In Vitro and In Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania infantum
Abstract
1. Introduction
2. Results
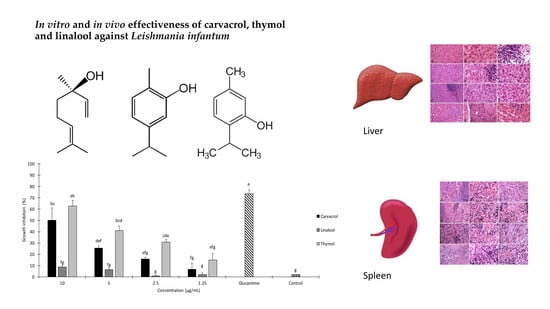
2.1. In Vitro Anti-leishmanial Activity
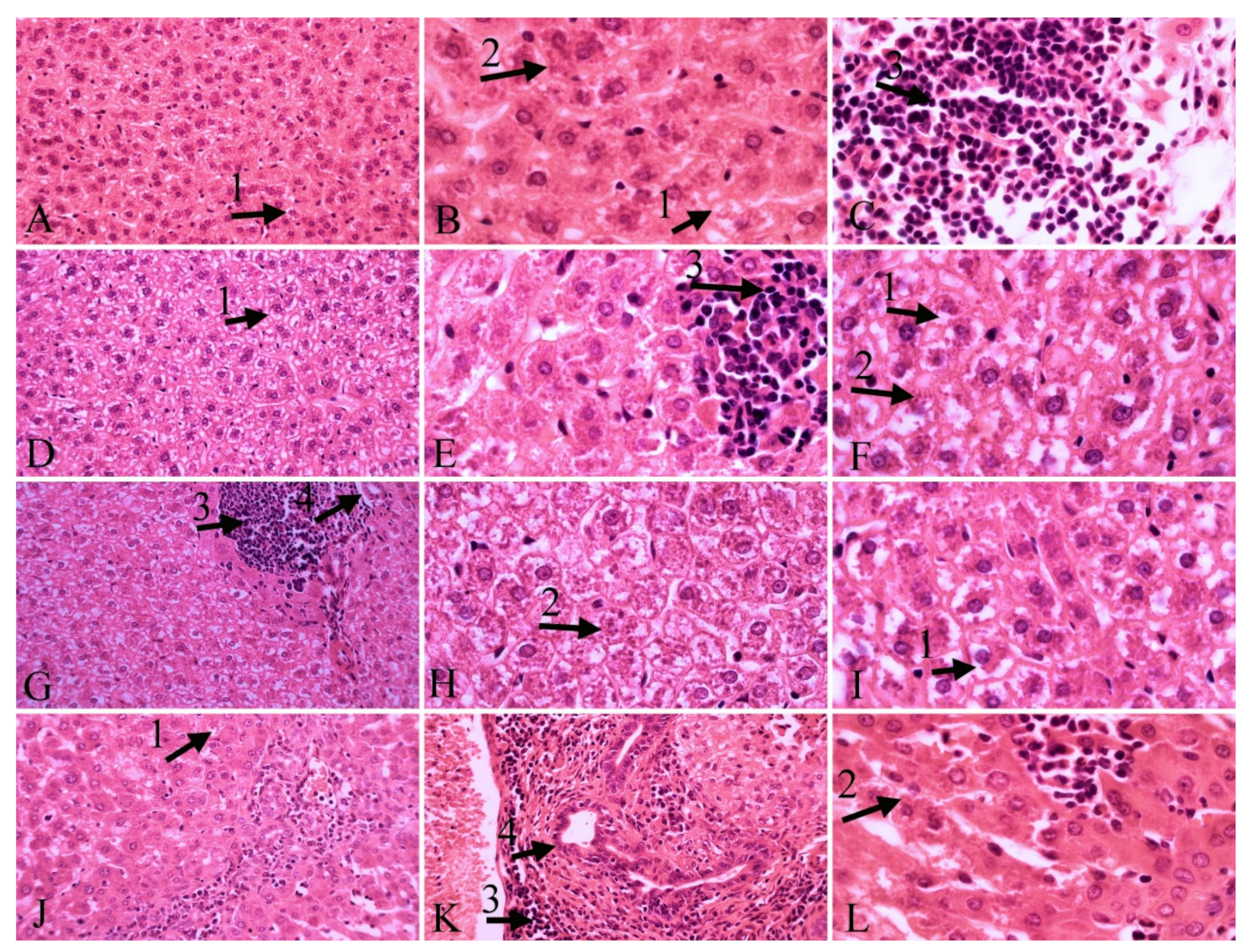
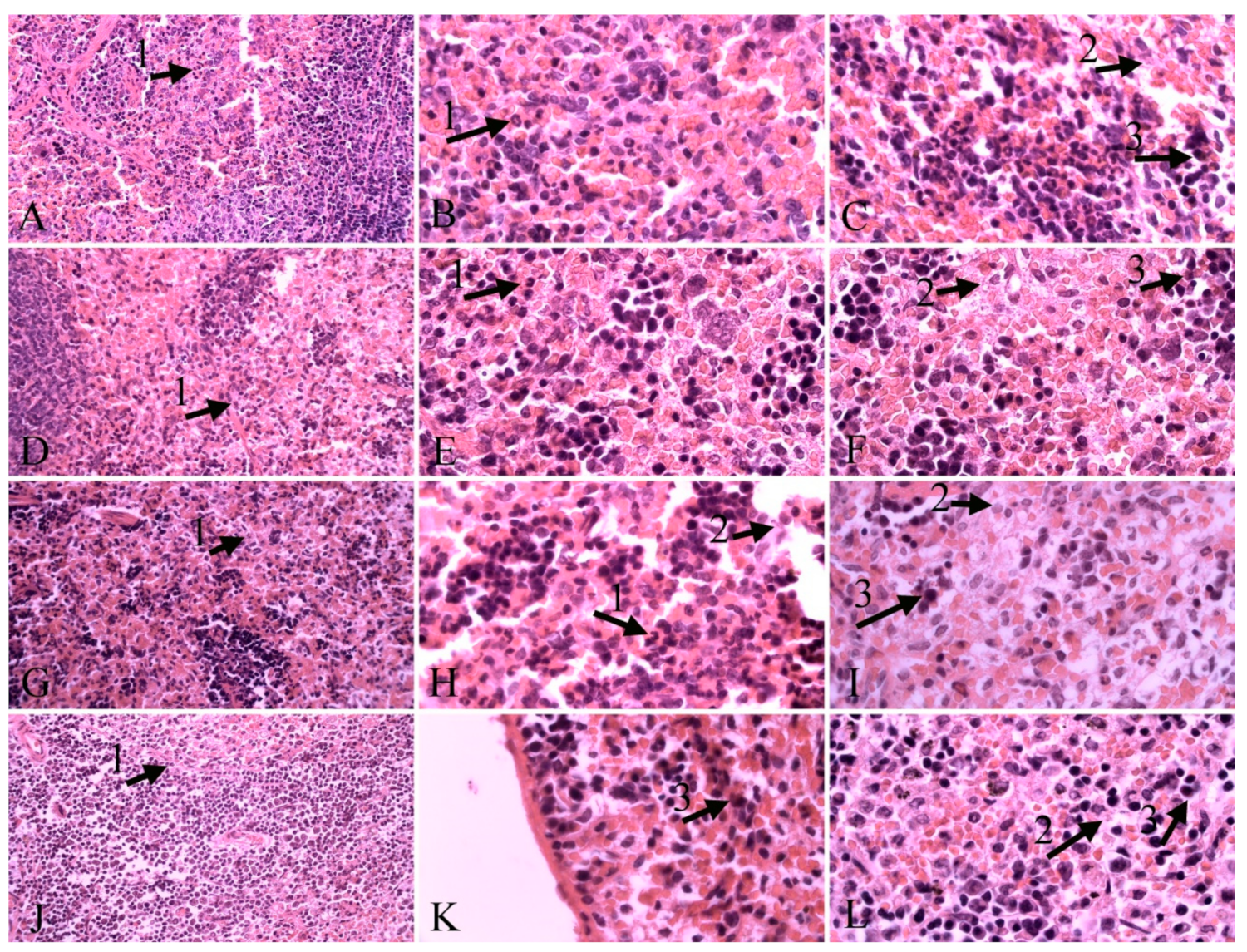
2.2. Histopathological Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Parasite Culture
4.3. In Vitro Experiments
4.4. In Vivo Experiments
4.5. Histopathological Examination
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Savoia, D. Recent updates and perspectives on leishmaniasis. J. Infect. Dev. Countries 2015, 9, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Tajebe, F.; Getahun, M.; Adem, E.; Hailu, A.; Lemma, M.; Fikre, H.; Diro, E. Disease severity in patients with visceral leishmaniasis is not altered by co-infection with intestinal parasites. PLoS Negl. Trop. Dis. 2017, 11, e0005727. [Google Scholar] [CrossRef]
- Freitas-Junior, L.H.; Chatelain, E.; Kim, H.A.; Siqueira-Neto, J.L. Visceral leishmaniasis treatment: What do we have, what do we need and how to deliver it? Int. J. Parasitol. Drugs Drug. Resist. 2012, 2, 11–19. [Google Scholar] [CrossRef]
- Yuan, M.; Vásquez-Valdivieso, M.G.; McNae, I.W.; Michels, P.A.; Fothergill-Gilmore, L.A.; Walkinshaw, M.D. Structures of Leishmania Fructose-1, 6-Bisphosphatase Reveal Species-Specific Differences in the Mechanism of Allosteric Inhibition. J. Mol. Biol. 2017, 429, 3075–3089. [Google Scholar] [CrossRef] [PubMed]
- Reithinger, R.; Dujardin, J.C. Molecular diagnosis of leishmaniasis: current status and future applications. J. Clin. Microbiol. 2007, 45, 21–25. [Google Scholar] [CrossRef]
- Sundar, S.; Sinha, P.K.; Rai, M.; Verma, D.K.; Nawin, K.; Alam, S.; Pandey, K. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: An open-label, non-inferiority, randomised controlled trial. Lancet 2011, 377, 477–486. [Google Scholar] [CrossRef]
