Dual-Responsive Alginate Hydrogels for Controlled Release of Therapeutics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of SA/pNIPAAm and OTC@SA/pNIPAAm
2.3. Physical Characterization
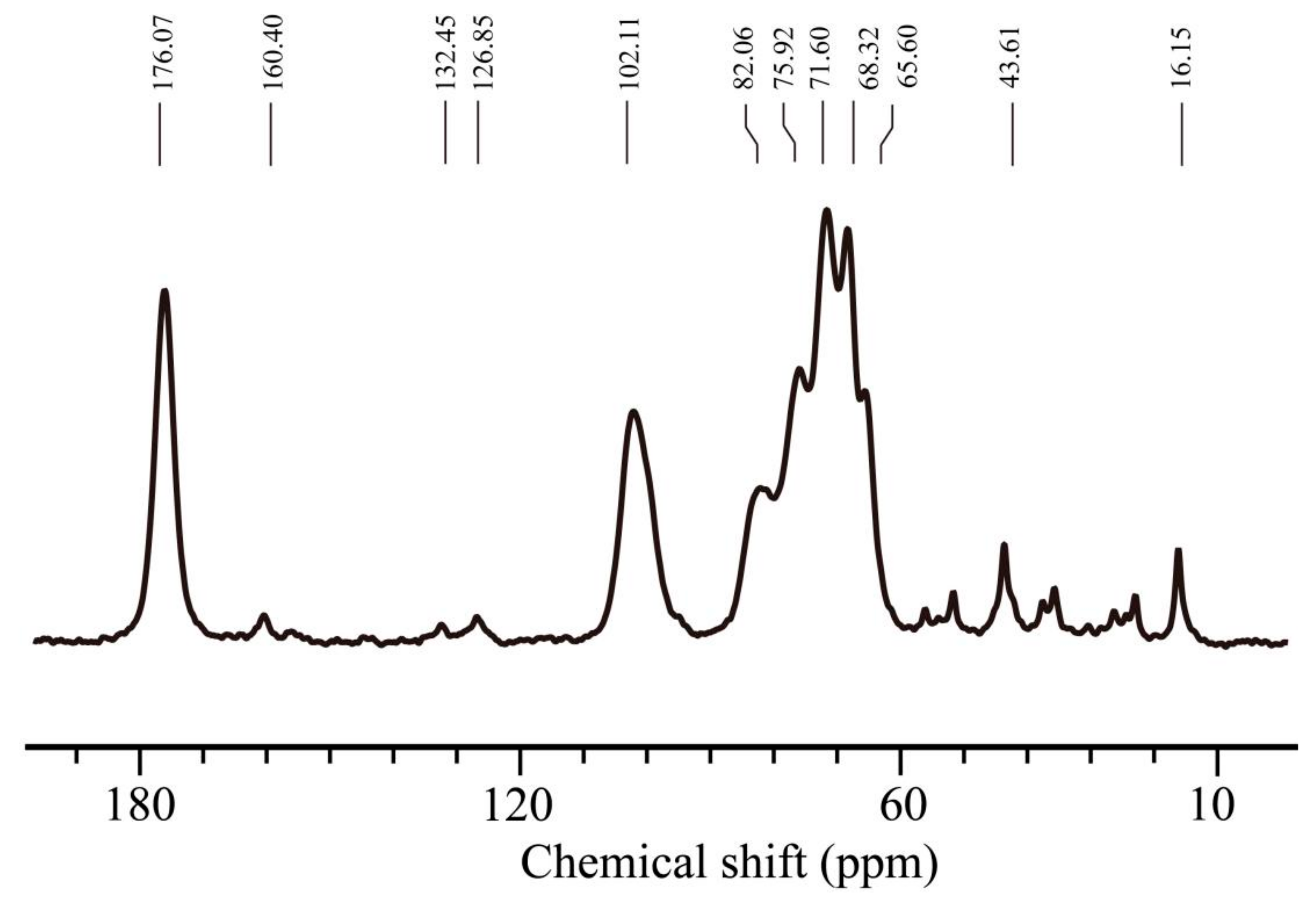
2.4. Characterization of SA/NIPAAm by NMR
2.5. Swelling-Deswelling and Degradation Behaviors of SA/pNIPAAm Hydrogels
2.6. Entrapment Efficiency In Vitro
2.7. OTC Release In Vitro
2.8. Cytotoxicity
2.9. Antimicrobial Studies
3. Results and Discussion
3.1. Physical Characterization
3.2. pH- and Thermos-Responsive Swelling Behavior
3.3. Swelling-Deswelling Kinetics of Hydrogels
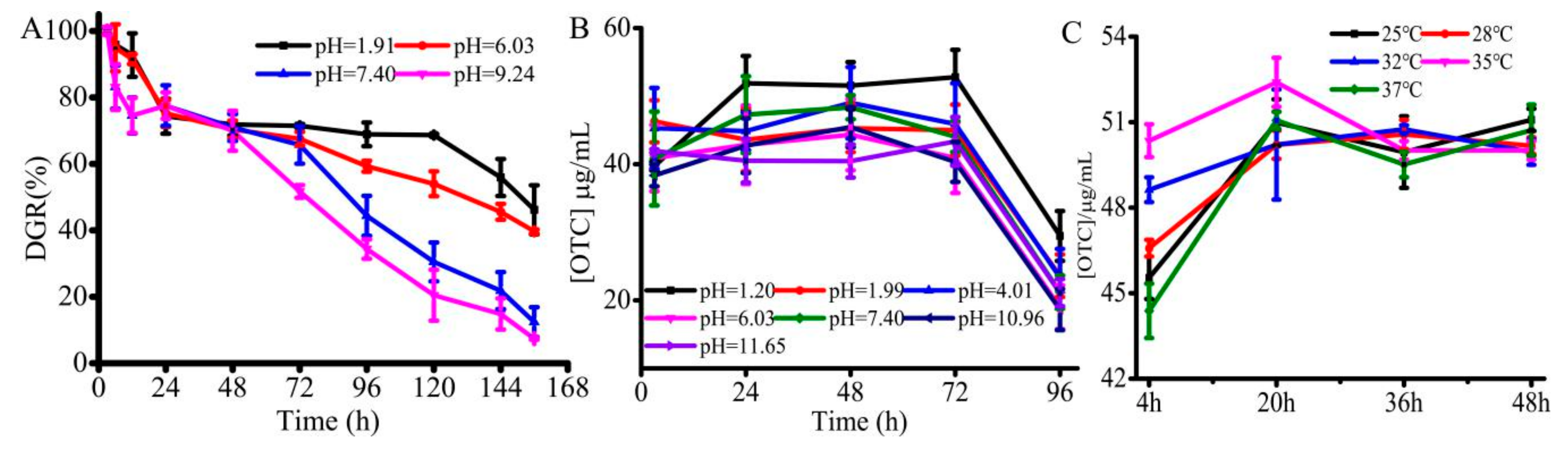
3.4. Evaluation of EP%, Degradation Efficiency and OTC Drug Release In Vitro
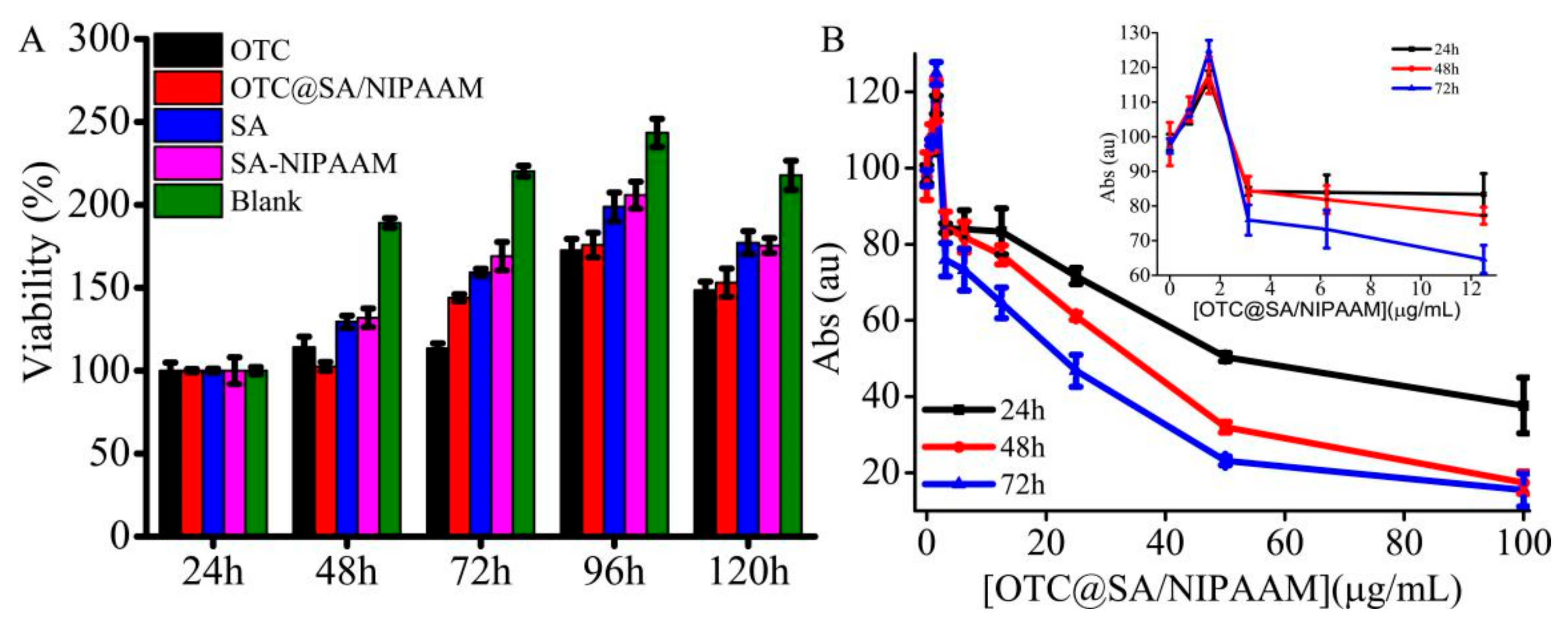
3.5. Cytotoxicity Studies and Antimicrobial Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pundir, S.; Badola, A.; Sharma, D. Sustained release matrix technology and recent advance in matrix drug delivery system: A review. Int. J. Drug Res. Tech. 2013, 3, 12–20. [Google Scholar]
- Hu, C.; Chen, Z.; Wu, S.; Han, Y.; Wang, H.; Sun, H.; Kong, D.; Leng, X.; Wang, C.; Zhang, L.; et al. Micelle or polymersome formation by PCL-PEG-PCL copolymers as drug delivery systems. Chinese Chem. Lett. 2017, 28, 1905–1909. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-G.; Han, Y.-H.; Zhang, J.-T.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Rerouting engineered metal-dependent shapes of mesoporous silica nanocontainers to biodegradable Janus-type (sphero-ellipsoid) nanoreactors for chemodynamic therapy. Chem. Eng. J. 2019, 370, 1188–1199. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Wei, Y.; Tao, L. Chitosan-based self-healing hydrogel for bioapplications. Chinese Chem. Lett. 2017, 28, 2053–2057. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Wang, G.; Li, M.; Liu, J.; Ding, X.; Huang, W.; Gao, W.; Wu, H. A photocleavable low molecular weight hydrogel for light-triggered drug delivery. Chinese Chem. Lett. 2018, 30, 485–488. [Google Scholar] [CrossRef]
