One-step Preparation of a VHH-based Immunoadsorbent for the Extracorporeal Removal of β2-microglobulin
Abstract
1. Introduction
2. Results and Discussion
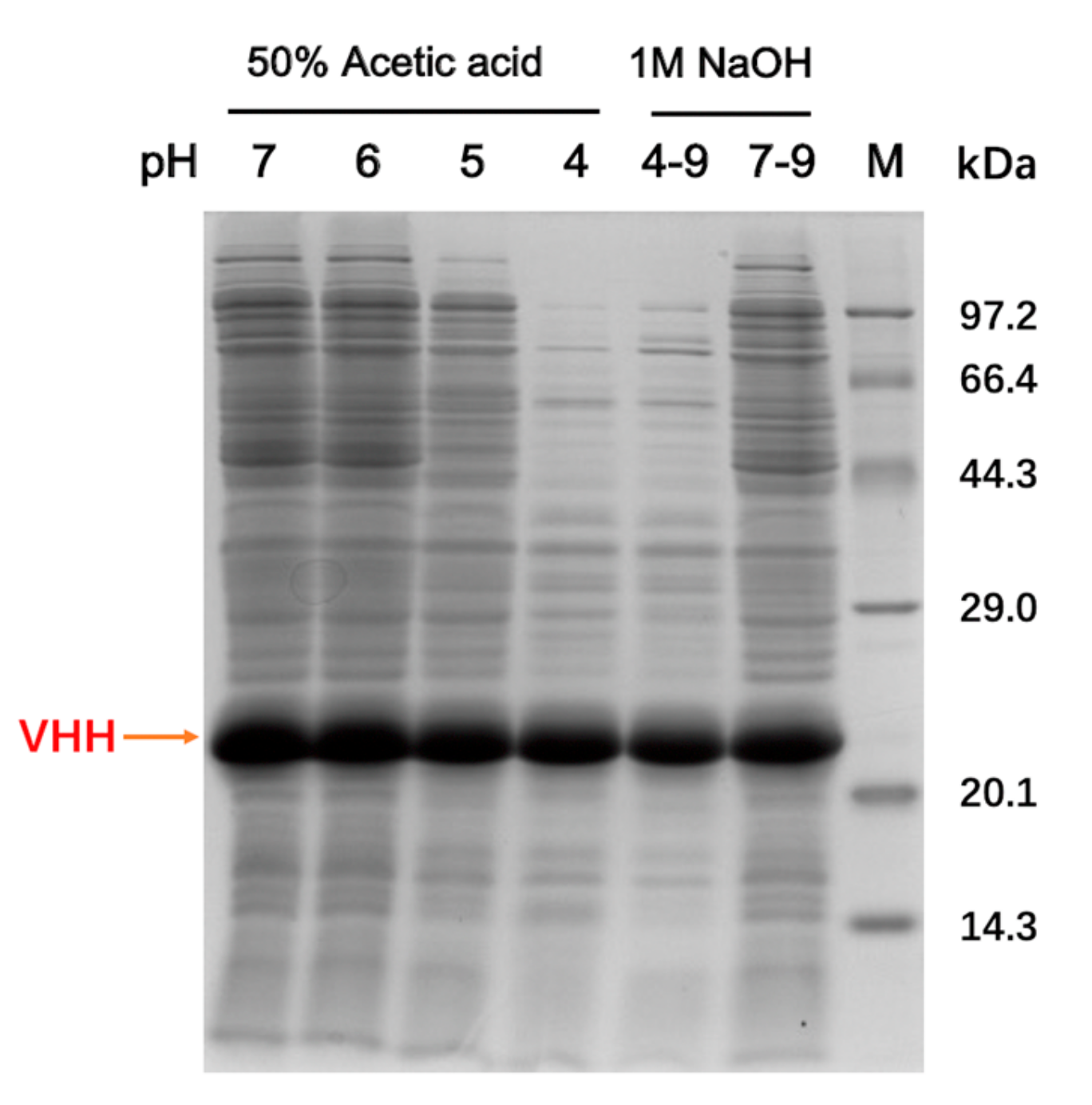
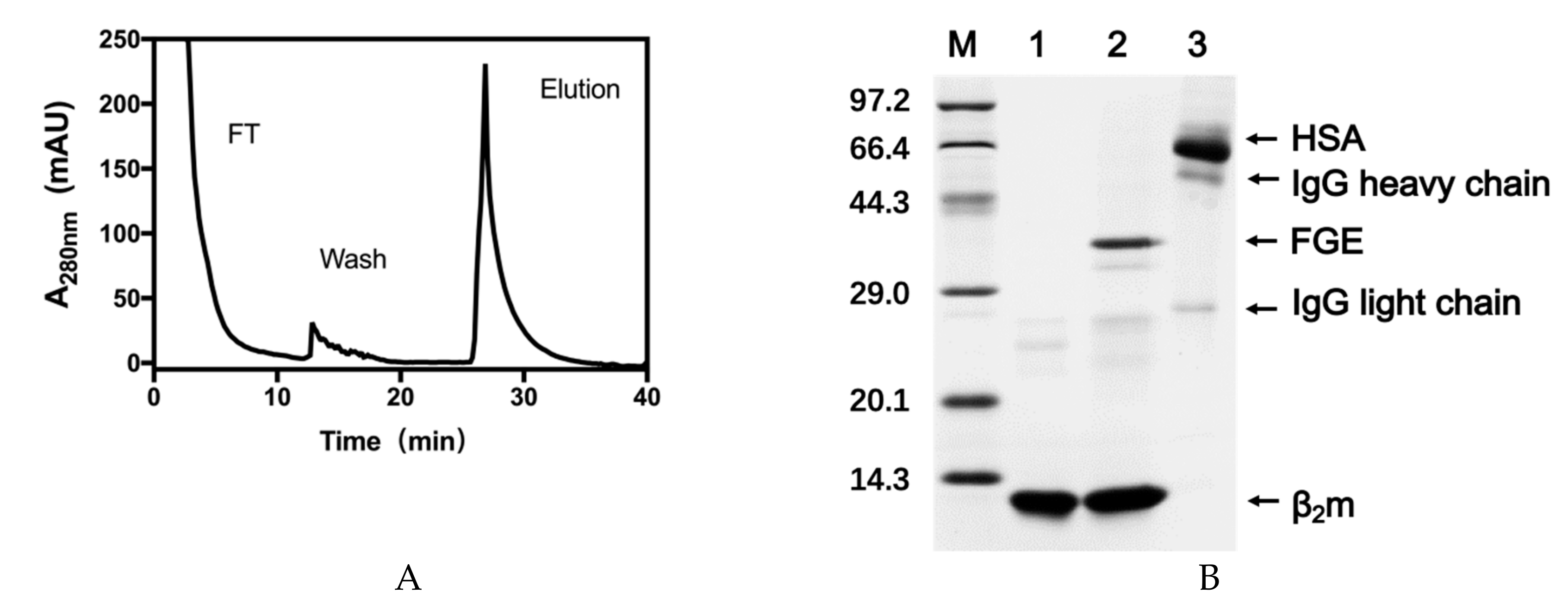
2.1. Expression of Recombinant VHHs and FGE
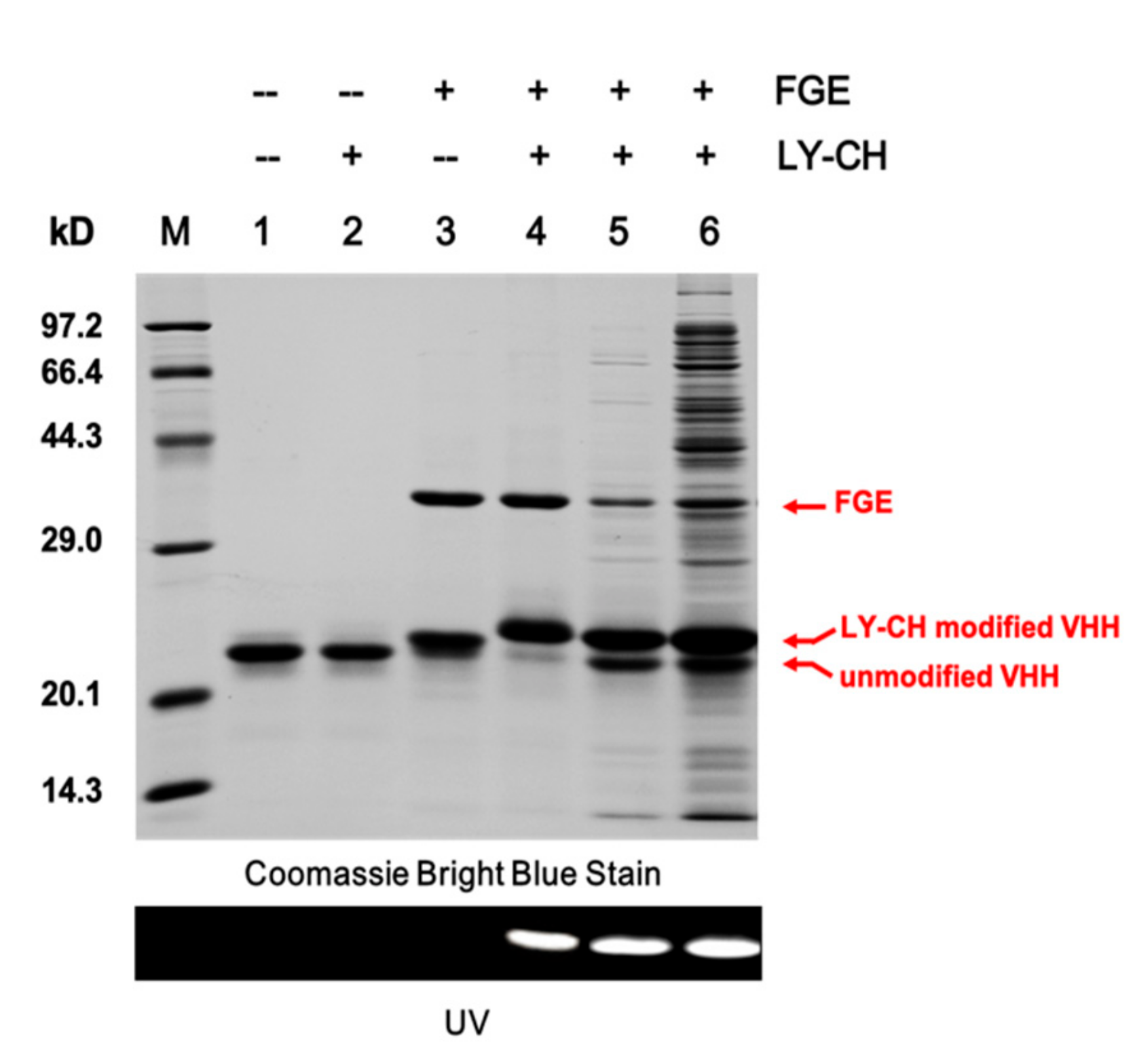
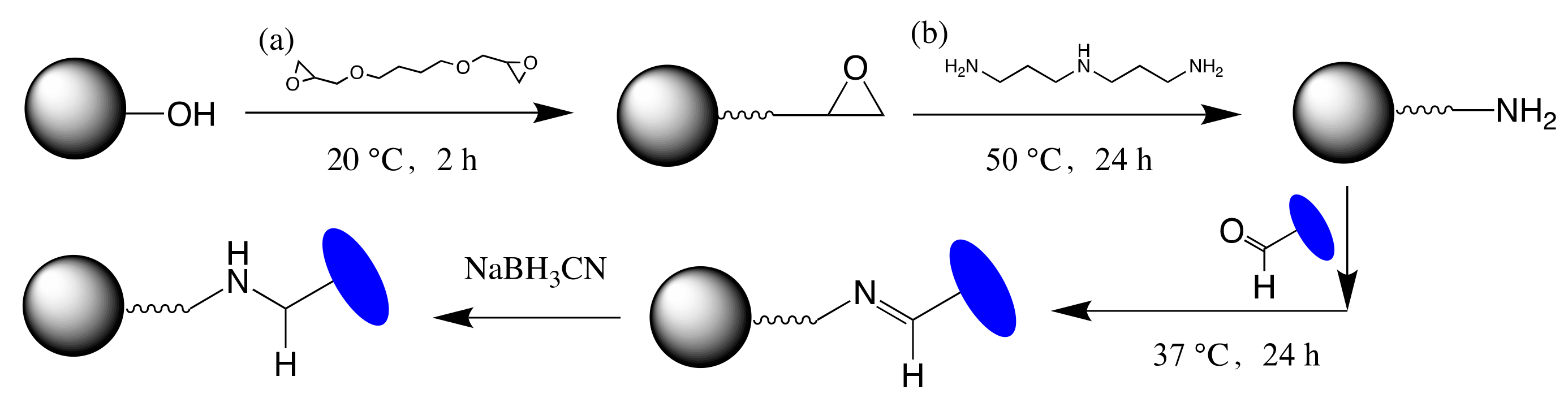
2.2. Validation of the Site-Specific Aldehyde Modification
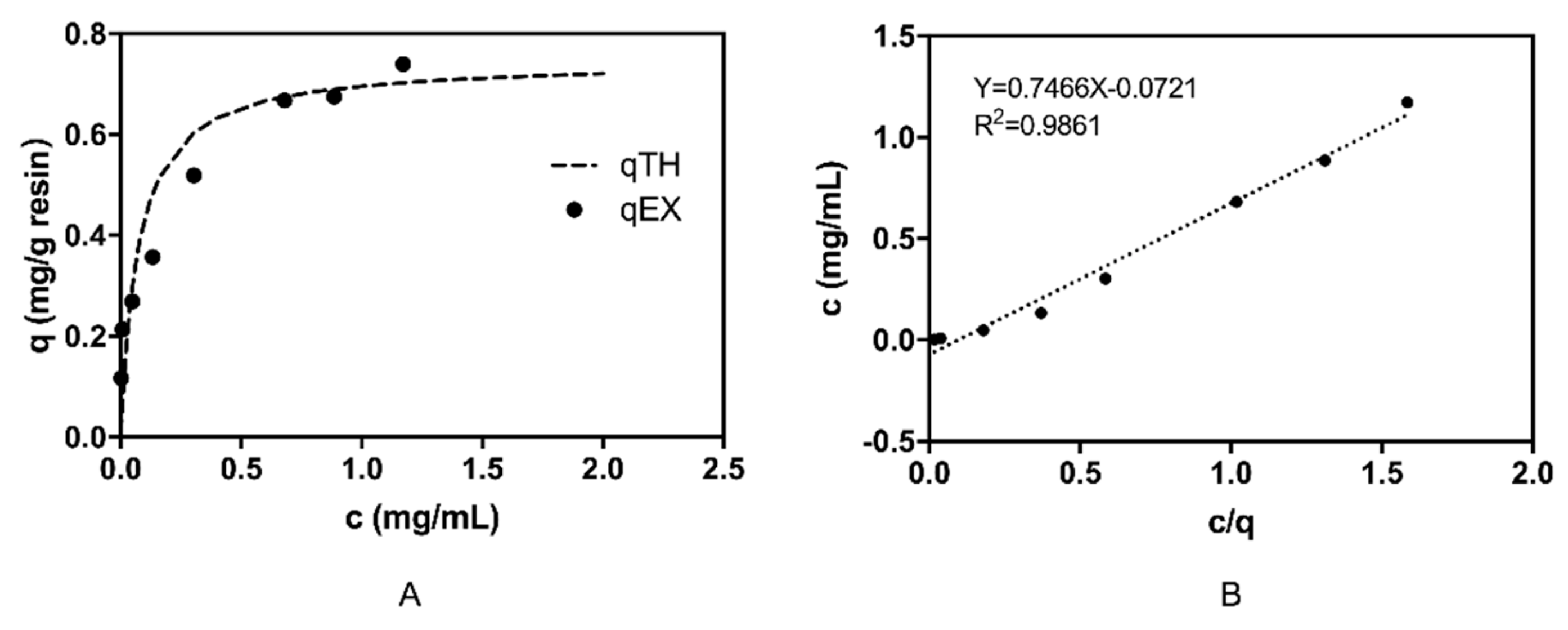
2.3. β2-m Adsorption Performance of the VHH Immunoadsorbent
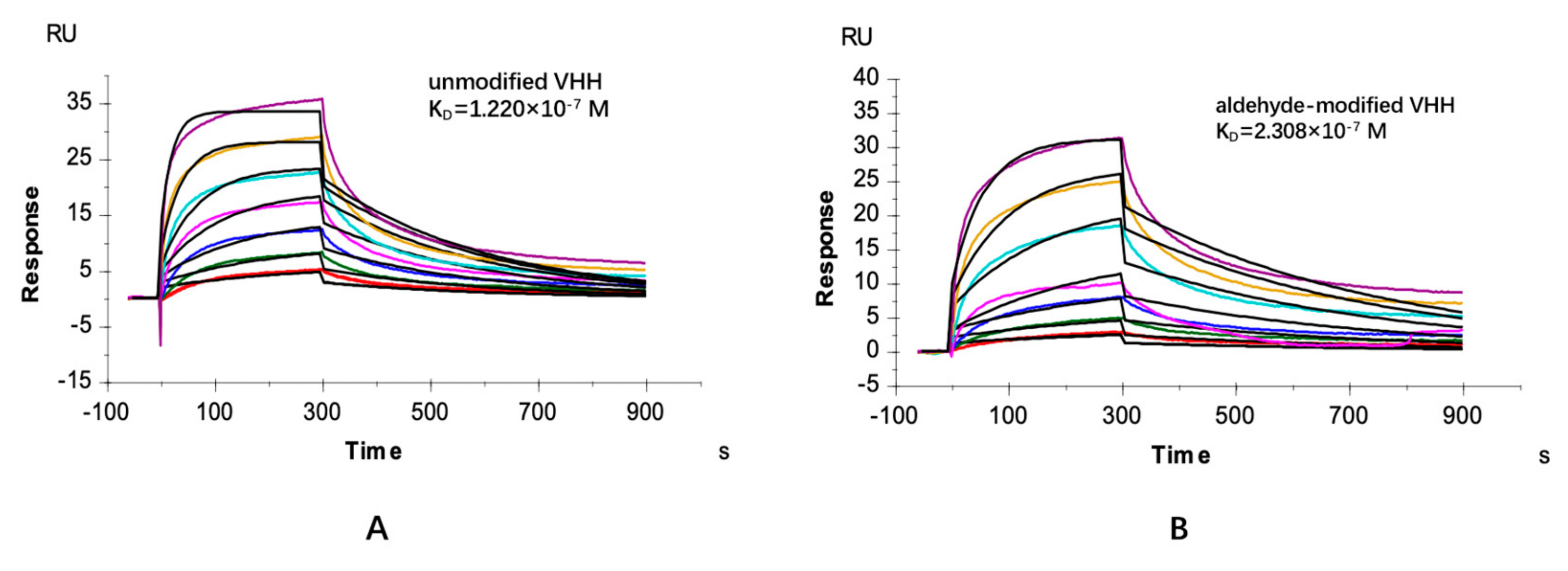
2.4. Specific Adsorption Performance of the VHH Immunoadsorbent
2.5. Storage Stability of the VHH-based Immunoadsorbent
3. Materials and Methods
3.1. Reagents and Equipment
3.2. Expression of VHH Antibody and FGE
3.3. Validation of Aldehyde on VHH
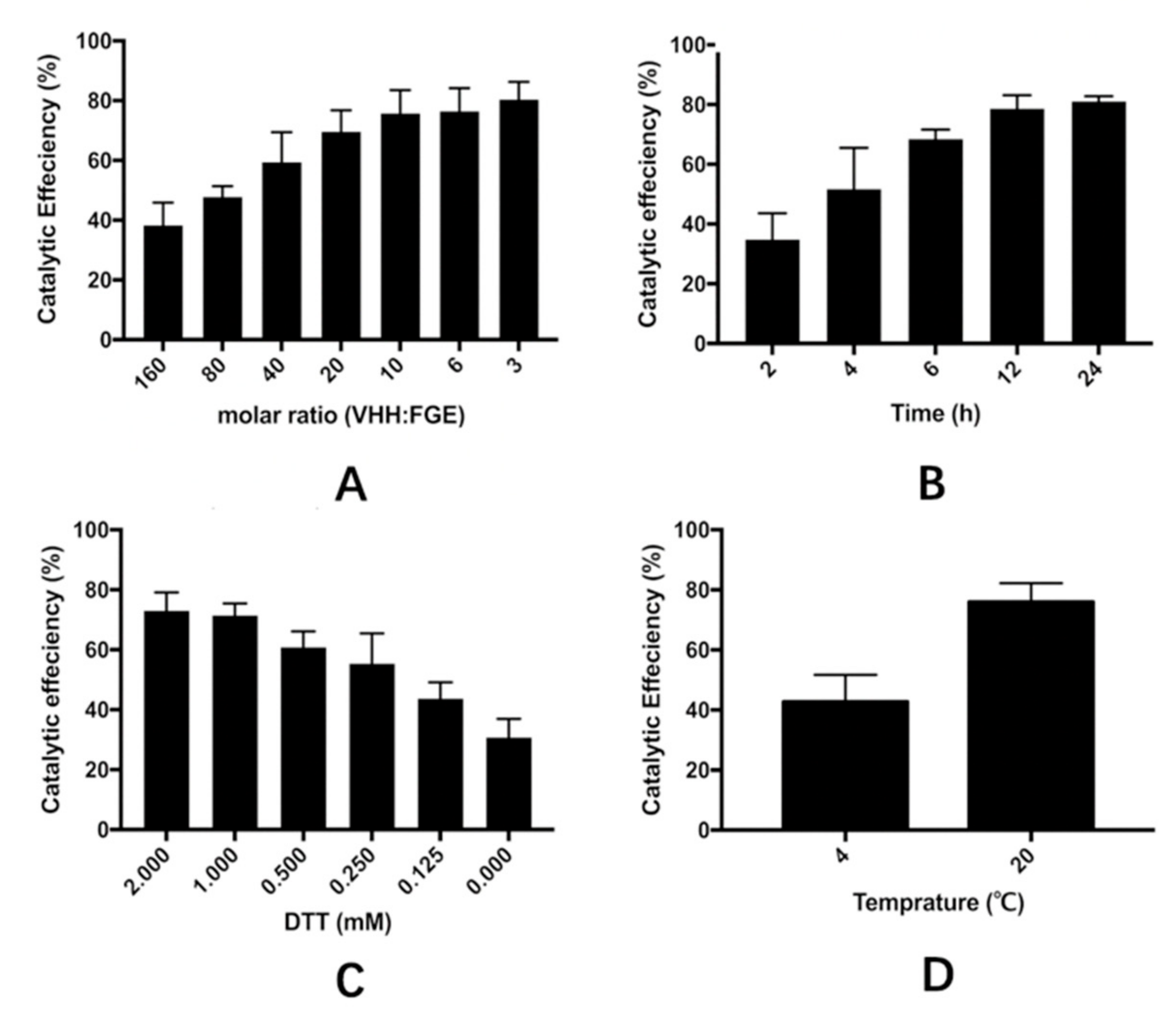
3.4. Optimization of the Catalytic Process of VHH in the Cell Extract
3.5. Fabrication of VHH-Based Immunoadsorbent
3.6. Adsorption Isotherm in β2-m Enriched Human Serum
3.7. Specific Adsorption
3.8. Storage Stability of the VHH-Based Immunoadsorbent
