New Phenolic Derivatives of Thiazolidine-2,4-dione with Antioxidant and Antiradical Properties: Synthesis, Characterization, In Vitro Evaluation, and Quantum Studies
Abstract
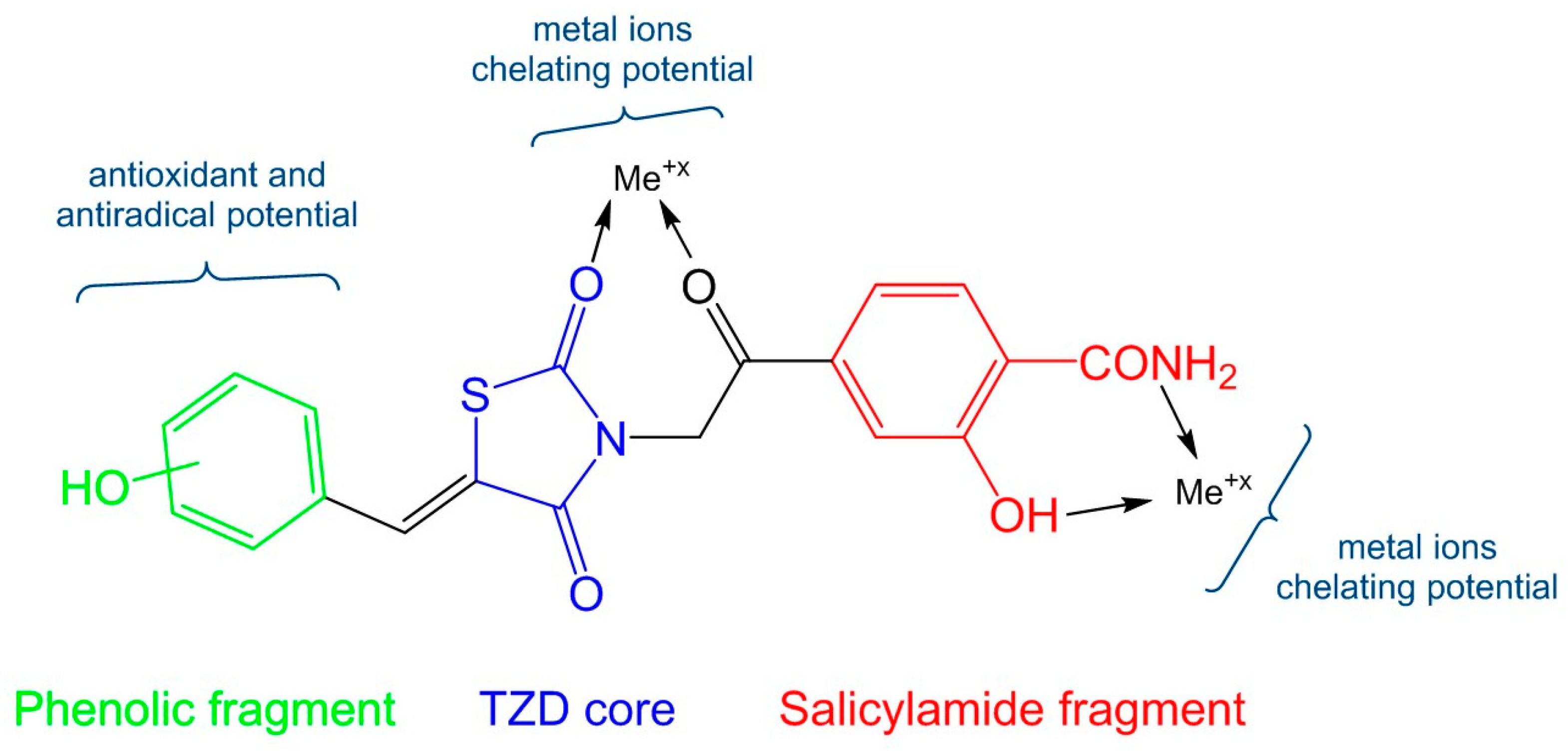
1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis
2.2. In Vitro Antioxidant, Antiradical and Chelation Assays
2.2.1. Antiradical Assays
ABTS·+ Radical Scavenging Assay
DPPH· Radical Scavenging Assay
2.2.2. Electron Transfer Assays
Ferric Reducing Antioxidant Potential (FRAP)
Phosphomolybdate Assay for Total Antioxidant Capacity (TAC)
Reducing Power Assay (RP)
2.2.3. Fe2+ Chelation Assay
2.3. Theoretical Quantum Calculation of Chemical Descriptors
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of Compound 3
3.1.2. Synthesis of Compounds 5a–l
3.2. In Vitro Antioxidant, Antiradical and Chelation Assays
3.2.1. Antiradical assays
ABTS·+ radical scavenging assay
DPPH· Radical Scavenging Assay
3.2.2. Electron Transfer assays
Ferric Reducing Antioxidant Potential (FRAP)
Phosphomolybdate Assay for Total Antioxidant Capacity (TAC)
Reducing Power Assay (RP)
3.2.3. Fe2+ Chelation Assay
3.3. Theoretical Quantum Calculation of Chemical Descriptors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of Total Content of Phenolic Compounds and Their Antioxidant Activity in VegetablesEvaluation of Spectrophotometric Methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Giacomelli, C.; Miranda, F. da S.; Gonçalves, N.S.; Spinelli, A. Antioxidant activity of phenolic and related compounds: A density functional theory study on the O–H bond dissociation enthalpy. Redox Rep. 2004, 9, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cruz, K.; Moncada-Basualto, M.; Morales-Valenzuela, J.; Barriga-González, G.; Navarrete-Encina, P.; Núñez-Vergara, L.; Squella, J.A.; Olea-Azar, C. Synthesis and antioxidant study of new polyphenolic hybrid-coumarins. Arab. J. Chem. 2018, 11, 525–537. [Google Scholar] [CrossRef]
- Al-Majedy, Y.K.; Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B. Antioxidant Activities of 4-Methylumbelliferone Derivatives. PLoS ONE 2016, 11, e0156625. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2012, 1, 106. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Saour, K.Y.; A-Duhaidahawi, D.L.; Al-Majedy, Y.K.; Kadhum, A.A.; Mohamad, A.B. Comparative Molecular Modelling Studies of Coumarin Derivatives as Potential Antioxidant Agents. Free Radicals Antioxid. 2016, 7, 31–35. [Google Scholar] [CrossRef]
