Carbazole Derivatives as Antiviral Agents: An Overview
Abstract
1. Introduction
2. Human Immunodeficiency Virus (HIV)
3. Human Cytomegalovirus (HCMV)
4. Chronic Hepatitis C Virus (HCV)
5. Herpes Simplex Virus (HSV)
6. Human Papilloma Virus (HPV)
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, M.Z.; Chen, Q.; Yang, G.F. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.; Brown, K.; Zhang, Q.; Gartland, M.; Walton, L.; Talarico, C.; Lawrence, W.; Selleseth, D.; Coffield, N.; Leary, J.; et al. GSK983: A novel compound with broad-spectrum antiviral activity. Antivir. Res. 2009, 82, 1–11. [Google Scholar] [CrossRef]
- Morens, D.M.; Fauci, A.S. Dengue and hemorrhagic fever: A potential threat to public health in the United States. JAMA 2008, 299, 214–216. [Google Scholar] [CrossRef]
- Dunne, E.F.; Unger, E.R.; Sternberg, M.; McQuillan, G.; Swan, D.C.; Patel, S.S.; Markowitz, L.E. Prevalence of HPV infection among females in the United States. JAMA 2007, 297, 813–819. [Google Scholar] [CrossRef]
- Saturnino, C.; Grande, F.; Aquaro, S.; Caruso, A.; Iacopetta, D.; Bonomo, M.G.; Longo, P.; Schols, D.; Sinicropi, M.S. Chloro-1,4-dimethyl-9H-carbazole Derivatives Displaying Anti-HIV Activity. Molecules 2018, 23, 286. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rossouw, J.E.; Siscovick, D.S.; Mouton, C.P.; Rifai, N.; Wallace, R.B.; Jackson, R.D.; Pettinger, M.B.; Ridker, P.M. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: Prospective analysis from the Women’s Health Initiative observational study. JAMA 2002, 288, 980–987. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Iacopetta, D.; Rosano, C.; Randino, R.; Caruso, A.; Saturnino, C.; Muia, N.; Ceramella, J.; Puoci, F.; Rodriquez, M.; et al. N-thioalkylcarbazoles derivatives as new anti-proliferative agents: Synthesis, characterisation and molecular mechanism evaluation. J. Enzym. Inhib. Med. Chem. 2018, 33, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, C.; Caruso, A.; Iacopetta, D.; Rosano, C.; Ceramella, J.; Muia, N.; Mariconda, A.; Bonomo, M.G.; Ponassi, M.; Rosace, G.; et al. Inhibition of Human Topoisomerase II by N,N,N-Trimethylethanammonium Iodide Alkylcarbazole Derivatives. ChemMedChem 2018, 13, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Voisin-Chiret, A.S.; Lancelot, J.C.; Sinicropi, M.S.; Garofalo, A.; Rault, S. Efficient and simple synthesis of 6-aryl-1,4-dimethyl-9H-carbazoles. Molecules 2008, 13, 1312–1320. [Google Scholar] [CrossRef]
- Caruso, A.; Lancelot, J.; El-Kashef, H.; Sinicropi, M.S.; Legay, R.; Lesnard, A.; Rault, S. A rapid and versatile synthesis of novel pyrimido[5,4-b]carbazoles. Tetrahedron 2009, 65, 10400–10405. [Google Scholar] [CrossRef]
- Panno, A.; Sinicropi, M.S.; Caruso, A.; El-Kashef, H.; Lancelot, J.C.; Aubert, G.; Lesnard, A.; Cresteil, T.; Rault, S. New Trimethoxybenzamides and Trimethoxyphenylureas Derived from Dimethylcarbazole as Cytotoxic Agents. Part I. J. Heterocycl. Chem. 2014, 51, 294–302. [Google Scholar] [CrossRef]
- Caruso, A.; Lancelot, J.C.; El-Kashef, H.; Panno, A.; Sinicropi, M.S.; Legay, R.; Lesnard, A.; Lepailleur, A.; Rault, S. Four Partners, Three-Step, One-Pot Reaction for a Library of New 2-Alkyl(dialkyl)aminoquinazolin-4(3H)-ones. J. Heterocycl. Chem. 2014, 51, 282–293. [Google Scholar] [CrossRef]
