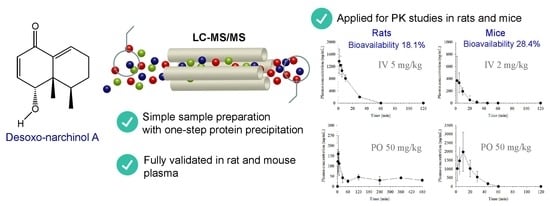
Liquid Chromatography-Tandem Mass Spectrometry of Desoxo-Narchinol a and Its Pharmacokinetics and Oral Bioavailability in Rats and Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Sample Preparation
2.2. Chromatography
2.3. Linearity and Sensitivity
2.4. Accuracy, Precision, and Recovery
2.5. Stability
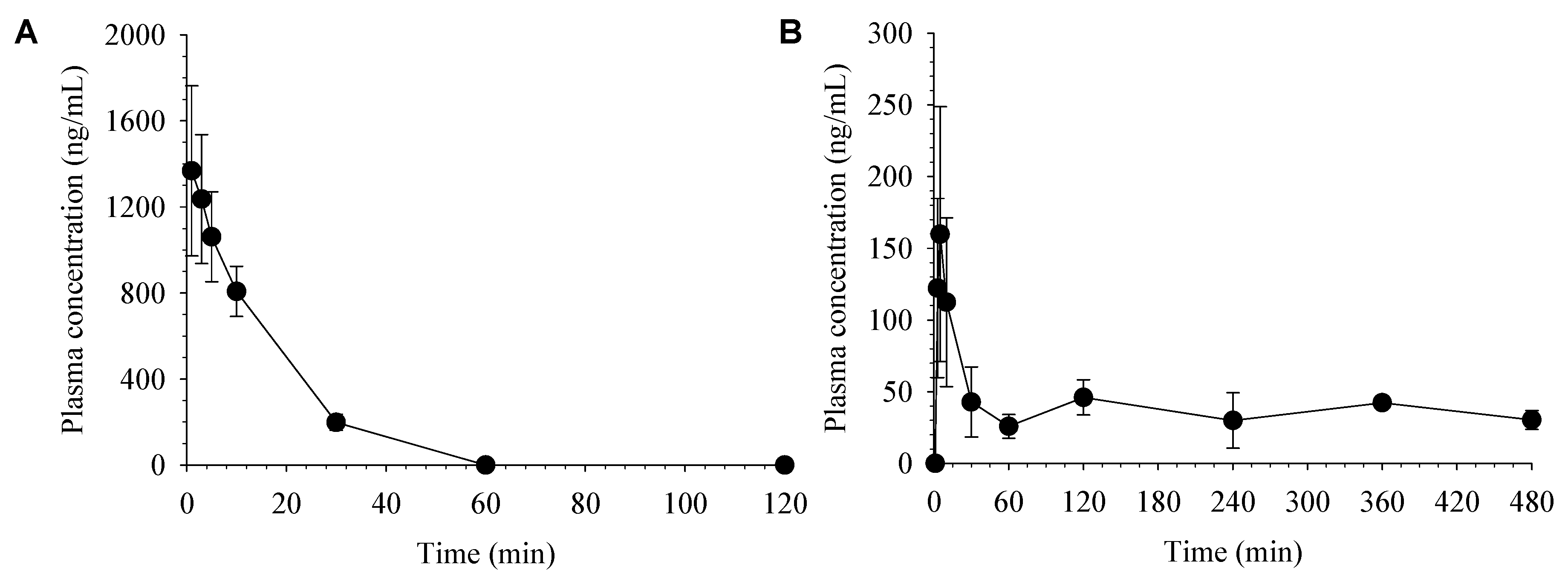
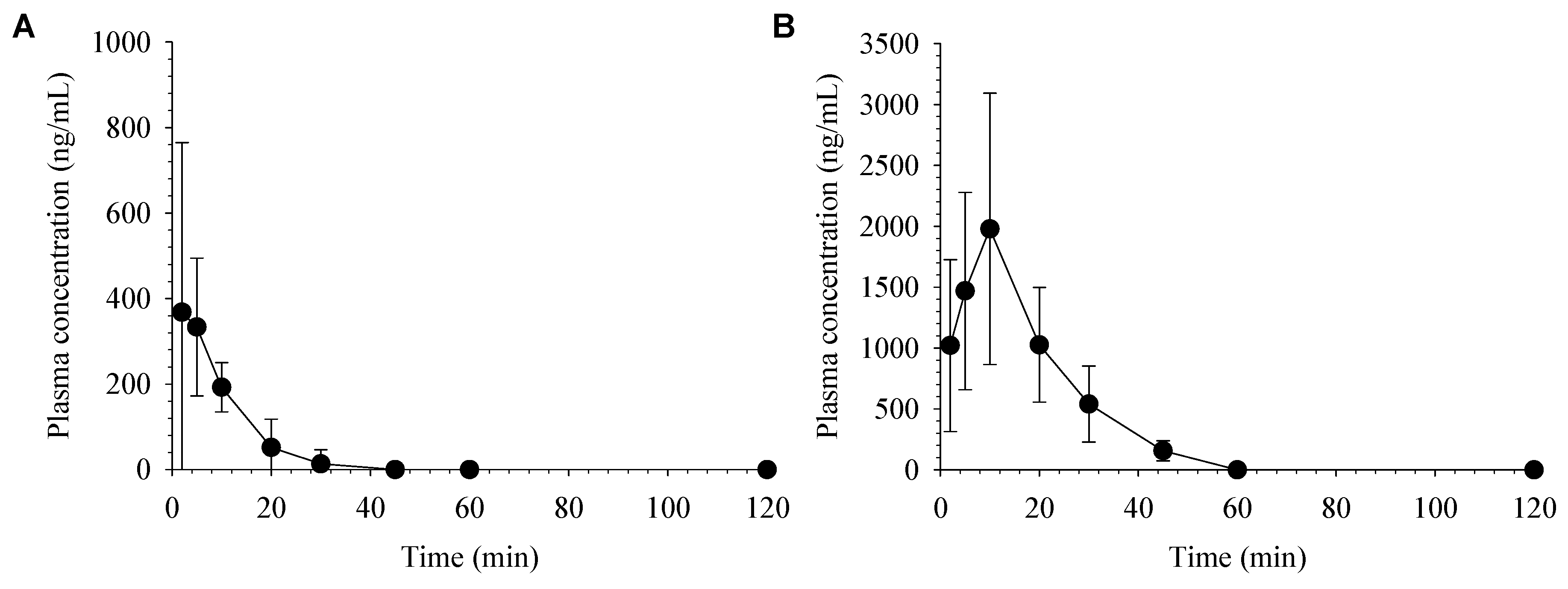
2.6. Pharmacokinetics of Desoxo-Narchinol A in Rats
2.7. Pharmacokinetics of Desoxo-Narchinol A in Mice
3. Materials and Methods
3.1. Materials
3.2. Animal Study
3.2.1. Rat Study
3.2.2. Mice Study
3.2.3. Noncompartmental Analysis
3.3. Calibration Standards and Quality Control Samples
3.4. LC-MS/MS Conditions
3.5. Assay Validation
3.5.1. Specificity, linearity, and Sensitivity
3.5.2. Accuracy and Precision
3.5.3. Recovery
3.5.4. Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ali, S.; Ansari, K.A.; Jafry, M.; Kabeer, H.; Diwakar, G. Nardostachys jatamansi protects against liver damage induced by thioacetamide in rats. J. Ethnopharmacol. 2000, 71, 359–363. [Google Scholar] [CrossRef]
- Bagchi, A.; Oshima, Y.; Hikino, H. Neolignans and lignans of nardostachys jatamansi roots1. Planta Medica. 1991, 57, 96–97. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chandrashekar, K.S.; Pai, K.S.R.; Setty, M.M.; Devkar, R.A.; Reddy, N.D.; Shoja, M.H. Evaluation of antioxidant and anticancer activity of extract and fractions of nardostachys jatamansi dc in breast carcinoma. Bmc Complementary Altern. Med. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Ahmad, M.; Zafar, K.S.; Ahmad, A.S.; Islam, F. Protective effect of nardostachys jatamansi in rat cerebral ischemia. Pharmacol. Biochem. Behav. 2003, 74, 481–486. [Google Scholar] [CrossRef]
