4.2. Syntheses and Spectral Data
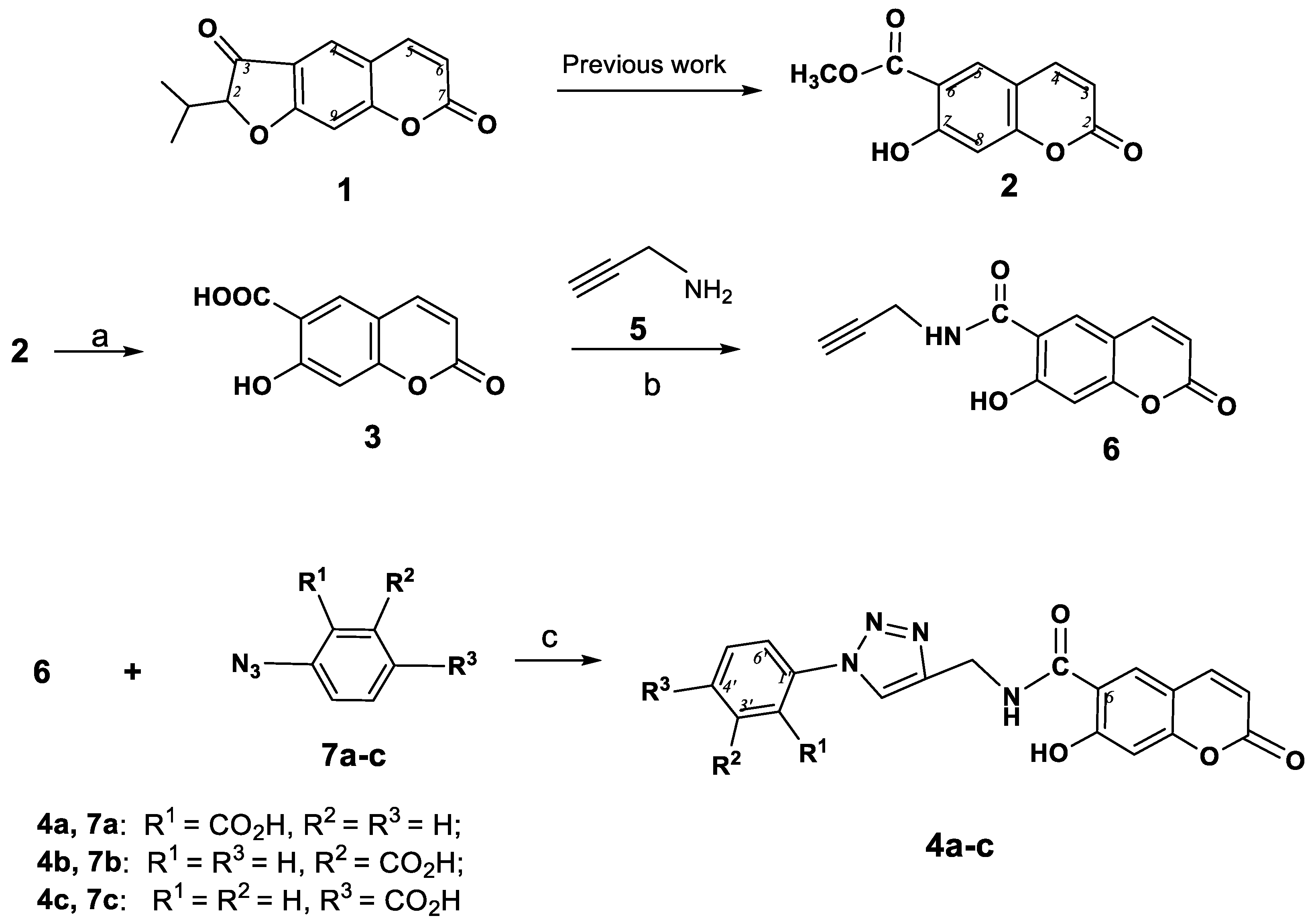
7-Hydroxy-2-oxo-N-(prop-2-ynyl)-2H-chromene-6-carboxamide (6) To a stirred solution of umbelliferone-6-carboxylic acid (3, 500 mg, 2 mmol) and hydroxybenzotriazole (135 mg, 1 mmol) in DMF (5 mL) was added propargylamine hydrochloride (5, 120 mg, 2.1 mmol) and Et3N (0.27 mL, 2 mmol) and the reaction mixture was left for 5 min. Then diisopropylcarbodiimide (300 mg, 2.4 mmol) was added and the mixture was stirred for 2 days. The reaction mixture was poured into a Petri dish to evaporate in air, the residue was dissolved in CH2Cl2 (10 mL) and acetone was added (5 mL). The precipitate was filtered, the solution was evaporated and residue was dried. The product was isolated by fractional precipitation from acetone. Compound 6 was obtained in 67% yield (320 mg). Colorless powder. М.р. 188 °С (dec.). IR (KBr, ν, cm−1): 3388, 3286, 3243, 3059, 2921, 2852, 2100, 1737, 1708, 1649, 1620, 1598, 1570, 1500, 1392, 1344, 1310, 1234, 1213, 1144, 911, 854, 837, 735, 688, 639, 628. UV (EtOH) λmax, (lgε): 222(3.22), 240 (3.35), 329 (2.89), 372 (2.88) nm. 1H-NMR (CDCl3 + CD3OD, 400 MHz, δH): 3.16 (s, 1Н, ≡СН), 4.20 (s, 2Н, СН2), 6.26 (d, J = 9.6 Hz, 1Н, Н-4), 6.83 (s, 1Н, Н-8), 7.76 (d, J = 9.6 Hz, 1Н, Н-3), 8.10 (s, 1Н, Н-5). 13C-NMR (CDCl3+CD3OD, 100 MHz, δC): 45.8, 71.0, 78.8, 103.9, 111.4, 112.6, 113.8, 129.3, 144.0, 157.1, 161.2, 162.4, 167.1 (С=O). Anal. calcd for C13H9NO4: C, 64.20; H, 3.73; N, 5.76; found: С 64.33; Н 3.77; N 5.65.
4.2.1. General Method for the Synthesis of Compounds 4a–c
A solution of the appropriate azidobenzoic acid 7a–с (35 mg, 0.14 mmol) in CH2Cl2 (10 mL) was mixed with a solution of sodium ascorbate (42 mg, 15 mol%, 0.021 mmol) and СuSO4·5H2O (1.2 mg, 5 mol%, 0.007 mmol) in Н2О (5 mL) and 6-(N-propynyl)carboxamidocoumarin (6, 20 mg, 0.14 mmol) was added while stirring. The reaction mixture was stirred for 3 h at room temperature and additional for 1 h at 40 °С. After the completion of the reaction, the mixture was diluted with Н2О (10 mL), the product was extracted with CH2Cl2 (4 × 10 mL), the combined extracts were dried over anhydrous MgSO4, and the solvent was removed at reduced pressure. The product was purified by column chromatography (eluent CHCl3).
2-(4-((7-Hydroxy-2-oxo-2H-chromene-6-carboxamido)methyl)-1H-1,2,3-triazol-1-yl)benzoic acid (4a). Colorless powder, m.p. 201–202 °C (ethanol). Yield 50%. IR (KBr, ν, cm−1): 3367, 3257, 3066, 2922, 2852, 2666, 1740, 1691, 1596, 1575, 1485, 1446, 1392, 1304, 1265, 1148, 1078, 1020, 908, 829, 796, 752, 687, 640. UV (EtOH) λmax, (lgε): 246 (3.45), 301 (2.76), 331 (3.51), 357 (3.23) nm. 1H-NMR (CDCl3+CD3OD, 400 MHz, δH): 4.93 (s, 2Н, СН2), 6.22 (d, J = 9.8 Hz, 1Н, Н-3), 6.82 (s, 1Н, Н-8), 7.13 (dd, J = 7.6, 7.2 Hz, 1Н, Н-4′′), 7.18 (1Н, d, J = 8.0 Hz, 1Н, Н-6′′), 7.48 (dd, J = 8.0, 7.2 Hz, 1Н, Н-5′′), 7.63 (d, J = 9.8 Hz, 1Н, Н-4), 7.78 (s, 1Н, Н-5), 7.88 (d, J = 7.6 Hz, 1Н, Н-3′′), 7.98 (s, 1Н, Н-5′). 13C-NMR (CDCl3+CD3OD, 100 MHz, δC): 44.5, 104.3, 111.8, 113.4, 119.8, 121.3, 123.0, 124.1, 125.9, 129.5, 130.7, 132.1, 135.9, 142.3, 143.8, 158.5, 160.5, 163.8, 167.6, 169.2. Anal. calcd for C20H14N4O6: C, 59.12; H, 3.47; N, 13.79; found: C, 58.88; H, 3.77; N, 14.14.
