Screening of Phenanthroquinolizidine Alkaloid Derivatives for Inducing Cell Death of L1210 Leukemia Cells with Negative and Positive P-glycoprotein Expression
Abstract
1. Introduction
2. Results
2.1. Characterization of L1210 Cell Variants
2.2. Characterization of QD Derivatives
2.3. Effectiveness of QDs to Induce Cell Death of S, R and T Cells
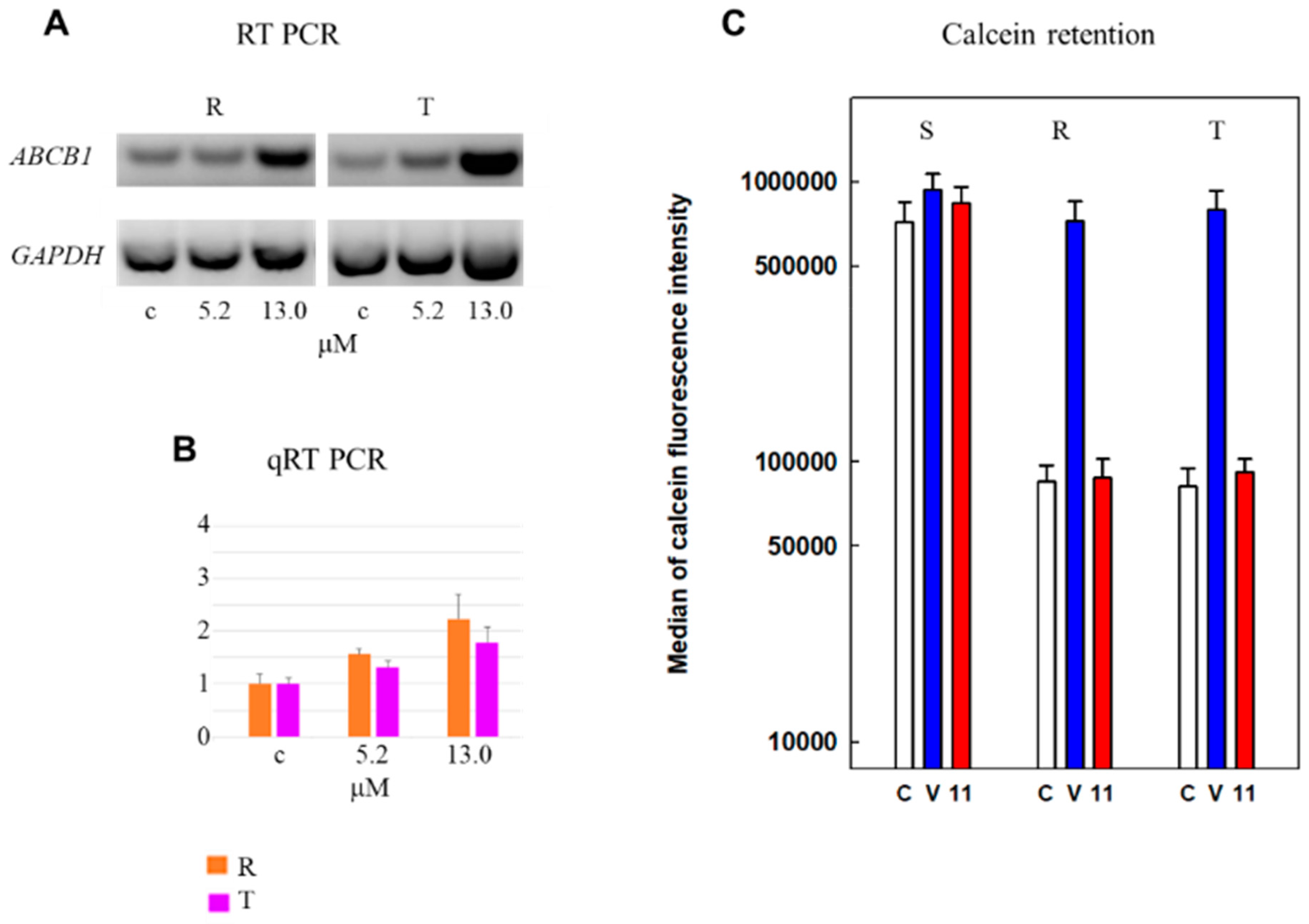
2.4. Effectiveness of Derivative 11 on Expression and Efflux Activity of P-gp in R and T Cells
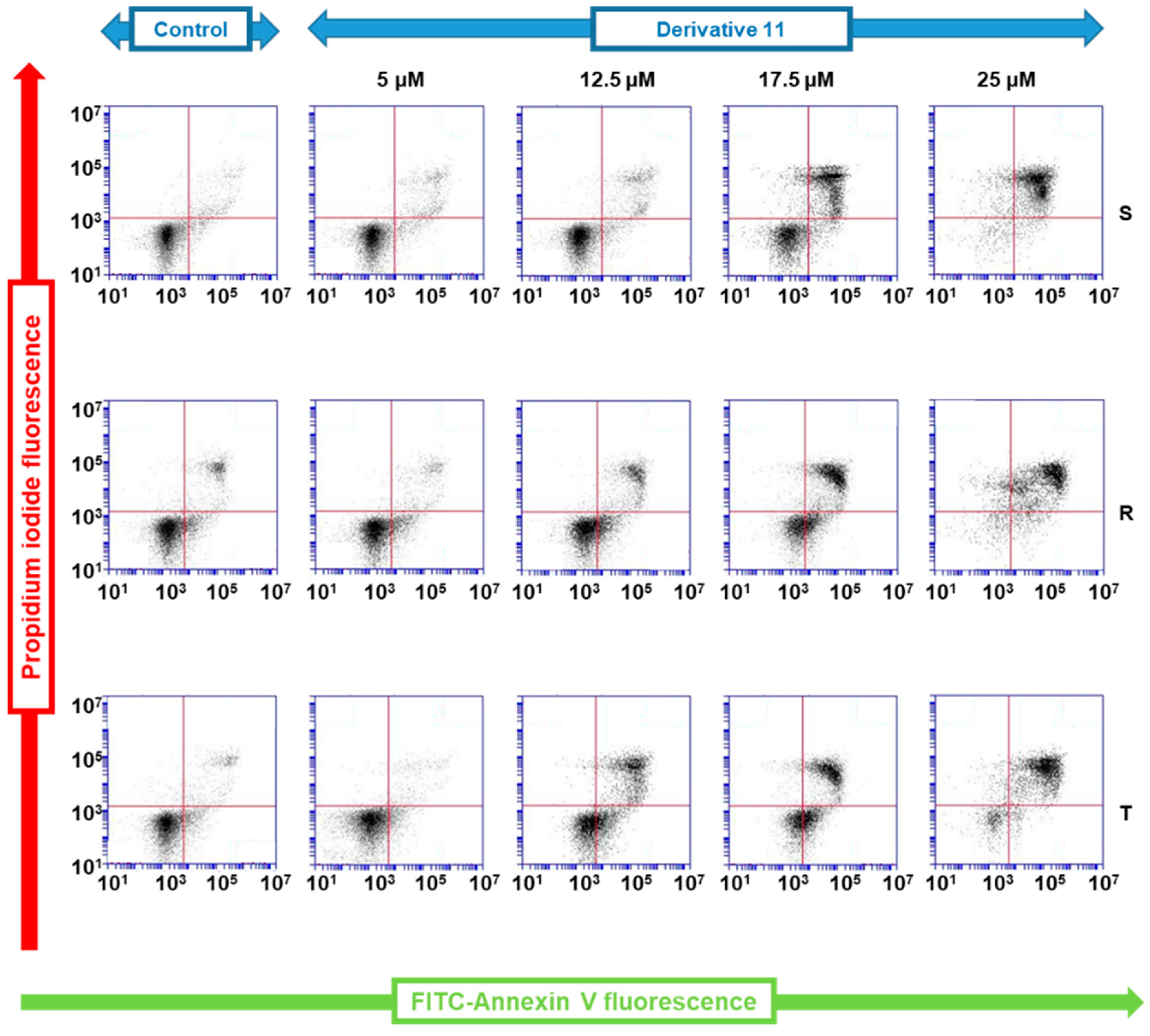
2.5. Measurements of the Effectiveness of Derivative 11 to Induce Cell Death Based on FITC-Annexin V and Propidium Iodide Double Staining
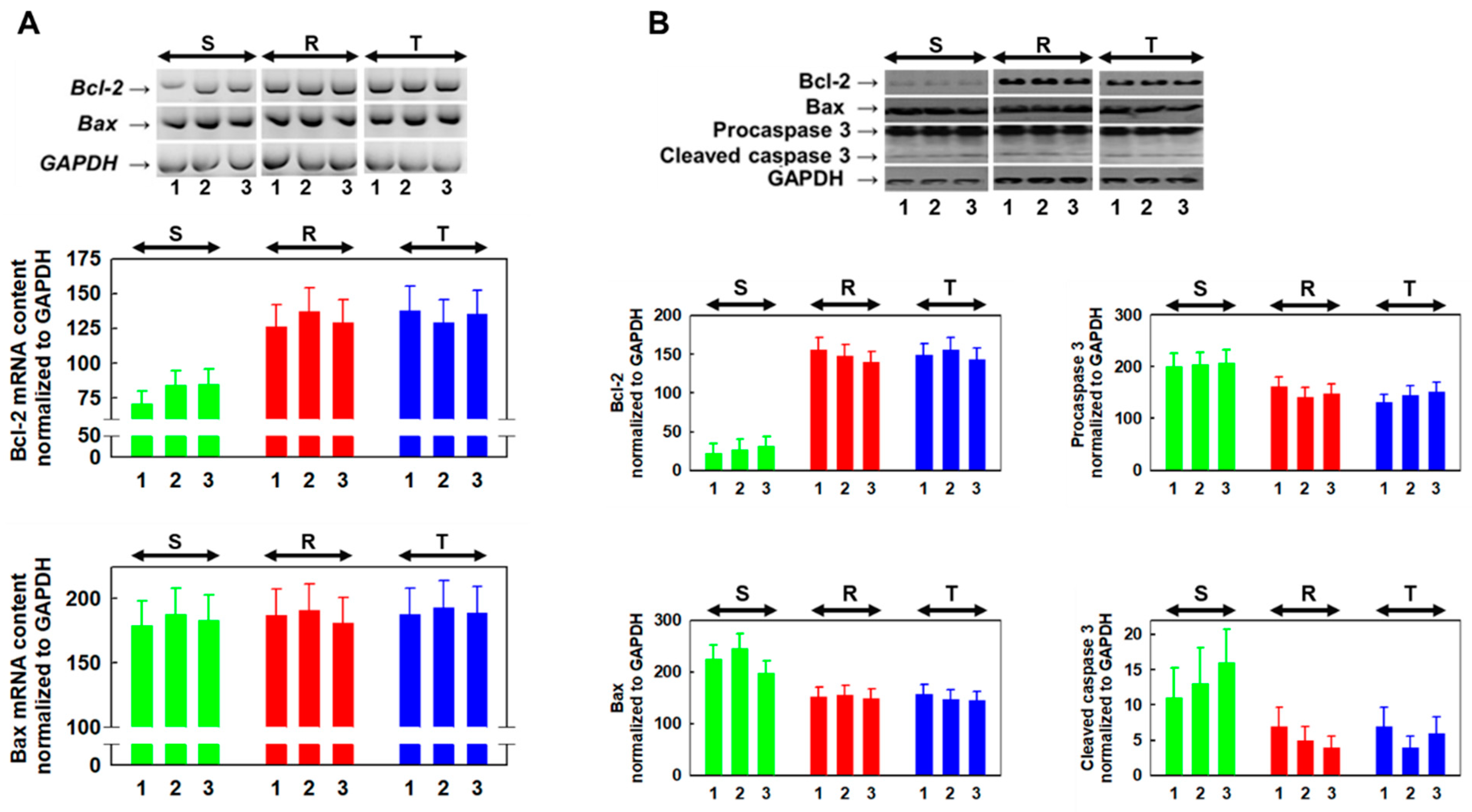
2.6. Derivative 11 Induced Changes Expression of Bcl-2 and Bax as Well as Expression and Activation of Caspase-3
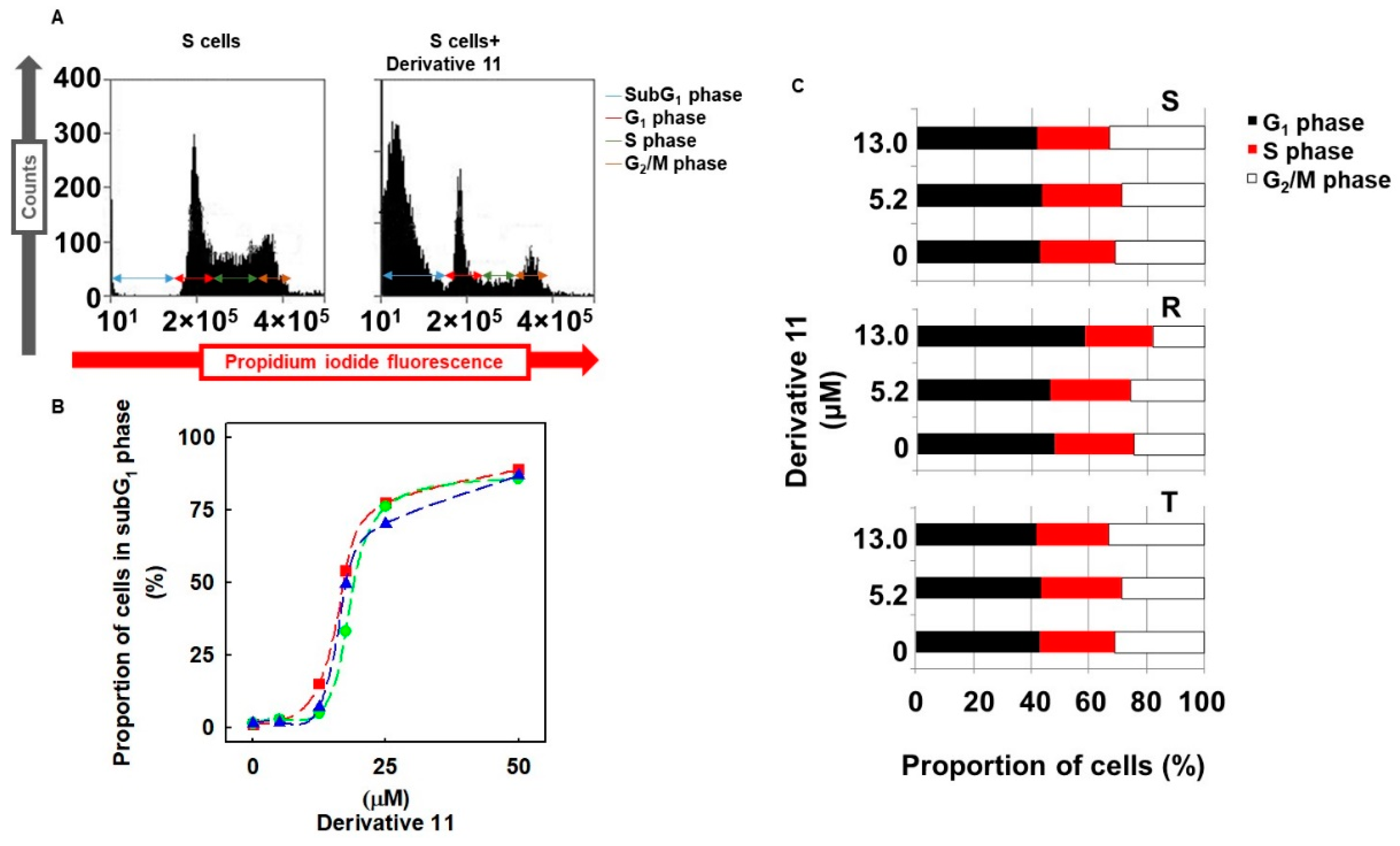
2.7. Effect of Derivative 11 on Cell Cycle Progression of S, R and T Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.1.1. Components of Cultivation Medium
4.1.2. Kits
4.1.3. PCR Primers
4.1.4. Antibodies
4.1.5. Other Chemicals
4.2. Cell Culture Conditions
4.3. Measurements of Cytotoxic Effects of Quinolizidine Derivatives on S, R and T Cells
4.4. Detection of the Effect of Derivative 11 on P-gp, Bcl-2 and Bax Transcription
4.4.1. RT-PCR
4.4.2. qRT-PCR
4.5. Western Blot Procedures
4.6. Estimation of P-gp Transport Activity by Calcein/AM Retention Assay
4.7. Measurement of Apoptosis/Necrosis Induced by Derivative 11 in S, R and T Cells
4.8. Detection of Cell Proportions in Different Phases of the Cell Cycle