- Ahmad, M.; Yousuf, S.; Khan, M.B.; Hoda, M.N.; Ahmad, A.S.; Ansari, M.A.; Ishrat, T.; Agrawal, A.K.; Islam, F. Attenuation by nardostachys jatamansi of 6-hydroxydopamine-induced parkinsonism in rats: Behavioral, neurochemical, and immunohistochemical studies. Pharmacol. Biochem. Behav. 2006, 83, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Rao, A.; Karanth, K.S. Anticonvulsant and neurotoxicity profile of nardostachys jatamansi in rats. J. Ethnopharmacol. 2005, 102, 351–356. [Google Scholar] [CrossRef]
- Dhingra, D.; Goyal, P.K. Inhibition of mao and gaba: Probable mechanisms for antidepressant-like activity of nardostachys jatamansi dc. In mice. Indian J. Exp. Biol. 2008, 46, 212–218. [Google Scholar]
- Joshi, H.; Parle, M. Nardostachys jatamansi improves learning and memory in mice. J. Med. Food 2006, 9, 113–118. [Google Scholar] [CrossRef]
- Song, M.Y.; Bae, U.J.; Lee, B.H.; Kwon, K.B.; Seo, E.A.; Park, S.J.; Kim, M.S.; Song, H.J.; Kwon, K.S.; Park, J.W.; et al. Nardostachys jatamansi extract protects against cytokine-induced beta-cell damage and streptozotocin-induced diabetes. World J. Gastroenterol. 2010, 16, 3249–3257. [Google Scholar] [CrossRef]
