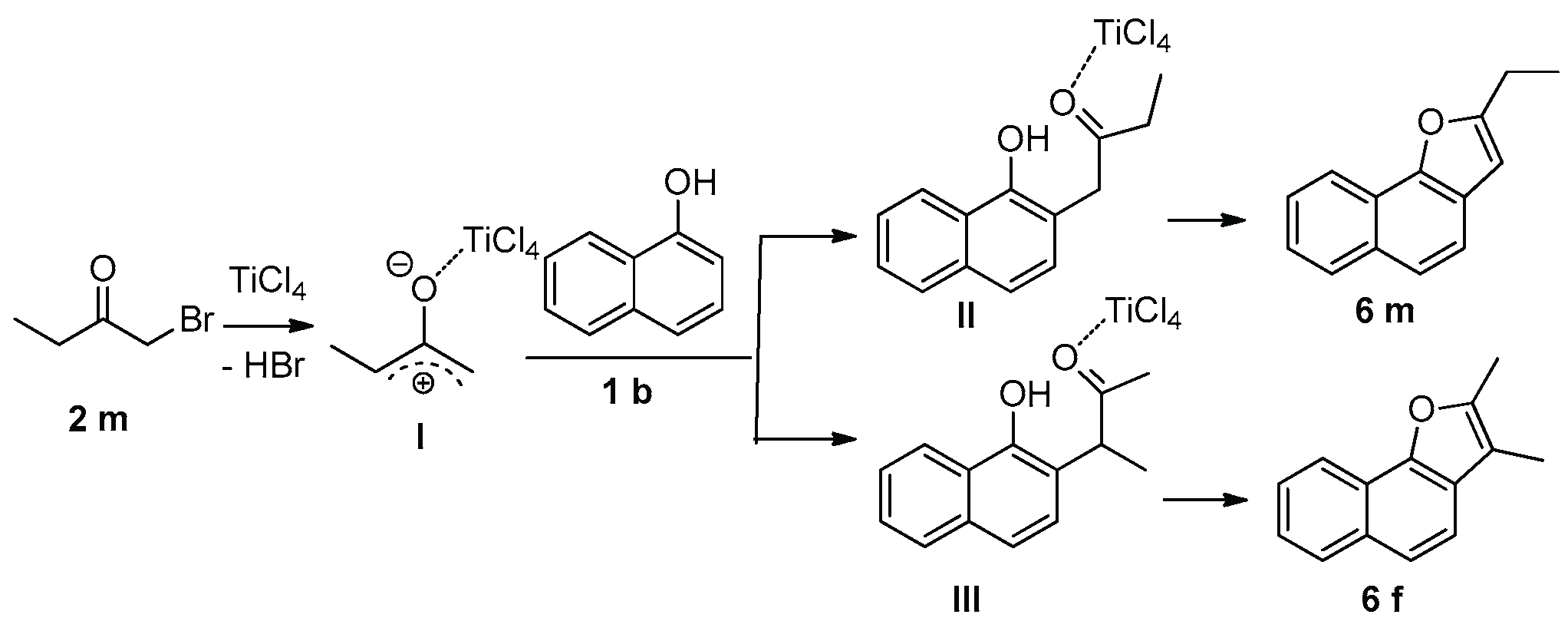
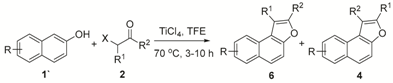
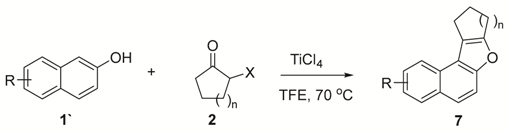
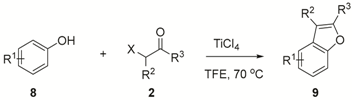
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. General Procedure for the Reaction between Phenol and α-Haloketone
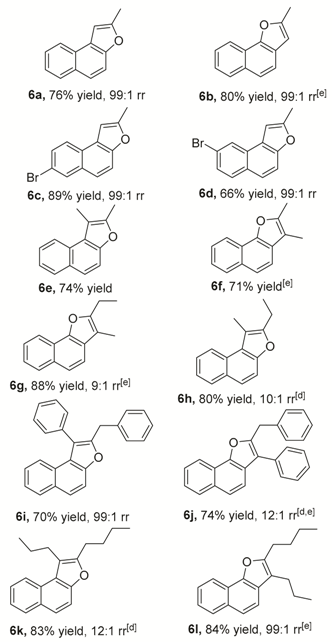
3.2.1. Characterizations of Naphthofuran 6 (Table 2)
3.2.2. Characterizations of Naphthofuran7 (Table 3)
3.2.3. Characterizations of Benzofuran 9 (Table 4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dawood, K.M. Benzofuran derivatives: A patent review. Expert Opin. Ther. Pat. 2013, 23, 1133–1156. [Google Scholar] [CrossRef] [PubMed]
- Boto, A.; Alvarez, L. Furan and its derivatives. In Heterocycles in Natural Product Synthesis; Wiley-VCHVerlagGmbH&Co.KGaA: Weinheim, Germany, 2011; pp. 97–152. [Google Scholar]
- Smith, D.T.; Vitaku, E.; Njardarson, J.T. Dearomatization approach to 2-trifluoromethylated benzofuran and dihydrobenzofuran products. Org. Lett. 2017, 19, 3508–3511. [Google Scholar] [CrossRef] [PubMed]
- Radadiya, A.; Shah, A. Bioactive benzofuran derivatives: An insight on lead developments, radioligands and advances of the last decade. Eur. J. Med. Chem. 2015, 97, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Negi, A.S.; Kumar, J.K.; Faridi, U.; Sisodia, B.S.; Darokar, M.P.; Luqman, S.; Khanuja, S.P.S. Synthesis of 1-(3′,4′,5′-trimethoxy) phenyl naphtho[2,1b]furan as a novel anticancer agent. Bioorg. Med. Chem. Lett. 2006, 16, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Mathiyazhagan, K.; Kumaran, A.; Arjun, P. Isolation of natural naphthoquinones from juglans regia and in vitro antioxidant and cytotoxic studies of naphthoquinones and the synthetic naphthofuran derivatives. Russ. J. Bioorg Chem 2018, 44, 346–353. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, G.; Srivastava, S.; Verma, S.; Negi, A.S.; Sharma, N. Investigation of naphthofuran moiety as potential dual inhibitor against BACE-1 and GSK-3β: Molecular dynamics simulations, binding energy, and network analysis to identify first-in-class dual inhibitors against Alzheimer’s disease. J. Mol. Model. 2017, 16, 911–914. [Google Scholar] [CrossRef]
- Cozza, G.; Sarno, S.; Ruzzene, M.; Girardi, C.; Orzeszko, A.; Kazimierczuk, Z.; Zagotto, G.; Bonaiuto, E.; Di Paolo, M.L.; Pinna, L.A. Exploiting the repertoire of CK2 inhibitors to target DYRK and PIM kinases. Biochim. Et Biophys. Acta Proteins Proteom. 2013, 1834, 1402–1409. [Google Scholar] [CrossRef]
- Le Guével, R.; Oger, F.; Lecorgne, A.; Dudasova, Z.; Chevance, S.; Bondon, A.; Barath, P.; Simonneaux, G.; Salbert, G. Identification of small molecule regulators of the nuclear receptor HNF4α based on naphthofuran scaffolds. Biorg. Med. Chem. 2009, 17, 7021–7030. [Google Scholar] [CrossRef]
- Phan, P.-T.T.; Nguyen, T.-T.T.; Nguyen, H.-N.T.; Le, B.-K.N.; Vu, T.T.; Tran, D.C.; Pham, T.-A.N. Synthesis and bioactivity evaluation of novel 2-salicyloylbenzofurans as antibacterial agents. Molecules 2017, 22, 687. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, X.; Gao, H.; Wan, C.; Rao, G.; Mao, Z. Design, synthesis, and biological evaluation of novel benzofuran derivatives bearing N-aryl piperazine moiety. Molecules 2016, 21, 1684. [Google Scholar] [CrossRef]