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mangal, M.; Sagar, P.; Singh, H.; Raghava, G.P.; Agarwal, S.M. NPACT: Naturally Occurring Plant-based Anti-cancer Compound-Activity-Target database. Nucleic Acids Res. 2013, 41, D1124–D1129. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, G.; Viel, A.; Tumiotto, L.; Biscontin, G.; Rossi, C.; Boiocchi, M. Pleiotropic-resistant phenotype is a multifactorial phenomenon in human colon carcinoma cell lines. Br. J. Cancer 1991, 63, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, M.; Konkar, S.; Funk, R.S.; Krise, J.M.; Krise, J.P. A chemical strategy to manipulate the intracellular localization of drugs in resistant cancer cells. Biochemistry 2005, 44, 15743–15749. [Google Scholar] [CrossRef] [PubMed]
- Genilloud, O. Current challenges in the discovery of novel antibacterials from microbial natural products. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Stoye, A.; Opatz, T. Synthesis of (–)-Cryptopleurine by Combining Gold (I) Catalysis with a Free Radical Cyclization. Eur. J. Org. Chem. 2015, 2015, 2149–2156. [Google Scholar] [CrossRef]
- Wang, K.; Hu, Y.; Liu, Y.; Mi, N.; Fan, Z.; Wang, Q. Design, synthesis, and antiviral evaluation of phenanthrene-based tylophorine derivatives as potential antiviral agents. J. Agric. Food Chem. 2010, 58, 12337–12342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, M.; Wang, Y.; Li, Z.; Wang, L.; Han, G.; Chen, F.; Liu, Y.; Wang, K.; Zhang, A.; et al. Synthesis and SAR studies of phenanthroindolizidine and phenanthroquinolizidine alkaloids as potent anti-tumor agents. Eur. J. Med. Chem. 2012, 51, 250–258. [Google Scholar] [CrossRef]