- Thiratmatrakul, S.; Yenjai, C.; Waiwut, P.; Vajragupta, O.; Reubroycharoen, P.; Tohda, M.; Boonyarat, C. Synthesis, biological evaluation and molecular modeling study of novel tacrine-carbazole hybrids as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 75, 21–30. [Google Scholar] [CrossRef]
- Kongkathip, B.; Kongkathip, N.; Sunthitikawinsakul, A.; Napaswat, C.; Yoosook, C. Anti-HIV-1 constituents from Clausena excavata: Part II. Carbazoles and a pyranocoumarin. Phytother. Res. 2005, 19, 728–731. [Google Scholar] [CrossRef]
- Saturnino, C.; Iacopetta, D.; Sinicropi, M.S.; Rosano, C.; Caruso, A.; Caporale, A.; Marra, N.; Marengo, B.; Pronzato, M.A.; Parisi, O.I.; et al. N-alkyl carbazole derivatives as new tools for Alzheimer’s disease: Preliminary studies. Molecules 2014, 19, 9307–9317. [Google Scholar] [CrossRef]
- Zimmermann, A.; Wilts, H.; Lenhardt, M.; Hahn, M.; Mertens, T. Indolocarbazoles exhibit strong antiviral activity against human cytomegalovirus and are potent inhibitors of the pUL97 protein kinase. Antivir. Res. 2000, 48, 49–60. [Google Scholar] [CrossRef]
- Gudmundsson, K.S.; Sebahar, P.R.; Richardson, L.D.; Catalano, J.G.; Boggs, S.D.; Spaltenstein, A.; Sethna, P.B.; Brown, K.W.; Harvey, R.; Romines, K.R. Substituted tetrahydrocarbazoles with potent activity against human papillomaviruses. Bioorg. Med. Chem. Lett. 2009, 19, 3489–3492. [Google Scholar] [CrossRef]
- Gopalsamy, A.; Shi, M.; Ciszewski, G.; Park, K.; Ellingboe, J.W.; Orlowski, M.; Feld, B.; Howe, A.Y. Design and synthesis of 2,3,4,9-tetrahydro-1H-carbazole and 1,2,3,4-tetrahydro-cyclopenta[b]indole derivatives as non-nucleoside inhibitors of hepatitis C virus NS5B RNA-dependent RNA polymerase. Bioorg. Med. Chem. Lett. 2006, 16, 2532–2534. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, M.; Li, M.; Luo, B.; Zhao, Y.; Huang, P.; Xue, F.; Rapposelli, S.; Pi, R.; Wen, S. Discovery of novel N-substituted carbazoles as neuroprotective agents with potent anti-oxidative activity. Eur. J. Med. Chem. 2013, 68, 81–88. [Google Scholar] [CrossRef]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef]
- Caruso, A.; Iacopetta, D.; Puoci, F.; Cappello, A.R.; Saturnino, C.; Sinicropi, M.S. Carbazole derivatives: A promising scenario for breast cancer treatment. Mini Rev. Med. Chem. 2016, 16, 630–643. [Google Scholar] [CrossRef]
- Caruso, A.; Chimento, A.; El-Kashef, H.; Lancelot, J.C.; Panno, A.; Pezzi, V.; Saturnino, C.; Sinicropi, M.S.; Sirianni, R.; Rault, S. Antiproliferative activity of some 1,4-dimethylcarbazoles on cells that express estrogen receptors: Part I. J. Enzym. Inhib. Med. Chem. 2012, 27, 609–613. [Google Scholar] [CrossRef]
- Bashir, M.; Bano, A.; Ijaz, A.S.; Chaudhary, B.A. Recent Developments and Biological Activities of N-Substituted Carbazole Derivatives: A Review. Molecules 2015, 20, 13496–13517. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Rosano, C.; Puoci, F.; Parisi, O.I.; Saturnino, C.; Caruso, A.; Longo, P.; Ceramella, J.; Malzert-Freon, A.; Dallemagne, P.; et al. Multifaceted properties of 1,4-dimethylcarbazoles: Focus on trimethoxybenzamide and trimethoxyphenylurea derivatives as novel human topoisomerase II inhibitors. Eur. J. Pharm. Sci. 2017, 96, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Rizza, P.; Pellegrino, M.; Caruso, A.; Iacopetta, D.; Sinicropi, M.S.; Rault, S.; Lancelot, J.C.; El-Kashef, H.; Lesnard, A.; Rochais, C.; et al. 3-(Dipropylamino)-5-hydroxybenzofuro[2,3-f]quinazolin-1(2H)-one (DPA-HBFQ-1) plays an inhibitory role on breast cancer cell growth and progression. Eur. J. Med. Chem. 2016, 107, 275–287. [Google Scholar] [CrossRef]