- Alvar, J.; Velez, I.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Bockman, M.R.; Kalinda, A.S.; Petrelli, R.; De La Mora-Rey, T.; Tiwari, D.; Liu, F.; Dawadi, S.; Nandakumar, M.; Rhee, K.Y.; Schnappinger, D.; et al. Targeting Mycobacterium tuberculosis Biotin Protein Ligase (MtBPL) with Nucleoside-Based Bisubstrate Adenylation Inhibitors. J. Med. Chem. 2015, 58, 7349–7369. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kamte, S.L.N.; Ranjbarian, F.; Cianfaglione, K.; Sut, S.; Dall’Acqua, S.; Bruno, M.; Afshar, F.H.; Iannarelli, R.; Benelli, G.; Cappellacci, L.; et al. Identification of highly effective antitrypanosomal compounds in essential oils from the Apiaceae family. Ecotoxicol. Environ. Saf. 2018, 156, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Mafud, A.C.; Silva, M.P.; Monteiro, D.C.; Oliveira, M.F.; Resende, J.G.; Coelho, M.L.; Freitas, R.M. Structural parameters, molecular properties, and biological evaluation of some terpenes targeting Schistosoma mansoni parasite. Chem. Biol. Interact. 2016, 244, 129–139. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Craker, L.E.; Salami, A.; Nazeri, V.; Sang, H.; Maggi, F. Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono-and sesquiterpene synthesis in two oregano (Origanum vulgare L.) subspecies. Plant Physiol. Biochem. 2017, 111, 119–128. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemi, M.; Maggi, F.; Morshedloo, M.R. Application of combined fertilizers improves biomass, essential oil yield, aroma profile, and antioxidant properties of Thymus daenensis Celak. Ind. Crops Prod. 2018, 121, 434–440. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nathan, S.; Mehlhorn, H.; Maggi, F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef]
- Vitali, L.A.; Beghelli, D.; Nya, P.C.B.; Bistoni, O.; Cappellacci, L.; Damiano, S.; Lupidi, G.; Maggi, F.; Orsomando, G.; Papa, F.; et al. Diverse biological effects of the essential oil from Iranian Trachyspermum ammi. Arab. J. Chem. 2016, 9, 775–786. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Maggi, F. Essential oil composition, total phenolic and flavonoids contents, and antioxidant activity of Oliveria decumbens Vent.(Apiaceae) at different phenological stages. J. Clean. Prod. 2018, 198, 91–95. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Kamte, S.N.; Rakotosaona, R.; Rasoanaivo, P.; Nicoletti, M.; Canale, A.; Benelli, G. Chemical composition of Cinnamosma madagascariensis (Cannelaceae) essential oil and its larvicidal potential against the filariasis vector Culex quinquefasciatus Say. S. Afr. J. Bot. 2017, 108, 359–363. [Google Scholar] [CrossRef]
- Duarte, A.; Luís, Â.; Oleastro, M.; Domingues, F.C. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Cecchini, C.; Silvi, S.; Cresci, A.; Piciotti, A.; Caprioli, G.; Papa, F.; Sagratini, G.; Vittori, S.; Maggi, F. Antimicrobial efficacy of Achillea ligustica All. (Asteraceae) essential oils against reference and isolated oral microorganisms. Chem. Biodivers. 2012, 9, 12–24. [Google Scholar] [CrossRef]
- Tabari, M.A.; Youssefi, M.R.; Maggi, F.; Benelli, G. Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae). Vet. Parasitol. 2017, 245, 86–91. [Google Scholar] [CrossRef]
- Ramos-Lopez, M.; Sanchez-Mir, E.; Fresan-Orozco, M.; Perez-Ramos, J. Antiprotozoa activity of some essential oils. J. Med. Plants Res. 2012, 6, 2901–2908. [Google Scholar]
- Bagamboula, C.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Rahimmalek, M.; Malekpoor, F.; Karimi, A. Variation in antibacterial activity, thymol and carvacrol contents of wild populations of Thymus daenensis subsp. daenensis Celak. Plant Omics 2011, 4, 209–214. [Google Scholar]
- Jesus, F.; Ferreiro, L.; Bizzi, K.; Loreto, É.; Pilotto, M.; Ludwig, A.; Santurio, J. In vitro activity of carvacrol and thymol combined with antifungals or antibacterials against Pythium insidiosum. J. Mycol. Med. 2015, 25, e89–e93. [Google Scholar] [CrossRef]
- Katiki, L.; Evangelista, A.; Canova, E.; Piza, A.; Fornazari, B.; Araujo, R.; Bueno, M. Anthelmintic activity of anethole, carvone, carvacrol, thymol, linalool, limonene, eucalyptol, vanillin, cinnamaldehyde and eugenol in in vitro tests. J. Med. Plants 2014, 80, 1415. [Google Scholar] [CrossRef]
- Lai, W.-L.; Chuang, H.-S.; Lee, M.-H.; Wei, C.-L.; Lin, C.-F.; Tsai, Y.-C. Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. J. Med. Plants 2012, 78, 1636–1638. [Google Scholar] [CrossRef]