- Jin, H.R.; Jin, S.Z.; Cai, X.F.; Li, D.; Wu, X.; Nan, J.X.; Lee, J.J.; Jin, X. Cryptopleurine targets NF-kappaB pathway, leading to inhibition of gene products associated with cell survival, proliferation, invasion, and angiogenesis. PLoS ONE 2012, 7, e40355. [Google Scholar]
- Yang, C.W.; Lee, Y.Z.; Hsu, H.Y.; Wu, C.M.; Chang, H.Y.; Chao, Y.S.; Lee, S.J. c-Jun-mediated anticancer mechanisms of tylophorine. Carcinogenesis 2013, 34, 1304–1314. [Google Scholar] [CrossRef]
- Kwon, Y.; Song, J.; Lee, H.; Kim, E.Y.; Lee, K.; Lee, S.K.; Kim, S. Design, Synthesis, and Biological Activity of Sulfonamide Analogues of Antofine and Cryptopleurine as Potent and Orally Active Antitumor Agents. J. Med. Chem. 2015, 58, 7749–7762. [Google Scholar] [CrossRef]
- Yang, X.; Shi, Q.; Lai, C.Y.; Chen, C.Y.; Ohkoshi, E.; Yang, S.C.; Wang, C.Y.; Bastow, K.F.; Wu, T.S.; Pan, S.L.; et al. Antitumor agents 295. E-ring hydroxylated antofine and cryptopleurine analogues as antiproliferative agents: Design, synthesis, and mechanistic studies. J. Med. Chem. 2012, 55, 6751–6761. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Peng, Z.G.; Zhang, X.; Cheng, X.Y.; Li, W.J.; Jiang, J.D.; Li, Y.H.; Song, D.Q. Synthesis and biological evaluation of 12-benzyl matrinic amide derivatives as a novel family of anti-HCV agents. Chin. Chem. Lett. 2016, 27, 1052–1057. [Google Scholar] [CrossRef]
- Olejníková, P.; Thomay, S.; Pagáč, T.; Ježíková, Z.; Marchalín, Š.; Šafař, P. Antimicrobial activity of newly synthesized thienoquinolizidines derivatives: Inspired by natural plant alkaloids. Chem. Pap. 2017, 71, 2375–2383. [Google Scholar] [CrossRef]