- Das, S.; Mitra, I.; Batuta, S.; Niharul Alam, M.; Roy, K.; Begum, N.A. Design, synthesis and exploring the quantitative structure–activity relationship of some antioxidant flavonoid analogues. Bioorg. Med. Chem. Lett. 2014, 24, 5050–5054. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Shaveta; Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 124, 500–536. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Singh, P.K.; Verma, H.; Singh, H.; Silakari, O. Success stories of natural product-based hybrid molecules for multi-factorial diseases. Eur. J. Med. Chem. 2018, 151, 62–97. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; An, L.; Dou, Y.; Liu, Y. Density functional theory study of the structure–antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar] [CrossRef]
- Chadha, N.; Bahia, M.S.; Kaur, M.; Silakari, O. Thiazolidine-2,4-dione derivatives: Programmed chemical weapons for key protein targets of various pathological conditions. Bioorg. Med. Chem. 2015, 23, 2953–2974. [Google Scholar] [CrossRef] [PubMed]
- Nanjan, M.J.; Mohammed, M.; Prashantha Kumar, B.R.; Chandrasekar, M.J.N. Thiazolidinediones as antidiabetic agents: A critical review. Bioorg. Chem. 2018, 77, 548–567. [Google Scholar] [CrossRef]
- Jain, V.S.; Vora, D.K.; Ramaa, C.S. Thiazolidine-2,4-diones: Progress towards multifarious applications. Bioorg. Med. Chem. 2013, 21, 1599–1620. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, S.; Jayaprakash, V. Thiazolidinediones and PPAR orchestra as antidiabetic agents: From past to present. Eur. J. Med. Chem. 2017, 126, 879–893. [Google Scholar] [CrossRef]
- Naim, M.J.; Alam, M.J.; Ahmad, S.; Nawaz, F.; Shrivastava, N.; Sahu, M.; Alam, O. Therapeutic journey of 2,4-thiazolidinediones as a versatile scaffold: An insight into structure activity relationship. Eur. J. Med. Chem. 2017, 129, 218–250. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. Thiazolidine-2,4-diones as multi-targeted scaffold in medicinal chemistry: Potential anticancer agents. Eur. J. Med. Chem. 2014, 87, 814–833. [Google Scholar] [CrossRef]