3-(4-((7-Hydroxy-2-oxo-2H-chromene-6-carboxamido)methyl)-1H-1,2,3-triazol-1-yl)benzoic acid (4b). Yellowish powder, m.p. 186–187 °C (hexane). Yield 64%. IR (KBr, ν, cm−1): 3427, 3095, 3062, 2923, 2852, 2665, 1738, 1687, 1631, 1600, 1581, 1456, 1305, 1236, 1153, 1113, 1097, 1056, 1008, 931, 912, 893, 800, 795, 754, 698, 673. UV (EtOH) λmax, (lgε): 225 (3.66), 246 (3.53), 301 (3.23), 336 (3.22), 347 (3.18), 357 (3.23) nm. 1H-NMR (CDCl3, 400 MHz, δH): 4.97 (s, 2Н, СН2), 6.30 (d, J = 9.8 Hz, 1Н, Н-3), 6.87 (s, 1Н, Н-8), 7.45 (d, J = 8.2 Hz, 1Н, Н-6′′), 7.47 (dd, J = 8.2, 7.6 Hz, 1Н, Н5′′), 7.62 (d, J = 9.8 Hz, 1Н, Н-4), 7.77 (s, 1Н, Н-5), 7.88 (d, J = 7.6 Hz, 1Н, Н-4′′), 8.01 (s, 2Н, Н-5′, 2′′), 11.19 (br.s, 3H, OH, NH). 13C-NMR (CDCl3, 100 MHz, δC): 44.2, 104.9, 111.9, 114.2, 120.4, 121.7, 123.4, 124.3, 126.9, 127.1, 130.0, 130.7, 135.7, 140.8, 143.1, 158.9, 160.2, 161.0 (С-7), 164.2, 170.8. Anal. calcd for C20H14N4O6: C, 59.12; H, 3.47; N, 13.79; found: C, 59.55; H, 3.49; N, 13.43.
4-(4-((7-Hydroxy-2-oxo-2H-chromene-6-carboxamido)methyl)-1H-1,2,3-triazol-1-yl)benzoic acid (4c). Grayish powder, m.p. 203–204 °C (hexane). Yield 60%. IR (KBr, ν, cm−1): 3433, 3062, 2958, 2924, 2852, 2673, 1734, 1676, 1630, 1600, 1579, 1448, 1427, 1392, 1288, 1236, 1215, 1143, 1113, 1076, 946, 904, 858, 827, 796, 768, 732. UV (EtOH) λmax, (lgε): 225 (3.66), 246 (3.78), 270 (3.65), 293 (3.53), 324 (3.36), 351 (3.21) nm. 1H-NMR (CDCl3, 400 MHz, δH): 4.95 (s, 2Н, СН2), 6.28 (d, J = 9.8 Hz, 1Н, Н-3), 6.87 (s, 1Н, Н-8), 7.10 (d, J = 8.4 Hz, 2Н, Н-2′′,6′′), 7.61 (d, J = 9.8 Hz, 1Н, Н-4), 7.98 (s, 1Н, Н-5), 8.02 (s, 1Н, Н-5′), 8.03 (br.s, 1Н, NН), 8.10 (d, J = 8.4 Hz, 2Н, Н-3′′,5′′), 11.19 (br.s, 2Н, ОН). 13C-NMR (CDCl3, 100 MHz, δC): 42.5, 104.9, 111.9, 114.1, 118.9 (2C), 121.8, 125.3, 125.7, 127.6, 130.7, 132.1 (2С), 143.0, 145.7, 158.9, 160.1, 164.2, 169.5, 170.7. Anal. calcd for C20H14N4O6: C, 59.12; H, 3.47; N, 13.79; found: C, 59.16; H, 3.14; N, 13.42.
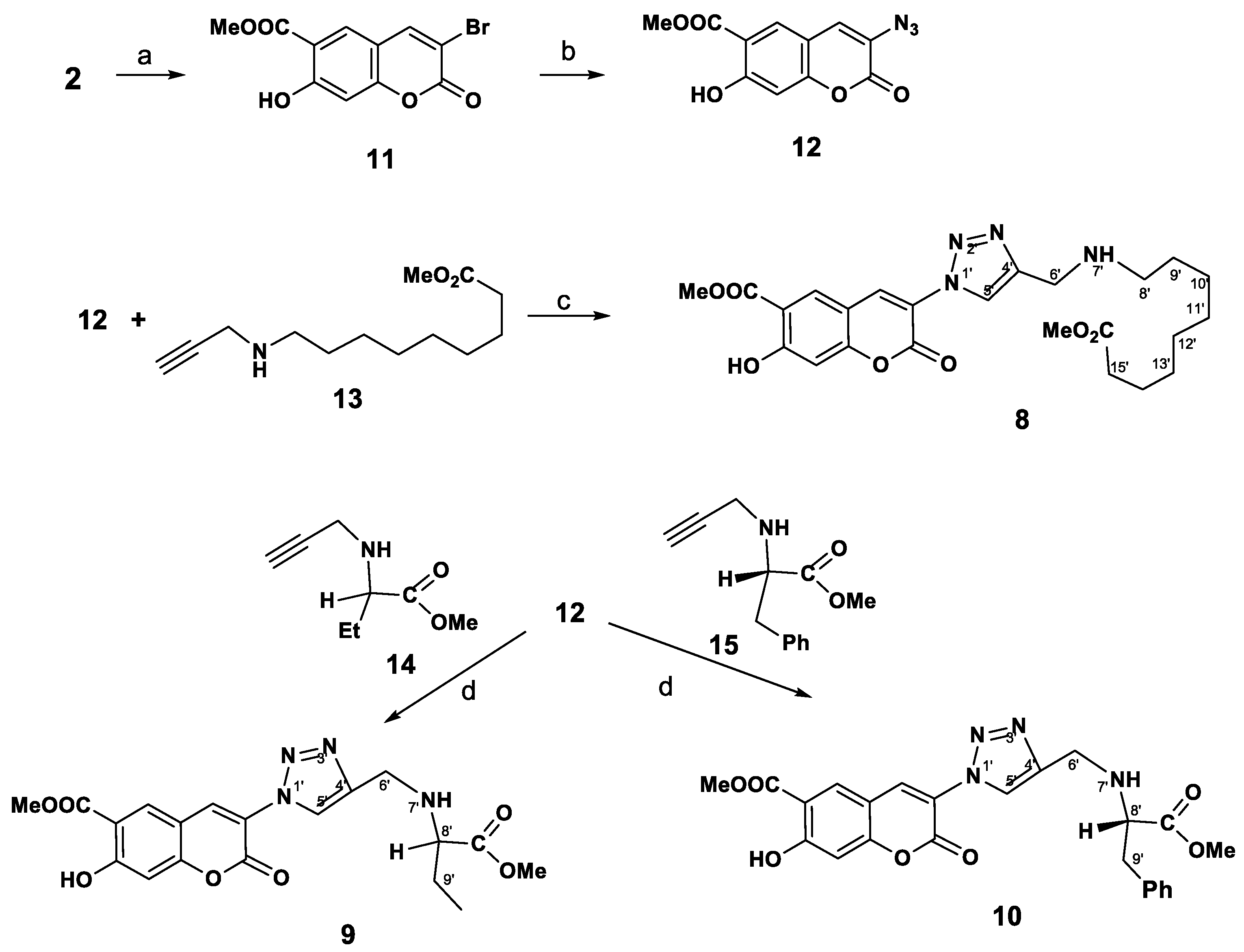
3-Bromopeuruthenicin (11). A solution of 1.0 g (4.5 mmol) of peuruthenicin (2) and 2.48 g (9 mmol) of dioxane dibromide in CH2Cl2 (10 mL) was stirred at rt for 16 h. Then the reaction mixture was filtered, and the filtrate was evaporated. 3-Bromopeuruthenicin (11, yield 86%, 1.14 g) was finally purified by crystallization from chloroform. White solid; m.p. 187–188 °C (ether). IR (KBr, ν, cm−1): 3480, 3350, 3116, 3050, 2956, 2853, 1720, 1675, 1621, 1616, 1598, 1440, 1311, 1293, 1231, 1163, 1124, 1089, 1030, 989, 964, 918, 845, 783, 750, 729, 719. UV (EtOH) λmax, (lgε): 244 (4.19), 314 (4.02), 334 (4.10), 400 (3.39) nm. 1H-NMR (600 MHz, CDCl3, δH): 3.99 (s, 3H, OCH3), 6.85 (s, 1H, H-8), 7.97 (s, 2H, H-4,5), 11.21 (s, 1H, OH). 13C-NMR (150 MHz, CDCl3, δC): 52.9, 104.8, 108.8, 110.5, 112.5, 129.9, 143.8, 156.4, 157.9, 164.3, 169.2. HRMS (ESI), m/z (Irel, %): 298(66), 269(15.9), 240(24), 220(18), 188(31), 160(16), 131(17), 103(19), 79(12); calcd for C11H7BrO5 [M]: 297.9471; found: 297.9468; Anal. Calcd. for C11H7BrO5: С, 44.18; Н, 2.36; Br, 26.72. Found: С, 44.08; Н, 2.16; Br, 26.70.