- Pagáč, T.; Šafář, P.; Marchalín, Š.; Ježíková, Z.; Balónová, B.; Šupolíková, M.; Nováková, E.; Kubíčková, J.; Šoral, M.; Sivý, J.; et al. Asymmetric synthesis and study of biological activity of (epi-) benzoanalogues of bioactive phenanthroquinolizidine alkaloids. Monatshefte Chem.-Chem. Mon. 2018, 149, 1865–1876. [Google Scholar]
- Dlugosz, A.; Janecka, A. ABC Transporters in the Development of Multidrug Resistance in Cancer Therapy. Curr. Pharm. Des. 2016, 22, 4705–4716. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Gibalova, L.; Seres, M.; Barancik, M.; Sulova, Z. New insight into p-glycoprotein as a drug target. Anticancer Agents Med. Chem. 2013, 13, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Sulova, Z.; Ditte, P.; Kurucova, T.; Polakova, E.; Rogozanova, K.; Gibalova, L.; Seres, M.; Skvarkova, L.; Sedlak, J.; Pastorek, J.; et al. The presence of P-glycoprotein in L1210 cells directly induces down-regulation of cell surface saccharide targets of concanavalin A. Anticancer Res. 2010, 30, 3661–3668. [Google Scholar] [PubMed]
- Polekova, L.; Barancik, M.; Mrazova, T.; Pirker, R.; Wallner, J.; Sulova, Z.; Breier, A. Adaptation of mouse leukemia cells L1210 to vincristine. Evidence for expression of P-glycoprotein. Neoplasma 1992, 39, 73–77. [Google Scholar]
- Pavlikova, L.; Seres, M.; Imrichova, D.; Hano, M.; Rusnak, A.; Zamorova, M.; Katrlik, J.; Breier, A.; Sulova, Z. The expression of P-gp in leukemia cells is associated with cross-resistance to protein N-glycosylation inhibitor tunicamycin. Gen. Physiol. Biophys. 2016, 35, 497–510. [Google Scholar] [CrossRef]
- Elefantova, K.; Lakatos, B.; Kubickova, J.; Sulova, Z.; Breier, A. Detection of the Mitochondrial Membrane Potential by the Cationic Dye JC-1 in L1210 Cells with Massive Overexpression of the Plasma Membrane ABCB1 Drug Transporter. Int. J. Mol. Sci. 2018, 19, 1985. [Google Scholar] [CrossRef]