- Kankala, R.K.; Wang, S.-B.; Chen, A.-Z.; Zhang, Y.S. Chapter 2 - Self-Assembled Nanogels: From Particles to Scaffolds and Membranes. In Handbook of Nanomaterials for Cancer Theranostics, 1; Conde, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 33–62. [Google Scholar]
- Ghaffari, R.; Eslahi, N.; Tamjid, E.; Simchi, A. Dual-sensitive hydrogel nanoparticles based on conjugated thermoresponsive copolymers and protein filaments for triggerable drug delivery. Acs Appl. Mater. Interfaces 2018, 10, 19336–19346. [Google Scholar] [CrossRef]
- Narendra, S.; Lee, K.; Sung, D. In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J. Control. Release 2014, 193, 214–227. [Google Scholar]
- Shang, J.; Theato, P. Smart composite hydrogel with pH-, ionic strength- and temperature-induced actuation. Soft Matter 2018, 14, 8401–8407. [Google Scholar] [CrossRef] [PubMed]
- Didehban, K.H.; Mohammadi, L.; Azimvand, J. Preparation of RGO/Fe3O4/poly (acrylic acid) hydrogel nanocomposites with improved magnetic, thermal and electrochemical properties. Mater. Chem. Phys. 2017, 195, 162–169. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Shi, X.; Jin, M.; Cheng, W.; Ren, L.; Wang, Y. Hierarchical and reversible assembly of graphene oxide/polyvinyl alcohol hybrid stabilized Pickering emulsions and their templating for macroporous composite hydrogels. Carbon 2017, 111, 38–47. [Google Scholar] [CrossRef]
- Rasib, S.Z.M.; Ahmad, Z.; Khan, A.; Akil, H.M.; Othman, M.B.H.; Hamid, Z.A.A.; Ullah, F. Synthesis and evaluation on pH- and temperature-responsive chitosan-p(MAA-co-NIPAM) hydrogels. Int. J. Biol. Macromol. 2018, 108, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Yang, P.; He, F.; Gai, S.; Yang, G.; Dai, Y. An imaging-guided platform for synergistic photodynamic/photothermal/chemo-therapy with pH/temperature-responsive drug release. Biomaterials 2015, 63, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Hiruta, Y.; Funatsu, T.; Matsuura, M.; Wang, J.; Ayano, E.; Kanazawa, H. pH/temperature-responsive fluorescence polymer probe with pH-controlled cellular uptake. Sensor Actuat. B Chem. 2015, 207, 724–731. [Google Scholar] [CrossRef]