3-Azidopeuruthenicin (12). To a stirred solution of 25 mg (0.84 mmol) of 3-bromopeuruthenicin (11) in DMF at room temperature was added NaN3 (82 mg, 1.26 mmol). The reaction mixture was heated at 40 °C for 14h. After consumption of the bromocoumarin, the mixture was cooled to room temperature, quenched with eq. NaCl (10 mL), and the products and were extracted with CH2Cl2 (5 × 5 mL). The combined organic phases were dried over anhydrous MgSO4, after filtration, the solvent was removed under reduced pressure and was further dried in a freeze drier at oil pump vacuum. (P = 1 mm). The product (0.15 g, 68%) was obtained as a yellow solid, m.p. 161–162 °C. IR (KBr, ν, cm−1): 3455, 3390, 3357, 3299, 3004, 2971, 2923, 2858, 2121, 1727, 1621, 1467, 1434, 1375, 1309, 1290, 1263, 1193, 975, 869, 728. UV (EtOH) λmax, (lgε): 223(4.51), 243(4.4), 329(4.2), 373(3.97). 1H-NMR (CDCl3, 400 MHz, δH): 3.99 (s, 3H, OCH3), 6.83 (s, 1H, H-8), 7.58 (s, 1Н, H-4), 8.00 (s, 1H, H-5), 11.19 (s, 1H, OH). 13C- NMR (CDCl3, 100 MHz, δC): 52.7, 104.8, 109.9, 111.9, 114.0, 120.6, 142.9, 158.8, 159.9, 164.2, 169.4. Anal. Calcd for C11H7N3O5: С, 50.58; Н, 2.70; N, 16.09; found С, 51.04; Н, 3.12; N, 15.74.
4.2.2. General Method for the N-Propargylation of Amino Acid Methyl Esters
Propargyl bromide (10.5 mmol, 80% in toluene solution) was added to a solution of the appropriate amino acid ether hydrochlorides (10 mmol) and potassium carbonate (21 mmol) in dry DMF at room temperature under argon. The mixture was stirred for 1–3 days (TLC control), the solvent was evaporated under reduced pressure and the residue partitioned between water (30 mL) and CH2Cl2 (30 mL). The organic phase was separated and the aqueous phase was extracted with CH2Cl2 (3 × 10 mL). The organic phases were combined, dried (MgSO4), filtered and the solvent evaporated under reduced pressure. The residue was purified by column chromatography to give secondary amines 13, 14, 15.
Methyl 9-(prop-2-ynylamino)nonanoate (13). Yellowish oil. 1H-NMR (CDCl3, 400 MHz, δH): 1.22 (m, 4H, CH2-11′,12′), 1.39 (2H, CH2-13′), 1.52 (2H, CH2-10′), 2.12 (4H, CH2-9′,14′), 2.21 (m, 2H, CH2-15′), 2.58 (2H, CH2-8′), 3.32 (s, 2H, CH2-6′), 3.57 (s, 3H, OCH3).13C-NMR (CDCl3, 100 MHz, δC): 25.2, 27.4, 29.3, 29.5, 30.0, 34.32, 38.40, 48.90 (CH2-12′,9′,5′), 51.62 (OCH3), 71.38, 73.02 (C≡CH). Anal. calcd for C13H23NO2: С, 69.29; Н, 10.29; N, 6.22; found С, 68.74; Н, 10.10; N, 5.94.
Methyl 7-hydroxy-3-(4-((9-methoxy-9-oxononylamino)methyl)-1H-1,2,3-triazol-1-yl)-2-oxo-2H-chromene-6-carboxylate (8). A solution of 3-azidopeuruthenicin (12, 50 mg, 0.19 mmol) in methylene chloride (10 mL) and a solution of sodium ascorbate (15 mol%, 0.255 mmol) and СuSO4 × 5H2O (5 mol%, 0.085 mmol) in water (10 mL) were mixed and the terminal alkyne 13 (63 mg, 0.28 mmol) was added with stirring. The reaction mixture was stirred for 3 h at room temperature and then for 1 h at 40 °С. The cooled mixture was diluted with water (10 mL) and the product was extracted with methylene chloride (4 × 10 mL). The combined extracts were dried over MgSO4, and the solvent was removed in vacuo. The residue was purified by column chromatography to give 73 mg (67%) of compound 8 as a yellow oil. IR (KBr, ν, cm−1): 3465, 3396, 3118, 3047, 2954, 2923, 2852, 1751, 1735, 1673, 1623, 1602, 1573, 1469, 1438, 1367, 1288, 1230, 1162, 1124, 1089, 1031, 962, 943, 865, 792. UV (EtOH) λmax, (lgε): 242 (4.49), 299 (4.17), 313 (4.26), 334 (4.34), 390 (3.39) nm. 1H-NMR (CDCl3, 400 MHz, δH): 1.25 (m, 4H, CH2-11′,12′), 1.66 (2H, CH2-13′), 2.18 (2H, CH2-10′), 2.40 (4H, CH2-9′,14′), 2.47 (m, 2H, CH2-15′), 2.72 (2H, CH2-8′), 3.65 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 4.02 (s, 2H, CH2-6′), 6.87 (s, 1H, H-8), 7.97 (s, 1Н, H-4), 7.98 (s, 1H, H-5), 8.01 (s, 1H, H-5′), 11.25 (br.s, 2H, OH, NH). 13C-NMR (CDCl3, 100 MHz, δC): 29.7, 30.8, 31.9, 35.5, 39.6, 40.1, 40.5, 45.1, 52.9, 61.9, 104.7, 108.7, 110.5, 112.5, 121.4, 124.9, 142.2, 143.8, 156.4, 157.8, 164.3, 169.2, 174.4. Anal. calcd. for C24H30N4O7: C, 59.25; H, 6.22; N, 11.52; found: C, 59.02; H, 6.12; N, 11.27.
4.2.3. Preparation of 3-(Triazolyl)coumarins 9, 10
A stirred solution of 3-azidopeuruthenicin (12, 90 mg, 0.35 mmol) in MeCN (15 mL) was successively treated with alkyne 14 or 15 (1.2 equiv.), CuI (0.1 equiv.) and Et3N (1.1 equiv.) and the reaction mixture was stirred at room temperature until complete consumption of the starting material. Then, water (5 mL) was added and the products were extracted with CH2Cl2 (3 × 10 mL). The combined organic phases were dried (MgSO4), filtered and concentrated in vacuo. The residue was purified by column chromatography, eluting with chloroform, chloroform:ethanol 100:1.
Methyl 7-hydroxy-3-(4-((1-methoxy-1-oxobutan-2-ylamino)methyl)-1H-1,2,3-triazol-1-yl)-2-oxo-2H-chromene-6-carboxylate (9). Yield 56%. Yellow oil. IR (KBr, ν, cm−1): 3463, 3398, 3307, 3050, 2954, 2923, 2852, 1733, 1677, 1625, 1575, 1465, 1440, 1367, 1309, 1292, 1232, 1220, 1164, 1124, 1089, 1012, 968, 865, 794, 948. UV (EtOH) λmax, (lgε): 207 (4.69), 244 (4.29), 300 (3.95), 315 (4.02), 336 (4.1), 387 (3.41) nm. 1H-NMR (CDCl3+CD3OD, 400 MHz, δH): 0.81 (t, 3H, J = 7.0 Нz, CH3-10′), 31.55 (m, 2H, CH2-9′), 3.27 (m, 1H, H-8′), 3.62 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 3.90 (m, 2H, H-6′), 6.76 (s, 1H, H-8), 7.90 (s, 1Н, H-4), 7.91 (s, 1H, H-5), 8.09 (s, 1H, H-5′). 13C-NMR (CDCl3, 100 MHz, δC): 9.5, 25.8, 36.3, 51.6, 51.8, 60.7, 104.4, 108.2, 110.3, 112.3, 121.3, 124.8, 141.0, 144.1, 156.5, 157.5, 164.0, 168.9, 174.8. Anal. calcd. for C19H20N4O7: C, 54.81; H, 4.84; N, 13.46; found, %: C, 55.13; H, 5.02; N, 13.59.