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VHH | variable region of the heavy chain antibody |
| FGE | formylglycine-generating enzyme |
| β2-m | β2-microglobulin |
| scFv | single-chain variable fragment |
| HSA | human serum albumin |
References
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; de Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National kidney foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Eknoyan, G.; Levin, N.W. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification-Foreword. Am. J. Kidney Dis. 2002, 39, S14–S266. [Google Scholar]
- Gejyo, F.; Homma, N.; Suzuki, Y.; Arakawa, M. Serum Levels of β-2-Microglobulin as a New Form of Amyloid Protein in Patients Undergoing Long-Term Hemodialysis. New Engl. J. Med. 1986, 314, 585–586. [Google Scholar] [PubMed]
- Ogawa, H.; Saito, A.; Hirabayashi, N.; Hara, K. Amyloid Deposition in Systemic Organs in Long-Term Hemodialysis-Patients. Clin. Nephrol. 1987, 28, 199–204. [Google Scholar] [PubMed]
- Gejyo, F.; Yamada, T.; Odani, S.; Nakagawa, Y.; Arakawa, M.; Kunitomo, T.; Kataoka, H.; Suzuki, M.; Hirasawa, Y.; Shirahama, T.; et al. A New Form of Amyloid Protein Associated with Chronic-Hemodialysis Was Identified as β-2-Microglobulin. Biochem. Bioph. Res. Co 1985, 129, 701–706. [Google Scholar] [CrossRef]
- Warren, D.J.; Otieno, L.S. Carpal-Tunnel Syndrome in Patients on Intermittent Hemodialysis. Postgrad. Med. J. 1975, 51, 450–452. [Google Scholar] [CrossRef]
- Vanholder, R.; Massy, Z.A. Progress in Uremic Toxin Research: An Introduction. Semin. Dial. 2009, 22, 321–322. [Google Scholar] [CrossRef]
- Kleinman, K.S.; Coburn, J.W. Amyloid Syndromes Associated with Hemodialysis. Kidney Int. 1989, 35, 567–575. [Google Scholar] [CrossRef][Green Version]
- Kazama, J.J.; Maruyama, H.; Gejyo, F. Reduction of circulating β2-microglobulin level for the treatment of dialysis-related amyloidosis. Nephrol. Dial. Transpl. 2001, 16, 31–35. [Google Scholar] [CrossRef]
- Ameer, G.A. Modalities for the removal of β(2)-microglobulin from blood. Semin. Dial. 2001, 14, 103–106. [Google Scholar] [CrossRef]