- Moghtader, M. Antifungal effects of the essential oil from Thymus vulgaris L. and comparison with synthetic thymol on Aspergillus niger. J. Yeast Fungal Res. 2012, 3, 83–88. [Google Scholar]
- Numpaque, M.; Oviedo, L.; Gil, J.; García, C.; Durango, D. Thymol and carvacrol: biotransformation and antifungal activity against the plant pathogenic fungi Colletotrichum acutatum and Botryodiplodia theobromae. Trop. Plant Pathol. 2011, 36, 3–13. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as eco-friendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Escobar, P.; Milena Leal, S.; Herrera, L.V.; Martinez, J.R.; Stashenko, E. Chemical composition and antiprotozoal activities of Colombian Lippia spp essential oils and their major components. Mem. Inst. Oswaldo Cruz. 2010, 105, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, C.; Melathopoulos, A.; Winston, M. Laboratory evaluation of miticides to control Varroa jacobsoni (Acari: Varroidae), a honey bee (Hymenoptera: Apidae) parasite. J. Econ. Entomol. 2000, 93, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.; das Graças Cardoso, M.; Guimarães, G.; Salgado, S.; Menna-Barreto, F.; Soares, M. Effect of oregano (Origanum vulgare L.) and thyme (Thymus vulgaris L.) essential oils on Trypanosoma cruzi (Protozoa: Kinetoplastida) growth and ultrastructure. Parasitol. Res. 2007, 100, 783–790. [Google Scholar] [CrossRef]
- Jeong, E.; Lim, J.; Kim, H.; Lee, H. Acaricidal activity of Thymus vulgaris oil and its main components against Tyrophagus putrescentiae, a stored food mite. J. Food Prot. 2008, 71, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M.A.; Youssefi, M.R.; Barimani, A.; Araghi, A. Carvacrol as a potent natural acaricide against Dermanyssus gallinae. Parasitol. Res. 2015, 114, 3801–3806. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 10, 996–1009. [Google Scholar] [CrossRef]
- Osorio, E.; Arango, G.; Robledo, S.; Munoz, D.; Jaramillo, L.; Velez, I. Antileishmanial and cytotoxic activity of synthetic aromatic monoterpens. Acta Farm. Bonaer. 2006, 25, 405–413. [Google Scholar]