- Bohacova, V.; Seres, M.; Pavlikova, L.; Kontar, S.; Cagala, M.; Bobal, P.; Otevrel, J.; Brtko, J.; Sulova, Z.; Breier, A. Triorganotin Derivatives Induce Cell Death Effects on L1210 Leukemia Cells at Submicromolar Concentrations Independently of P-glycoprotein Expression. Molecules 2018, 23, 1053. [Google Scholar] [CrossRef] [PubMed]
- Pavlikova, L.; Seres, M.; Hano, M.; Bohacova, V.; Sevcikova, I.; Kyca, T.; Breier, A.; Sulova, Z. L1210 Cells Overexpressing ABCB1 Drug Transporters Are Resistant to Inhibitors of the N- and O-glycosylation of Proteins. Molecules 2017, 22, 1104. [Google Scholar] [CrossRef] [PubMed]
- Šafař, P.; Marchalín, Š.; Prónayová, N.; Vrábel, V.; Lawson, A.M.; Othman, M.; Daich, A. Use of chiral-pool approach into epi-thieno analogues of the scarce bioactive phenanthroquinolizidine alkaloids. Tetrahedron 2016, 72, 3221–3231. [Google Scholar] [CrossRef]
- Seres, M.; Cholujova, D.; Bubencikova, T.; Breier, A.; Sulova, Z. Tunicamycin depresses P-glycoprotein glycosylation without an effect on its membrane localization and drug efflux activity in L1210 cells. Int. J. Mol. Sci. 2011, 12, 7772–7784. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, R.; Deng, J.; Elia, J.; McIntyre, C.; Mittar, D. Detection of Apoptosis Using the BD Annexin V FITC Assay on the BD FACSVerse™ System; BD Bioscience Application Note; BD Bioscience: San Jose, CA, USA, 2011; pp. 1–12. Available online: https://www.bdbiosciences.com/documents/BD_FACSVerse_Apoptosis_Detection_AppNote.pdf (accessed on 05 June 2019).