- Kotoku, N.; Higashimoto, K.; Kurioka, M.; Arai, M.; Fukuda, A.; Sumii, Y.; Sowa, Y.; Sakai, T.; Kobayashi, M. Xylarianaphthol-1, a novel dinaphthofuran derivative, activates p21 promoter in a p53-independent manner. Bioorg. Med. Chem. Lett. 2014, 24, 3389–3391. [Google Scholar] [CrossRef] [PubMed]
- Lumb, J.-P.; Trauner, D. Biomimetic synthesis and structure elucidation of rubicordifolin, a cytotoxic natural product from rubia cordifolia. J. Am. Chem. Soc. 2005, 127, 2870–2871. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, C.; Mangalagiu, I.; Isac, D.L.; Airinei, A.; Zbancioc, G. A new pathway for the synthesis of a new class of blue fluorescent benzofuran derivatives. Molecules 2018, 23, 1968. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jiang, P.; Guo, M.; Yang, J. Synthesis of new 2-arylbenzo[b]furan derivatives via palladium-catalyzed Suzuki cross-coupling reactions in aqueous media. Molecules 2018, 23, 2450. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.-J.; Xie, J.-H.; Li, Y.-L.; Liu, S.; Zhou, Q.-L. Enantioselective synthesis of chiral β-aryloxy alcohols by ruthenium-catalyzed ketone hydrogenation via dynamic kinetic resolution (DKR). Adv. Synth. Catal. 2010, 352, 81–84. [Google Scholar] [CrossRef]
- Ando, K.; Kawamura, Y.; Akai, Y.; Kunitomo, J.-I.; Yokomizo, T.; Yamashita, M.; Ohta, S.; Ohishi, T.; Ohishi, Y. Preparation of 2-, 3-, 4- and 7-(2-alkylcarbamoyl-1-alkylvinyl)benzo[b]furans and their BLT1 and/or BLT2 inhibitory activities. Org. Biomol. Chem. 2008, 6, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, J.; Wang, B.; Xiao, X.; Gan, Z.; Tang, Q. Titanium tetrachloride promoted cyclodehydration of aryloxyketones: Facile synthesis of benzofurans and naphthofurans with high regioselectivity. Tetrahedron Lett. 2019, 60, 1337–1340. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, J.; Jing, H.; Liang, Y. Novel one-pot synthesis of 3-phenylnaphtho[2,3-b]furan and 3-phenylbenzofurans under microwave irradiation and solvent-free conditions. Synth. Commun. 2009, 39, 4079–4087. [Google Scholar] [CrossRef]
- Wang, H.-S.; Chan, C.-K.; Chang, M.-Y. Ga(OTf)3-mediated synthesis of substituted benzofurans. Tetrahedron 2016, 72, 5132–5141. [Google Scholar] [CrossRef]
- Erian, A.; Sherif, S.; Gaber, H. The chemistry of α-haloketones and their utility in heterocyclic synthesis. Molecules 2003, 8, 793. [Google Scholar] [CrossRef]
- Arias, L.; Vara, Y.; Cossío, F.P. Regioselective preparation of benzo[b]furans from phenols and α-bromoketones. J. Org. Chem. 2012, 77, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Satake, K.; Okamoto, H.; Kimura, M. Electrophilic behavior of the π delocalized azepinium ion: Friedel–Crafts reactions with benzenes and five-membered aromatic heterocycles. Org. Lett. 2005, 7, 5215–5218. [Google Scholar] [CrossRef] [PubMed]