- Moukette, B.M.; Pieme, A.C.; Biapa, P.C.N.; Njimou, J.R.; Stoller, M.; Bravi, M.; Yonkeu Ngogang, J. In Vitro Ion Chelating, Antioxidative Mechanism of Extracts from Fruits and Barks of Tetrapleura tetraptera and Their Protective Effects against Fenton Mediated Toxicity of Metal Ions on Liver Homogenates. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Vo, Q.V.; Nam, P.C.; Van Bay, M.; Thong, N.M.; Cuong, N.D.; Mechler, A. Density functional theory study of the role of benzylic hydrogen atoms in the antioxidant properties of lignans. Sci. Rep. 2018, 8, 12361. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, H.; Zheng, J.; Liang, G. Structure-Thermodynamics-Antioxidant Activity Relationships of Selected Natural Phenolic Acids and Derivatives: An Experimental and Theoretical Evaluation. PLoS ONE 2015, 10, e0121276. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Anouar, E. A Quantum Chemical and Statistical Study of Phenolic Schiff Bases with Antioxidant Activity against DPPH Free Radical. Antioxidants 2014, 3, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Stana, A.; Tiperciuc, B.; Duma, M.; Pîrnău, A.; Verité, P.; Oniga, O. Synthesis and antimicrobial activity of some new N-(aryl-oxo-alkyl)-5-arylidene-thiazolidine-2,4-diones. J. Serbian Chem. Soc. 2014, 79, 115–123. [Google Scholar] [CrossRef]
- Marc, G.; Araniciu, C.; Oniga, S.; Vlase, L.; Pîrnău, A.; Duma, M.; Măruțescu, L.; Chifiriuc, M.; Oniga, O. New N-(oxazolylmethyl)-thiazolidinedione Active against Candida albicans Biofilm: Potential Als Proteins Inhibitors. Molecules 2018, 23, 2522. [Google Scholar] [CrossRef]
- Marc, G.; Stana, A.; Pîrnău, A.; Vlase, L.; Vodnar, D.C.; Duma, M.; Tiperciuc, B.; Oniga, O. 3,5-Disubstituted Thiazolidine-2,4-Diones: Design, Microwave-Assisted Synthesis, Antifungal Activity, and ADMET Screening. SLAS Discov. Adv. Life Sci. R&D 2018, 247255521875903. [Google Scholar]
- Marc, G.; Ionuț, I.; Pirnau, A.; Vlase, L.; Vodnar, D.C.; Duma, M.; Tiperciuc, B.; Oniga, O. Microwave assisted synthesis of 3,5-disubstituted thiazolidine-2,4-diones with antifungal activity. Design, synthesis, virtual and in vitro antifungal screening. Farmacia 2017, 65, 414–422. [Google Scholar]