- Tsuchida, L.; Takemoto, Y.; Sugimura, K.; Yoshimura, R.; Yamamoto, K.; Nakatani, T. Adsorption of endotoxin by β(2)-microglobulin adsorbent column (Lixelle): The new approach for endotoxinemia. Ther. Apher. 2002, 6, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Reiter, K.; Bordoni, V.; Dall’Olio, G.; Ricatti, M.G.; Soli, M.; Ruperti, S.; Soffiati, G.; Galloni, E.; D’Intini, V.; Bellomo, R.; et al. In vitro removal of therapeutic drugs with a novel adsorbent system. Blood Purif. 2002, 20, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Furuyoshi, S.; Nakatani, M.; Taman, J.; Kutsuki, H.; Takata, S.; Tani, N. New adsorption column (Lixelle) to eliminate β2-microglobulin for direct hemoperfusion. Ther. Apher. 1998, 2, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kutsuki, H. β(2)-microglobulin-selective direct hemoperfusion column for the treatment of dialysis-related amyloidosis. Bba-Proteins Proteom. 2005, 1753, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, K.; Takemoto, Y.; Nakamura, T.; Fu, O.; Okada, C.; Yamagami, S.; Kishimoto, T. Lixelle adsorbent to remove inflammatory cytokines. Artif. Organs 