- Bae, G.-S.; Heo, K.-H.; Choi, S.B.; Jo, I.-J.; Kim, D.-G.; Shin, J.-Y.; Seo, S.-H.; Park, K.-C.; Lee, D.-S.; Oh, H. Beneficial effects of fractions of nardostachys jatamansi on lipopolysaccharide-induced inflammatory response. Evid. Based Complementary Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Bose, B.; Tripathy, D.; Chatterjee, A.; Tandon, P.; Kumaria, S. Secondary metabolite profiling, cytotoxicity, anti-inflammatory potential and in vitro inhibitory activities of nardostachys jatamansi on key enzymes linked to hyperglycemia, hypertension and cognitive disorders. Phytomedicine: Int. J. Phytother. Phytopharm. 2018, 55, 58–69. [Google Scholar] [CrossRef]
- Bae, G.S.; Park, K.C.; Koo, B.S.; Jo, I.J.; Choi, S.B.; Song, H.J.; Park, S.J. Nardostachys jatamansi inhibits severe acute pancreatitis via mitogen-activated protein kinases. Exp. Med. 2012, 4, 533–537. [Google Scholar] [CrossRef]
- Shin, J.Y.; Bae, G.S.; Choi, S.B.; Jo, I.J.; Kim, D.G.; Lee, D.S.; An, R.B.; Oh, H.; Kim, Y.C.; Shin, Y.K.; et al. Anti-inflammatory effect of desoxo-narchinol-a isolated from nardostachys jatamansi against lipopolysaccharide. Int. Immunopharmacol. 2015, 29, 730–738. [Google Scholar] [CrossRef]
- Hwang, J.S.; Lee, S.A.; Hong, S.S.; Han, X.H.; Lee, C.; Lee, D.; Lee, C.-K.; Hong, J.T.; Kim, Y.; Lee, M.K. Inhibitory constituents of nardostachys chinensis on nitric oxide production in raw 264.7 macrophages. Bioorganic Med. Chem. Lett. 2012, 22, 706–708. [Google Scholar] [CrossRef]
- Yoon, C.S.; Kim, K.W.; Lee, S.C.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory effects of sesquiterpenoids isolated from nardostachys jatamansi. Bioorg. Med. Chem. Lett. 2018, 28, 140–144. [Google Scholar] [CrossRef]