- Silva, I.M.; Filho, J.; Santiago, P.B.G.; Egito, M.S.; Souza, C.A.; Gouveia, F.L. Synthesis and Antimicrobial Activities of 5-Arylidene-thiazolidine-2,4-dione Derivatives. Biomed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Čačić, M.; Molnar, M. Design, Synthesis and Characterization of Some Novel 3-Coumarinyl- 5-aryliden-1,3-thiazolidine-2,4-diones and Their Antioxidant Activity. Zeitschrift für Naturforsch. B 2011, 66, 177–183. [Google Scholar] [CrossRef]
- Ha, Y.M.; Park, Y.J.; Kim, J.A.; Park, D.; Park, J.Y.; Lee, H.J.; Lee, J.Y.; Moon, H.R.; Chung, H.Y. Design and synthesis of 5-(substituted benzylidene)thiazolidine-2,4-dione derivatives as novel tyrosinase inhibitors. Eur. J. Med. Chem. 2012, 49, 245–252. [Google Scholar] [CrossRef]
- Pratap, U.R.; Jawale, D.V.; Waghmare, R.A.; Lingampalle, D.L.; Mane, R.A. Synthesis of 5-arylidene-2,4-thiazolidinediones by Knoevenagel condensation catalyzed by baker’s yeast. New J. Chem. 2011, 35, 49–51. [Google Scholar] [CrossRef]
- Tuncbilek, M.; Altanlar, N. Synthesis of New 3-(Substituted Phenacyl)-5-[3′-(4H-4-oxo-1-benzopyran-2-yl)-benzylidene]-2,4-thiazolidinediones and their Antimicrobial Activity. Arch. Pharm. (Weinheim). 2006, 339, 213–216. [Google Scholar] [CrossRef]
- Javed, F.; Sirajuddin, M.; Ali, S.; Khalid, N.; Tahir, M.N.; Shah, N.A.; Rasheed, Z.; Khan, M.R. Organotin(IV) derivatives of o-isobutyl carbonodithioate: Synthesis, spectroscopic characterization, X-ray structure, HOMO/LUMO and in vitro biological activities. Polyhedron 2016, 104, 80–90. [Google Scholar] [CrossRef]
- Rajaraman, D.; Sundararajan, G.; Rajkumar, R.; Bharanidharan, S.; Krishnasamy, K. Synthesis, crystal structure investigation, DFT studies and DPPH radical scavenging activity of 1-(furan-2-ylmethyl)-2,4,5-triphenyl- 1H -imidazole derivatives. J. Mol. Struct. 2016, 1108, 698–707. [Google Scholar] [CrossRef]
- Al-Majedy, Y.; Al-Duhaidahawi, D.; Al-Azawi, K.; Al-Amiery, A.; Kadhum, A.; Mohamad, A. Coumarins as Potential Antioxidant Agents Complemented with Suggested Mechanisms and Approved by Molecular Modeling Studies. Molecules 2016, 21, 135. [Google Scholar] [CrossRef]