- Rondon, F.; Bevilaqua, C.; Accioly, M.; Morais, S.; Andrade-Júnior, H.; Carvalho, C.; Magalhães, H. In vitro efficacy of Coriandrum sativum, Lippia sidoides and Copaifera reticulata against Leishmania chagasi. Rev. Bras. Parasitol. Vet. 2012, 21, 185–191. [Google Scholar] [CrossRef]
- Oliveira, C.; Moura, M.; Lopes, A.; de Andrade, P.; da Silva, H.; Figueiredo, C. Effects of essential oils from Cymbopogon citratus (DC) Stapf., Lippia sidoides Cham., and Ocimum gratissimum L. on growth and ultrastructure of Leishmania chagasi promastigotes. Parasitol. Res. 2009, 104, 1053–1059. [Google Scholar] [CrossRef]
- De Medeiros, M.; Da Silva, A.; Citó, A.; Borges, A.; De Lima, S.; Lopes, A.; Figueiredo, R. In vitro antileishmanial activity and cytotoxicity of essential oil from Lippia sidoides Cham. Parasitol. Int. 2011, 60, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Farias-Junior, P.; Rios, M.; Moura, T.; Almeida, R.; Alves, P.; Blank, A.; Scher, R. Leishmanicidal activity of carvacrol-rich essential oil from Lippia sidoides Cham. Biol. Res. 2012, 45, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Navarro, M.C.; Martin, J.; Valero, A.; Lara, A.M.; Barón, S.; Morillas, F. Activity of different monoterpenic derivatives of natural origin against Leishmania infantum promatisgotes. Revista Ibero-Latinoamericana de Parasitología 2009, 68, 65–72. [Google Scholar]
- Robledo, S.; Osorio, E.; Muñoz, D.; Jaramillo, L.M.; Restrepo, A.; Arango, G.; Vélez, I. In vitro and in vivo cytotoxicities and antileishmanial activities of thymol and hemisynthetic derivatives. Antimicrob. Agents Chemother. 2005, 49, 1652–1655. [Google Scholar] [CrossRef] [PubMed]
- El Hag, I.; Hashim, F.; El Toum, I.; Homeida, M.; El Kalifa, M.; El Hassan, A. Liver morphology and function in visceral leishmaniasis (Kala-azar). J. Clin. Pathol. 1994, 47, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Vianna, V.; Takiya, C.; de Brito-Gitirana, L. Histopathologic analysis of hamster hepatocytes submitted to experimental infection with Leishmania donovani. Parasitol. Res. 2002, 88, 829–836. [Google Scholar] [PubMed]