- Satake, K.; Kubota, Y.; Cordonier, C.E.J.; Okamoto, H.; Kimura, M. Synthesis of a delocalized azepinium ion and investigation of its electrophilic character. Angew Chem. Int. Ed. 2004, 43, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Lu, D.; Peng, Y.; Tang, Q. Paal-Knorr furan synthesis using titanium tetrachloride as dehydrating agent: A concise furan synthesis from α-haloketones and β-dicarbonyl compounds. Asian J. Org. Chem. 2017, 6, 1546–1550. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, H.; Hu, J.; Wang, R.; Tang, Q. Efficient catalytic-free method to produce alpha-aryl cycloalkanones through highly chemoselective coupling of aryl compounds with oxyallyl cations. RSC Adv. 2014, 4, 17370–17377. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, X.; Tiwari, B.; Chi, Y.R. Addition of indoles to oxyallyl cations for facile access to α-indole carbonyl compounds. Org. Lett. 2012, 14, 1922–1925. [Google Scholar] [CrossRef] [PubMed]
- Laskovics, F.M.; Schulman, E.M. Mechanism of the reaction of hexachloroacetone with enamines. A new, convenient synthesis of .alpha.-chloro ketones, .beta.-chloro enamines, and allylic chloro enamines. J. Am. Chem Soc. 1977, 99, 6672–6677. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. A mild and efficient procedure for alpha-bromination of ketones using N-bromosuccinimide catalysed by ammonium acetate. Chem. Commun. 2004, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Li, Z. Direct synthesis of 2-methylbenzofurans from calcium carbide and salicylaldehyde p-tosylhydrazones. Org. Lett. 2018, 20, 2342–2345. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Matsui, K.; Shibuya, M. A combined experimental and computational study on the cycloisomerization of 2-ethynylbiaryls catalyzed by dicationic arene ruthenium complexes. Chem. A Eur. J. 2015, 21, 7245–7255. [Google Scholar] [CrossRef]
- Sharma, U.; Naveen, T.; Maji, A.; Manna, S.; Maiti, D. Palladium-catalyzed synthesis of benzofurans and coumarins from phenols and olefins. Angew Chem. Int. Ed. 2013, 52, 12669–12673. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Biswas, S. Brønsted acid catalyzed functionalization of aromatic alcohols through Nnucleophilic substitution of hydroxyl group. J. Org. Chem. 2016, 81, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-T.; Nan, W.-H.; Luo, Q.-L. Metal-free sequential reaction via a propargylation, annulation and isomerization sequence for the one-pot synthesis of 2,3-disubstituted benzofurans. Rsc Adv. 2014, 4, 34774–34779. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Shafiq, Z.; Wu, Y.-C.; Wang, D.; Chen, Y.-J. InCl3-catalyzed propargylation of indoles and phenols with propargylic acetates: Application to the syntheses of benzofurans and naphthofurans. Synthesis 2007, 2007, 1961–1969. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kwon, K.-H.; Yi, C.S. Dehydrative C–H alkylation and alkenylation of phenols with alcohols: Expedient synthesis for substituted phenols and benzofurans. J. Am. Chem. Soc. 2012, 134, 7325–7328. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Santra, S.; Mondal, P.; Kundu, D.; Hajra, A. Diversified synthesis of furans by coupling between enols/1,3-dicarbonyl compounds and nitroolefins: Direct access to dioxa[5]helicenes. Chem. Asian J. 2015, 10, 2525–2536. [Google Scholar] [CrossRef]