(S)-Methyl 7-hydroxy-3-(4-((1-methoxy-1-oxo-3-phenylpropan-2-ylamino)methyl)-1H-1,2,3-triazol-1-yl)-2-oxo-2H-chromene-6-carboxylate (10). Yield 62% (by method for snthesis of compound 8–34%). Yellow oil. [α]D +12.04 (с 1.00, CHCl3). IR (ν, cm−1): 3459, 3395, 3290, 3049, 2954, 2925, 2854, 1737, 1673, 1623, 1602, 1573, 1537, 1490, 1438, 1367, 1288, 1232, 1162, 1124, 1087, 962, 943, 865, 792. UV (EtOH) λmax, (lgε): 244 (4.22), 312 (3.94), 334 (4.02), 399 (3.26) nm. 1H-NMR (CDCl3, 400 MHz, δH): 2.90-3.03 (m, 2H, H-9′), 3.36 (dd, J = 16.3, 1.8 Hz, 2H, H-6′), 3.65 s (3H, OCH3), 3.73 (dd, J = 6.4, 7.0 Hz, 1H, H-8′), 3.98 (s, 3H, OCH3), 6.84 s (1H, H8), 7.16-7.18 (m, 2H, o-Ph), 7.19-7.22 (m, 1H, p-Ph), 7.26-7.32 (m, 2H, m-Ph), 7.93 s (1Н, H-4), 7.95 (s, 1H, H-5), 8.08 (s, 1H, H-5′), 11.13 (br s, 2H, OH, NH). 13C-NMR (CDCl3, 100 MHz, δC): 36.7, 39.3, 51.8, 52.8, 61.0, 104.9, 108.4, 110.3, 112.4, 120.5 (C-4), 125.1, 126.9, 129.1 (2C), 129.5 (2C), 132.5, 141.3, 143.8, 156.1, 157.7, 164.2, 169.2, 174.2. Anal. calcd. for C24H22N4O7: C, 60.25; H, 4.63; N, 11.71; found: C, 60.16; H, 4.96; N, 11.55.
4.2.4. General Procedure for the Sonogashira Cross-coupling Reactions of 3-Bromopeuruthenicin 11 with Aryl Alkynes 17, 19–24 or 2-methylbut-3-yn-2-ol 31
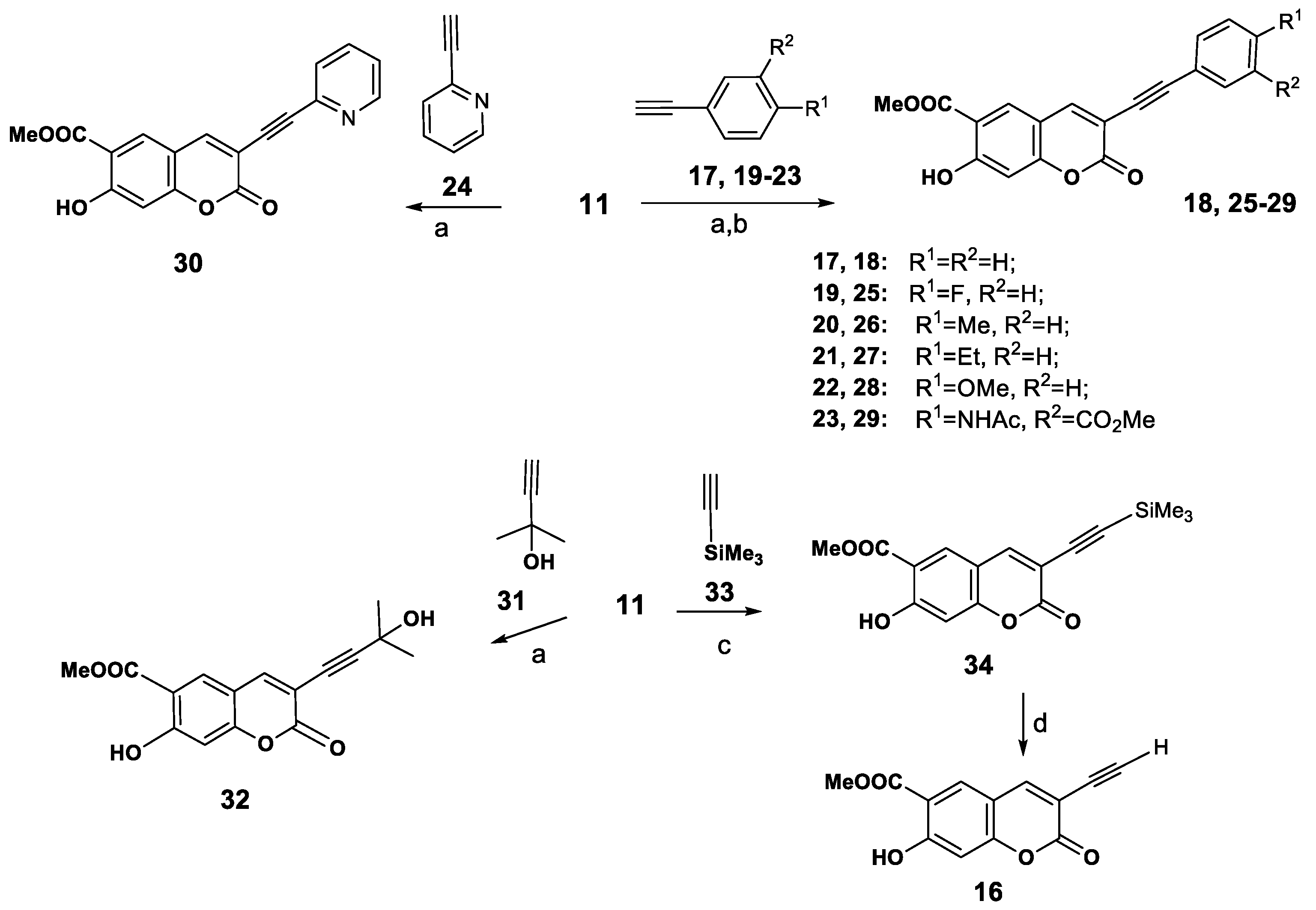
To a solution of 3-bromopeurutenicin (11, 100 mg, 0.34 mmol) and terminal alkynes 17, 19–24 or 31 (0.54 mmol) in benzene (5 mL) was added CuI (6 mg, 10 mol%), Pd(PPh3)2Cl2 (12 mg, 5 mol%), and Et3N (0.076 mL, 0.44 mmol; 1.3 (eq. to 11) under argon. The reaction mixture was stirred at 80 °C for 12–14 h (TLC). The mixture was cooled and 5 mL of water was added. The separated water layer was extracted with СН2Cl2 (5 × 4 mL). The combined organic extracts was washed with water, dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was subjected to column chromatography on silica gel. Eluting with chloroform and crystallization from ether afforded the corresponding 3-(R)-alkynylcoumarins 18, 25–30, 32.
Methyl 7-hydroxy-2-oxo-3-(phenylethynyl)-2H-chromene-6-carboxylate (18). Yield 65% (70 mg). Yellow oil. IR (ν, cm−1): 3430, 3350, 3052, 2954, 2925, 2852, 2112 (С≡С), 1736, 1682, 1616, 1571, 1442, 1367, 1290, 1224, 1160, 1128, 1091, 966, 873, 792, 750. UV (EtOH) λmax, (lgε): 237 (4.14), 272 (3.86), 280 (3.88), 315 (3.78), 335 (3.83) nm. 1H-NMR (CDCl3, 400 MHz, δH): 3.85 (s, 3H, OCH3), 6.79 (s, 1H, H-8), 7.48–7.56 (m, 5H, H-Ph), 7.92 s(1H, H-4), 7.93 (s, 1H, H5), 11.21 (1H, OH). 13C-NMR (CDCl3, 100 MHz, δC): 52.1, 83.3, 95.2, 108.1, 112.0, 113.1, 114.7, 129.8 (2C), 130.0, 130.8, 132.8, 133.6 (2C), 144.4, 157.6, 158.4, 164.6, 169.7. Anal. Calcd for C19H12O5: 320.0745; found: 320.0743.