1998, 22, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Grovender, E.A.; Kellogg, B.; Singh, J.; Blom, D.; Ploegh, H.; Wittrup, K.D.; Langer, R.S.; Ameer, G.A. Single-chain antibody fragment-based adsorbent for the extracorporeal removal of β(2)-microglobulin. Kidney Int. 2004, 65, 310–322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Floege, J.; Ketteler, M. β(2)-microglobulin-derived amyloidosis: An update. Kidney Int. 2001, 59, S164–S171. [Google Scholar] [CrossRef]
- Wesolowski, J.; Alzogaray, V.; Reyelt, J.; Unger, M.; Juarez, K.; Urrutia, M.; Cauerhff, A.; Danquah, W.; Rissiek, B.; Scheuplein, F.; et al. Single domain antibodies: Promising experimental and therapeutic tools in infection and immunity. Med. Microbiol. Immun. 2009, 198, 157–174. [Google Scholar] [CrossRef]
- De Meyer, T.; Muyldermans, S.; Depicker, A. Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 2014, 32, 263–270. [Google Scholar] [CrossRef]
- Dumoulin, M.; Conrath, K.; van Meirhaeghe, A.; Meersman, F.; Heremans, K.; Frenken, L.G.J.; Muyldermans, S.; Wyns, L.; Matagne, A. Single-domain antibody fragments with high conformational stability. Protein. Sci. 2002, 11, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Zang, B.; Ren, J.; Li, D.; Huang, C.; Ma, H.; Peng, Q.; Ji, F.; Han, L.; Jia, L. Freezing-assisted synthesis of covalent C-C linked bivalent and bispecific nanobodies. Org. Biomol. Chem. 2019, 17, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Meury, M.; Knop, M.; Seebeck, F.P. Structural Basis for Copper-Oxygen Mediated C-H Bond Activation by the Formylglycine-Generating Enzyme. Angew. Chem. Int. Edit 2017, 56, 8115–8119. [Google Scholar] [CrossRef] [PubMed]
- Knop, M.; Engi, P.; Lemnaru, R.; Seebeck, F.P. In Vitro Reconstitution of Formylglycine-Generating Enzymes Requires Copper(I). Chembiochem 2015, 16, 2147–2150. [Google Scholar] [CrossRef] [PubMed]
- Knop, M.; Dang, T.Q.; Jeschke, G.; Seebeck, F.P. Copper is a Cofactor of the Formylglycine-Generating Enzyme. Chembiochem 2017, 18, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Barcia, S.; Kastner, J. Copper coordination in formylglycine generating enzymes. Eur. Phys. J. Spec. Top. 2019, 227, 1657–1664. [Google Scholar] [CrossRef]