- Wilson, M.E.; Innes, D.J.; Sousa, A.Q.; Pearson, R.D. Early histopathology of experimental infection with Leishmania donovani in hamsters. J. Parasitol. 1987, 73, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.M.; Zhang, J.X.; Luo, J.; Guo, H.X.; Deng, H.; Chen, J.Y.; Sun, S.L. A role of cell apoptosis in lipopolysaccharide (LPS)-induced nonlethal liver injury in D-galactosamine (D-GalN)-sensitized rats. Diges. Dis. Sci. 2008, 53, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Bandyopadhyay, S.; Mandal, C.; Chatterjee, M. Development of a modified MTT assay for screening antimonial resistant field isolates of Indian visceral leishmaniasis. Parasit. Int. 2005, 54, 119–122. [Google Scholar] [CrossRef]
- De Almeida, L.; Passalacqua, T.G.; Dutra, L.A.; Varonez da Fonseca, J.N.; Queiroz Nascimento, R.F.; Imamura, K.B.; de Andrade, C.R.; dos Santos, J.L.; Graminha, M.A.S. In vivo antileishmanial activity and histopathological evaluation in Leishmania infantum infected hamsters after treatment with a furoxan derivative. Biomed. Pharmacother. 2017, 95, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Maggi, F.; Petrelli, R.; Canale, A.; Nicoletti, M.; Rakotosaona, R.; Rasoanaivo, P. Not ordinary antimalarial drugs: Madagascar plant decoctions potentiating the chloroquine action against Plasmodium parasites. Ind. Crops Prod. 2017, 103, 19–38. [Google Scholar] [CrossRef]
- Jain, K.; Jain, N.K. Novel therapeutic strategies for treatment of visceral leishmaniasis. Drug Discov. Today 2013, 18, 1272–1281. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
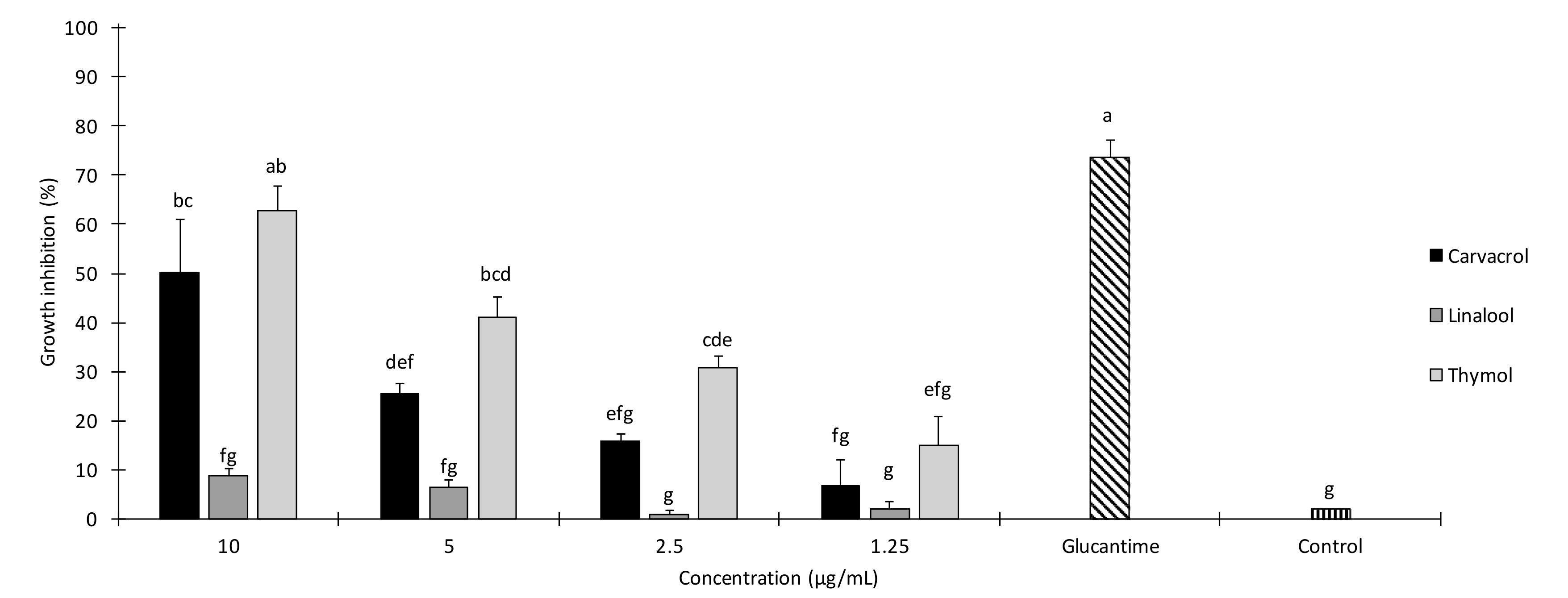
| Treatment | Concentration (µg/mL) | Growth Inhibition after 24 h (%) ± SE a | IC50 (µg/mL) (95% LCL–UCL) | χ2 b |
|---|---|---|---|---|
| Carvacrol | 10 | 50.3 ± 10.7 | 9.8 (65 μM) (8.51–11.7) | 1.29 n.s. |
| 5 | 25.6 ± 2.4 | |||
| 2.5 | 15.76 ± 1.6 | |||
| 1.25 | 6.66 ± 5.2 | |||
| Thymol | 10 | 62.76 ± 5.09 | 7.22 (48 μM) (6.22–8.62) | 3.37 n.s. |
| 5 | 41.13 ± 4.02 | |||
| 2.5 | 30.83 ± 2.24 | |||