- Suresh Kumar, G.S.; Antony Muthu Prabhu, A.; Bhuvanesh, N. Studies on the self-catalyzed Knoevenagel condensation, characterization, DPPH radical scavenging activity, cytotoxicity, and molecular properties of 5-arylidene-2,2-dimethyl-1,3-dioxane-4,6-diones using single crystal XRD and DFT techniques. J. Mol. Struct. 2014, 1075, 166–177. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Samadi, A.; Soriano, E.; Revuelta, J.; Valderas, C.; Chioua, M.; Garrido, I.; Bartolomé, B.; Tomassolli, I.; Ismaili, L.; González-Lafuente, L.; et al. Synthesis, structure, theoretical and experimental in vitro antioxidant/pharmacological properties of α-aryl, N-alkyl nitrones, as potential agents for the treatment of cerebral ischemia. Bioorg. Med. Chem. 2011, 19, 951–960. [Google Scholar] [CrossRef]
- Detsi, A.; Majdalani, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Kefalas, P. Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg. Med. Chem. 2009, 17, 8073–8085. [Google Scholar] [CrossRef]
- Ahmed, D.; Khan, M.; Saeed, R. Comparative Analysis of Phenolics, Flavonoids, and Antioxidant and Antibacterial Potential of Methanolic, Hexanic and Aqueous Extracts from Adiantum caudatum Leaves. Antioxidants 2015, 4, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method. Enzymol. 1999, 299, 15–27. [Google Scholar]
- Grozav, A.; Porumb, I.-D.; Găină, L.; Filip, L.; Hanganu, D. Cytotoxicity and Antioxidant Potential of Novel 2-(2-((1H-indol-5yl)methylene)-hydrazinyl)-thiazole Derivatives. Molecules 2017, 22, 260. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Baig, H.; Ahmed, D.; Zara, S.; Asghar, M.N.; Aujla, M.I. In vitro Evaluation of Antioxidant Properties of Different Solvent Extracts of Rumex acetosella Leaves. Orient. J. Chem. 2011, 27, 1509–1516. [Google Scholar]
- Stana, A.; Vodnar, D.C.; Marc, G.; Benedec, D.; Tiperciuc, B.; Tamaian, R.; Oniga, O. Antioxidant activity and antibacterial evaluation of new thiazolin-4-one derivatives as potential tryptophanyl-tRNA synthetase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 898–908. [Google Scholar] [CrossRef]
- Qureshi, N.N.; Kuchekar, B.S.; Logade, N.A.; Haleem, M.A. Antioxidant and hepatoprotective activity of Cordia macleodii leaves. Saudi Pharm. J. 2009, 17, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Cesari, L.; Mutelet, F.; Canabady-Rochelle, L. Antioxidant properties of phenolic surrogates of lignin depolymerisation. Ind. Crops Prod. 2019, 129, 480–487. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the compounds are available from the authors. |