Methyl 3-((4-fluorophenyl)ethynyl)-7-hydroxy-2-oxo-2H-chromene-6-carboxylate (25). Yield 68% (78 mg), yellowish powder, m.p. 167–168 °C (ether). IR (KBr, ν, cm−1): 3425, 2933, 2836, 2145, 1733, 1673, 1608, 1517, 1463, 1450, 1373, 1346, 1257, 1228, 1124, 1012, 939, 873, 852, 840, 792, 753. UV (EtOH) λmax, (lgε): 218 (4.56), 225 (4.54), 245 (4.64), 305 (4.3), 329 (4.41) nm. 1H-NMR (CDCl3, 400 MHz, δH): 3.91 (s, 3H, OCH3), 6.77 (s, 1H, H-8), 6.93 (dd, J = 8.2, 7.2 Нz, 2H, H-3′,5′), 7.40 (dd, J = 8.2, 3.4 Нz, 2H, H-2′,6′), 7.74 (s, 1H, H-4), 7.92 (s, 1H, H-5), 11.26 (1H, OH). 13C-NMR (CDCl3, 100 MHz, δC): 53.3, 82.0, 95.7, 104.7, 110.7, 111.2, 112.1, 118.9 (2C), 128.6, 130.5, 131.8 (2C), 143.4, 158.0, 160.1(C-4′, JC-F 259.1 Hz), 161.3, 164.4, 169.4. Anal. calcd for C19H11FO5: С, 67.46; Н, 3.28; F, 5.62; found: С, 68.08; Н, 3.50; F, 5.12.
Methyl 7-hydroxy-2-oxo-3-(p-tolylethynyl)-2H-chromene-6-carboxylate (26). Yield 61% (70 mg). brownish powder, m.p. 132–133 °C (ether). IR (KBr, ν, cm−1): 3430, 3299, 3259, 3049, 2956, 2852, 2121, 1753, 1735, 1697, 1677, 1594, 1516, 1436, 1367, 1321, 1294, 1234, 1203, 1162, 1087, 960, 848, 794, 748, 734. UV (EtOH) λmax, (lgε): 237 (4.41), 251 (4.21), 272 (4.13), 281 (4.16), 314 (4.04), 338 (4.11) nm. 1H-NMR (CDCl3, 400 MHz, δH) 2.36 (s, 3H, CH3), 4.00 (s, 3H, OCH3), 6.88 (s, 1H, H-8), 7.15 (d, J = 8.2 Нz, 2H, H3′,5′), 7.45 (d, J = 8.2 Нz, 2H, H-2′,6′), 7.81 (s, 1H, H-4), 8.00 (s, 1H, H-5), 11.24 (1H, OH). 13C-NMR (CDCl3, 100 MHz, δC): 21.7, 52.9, 82.4, 95.9, 104.8, 110.5, 111.2, 112.1, 119.00, 129.2 (2C), 130.5, 131.8 (2C), 139.3, 143.9, 158.0, 158.8, 164.4, 169.5. Anal. calcd for C20H14O5: С 71.85, Н 4.22, found: С 71.36, Н 3.94.
Methyl 3-((4-ethylphenyl)ethynyl)-7-hydroxy-2-oxo-2H-chromene-6-carboxylate (27). Yield 52% (61 mg) brownish powder, m.p. 130–131 °C (ether). IR (KBr, ν, cm−1): 3380, 3139, 3049, 2929, 2856, 2185, 1736, 1673, 1625, 1579, 1481, 1439, 1389, 1351, 1288, 1230, 1162, 1126, 1091, 1037, 962, 946, 865, 825, 750, 736. UV (EtOH) λmax, (lgε): 225 (4.1), 249 (3.67), 259 (3.59), 335 (4.18) nm. 1H-NMR (CDCl3, 400 MHz, δH): 1.21 (t, J = 6.8 Нz, 3H, Et), 2.62 (q, J = 6.8 Нz, 2H, Et), 3.97 (s, 3H, OCH3), 6.85 (s, 1H, H-8), 7.13 (dd, J = 8.4 Нz, 2H, H-3′,5′), 7.40 (d, J = 8.4 Нz, 2H, H-2′,6′), 7.96 (s, 1H, H4), 7.97 (s, 1H, H5), 11.21 (1H, OH). 13C-NMR (CDCl3, 100 MHz, δC): 17.3, 28.2, 53.5, 81.9, 97.3, 105.6, 112.1, 113.5, 114.8, 122.9, 129.2, 129.9 (2C), 132.6 (2C), 144.2, 144.5, 158.8, 159.5, 165.2, 170.2. Anal. calcd for C21H16O5: С, 72.41; Н, 4.63; found: С, 72.18; Н, 4.90.
Methyl 7-hydroxy-3-((4-methoxyphenyl)ethynyl)-2-oxo-2H-chromene-6-carboxylate (28). Yield 58% (70 mg) brownish powder, m.p. 134–135 °C (ether). IR (KBr, ν, cm−1): 3430, 3303, 3216, 3049, 3014, 2956, 2129, 1732, 1677, 1594, 1516, 1491, 1436, 1367, 1321, 1294, 1267, 1234, 1203, 1163, 1124, 1088, 960, 865, 849, 794, 748, 734. UV (EtOH) λmax, (lgε): 237 (4.18), 265 (3.78), 277 (3.91), 280 (3.92), 324 (3.84), 335 (3.88) nm. 1H-NMR (CDCl3, 400 MHz, δH): 3.91 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 6.86 (s, 1H, H-8), 7.13 (d, J = 8.6 Нz, 2H, H-3′,5′), 7.41 (d, J = 8.6 Нz, 2H, H-2′,6′), 7.97 (s, 1H, H-4), 7.98 (s, 1H, H-5), 11.18 (1H, OH). 13C-NMR (CDCl3, 100 MHz, δC): 53.2, 53.6, 81.6, 95.4, 105.1, 111.1, 111.7, 112.8, 118.7, 119.4 (2C), 129.5, 133.3 (2C), 144.1, 157.9, 158.7, 161.6, 164.7, 169.8. Anal. calcd for C20H14O6: С, 68.57; Н, 4.03; found: С, 68.88; Н, 3.90.
Methyl 3-((4-acetylamino-3-(methoxycarbonyl)phenyl)ethynyl)-7-hydroxy-2-oxo-2H-chromene-6-carboxylate (29). Yield 45% (66 mg) yellowish powder, m.p. 154–155 °C (ethanol). IR (KBr, ν, cm−1): 3430, 3299, 3259, 3216, 3049, 2956, 2925, 2852, 2208, 1753, 1735, 1697, 1677, 1620, 1594, 1515, 1491, 1437, 1367, 1321, 1294, 1234, 1203, 1162, 1124, 1088, 960, 920, 866, 849, 795, 748, 735. UV (EtOH) λmax, (lgε): 237 (4.28), 272 (4.00), 280 (4.01), 316 (3.92), 335 (3.96) nm. 1H-NMR (CDCl3, 400 MHz, δH): 2.19 (s, 3H, CH3-CO), 3.88 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 6.82 (s, 1H, H-8), 7.55 (dd, J = 8.8, 2.0 Нz, 1H, H-6′), 7.94 (s, 1H, H-4), 7.95 (s, 1H, H-5), 8.09 (d, J = 2.0 Нz, 1H, H-2′), 8.58 (d, J = 8.8 Нz, 1H, H-5′), 11.16 (br.s, 2H, NH, OH). 13C-NMR (CDCl3+CD3OD, 100 MHz, δC): 25.2, 52.5, 52.8, 71.3, 82.2, 104.6, 108.5, 112.4, 116.2, 120.0, 130.0, 130.5, 133.7, 134.6, 137.7, 141.2, 143.9, 156.5, 157.7, 166.4, 167.9, 169.1, 169.3. Anal. calcd for C23H17NO8: С, 63.45; Н, 3.94; N, 3.22; found: С, 63.18; Н, 3.92; N, 3.12.
Methyl 7-hydroxy-2-oxo-3-(pyridin-2-ylethynyl)-2H-chromene-6-carboxylate (30). Yield 55% (60 mg) brown oil. IR (ν, cm−1): 3444, 2971, 2929, 2856, 2133, 1735, 1674, 1626, 1579, 1481, 1438, 1388, 1352, 1300, 1230, 1163, 1126, 1092, 1038, 962, 947, 865, 825, 750, 736, 723. UV (EtOH) λmax, (lgε): 227 (4.25), 238 (4.15), 294 (3.93), 306 (3.92), 351 (4.13) nm. 1H-NMR (CDCl3, 400 MHz, δH): 3.76 (s, 3H, OCH3), 6.72 (s, 1H, H-8), 7.18 (ddd, J = 7.4, 5.2, 1.4 Нz, 1H, H-4′), 7.31 (dd, J = 6.6, 1.4 Нz, 1H, H-6′), 7.54 (ddd, J = 7.4, 6.6, 1.8 Нz, 1H, H-5′), 7.69 (s, 1H, H-4), 7.77 (s, 1H, H-5), 8.49 (ddd, J = 7.2, 1.8 Нz, 1H, H-3′), 11.20 (1H, OH). 13C-NMR (CDCl3, 100 MHz, δC): 52.9, 82.6, 94.8, 105.0, 112.8, 115.1, 116.6, 123.5, 129.4, 130.4, 134.1, 138.1, 141.6, 144.4, 156.9, 158.1, 164.4, 169.7. Anal. calcd for C18H11NO5: С, 67.29; Н, 3.45; N, 4.36; found: С, 67.08; Н, 3.38; N, 4.22.