- Fruci, D.; Cho, W.C.; Nobili, V.; Locatelli, F.; Alisi, A. Drug Transporters and Multiple Drug Resistance in Pediatric Solid Tumors. Curr. Drug Metab. 2016, 17, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Salama, N.N.; Azab, A.K. The role of P-glycoprotein in drug resistance in multiple myeloma. Leuk. Lymphoma 2015, 56, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Manallack, D.T. The pK(a) Distribution of Drugs: Application to Drug Discovery. Perspect. Med. Chem. 2007, 1, 25–38. [Google Scholar]
- Bennion, B.J.; Be, N.A.; McNerney, M.W.; Lao, V.; Carlson, E.M.; Valdez, C.A.; Malfatti, M.A.; Enright, H.A.; Nguyen, T.H.; Lightstone, F.C.; et al. Predicting a Drug’s Membrane Permeability: A Computational Model Validated With in Vitro Permeability Assay Data. J. Phys. Chem. B 2017, 121, 5228–5237. [Google Scholar] [CrossRef]
- Ueno, S.; Yamazaki, R.; Ikeda, T.; Yaegashi, T.; Matsuzaki, T. Antitumor effect of a novel phenanthroindolizidine alkaloid derivative through inhibition of protein synthesis. Anticancer Res. 2014, 34, 3391–3397. [Google Scholar] [PubMed]
- Hano, M.; Tomasova, L.; Seres, M.; Pavlikova, L.; Breier, A.; Sulova, Z. Interplay between P-Glycoprotein Expression and Resistance to Endoplasmic Reticulum Stressors. Molecules 2018, 23, 337. [Google Scholar] [CrossRef] [PubMed]
- Tainton, K.M.; Smyth, M.J.; Jackson, J.T.; Tanner, J.E.; Cerruti, L.; Jane, S.M.; Darcy, P.K.; Johnstone, R.W. Mutational analysis of P-glycoprotein: Suppression of caspase activation in the absence of ATP-dependent drug efflux. Cell Death Differ. 2004, 11, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Gibalova, L.; Seres, M.; Rusnak, A.; Ditte, P.; Labudova, M.; Uhrik, B.; Pastorek, J.; Sedlak, J.; Breier, A.; Sulova, Z. P-glycoprotein depresses cisplatin sensitivity in L1210 cells by inhibiting cisplatin-induced caspase-3 activation. Toxicol. In Vitro 2012, 26, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Del Principe, M.I.; Del Poeta, G.; Maurillo, L.; Buccisano, F.; Venditti, A.; Tamburini, A.; Bruno, A.; Cox, M.C.; Suppo, G.; Tendas, A.; et al. P-glycoprotein and BCL-2 levels predict outcome in adult acute lymphoblastic leukaemia. Br. J. Haematol. 2003, 121, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Murad, H.; Hawat, M.; Ekhtiar, A.; AlJapawe, A.; Abbas, A.; Darwish, H.; Sbenati, O.; Ghannam, A. Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int. 2016, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Pastan, I.; Gottesman, M.M.; Ueda, K.; Lovelace, E.; Rutherford, A.V.; Willingham, M.C. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells. Proc. Natl. Acad. Sci. USA 1988, 85, 4486–4490. [Google Scholar] [CrossRef] [PubMed]
- Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 1986, 94, 57–63. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Turakova, K.; Pavlikova, L.; Messingerova, L.; Lakatos, B.; Breier, A.; Sulova, Z. Reduced UDP-glucose Levels Are Associated with P-glycoprotein Over-expression in L1210 Cells and Limit Glucosylceramide Synthase Activity. Anticancer Res. 2015, 35, 2627–2634. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
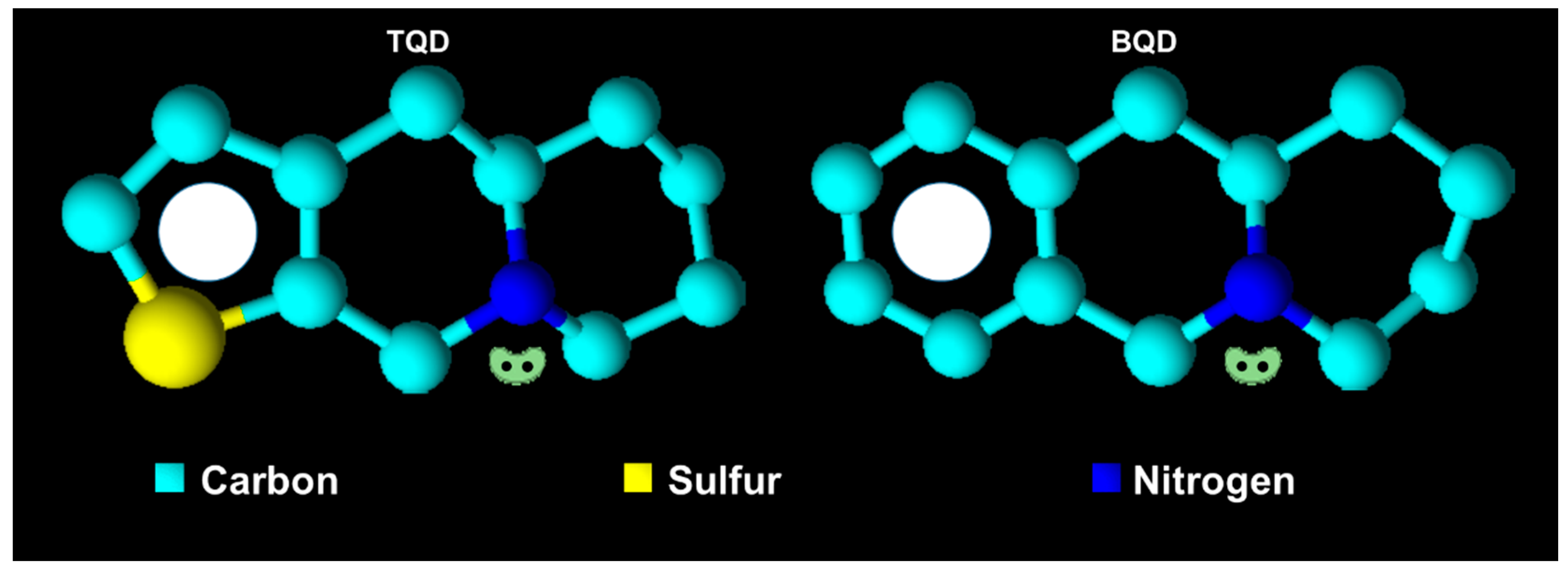
| (epi)-thieno analogs of phenanthroquinolizidine alkaloids (TQDs) | |||
| Derivative | Structure | Derivative | Structure |
| 11Ba1 Mw = 193.31 g/mol | 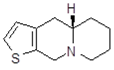 | 11Bb1 Mw = 192.31 g/mol | 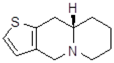 |
| 11Aa1 Mw = 207.29 g/mol | 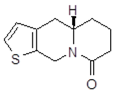 | 11Ab1 Mw = 207.29 g/mol | 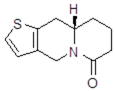 |
| A42 Mw = 335.25 g/mol | 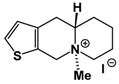 | B102 Mw = 235.25 g/mol | 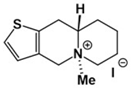 |
| B92 Mw = 351.25 g/mol | 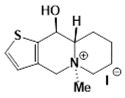 | trans10Bb1 Mw = 209.31 g/mol | 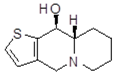 |
| trans10Aa1 Mw = 223.29 g/mol |  | trans10Ab1 Mw = 223.29 g/mol | 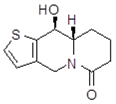 |
| cis10Aa1 Mw = 223.29 g/mol | 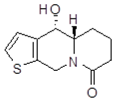 | cis10Ab1 Mw = 223.29 g/mol | 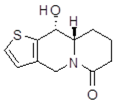 |
| 15a1 Mw = 221.28 g/mol | 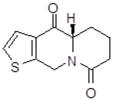 | 15b1 Mw = 221.29 g/mol | 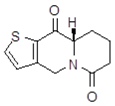 |
| 18Aa1 Mw = 263.33 g/mol |  | 18Ab1 Mw=265.33 g/mol | 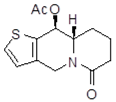 |
| (epi)benzo analogs of phenanthroquinolizidine alkaloids (BQDs) | |||
| 5a3 Mw = 217,27 g/mol | 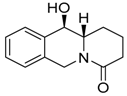 | 5b3 Mw = 217.27 g/mol |  |
| 6b3 Mw = 259.31 g/mol |  | 93 Mw = 307.43 g/mol | 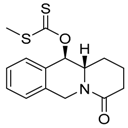 |
| 7a3 Mw = 203.29 g/mol | 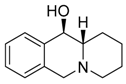 | 7b3 Mw = 203.29 |  |
| 103 Mw = 201.27 g/mol | 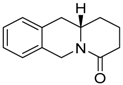 | 113 Mw = 187.29 g/mol | 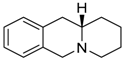 |
| (epi)-thieno analogs of phenanthroquinolizidine | |||
|---|---|---|---|
| Derivative | IC50 (µmol/l) | ||
| S | R | T | |
| 11Ba | 130 ± 9 | na | na |
| 11Bb | na | na | na |
| 11Aa | na | na | na |
| 11Ab | na | na | na |