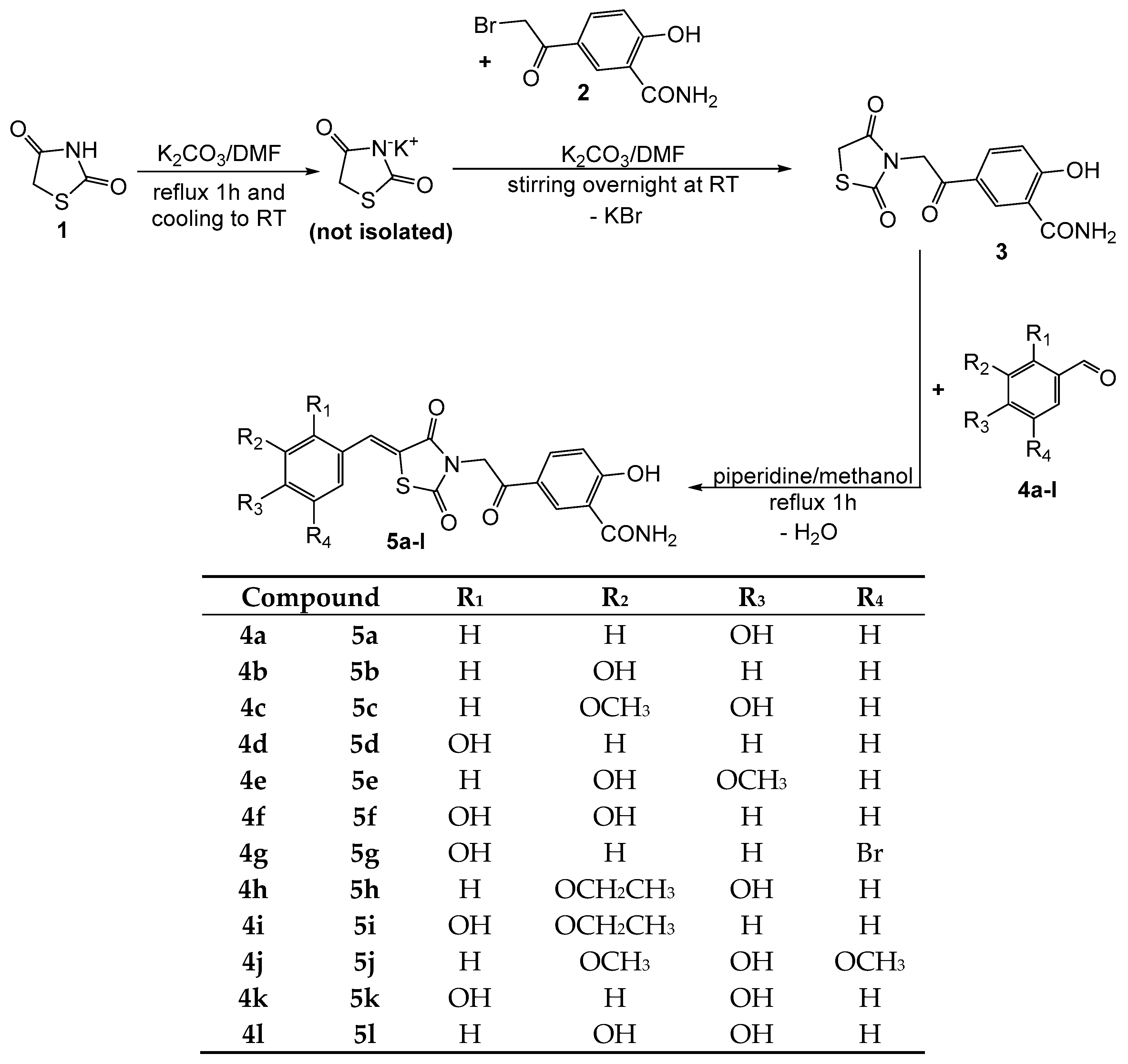
| Compound | % of Radical Scavenging | |
|---|---|---|
| ABTS·+ | DPPH· | |
| 3 | - | - |
| 5a | - | - |
| 5b | 12.11 | - |
| 5c | - | - |
| 5d | 15.81 | - |
| 5e | - | - |
| 5f | 58.27 | 89.61 |
| 5g | - | 12.36 |
| 5h | - | - |
| 5i | - | - |
| 5j | - | - |
| 5k | 22.75 | 18.13 |
| 5l | 70.66 | 92.55 |
| Ascorbic acid | N.T. | 77.20 |
| BHT | N.T. | 63.50 |
| Trolox | 54.35 | 73.62 |
| Compound | FRAP | TAC | RP |
|---|---|---|---|
| 3 | 11.86 | - | - |
| 5a | - | - | 14.75 |
| 5b | - | - | 23.82 |
| 5c | - | 23.05 | 15.20 |
| 5d | 23.69 | 17.61 | 16.54 |
| 5e | 16.26 | - | 18.32 |
| 5f | 86.12 | 96.94 | 71.22 |
| 5g | 15.01 | - | 19.34 |
| 5h | 18.68 | 26.59 | 18.49 |
| 5i | 28.63 | 31.46 | 19.42 |
| 5j | 20.15 | 36.24 | 23.81 |
| 5k | 37.20 | 51.94 | 46.36 |
| 5l | 91.28 | 102.70 | 104.75 |
| Ascorbic acid | 100.00 | 100.00 | 100.00 |
| BHT | 86.03 | 92.83 | 64.87 |
| Trolox | 85.87 | 88.95 | 56.83 |
| Compound | Fe2+ Chelation Capacity (%) |
|---|---|
| 3 | - |
| 5a | - |
| 5b | - |
| 5c | - |
| 5d | - |
| 5e | - |
| 5f | 12.16 |
| 5g | - |
| 5h | - |
| 5i | - |
| 5j | 10.06 |
| 5k | - |
| 5l | 14.58 |
| EDTA | 92.78 |
| Compound | Frontier Orbitals (eV) | Enthalpy (Ha) | ||
|---|---|---|---|---|
| EHOMO | ELUMO | Egap | ||
| 5a | −5.83 | −2.3 | 3.53 | −1691.18 |
| 5b | −6.24 | −1.97 | 4.27 | −1691.19 |
| 5c | −4.67 | −2.21 | 2.46 | −1805.25 |
| 5d | −6.20 | −1.84 | 4.36 | −1691.19 |
| 5e | −5.84 | −1.82 | 4.02 | −1805.68 |
| 5f | −6.29 | −1.93 | 4.36 | −1766.38 |
| 5g | −6.28 | −2.01 | 4.27 | −4264.48 |
| 5h | −5.76 | −1.80 | 3.96 | −1844.96 |
| 5i | −5.87 | −1.75 | 4.12 | −1844.97 |
| 5j | −5.66 | −1.83 | 3.83 | −1920.16 |
| 5k | −6.01 | −1.74 | 4.27 | −1766.40 |
| 5l | −5.90 | −1.89 | 4.01 | −1766.40 |
| Radical of Compound | Position of the Radical | Frontier Orbitals (eV) | Enthalpy (Ha) | |
|---|---|---|---|---|
| EHOMO | ELUMO | |||
| 5a | - | −6.09 | −2.44 | −1690.54 |
| 5b | - | −6.68 | −2.32 | −1690.55 |
| 5c | - | −6.12 | −2.19 | −1804.61 |
| 5d | - | −6.62 | −1.94 | −1690.56 |
| 5e | - | −6.13 | −2.15 | −1805.04 |
| 5f | ortho | −6.34 | −1.91 | −1765.77 |
| meta | −6.39 | −2.23 | −1765.77 | |
| 5g | - | −6.63 | −2.02 | −4263.84 |