- Zeng, J.; Du, P.; Liu, L.; Li, J.; Tian, K.; Jia, X. Superparamagnetic Reduction/pH/Temperature Multistimuli-Responsive Nanoparticles for Targeted and Controlled Antitumor Drug Delivery. Mol. Pharm. 2015, 12, 4188–4199. [Google Scholar] [CrossRef] [PubMed]
- Raguvaran, R.; Manuja, B.K.; Chopra, M.; Thakur, R.; Anand, T.; Kalia, A.; Manuja, A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017, 96, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Treenate, P.; Monvisade, P. In vitro drug release profiles of pH-sensitive hydroxyethylacryl chitosan / sodium alginate hydrogels using paracetamol as a soluble model drug. Int. J. Biol. Macromol. 2017, 99, 71–78. [Google Scholar] [CrossRef]
- Marrella, A.; Lagazzo, A.; Barberis, F.; Catelani, T.; Quarto, R.; Scaglione, S. Enhanced mechanical performances and bioactivity of cell laden-graphene oxide/alginate hydrogels open new scenario for articular tissue engineering applications. Carbon 2017, 115, 608–616. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.-L.; Li, J. Injectable thermoresponsive hydrogel formed by alginate-g-poly(N-isopropylacrylamide) that releases doxorubicin-encapsulated micelles as a smart Drug Delivery System. Acs Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Sun, L.; He, X.; Li, J.; Liu, Y.; Xiong, F.; Ge, L.; Webster, T.J.; Zheng, C. Doxorubicin-loaded poly (lactic-co-glycolic acid) nanoparticles coated with chitosan/alginate by layer by layer technology for antitumor applications. Int. J. Nanomed. 2017, 12, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Kang, H.; Bielec, M.; Wu, X.; Cheng, Q.; Wei, W.; Dai, H. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohyd. Polym. 2018, 197, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, P.M.; Peighambardoust, S.J. A review on acrylic based hydrogels and their applications in wastewater treatment. J. Environ. Manage. 2018, 217, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-J.; Yao, M.; Gao, S.; Zhang, A.P.; Tam, H.