- Kim, K.W.; Yoon, C.S.; Kim, Y.C.; Oh, H. Desoxo-narchinol a and narchinol b isolated from nardostachys jatamansi exert anti-neuroinflammatory effects by up-regulating of nuclear transcription factor erythroid-2-related factor 2/heme oxygenase-1 signaling. Neurotox. Res. 2019, 35, 230–243. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, P.; Zhan, Y.; Su, J.; Yi, D. Quantification of nardosinone in rat plasma using liquid chromatography–tandem mass spectrometry and its pharmacokinetics application. J. Chromatogr. Sci. 2015, 53, 1725–1729. [Google Scholar] [CrossRef]
- Le, V.N.H.; Zhao, Y.; Cho, C.W.; Na, M.; Quan, K.T.; Kim, J.H.; Hwang, S.Y.; Kim, S.W.; Kim, K.T.; Kang, J.S. Pharmacokinetic study comparing pure desoxo-narchinol a and nardosinonediol with extracts from nardostachys jatamansi. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1102–1103, 152–158. [Google Scholar] [CrossRef]
- Shin, S.; Jeong, H.M.; Chung, S.E.; Kim, T.H.; Thapa, S.K.; Lee, D.Y.; Song, C.H.; Lim, J.Y.; Cho, S.M.; Nam, K.Y.; et al. Simultaneous analysis of acetylcarnitine, proline, hydroxyproline, citrulline, and arginine as potential plasma biomarkers to evaluate nsaids-induced gastric injury by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 165, 101–111. [Google Scholar] [CrossRef]
- Thapa, S.K.; Weon, K.Y.; Jeong, S.W.; Kim, T.H.; Upadhyay, M.; Han, Y.H.; Jin, J.S.; Hong, S.H.; Youn, Y.S.; Shin, B.; et al. Simple and rapid liquid chromatography-tandem mass spectrometry analysis of arctigenin and its application to a pharmacokinetic study. Mass Spectrom. Lett. 2017, 8, 23–28. [Google Scholar]