| 5h | - | −6.32 | −2.25 | −1844.32 |
| 5i | - | −6.59 | −1.92 | −1844.33 |
| 5j | - | −5.78 | −2.14 | −1919.52 |
| 5k | ortho | −6.54 | −1.9 | −1765.76 |
| para | −6.42 | −2.49 | −1765.76 | |
| 5l | meta | −6.65 | −2.33 | −1765.75 |
| para | −6.12 | −2.25 | −1765.77 | |
| Compound | Position of the Phenol Group | O−H BDE | ||
|---|---|---|---|---|
| Hartrees | Kcal/mol | KJ/mol | ||
| 5a | - | 0.145 | 90.826 | 380.014 |
| 5b | - | 0.145 | 90.801 | 379.909 |
| 5c | - | 0.142 | 88.818 | 371.613 |
| 5d | - | 0.135 | 84.431 | 353.260 |
| 5e | - | 0.142 | 89.326 | 373.739 |
| 5f | ortho | 0.114 | 71.561 | 299.411 |
| meta | 0.114 | 71.668 | 299.858 | |
| 5g | - | 0.146 | 91.610 | 383.296 |
| 5h | - | 0.140 | 87.657 | 366.755 |
| 5i | - | 0.143 | 89.627 | 374.999 |
| 5j | - | 0.141 | 88.344 | 369.633 |
| 5k | ortho | 0.143 | 89.684 | 375.236 |
| para | 0.140 | 87.826 | 367.464 | |
| 5l | meta | 0.153 | 95.971 | 401.543 |
| para | 0.132 | 82.963 | 347.117 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marc, G.; Stana, A.; Oniga, S.D.; Pîrnău, A.; Vlase, L.; Oniga, O. New Phenolic Derivatives of Thiazolidine-2,4-dione with Antioxidant and Antiradical Properties: Synthesis, Characterization, In Vitro Evaluation, and Quantum Studies. Molecules 2019, 24, 2060. https://doi.org/10.3390/molecules24112060
Marc G, Stana A, Oniga SD, Pîrnău A, Vlase L, Oniga O. New Phenolic Derivatives of Thiazolidine-2,4-dione with Antioxidant and Antiradical Properties: Synthesis, Characterization, In Vitro Evaluation, and Quantum Studies. Molecules. 2019; 24(11):2060. https://doi.org/10.3390/molecules24112060
Chicago/Turabian StyleMarc, Gabriel, Anca Stana, Smaranda Dafina Oniga, Adrian Pîrnău, Laurian Vlase, and Ovidiu Oniga. 2019. "New Phenolic Derivatives of Thiazolidine-2,4-dione with Antioxidant and Antiradical Properties: Synthesis, Characterization, In Vitro Evaluation, and Quantum Studies" Molecules 24, no. 11: 2060. https://doi.org/10.3390/molecules24112060
APA StyleMarc, G., Stana, A., Oniga, S. D., Pîrnău, A., Vlase, L., & Oniga, O. (2019). New Phenolic Derivatives of Thiazolidine-2,4-dione with Antioxidant and Antiradical Properties: Synthesis, Characterization, In Vitro Evaluation, and Quantum Studies. Molecules, 24(11), 2060. https://doi.org/10.3390/molecules24112060