Methyl 7-hydroxy-3-(3-hydroxy-3-methylbut-1-ynyl)-2-oxo-2H-chromene-6-carboxylate (32). Yield 66% (68 mg), m.p.114–115 °C (ethanol). IR (KBr, ν, cm−1): 3282, 3221, 2981, 2932, 2868, 2250, 1741, 1679, 1612, 1600, 1584, 1462, 1446, 1363, 1296, 1269, 1210, 1170, 1120, 1096, 1058, 998, 964, 954, 900, 889, 842, 792, 735, 723. UV (EtOH) λmax, (lgε): 223 (4.04), 246 (3.84), 338 (3.67), 365 (3.57) nm. 1H-NMR (CDCl3, 400 MHz, δH): 1.34 (s, 6H, 2 × CH3), 3.95 (s, 3H, OCH3), 6.78 (s, 1H, H-8), 7.76 (br. s, 1H, H-4), 7.93 (s, 1H, H-5). 3C-NMR (CDCl3, 100 MHz, δc): 30.9, 31.0, 52.7, 65.3, 66.2, 84.1, 104.8, 109.9, 110.5, 114.0, 130.6, 143.1, 157.8, 160.0, 164.1, 169.4. Anal. calcd for C16H14O6: С, 63.57; Н, 4.67; found: С, 63.22; Н, 4.44. HRMS, m/z calcd.: 302.0785; found 302.0783 [M].
Methyl 7-hydroxy-2-oxo-3-((trimethylsilyl)ethynyl)-2H-chromene-6-carboxylate (34). A sealed 10 mL glass tube containing 3-bromopeuruthenicin (11, 100 mg (0.34 mmol), toluene (3 mL), trimethylsilylacetylene (33, 0.1 mL, 0.68 mmol), CuI (3 mg, 5 mol %), Pd(PPh3)2Cl2 (23 mg, 10 mol. %), and Et3N (0.061 mL, 1.3 eq.) was placed in the cavity of a microwave reactor and irradiated for 2 h at 100 °C and power 50 W in an Anton Paar Microwave 50 reactor. After cooling to 25 °C, the tube was removed from the reactor. Then the mixture was cooled and 5 mL of water was added. The separated water layer was extracted with СН2Cl2 (5 × 4 mL). The combined organic extracts was washed with water, dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was crystallized from ether, yield 64% (68 mg), m.p. 104–107 °C. IR (KBr, ν, cm−1): 3437, 2924, 2852, 2223, 1735, 1672, 1314, 1597, 1508, 1425, 1388, 1328, 1236, 1222, 1159, 1107, 908, 839, 752, 694, 680. UV (EtOH) λmax, (lgε): 325 (3.97), 304 (3.85), 241 (4.01) nm. 1H-NMR (CDCl3, 400 MHz, δH) 0.20 (s, 9H, SiMe3), 3.93 (s, 3H, OCH3), 6.76 (s, 1H, H-8), 7.74 (s, 1H, H-4), 7.91 (s, 1H, H-5), 10.97 (s, 1H, OH). 13C-NMR (CDCl3, 100 MHz, δC): 8.6, 52.7, 73.8, 81.5, 101.6, 110.2, 110.4, 111.7, 128.4, 145.1, 158.0, 158.3, 164.45, 169.2. Anal. calcd for C16H16O5Si: С, 60.74; Н, 5.10; Si, 8.88; found: С, 60.55; Н, 5.18; Si, 8.54.
Methyl 3-ethynyl-7-hydroxy-2-oxo-2H-chromene-6-carboxylate (16). To a solution of compound 34 (60 mg, 0.00019 mol) in methanol (3 mL) were added CsF (14 mg, 0.00095 mol) and TEBA (5 mg). The mixture was stirred at rt for 10 h in an argon flow (TLC). Then 10 mL of water was added and the mixture was extracted with methylene chloride (5 × 4 mL). The combined extract was washed with water, dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was subjected to column chromatography on silica gel. Eluting with chloroform and crystallization from ether gave compound 16, yield 85%, m.p. 84- 88 oC. IR (KBr, ν, cm−1): 3463, 2956, 2923, 2854, 2102, 1741, 1677, 1621, 1581, 1494, 1444, 1416, 1367, 1295, 1234, 1186, 1157, 1120, 1099, 1024, 952, 914, 856, 721. UV (EtOH) λmax, (lgε): 225 (4.1), 246 (3.96), 321 (3.84), 344 (3.92). 1H-NMR (CDCl3, 400 MHz) 2.16 (s, 1H, CH), 3.99 (s, 3H, OCH3), 6.86 (s, 1H, H-8), 7.83 (s, 1H, H-4), 8.00 (s, 1H, H-5), 11.29 (s, 1H, OH). 13C- NMR (CDCl3, 100 MHz, δC): 52.3, 75.4, 82.2, 105.1, 112.6, 114.0, 114.8, 128.5, 143.3, 159.4, 159.7, 164.7, 168.9. Anal. calcd for C13H8O5 С 63.94, Н 3.30 found: С 63.65, Н 3.84.
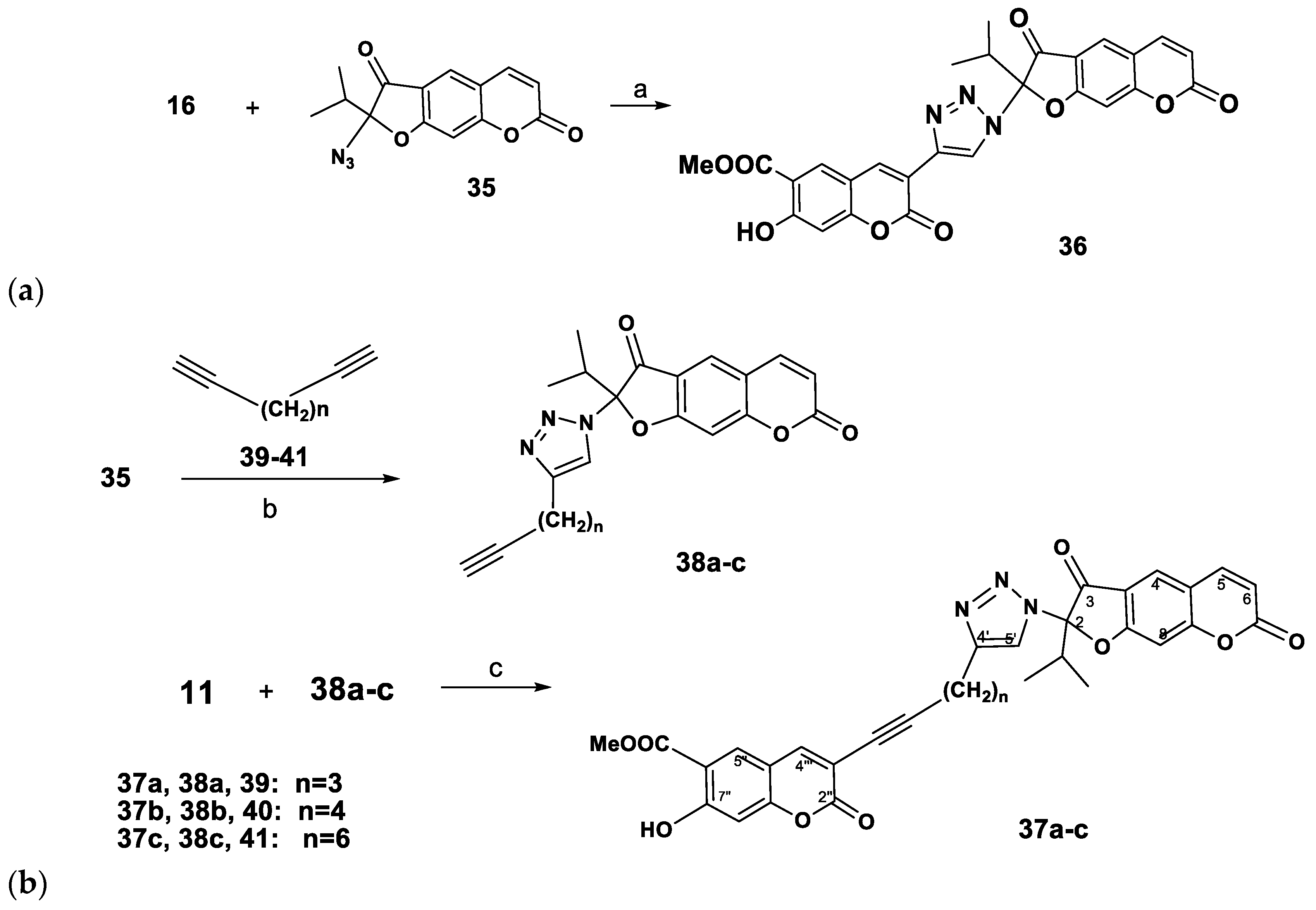
Methyl 7-hydroxy-3-[1-(2-isopropyl-3,7-dioxo-2,3-dihydro-7H-furo[3,2-g]chromen-2-yl)-1H-1,2,3-triazol-4-yl]-2-oxo-2H-chromene-6-carboxylate (36). A solution of 2-azidooreoselone (35, 100 mg, 0.35 mmol) and 3-ethynylpeurutenicin (16, 85 mg, 0.35 mmol) in CH2Cl2 (10 mL) was mixed with a solution of sodium ascorbate (10 mg, 15 mol%) and СuSO4·5H2O (4 mg, 5 mol%) in Н2О (5 mL). The reaction mixture was stirred for 3 h at rt and additional for 1 h at 40 °С. After the completion of the reaction, the mixture was diluted with Н2О (10 mL), the product was extracted with CH2Cl2 (4 × 10 mL), the combined extracts were dried over anhydrous MgSO4, filtered, and the solvent was removed at reduced pressure. Yield 70% (0.130 g), yellowish solid. IR (KBr, ν, cm−1): 3469, 3049, 2983, 2956, 2883, 2850, 1739, 1675, 1629, 1556, 1467, 1438, 1390, 1353, 1284, 1132, 1162, 1128, 1093, 1025, 964, 944, 869, 827, 736. UV (EtOH) λmax, (lgε): 217 (4.57), 249 (4.59), 294 (4.26), 307 (4.27), 337 (4.27), 381 (3.76), 393 (3.36) nm. 1H-NMR (CDCl3, 400 MHz, δH): 0.89 (d, J = 7.0 Нz, 3H, CH3), 1.11 (d, J = 7.0 Нz, 3H, CH3), 3.30 (m, 1H, CH(Me)2), 3.99 (s, 3H, OCH3), 6.41 (d, J = 9.6 Нz, 1H, H-6), 6.88 (s, 1H, H-8′), 7.24 (s, 1H, H-9), 7.71 (d, J = 9.6 Нz, 1H, H-5), 7.87 (s, 1H, H-4), 7.97 (s, 1H, H-4′), 7.98 (s, 1H, H-5′), 8.13 (s, 1H, H-1′′), 11.29 (s, 1H, OH). 