-Y.; Wai, P.K. Rapid 3D Patterning of Poly (acrylic acid) Ionic Hydrogel for Miniature pH Sensors. Adv. Mater. 2016, 28, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Su, J.; Lin, H.; Zhou, S.-F.; Sun, X.; Liu, B.; Zeng, M. Carbon nanoparticles with oligonucleotide probes for a label-free sensitive antibiotic residues detection based on competitive analysis. Sci. Rep. UK 2019, 9, 3489. [Google Scholar] [CrossRef]
- Peppas, N.A.; Merrill, E.W. Crosslinked poly(vinyl alcohol) hydrogels as swollen elastic networks. J. Appl. Polym. Sci. 1977, 21, 1763–1770. [Google Scholar] [CrossRef]
- Deak, A.; Csapo, E.; Juhasz, A.; Dekany, I.; Janovak, L. Anti-ulcerant kynurenic acid molecules intercalated Mg/Al-layered double hydroxide and its release study. Appl. Clay Sci. 2018, 156, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Vergara, A.C.; Lewis, J.; Hahn, M.S.; Munoz-Pinto, D.J. An improved correlation to predict molecular weight between crosslinks based on equilibrium degree of swelling of hydrogel networks. J. Biomed. Mater. Res. B 2018, 106, 1339–1348. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Ma, Q.; Su, J.; Wang, C.; Kankala, R.K.; Zeng, M.; Lin, H.; Zhou, S.-F. Dual-Responsive Alginate Hydrogels for Controlled Release of Therapeutics. Molecules 2019, 24, 2089. https://doi.org/10.3390/molecules24112089
Lin X, Ma Q, Su J, Wang C, Kankala RK, Zeng M, Lin H, Zhou S-F. Dual-Responsive Alginate Hydrogels for Controlled Release of Therapeutics. Molecules. 2019; 24(11):2089. https://doi.org/10.3390/molecules24112089
Chicago/Turabian StyleLin, Xuexia, Qiaoqiao Ma, Jianlong Su, Cui Wang, Ranjith Kumar Kankala, Mingrong Zeng, Honggui Lin, and Shu-Feng Zhou. 2019. "Dual-Responsive Alginate Hydrogels for Controlled Release of Therapeutics" Molecules 24, no. 11: 2089. https://doi.org/10.3390/molecules24112089
APA StyleLin, X., Ma, Q., Su, J., Wang, C., Kankala, R. K., Zeng, M., Lin, H., & Zhou, S.-F. (2019). Dual-Responsive Alginate Hydrogels for Controlled Release of Therapeutics. Molecules, 24(11), 2089. https://doi.org/10.3390/molecules24112089