- U.S. FDA. Guidance for industry: Bioanalytical method validation. Center for Drug Evaluation and Research (CDER); U.S. FDA: Rockville, MD, USA, 2018.
- Roberts, M.S.; Magnusson, B.M.; Burczynski, F.J.; Weiss, M. Enterohepatic circulation: Physiological, pharmacokinetic and clinical implications. Clin. Pharm. 2002, 41, 751–790. [Google Scholar] [CrossRef]
- Kim, T.H.; Shin, S.; Landersdorfer, C.B.; Chi, Y.H.; Paik, S.H.; Myung, J.; Yadav, R.; Horkovics-Kovats, S.; Bulitta, J.B.; Shin, B.S. Population pharmacokinetic modeling of the enterohepatic recirculation of fimasartan in rats, dogs, and humans. Aaps. J. 2015, 17, 1210–1223. [Google Scholar] [CrossRef]
- Kim, T.H.; Shin, S.; Bulitta, J.B.; Youn, Y.S.; Yoo, S.D.; Shin, B.S. Development of a physiologically relevant population pharmacokinetic in vitro-in vivo correlation approach for designing extended-release oral dosage formulation. Mol. Pharm. 2017, 14, 53–65. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
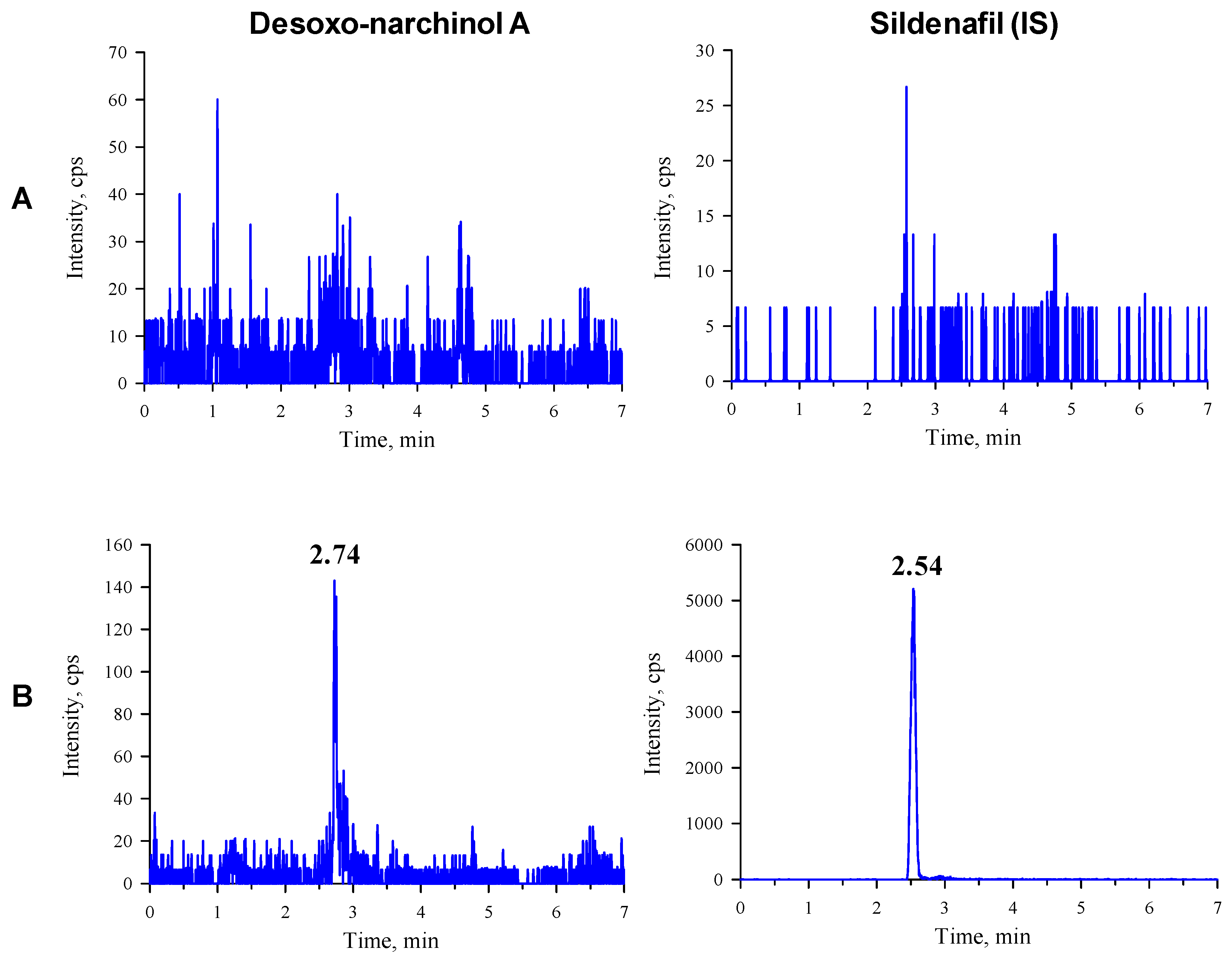
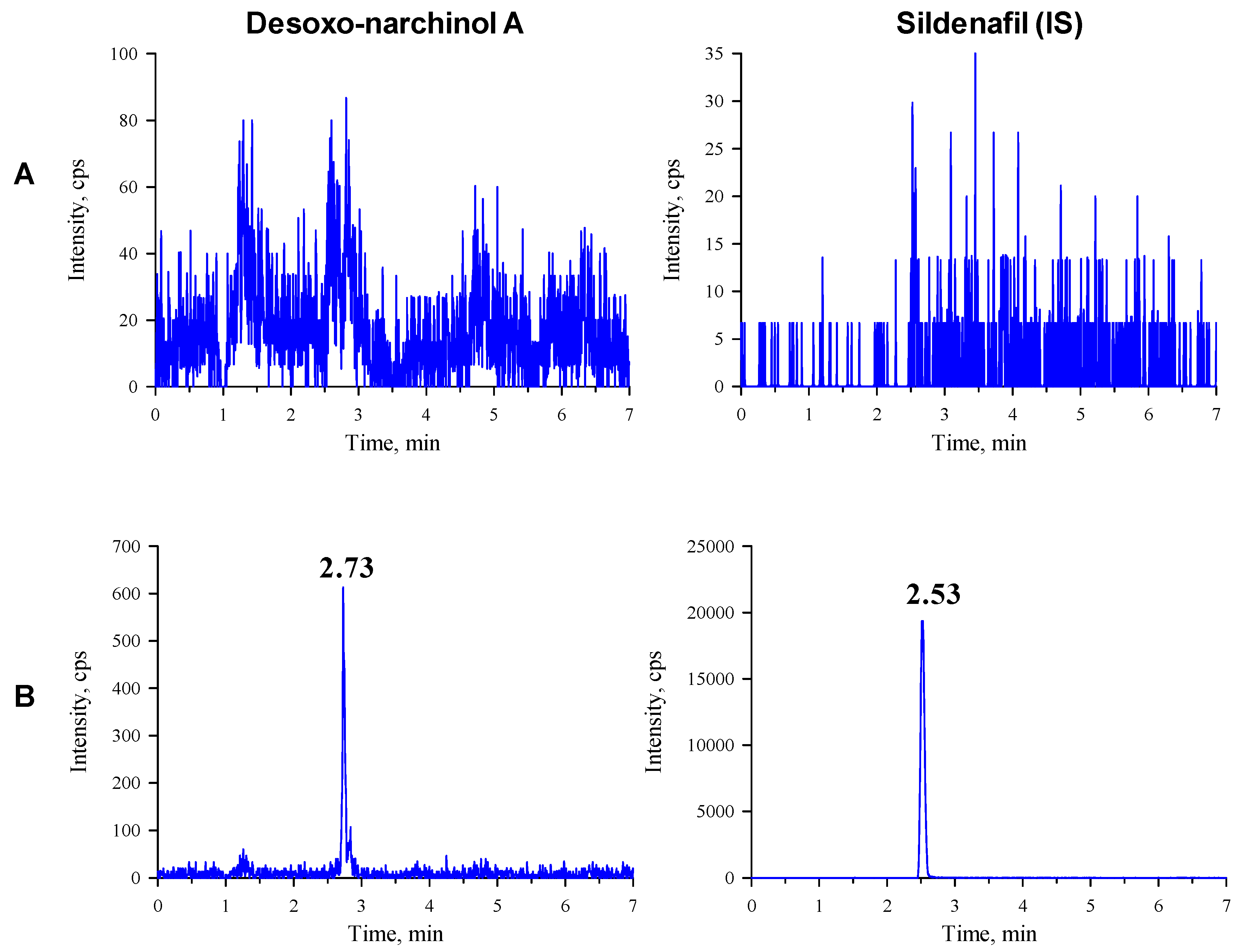
| Compounds | MRM Transition (m/z) | Retention Time (min) | DP (V) | FP (V) | CE (eV) | CXP (V) | |
|---|---|---|---|---|---|---|---|
| Rat Plasma | Mouse Plasma | ||||||
| Desoxo-narchinol A | 192.94 → 99.00 | 2.74 | 2.73 | 56 | 310 | 19 | 2 |
| IS | 474.76 → 58.10 | 2.54 | 2.53 | 96 | 370 | 67 | 0 |
| Matrix | Concentration (ng/mL) | Intra-Day (n = 5) | Inter-Day (n = 5) | ||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (%) | Accuracy (%) | Precision (%) | ||