13C-NMR (CDCl3, 100 MHz, δC): 16.2, 16.3, 30.1, 53.3, 96.5, 101.4, 105.2, 110.7, 111.8, 112.9, 115.7, 117.3, 117.5, 125.7, 128.0, 130.3, 142.9, 143.5, 144.3, 158.5, 159.3, 162.1, 164.7, 165.8, 169.6, 172.4, 194.7. Anal. calcd for C27H19N3O9: С, 61.25; Н, 3.62; N, 7.94; found: С, 61.01; Н, 3.54; N, 7.62.
4.2.5. General Method for the Preparation of Monoalkynes 38a–c
A solution of 2-azidooreoselone (35, 1.7 mmol) in methylene chloride (10 mL) and a solution of sodium ascorbate (15 mol%, 0.255 mmol) and СuSO4∙5H2O (5 mol%, 0.085 mmol) in water (10 mL) were mixed and the appropriate terminal alkyne 39–41 (0.85 mmol) was added with stirring. The reaction mixture was stirred for 5 h at 40 °С. The cooled mixture was diluted with water (10 mL) and the product was extracted with methylene chloride (4 × 10 mL). The combined extracts were dried over MgSO4, filtered, the solvent was removed to give the desired products 38a–с.
2-Isopropyl-2-(4-(pent-4-ynyl)-1H-1,2,3-triazol-1-yl)-2H-furo[3,2-g]chromene-3,7-dione (38a). Yield 66% (0.420 g), brownish oil. IR (ν, cm−1): 3120, 2981, 2932, 2867, 2140, 1741, 1678, 1600, 1584, 1461, 1446, 1381, 1363, 1295, 1269, 1210, 1169, 1120, 1058, 964, 953, 909, 889, 792, 749. UV (EtOH) λmax, (lgε): 231 (3.91), 282 (3.58), 320 (3.52), 348 (3.43) nm. 1H-NMR (CDCl3, 400 MHz, δH): 0.89 (d, J = 7.0 Hz, 3Н, СН3), 0.95 (d, J = 7.0 Hz, 3Н, СН3), 1.40 (m, 2H, CH2-7′), 1.85 (s, 1H, H-10′), 2.06 (m, 2H, CH2-6′), 2.55 (m, 2H, CH2-8′), 3.06 (m, 1H, CH(CH3)2), 6.28 (d, J = 9.6 Нz, 1H, H-6), 6.99 (s, 1H, H-9), 7.39 (s, 1H, H-4), 7.67 (d, J = 9.6 Нz, 1H, H-5), 7.82 (s, 1H, H-5′). 13C-NMR (CDCl3, 100 MHz, δC): 15.4, 15.8, 19.5, 28.1, 28.2, 33.7, 69.2, 82.3, 98.4, 100.4, 115.4, 115.8, 117.0, 126.7, 127.5, 137.4, 145.9, 161.2, 164.0, 173.6, 191.5. Anal. calcd for C21H19N3O4: С, 66.83; Н, 5.07; N, 11.13; found: С, 67.01; Н, 5.12; N, 10.88.
2-(4-(Hex-5-ynyl)-1H-1,2,3-triazol-1-yl)-2-isopropyl-2H-furo[3,2-g]chromene-3,7-dione (38b). Yield 62% (0.415 g). Yellowish powder, m.p. 111–112 °C (ether). IR (KBr, ν, cm−1): 2971, 2828, 2856, 2115, 1735, 1673, 1625, 1579, 1481, 1438, 1388, 1351, 1230, 1126, 1091, 1037, 997, 962, 946, 865, 825, 750. UV (EtOH) λmax, (lgε): 221 (4.12), 255 (4.47), 292 (3.98), 308 (3.89), 351 (4.03) nm. 1H-NMR (CDCl3, 400 MHz, δH): 0.89 (d, J = 7.0 Hz, 3Н, СН3), 1.03 (m, 2H, CH2-8′), 1.12 (d, J = 7.0 Hz, 3Н, СН3), 1.24 (m, 2H, CH2-7′), 1.92 (s, 1H, H-11′), 2.14 (m, 2H, CH2-6′), 2.30 (m, 1H, CH(СH3)2), 3.40 (m, 2H, CH2-9′ ), 6.35 (d, J = 9.6 Нz, 1H, H-6), 7.02 (s, 1H, H-9), 7.43 (s, 1H, H-4), 7.69 (d, J = 9.6 Нz, 1H, H-5), 7.84 (s, 1H, H-5′). 13C- NMR (CDCl3, 100 MHz, δC): 14.9, 15.3, 17.8, 27.6, 27.7, 27.73, 33.2, 68.7, 81.8, 97.9, 100.6, 114.9, 115.3, 116.1, 125.4, 126.9, 136.8, 144.7, 162.4, 165.6, 173.1, 191.0. Anal. calcd for C22H21N3O4: С, 67.51; Н, 5.41; N, 10.74; found: С, 67.17; Н, 5.21; N, 10.72.
2-Isopropyl-2-(4-(oct-7-ynyl)-1H-1,2,3-triazol-1-yl)-2H-furo[3,2-g]chromene-3,7-dione (38c). Yield 74% (0.527 g). Yellowish powder, m.p. 121–122 °C (ether). IR (KBr, ν, cm−1): 3143, 3084, 2976, 2935, 2858, 2113, 1735, 1627, 1581, 1481, 1419, 1390, 1352, 1286, 1226, 1128, 1093, 1039, 997, 947, 866, 825, 739. UV (EtOH) λmax, (lgε): 221 (4.16), 255 (4.5), 294 (4.00), 307 (3.94), 339 (4.05), 351 (4.06) nm. 1H-NMR (CDCl3, 400 MHz, δH): 0.94 (d, J = 7.0 Hz, 3Н, СН3), 0.97 (d, J = 7.0 Hz, 3Н, СН3), 1.27 (m, 2H, CH2-8′), 1.34 (m, 2H, CH2-9′),1.46 (m, 2H, CH2-10′), 1.55 (m, 2H, (m, 2H, CH2-7′), 1.89 (s, 1H, H-13′), 2.12 (m, 2H, CH2-6′), 2.60 (m, 2H, CH2-11′), 3.09 (m, 1H, CH(CH3)2), 6.32 (d, J = 9.6 Нz, 1H, H-6), 7.04 (s, 1H, H-9), 7.44 (s, 1H, H-4), 7.72 (d, J = 9.6 Нz, 1H, H-5), 7.87 (s, 1H, H-5′). 13C-NMR (CDCl3, 100 MHz, δC): 15.1, 15.5, 18.1, 27.9, 28.0, 28.4, 28.67, 28.73, 33.5, 68.1, 84.4, 98.1, 100.8, 115.1, 115.5, 116.3, 125.7, 128.4, 137.7, 143.1, 158.5, 161.4, 171.4, 191.6. Anal. calcd for C24H25N3O4: С, 68.72; Н, 6.01; N, 10.02; [M] 419; found: С, 69.08; Н, 6.06; N, 10.27; М = 419.