| 1.25 | 14.93 ± 5.92 |
| Necrosis | Infiltration of Inflammatory Cells | Hyperemia | Vacuolar Degeneration of the Liver | Kupffer’s Cell Hyperplasia | Bile Duct Hyperplasia | Presence of Parasite | |
|---|---|---|---|---|---|---|---|
| Control | ++ | +++ | - | + | ++ | - | +++ |
| Carvacrol | ++ | ++ | ++ | ++ | ++ | - | +++ |
| Thymol | + | +++ | ++ | + | ++ | ++ | + |
| Glucantime | ++ | ++ | + | +++ | ++ | + | +++ |
| Necrosis | Neutrophil Infiltration | Presence of Hemosiderin | Presence of the Parasite | |
|---|---|---|---|---|
| Control | + | ++ | + | ++ |
| Carvacrol | + | + | + | + |
| Thymol | + | + | ++ | + |
| Glucantime | + | ++ | + | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssefi, M.R.; Moghaddas, E.; Tabari, M.A.; Moghadamnia, A.A.; Hosseini, S.M.; Farash, B.R.H.; Ebrahimi, M.A.; Mousavi, N.N.; Fata, A.; Maggi, F.; et al. In Vitro and In Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania infantum. Molecules 2019, 24, 2072. https://doi.org/10.3390/molecules24112072
Youssefi MR, Moghaddas E, Tabari MA, Moghadamnia AA, Hosseini SM, Farash BRH, Ebrahimi MA, Mousavi NN, Fata A, Maggi F, et al. In Vitro and In Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania infantum. Molecules. 2019; 24(11):2072. https://doi.org/10.3390/molecules24112072
Chicago/Turabian StyleYoussefi, Mohammad Reza, Elham Moghaddas, Mohaddeseh Abouhosseini Tabari, Ali Akbar Moghadamnia, Seyed Mohammad Hosseini, Bibi Razieh Hosseini Farash, Mohammad Amin Ebrahimi, Niki Nabavi Mousavi, Abdolmajid Fata, Filippo Maggi, and et al. 2019. "In Vitro and In Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania infantum" Molecules 24, no. 11: 2072. https://doi.org/10.3390/molecules24112072
APA StyleYoussefi, M. R., Moghaddas, E., Tabari, M. A., Moghadamnia, A. A., Hosseini, S. M., Farash, B. R. H., Ebrahimi, M. A., Mousavi, N. N., Fata, A., Maggi, F., Petrelli, R., Dall’Acqua, S., Benelli, G., & Sut, S. (2019). In Vitro and In Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania infantum. Molecules, 24(11), 2072. https://doi.org/10.3390/molecules24112072