| Rat plasma | 10 | 97.23 | 8.65 | 104.54 | 8.30 |
| 25 | 99.80 | 3.90 | 100.81 | 1.97 | |
| 125 | 102.24 | 5.26 | 99.54 | 1.66 | |
| 800 | 102.64 | 1.10 | 98.28 | 3.23 | |
| Mouse plasma | 10 | 98.41 | 1.59 | 110.11 | 6.46 |
| 25 | 97.81 | 0.67 | 104.20 | 3.64 | |
| 125 | 97.31 | 0.78 | 100.85 | 2.13 | |
| 800 | 95.90 | 0.46 | 99.93 | 2.93 | |
| Concentration (ng/mL) | Rat Plasma (%) | Mouse Plasma (%) | |
|---|---|---|---|
| Desoxo-narchinol A | 10 | 98.77 ± 3.18 | 99.37 ± 0.65 |
| 25 | 99.10 ± 2.00 | 100.07 ± 0.88 | |
| 125 | 95.15 ± 1.63 | 95.53 ± 1.33 | |
| 800 | 96.10 ± 1.56 | 96.22 ± 1.29 | |
| IS | 200 | 103.90 ± 2.32 | 100.12 ± 3.48 |
| Matrix | Conc (ng/mL) | Percentage over Theoretical Concentration (%) | |||
|---|---|---|---|---|---|
| Autosampler Stability (24 h, 4 °C) | Freeze-Thaw Stability (3 cycles, −20 °C) | Short-Term Stability (4 h, 20 °C) | Long-Term Stability (2 wk, −20 °C) | ||
| Rat plasma | 25 | 102.34 ± 1.75 | 96.62 ± 0.89 | 98.23 ± 1.63 | 104.98 ± 1.05 |
| 800 | 99.06 ± 1.76 | 98.45 ± 1.15 | 99.68 ± 1.49 | 102.86 ± 1.66 | |
| Mouse plasma | 25 | 104.24 ± 0.50 | 106.11 ± 0.70 | 105.23 ± 0.49 | 98.61 ± 0.84 |
| 800 | 99.59 ± 0.43 | 103.46 ± 0.80 | 100.00 ± 0.56 | 97.43 ± 1.07 | |
| Pharmacokinetic Parameters | Rat | Mouse | ||
|---|---|---|---|---|
| IV (5 mg/kg, n = 4) | PO (50 mg/kg, n = 4) | IV (2 mg/kg, n = 6) | PO (50 mg/kg, n = 5) | |
| t1/2 (min) | 10.2 ± 0.7 | 516.9 ± 99.4 a | 7.4 ± 5.0 | 9.8 ± 2.3 |
| Tmax (min) | - | 5.0 ± 0.0 | - | 10.0 ± 0.0 |
| C0 or Cmax (ng/mL) | 1442.5 ± 463.6 | 159.8 ± 88.8 | 624.9 ± 434.8 | 1978.5 ± 1114.4 |