4.2.6. Sonogashira Cross-Coupling Reactions of Bromide 11 with Alkynes 38a–c
To a solution of 3-bromopeurutenicin (11, 100 mg, 0.34 mmol) and a terminal alkyne 38a–c (0.54 mmol) in benzene (5 mL) was added CuI (6 mg, 10 mol%), Pd(PPh3)2Cl2 (12 mg, 5 mol%), and Et3N (0.076 mL, 0.44 mmol; 1.3 equiv) under argon. The reaction mixture was stirred at 80 °C for 14 h (TLC). The mixture was cooled and 5 mL of water was added. The separated water layer was extracted with СН2Cl2 (5 × 4 mL). The combined organic extracts was washed with water, dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was subjected to column chromatography on silica gel (eluent—chloroform).
Methyl 7-hydroxy-3-(5-(1-(2-isopropyl-3,7-dioxo-3,7-dihydro-2H-furo[3,2-g]chromen-2-yl)-1H-1,2,3-triazol-4-yl)-pent-1-ynyl)-2-oxo-2H-chromene-6-carboxylate (37a). Yield 52% (0.105 g). Brownish oil. IR (ν, cm−1): 3350, 3200, 2981, 2932, 2867, 2254, 1741, 1678, 1620, 1600, 1580, 1461, 1446, 1381, 1363, 1295, 1269, 1210, 1169, 1120, 1096, 1058, 964, 953, 889, 792, 735, 723. UV (EtOH) λmax, (lgε): 234 (4.41), 256 (4.44), 273 (4.31), 310 (4.09), 339 (4.14), 354 (4.08) nm. 1H-NMR (CDCl3, 400 MHz, δH): 0.99 (d, J = 7.0 Hz, 3Н, СН3), 1.02 (d, J = 7.0 Hz, 3Н, СН3), 1.60 (m, 2H, CH2-7′), 2.18 (m, 2H, CH2-6′), 2.65 (m, 2H, CH2-8′), 3.13 (m, 1H, CH(CH3)2), 3.97 (s, 3H, OCH3), 6.37 (d, J = 9.8 Hz, 1H, H-6), 6.84 (s, 1H, H-8′′), 7.08 (s, 1H, H-9), 7.48 (d, 1H, J = 9.8 Hz, H-5), 7.76 (s, 1H, H-4), 7.71, 7.74 (both s, 2H, H-4′′,5′′), 7.97 (br.s, 2H, H-5′, OH). 13C-NMR (CDCl3, 100 MHz, δC): 15.0, 15.4, 20.0, 23.5, 32.4, 34.7, 55.7, 70.0, 85.7, 98.1, 99.9, 104.3, 107.2, 110.2, 112.3, 115.1, 116.0, 116.3, 125.5, 128.2, 130.7, 139.7, 142.9, 143.8, 156.1, 157.5, 158.5, 161.4, 162.0, 169.0, 171.4, 192.5. Anal. calcd for C32H25N3O9: С, 64.54; Н, 4.23; N, 7.06; [M] 598; found: С, 64.58, Н, 4.36, N, 7.07; М = 595.
Methyl 7-hydroxy-3-(6-(1-(2-isopropyl-3,7-dioxo-3,7-dihydro-2H-furo[3,2-g]chromen-2-yl)-1H-1,2,3-triazol-1-yl)-hex-1-ynyl)-2-oxo-2H-chromene-6-carboxylate (37b). Yield 57% (0.118 g). Yellowish powder, m.p. 126–127 °C (ether). IR (KBr, ν, cm−1): 3388, 3139, 3049, 2929, 2856, 2235, 1735, 1673, 1626, 1579, 1481, 1439, 1388, 1352, 1288, 1230, 1163, 1126, 1091, 1037, 997, 962, 946, 865, 825, 750, 737. UV (EtOH) λmax, (lgε): 234 (4.39), 256 (4.43), 273 (3.81), 309 (4.09), 339 (4.13), 352 (3.62) nm. 1H-NMR (CDCl3, 400 MHz, δH): 0.98 (d, J = 7.0 Hz, 3Н, СН3), 1.04 (d, J = 7.0 Hz, 3Н, СН3), 1.44–1.53 (m, 4H, CH2-7′,8′), 2.14 (m, 2H, CH2-6′), 2.64 (m, 2H, CH2-9′), 3.13 (m, 1H, CH(CH3)2), 3.95 (s, 3H, OCH3), 6.35 (d, J = 9.8 Hz, 1H, H-6), 6.92 (s, 1H, H-8′′), 7.07 (s, 1H, H-9), 7.64 (d, J = 9.8 Hz, 1H, H-5), 7.71, 7.74 (both s, 2H, H-4′′,5′′), 7.88 (s, 1H, H-4), 7.97 (s, 1H, H5′). 13C-NMR (CDCl3, 100 MHz, δC): 15.6, 16.0, 19.4, 32.5, 33.0, 36.7, 25.7, 54.2, 70.6, 88.5, 98.7, 100.6, 104.9, 108.8, 110.8, 112.8, 115.7, 116.6, 116.9, 126.1, 128.8, 132.3, 141.1, 143.5, 144.4, 156.6, 158.1, 159.1, 162.0, 162.6, 169.5, 172.0, 192.4. Anal. calcd for C33H27N3O9: С, 65.02; Н, 4.46; N, 6.89; [M] 609; found: С, 65.08; Н, 4.06; N, 6.37; M = 605.
Methyl 7-hydroxy-3-(8-(1-(2-isopropyl-3,7-dioxo-3,7-dihydro-2H-furo[3,2-g]chromen-2-yl)-1H-1,2,3-triazol-4-yl)-oct-1-ynyl)-2-oxo-2H-chromene-6-carboxylate (37c). Brownish powder, m.p. 152–153 °C (ether). Yield 55% (0.119 g). IR (KBr, ν, cm−1): 3440, 3141, 3049, 2971, 2929, 2856, 2213, 1735, 1673, 1625, 1579, 1481, 1388, 1351, 1288, 1230, 1126, 1091, 1037, 962, 946, 865, 825, 750. UV (EtOH) λmax, (lgε): 234 (4.63), 251 (4.67), 256 (4.67), 309 (4.33), 339 (4.37), 350 (4.34) nm. 1H-NMR (CDCl3, 400 MHz, δH): 0.91 (d, J = 7.0 Hz, 3Н, СН3), 0.94 (d, J = 7.0 Hz, 3Н, СН3), 1.23, 1.29 (both m, 4H, CH2-8′,9′), 1.42 (m, 2H, CH2-10′), 1.51 (m, 2H, CH2-7′), 2.07 (m, 2H, CH2-6′), 2.56 (m, 2H, CH2-11′), 3.07 (m, 1H, CH(CH3)2), 3.92 (s, 3H, OCH3), 6.31 (d, J = 9.8 Hz, 1H, H-6), 6.78 (s, 1H, H-8′′), 7.03 (s, 1H, H-9), 7.46 (s, 1H, H-4), 7.68 (d, J = 9.8 Hz, 1H, H-5), 7.83 (s, 1H, H-5′). 7.89, 7.90 (both s, 2H, H-4′′,5′′). 13C-NMR (CDCl3, 100 MHz, δC): 15.2, 15.6, 19.0, 25.2, 29.9, 30.7, 31.2, 33.6, 36.3, 52.7, 70.2, 87.4, 98.2, 101.0, 104.5, 108.4, 110.4, 112.4, 115.2, 115.6, 116.5, 125.7, 128.2, 131.8, 141.3, 143.0, 144.0, 156.2, 157.7, 158.9, 161.6, 162.2, 169.2, 171.5, 191.8. Anal. calcd for C35H31N3O9: С, 65.93; Н, 4.90; N 6.59; [M] 637; found: С, 65.38; Н, 4.66; N, 6.87; М = 635.