| A4 | na | na | na |
| B10 | na | na | na |
| B9 | na | na | na |
| trans10Bb | na | na | na |
| trans10Aa | na | na | na |
| trans10Ab | na | na | na |
| cis10Aa | na | na | na |
| cis10Ab | na | na | na |
| 15a | na | na | na |
| 15b | na | na | na |
| 18Aa | na | na | na |
| 18Ab | na | na | na |
| (epi)-benzo analogs of phenanthroquinolizidine alkaloids | |||
| 5a | na | na | na |
| 5b | na | na | na |
| 6b | 192 ± 12 | 385 ± 16 | 385 ± 19 |
| 7a | na | na | na |
| 7b | na | na | na |
| 9 | 81 ± 5 | 81 ± 4 | 74 ± 3 |
| 10 | 490 ± 43 | na | na |
| 11 | 13,1 ± 2,5 | 13,4 ± 3,1 | 12,8 ± 2,7 |
| Derivative | Effective on (μΜ) | pKa1 | Log P2 | ||
|---|---|---|---|---|---|
| S | R | T | |||
| 11Ba | ≈130 | Na | Na | 8.89 | 2.93 ± 0.35 |
| 6b | ≈190 | ≈385 | ≈385 | - | 1.05 ± 0.62 |
| 9 | ≈80 | ≈80 | ≈74 | - | 2.52 ± 0.69 |
| 10 | ≈490 | Na | Na | - | 1.62 ± 0.56 |
| 11 | ≈13 | ≈13 | ≈13 | 9.20 | 3.25 ± 0.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubíčková, J.; Elefantová, K.; Pavlikova, L.; Cagala, M.; Šereš, M.; Šafář, P.; Marchalín, Š.; Ďurišová, K.; Boháčová, V.; Sulova, Z.; et al. Screening of Phenanthroquinolizidine Alkaloid Derivatives for Inducing Cell Death of L1210 Leukemia Cells with Negative and Positive P-glycoprotein Expression. Molecules 2019, 24, 2127. https://doi.org/10.3390/molecules24112127
Kubíčková J, Elefantová K, Pavlikova L, Cagala M, Šereš M, Šafář P, Marchalín Š, Ďurišová K, Boháčová V, Sulova Z, et al. Screening of Phenanthroquinolizidine Alkaloid Derivatives for Inducing Cell Death of L1210 Leukemia Cells with Negative and Positive P-glycoprotein Expression. Molecules. 2019; 24(11):2127. https://doi.org/10.3390/molecules24112127
Chicago/Turabian StyleKubíčková, Jana, Katarína Elefantová, Lucia Pavlikova, Martin Cagala, Mário Šereš, Peter Šafář, Štefan Marchalín, Kamila Ďurišová, Viera Boháčová, Zdena Sulova, and et al. 2019. "Screening of Phenanthroquinolizidine Alkaloid Derivatives for Inducing Cell Death of L1210 Leukemia Cells with Negative and Positive P-glycoprotein Expression" Molecules 24, no. 11: 2127. https://doi.org/10.3390/molecules24112127
APA StyleKubíčková, J., Elefantová, K., Pavlikova, L., Cagala, M., Šereš, M., Šafář, P., Marchalín, Š., Ďurišová, K., Boháčová, V., Sulova, Z., Lakatoš, B., Breier, A., & Olejníková, P. (2019). Screening of Phenanthroquinolizidine Alkaloid Derivatives for Inducing Cell Death of L1210 Leukemia Cells with Negative and Positive P-glycoprotein Expression. Molecules, 24(11), 2127. https://doi.org/10.3390/molecules24112127