- Nakano, H.; Gomi, H.; Shibasaki, T.; Ishimoto, F.; Sakai, O. An Experimental-Study on Selective Elimination of β-2-Microglobulin Using Immunoadsorption Method in Patients with Chronic-Renal-Failure. Biomat. Artif. Cell Im. 1991, 19, 61–69. [Google Scholar] [CrossRef]
- Davankov, V.; Pavlova, L.; Tsyurupa, M.; Brady, J.; Balsamo, M.; Yousha, E. Polymeric adsorbent for removing toxic proteins from blood of patients with kidney failure. J. Chromatogr. B 2000, 739, 73–80. [Google Scholar] [CrossRef]
- Vallar, L.; Costa, P.M.P.; Teixeira, A.; Pfister, M.; Barrois, R.; Costa, P.P.; Rivat, C. Immunoadsorption Procedure as a Potential Method for the Specific β-2-Microglobulin Removal from Plasma of Patients with Chronic-Renal-Failure. J. Chromatogr. B Biomed. App. 1995, 664, 97–106. [Google Scholar] [CrossRef]
- Ameer, G.A.; Grovender, E.A.; Ploegh, H.; Ting, D.; Owen, W.F.; Rupnick, M.; Langer, R. A novel immunoadsorption device for removing beta(2)-microglobulin from whole blood. Kidney Int. 2001, 59, 1544–1550. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors, except the anti-β2-m VHH antibody. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zang, B.; Huang, C.; Ren, J.; Jia, L. One-step Preparation of a VHH-based Immunoadsorbent for the Extracorporeal Removal of β2-microglobulin. Molecules 2019, 24, 2119. https://doi.org/10.3390/molecules24112119
Zhang L, Zang B, Huang C, Ren J, Jia L. One-step Preparation of a VHH-based Immunoadsorbent for the Extracorporeal Removal of β2-microglobulin. Molecules. 2019; 24(11):2119. https://doi.org/10.3390/molecules24112119
Chicago/Turabian StyleZhang, Lijun, Berlin Zang, Chundong Huang, Jun Ren, and Lingyun Jia. 2019. "One-step Preparation of a VHH-based Immunoadsorbent for the Extracorporeal Removal of β2-microglobulin" Molecules 24, no. 11: 2119. https://doi.org/10.3390/molecules24112119
APA StyleZhang, L., Zang, B., Huang, C., Ren, J., & Jia, L. (2019). One-step Preparation of a VHH-based Immunoadsorbent for the Extracorporeal Removal of β2-microglobulin. Molecules, 24(11), 2119. https://doi.org/10.3390/molecules24112119