| AUCall (ng·h/mL) | 399.8 ± 73.0 | 317.9 ± 46.6 | 80 ± 25.8 | 688.9 ± 279.5 |
| AUCinf (ng·h/mL) | 400.8 ± 73.1 | 725.1 ± 213.3 a | 102.2 ± 27.2 | 726.6 ± 303.4 |
| CL or CL/F (mL/min/kg) | 213.8 ± 39.3 | 950.1 ± 574.0 a | 343.9 ± 83.1 | 1302.5 ± 505.3 |
| Vss (L/kg) | 2.9 ± 0.6 | - | 3.6 ± 2.7 | - |
| Bioavailability (F) | - | 18.1% | - | 28.4% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, S.K.; Upadhyay, M.; Kim, T.H.; Shin, S.; Park, S.-J.; Shin, B.S. Liquid Chromatography-Tandem Mass Spectrometry of Desoxo-Narchinol a and Its Pharmacokinetics and Oral Bioavailability in Rats and Mice. Molecules 2019, 24, 2037. https://doi.org/10.3390/molecules24112037
Thapa SK, Upadhyay M, Kim TH, Shin S, Park S-J, Shin BS. Liquid Chromatography-Tandem Mass Spectrometry of Desoxo-Narchinol a and Its Pharmacokinetics and Oral Bioavailability in Rats and Mice. Molecules. 2019; 24(11):2037. https://doi.org/10.3390/molecules24112037
Chicago/Turabian StyleThapa, Subindra Kazi, Mahesh Upadhyay, Tae Hwan Kim, Soyoung Shin, Sung-Joo Park, and Beom Soo Shin. 2019. "Liquid Chromatography-Tandem Mass Spectrometry of Desoxo-Narchinol a and Its Pharmacokinetics and Oral Bioavailability in Rats and Mice" Molecules 24, no. 11: 2037. https://doi.org/10.3390/molecules24112037
APA StyleThapa, S. K., Upadhyay, M., Kim, T. H., Shin, S., Park, S.-J., & Shin, B. S. (2019). Liquid Chromatography-Tandem Mass Spectrometry of Desoxo-Narchinol a and Its Pharmacokinetics and Oral Bioavailability in Rats and Mice. Molecules, 24(11), 2037. https://doi.org/10.3390/molecules24112037