- Orovecz, O.; Kovács, P.; Kolonits, P.; Kaleta, Z.; Párkányi, L.; Szabó, É.; Novák, L. Rearrangement of allyl aryl ethers; VI: Reaction of naphthols with cycloalkadienes. Synthesis 2003, 2003, 1043–1048. [Google Scholar]
- Gao, H.; Xu, Q.-L.; Keene, C.; Kürti, L. Scalable, Transition-metal-free direct oxime O-arylation: Rapid access to O-arylhydroxylamines and substituted benzo[b]furans. Chem. A Eur. J. 2014, 20, 8883–8887. [Google Scholar] [CrossRef]
- Alemagna, A.; Baldoli, C.; Del Buttero, P.; Licandro, E.; Maiorana, S. Nucleophilic aromatic substitution on tricarbonylchromium-complexed haloarenes: Synthesis of O-aryloximes and their cyclization to benzofurans. Synthesis 1987, 1987, 192–196. [Google Scholar] [CrossRef]
- Yagoubi, M.; Cruz, A.C.F.; Nichols, P.L.; Elliott, R.L.; Willis, M.C. Cascade palladium-catalyzed direct intramolecular arylation/alkene isomerization sequences: Synthesis of indoles and benzofurans. Angew Chem. Int. Ed. 2010, 49, 7958–7962. [Google Scholar] [CrossRef]
Sample Availability: All data generated and/or compound samples analyzed during this study are included in this article and are available from the corresponding author on reasonable request. |
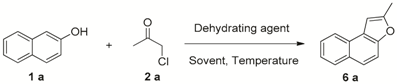
| Entry | Acid | Solvent | T (°C) | Yield of 6a b |
|---|---|---|---|---|
| 1 | HCl | TFE | 70 | NA |
| 2 | TsOH | TFE | 70 | NA |
| 3 | ZnCl2 | TFE | 70 | NA |
| 4 | AlCl3 | TFE | 70 | NA |
| 5 | TMSOTf | TFE | 70 | 14 |
| 6 | BF3.Et2O | TFE | 70 | 26 |
| 7 | Al2O3 | xylene | 150 | -- d |
| 8 | TiCl4 (1.0 eq) | TFE | 70 | 76% |
| 9 | TiCl4 (0.2 eq) | TFE | 70 | 16% |
| 10 | TiCl4 (0.5 eq) | TFE | 70 | 38% |
| 11 | TiCl4 (1.5 eq) | TFE | 70 | 71% |
| 12 | TiCl4 | TFE | 50 | 45% |
| 13 | TiCl4 | TFE | 25 | <10% |
| 14 | TiCl4 | CH2Cl2 | 40 | 34% |
| 15 | TiCl4 | Toluene | 110 | 38% |
| 16 | TiCl4 | Solvent c | reflux | NA |
 |
 |
 |
 |
 |
 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhang, Q.; Luo, J.; Gan, Z.; Jiang, W.; Tang, Q. One-Step Regioselective Synthesis of Benzofurans from Phenols and α-Haloketones. Molecules 2019, 24, 2187. https://doi.org/10.3390/molecules24112187
Wang B, Zhang Q, Luo J, Gan Z, Jiang W, Tang Q. One-Step Regioselective Synthesis of Benzofurans from Phenols and α-Haloketones. Molecules. 2019; 24(11):2187. https://doi.org/10.3390/molecules24112187
Chicago/Turabian StyleWang, Bingqiao, Qiu Zhang, Juan Luo, Zongjie Gan, Wengao Jiang, and Qiang Tang. 2019. "One-Step Regioselective Synthesis of Benzofurans from Phenols and α-Haloketones" Molecules 24, no. 11: 2187. https://doi.org/10.3390/molecules24112187
APA StyleWang, B., Zhang, Q., Luo, J., Gan, Z., Jiang, W., & Tang, Q. (2019). One-Step Regioselective Synthesis of Benzofurans from Phenols and α-Haloketones. Molecules, 24(11), 2187. https://doi.org/10.3390/molecules24112187