- Saturnino, C.; Palladino, C.; Napoli, M.; Sinicropi, M.S.; Botta, A.; Sala, M.; Carcereri de Prati, A.; Novellino, E.; Suzuki, H. Synthesis and biological evaluation of new N-alkylcarbazole derivatives as STAT3 inhibitors: Preliminary study. Eur. J. Med. Chem. 2013, 60, 112–119. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Lappano, R.; Caruso, A.; Santolla, M.F.; Pisano, A.; Rosano, C.; Capasso, A.; Panno, A.; Lancelot, J.C.; Rault, S.; et al. (6-bromo-1,4-dimethyl-9H-carbazol-3-yl-methylene)-hydrazine (carbhydraz) acts as a GPER agonist in breast cancer cells. Curr. Top. Med. Chem. 2015, 15, 1035–1042. [Google Scholar] [CrossRef]
- Parisi, O.I.; Morelli, C.; Puoci, F.; Saturnino, C.; Caruso, A.; Sisci, D.; Trombino, G.E.; Picci, N.; Sinicropi, M.S. Magnetic molecularly imprinted polymers (MMIPs) for carbazole derivative release in targeted cancer therapy. J. Mater. Chem. B 2014, 2, 6619–6625. [Google Scholar] [CrossRef]
- Saturnino, C.; Caruso, A.; Longo, P.; Capasso, A.; Pingitore, A.; Caroleo, M.C.; Cione, E.; Perri, M.; Nicolo, F.; Nardo, V.M.; et al. Crystallographic study and biological evaluation of 1,4-dimethyl-N-alkylcarbazoles. Curr. Top. Med. Chem. 2015, 15, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Sinicropi, M.S.; Lancelot, J.C.; El-Kashef, H.; Saturnino, C.; Aubert, G.; Ballandonne, C.; Lesnard, A.; Cresteil, T.; Dallemagne, P.; et al. Synthesis and evaluation of cytotoxic activities of new guanidines derived from carbazoles. Bioorg. Med. Chem. Lett. 2014, 24, 467–472. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Caruso, A.; Conforti, F.; Marrelli, M.; El Kashef, H.; Lancelot, J.C.; Rault, S.; Statti, G.A.; Menichini, F. Synthesis, inhibition of NO production and antiproliferative activities of some indole derivatives. J. Enzym. Inhib. Med. Chem. 2009, 24, 1148–1153. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse (NIDA). NIDA’s 2018 Avant-Garde awards Highlight Immune Response and Killer Cells. Available online: https://www.drugabuse.gov/news-events/news-releases/2018/03/nidas-2018-avant-garde-awards-highlight-immune-response-killer-cells (accessed on 11 February 2019).
- Maartens, G.; Celum, C.; Lewin, S.R. HIV infection: Epidemiology, pathogenesis, treatment, and prevention. Lancet 2014, 384, 258–271. [Google Scholar] [CrossRef]
- Palella, F.J., Jr.; Baker, R.K.; Moorman, A.C.; Chmiel, J.S.; Wood, K.C.; Brooks, J.T.; Holmberg, S.D.; HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J. Acquir. Immune Defic. Syndr. 2006, 43, 27–34. [Google Scholar] [CrossRef]
- Ho, D.D.; Neumann, A.U.; Perelson, A.S.; Chen, W.; Leonard, J.M.; Markowitz, M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 1995, 373, 123–126. [Google Scholar] [CrossRef]
- Palella, F.J., Jr.; Delaney, K.M.; Moorman, A.C.; Loveless, M.O.; Fuhrer, J.; Satten, G.A.; Aschman, D.J.; Holmberg, S.D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 1998, 338, 853–860. [Google Scholar] [CrossRef]
- Barre-Sinoussi, F.; Ross, A.L.; Delfraissy, J.F. Past, present and future: 30 years of HIV research. Nat. Rev. Microbiol. 2013, 11, 877–883. [Google Scholar] [CrossRef]
- Owen, A.; Rannard, S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Adv. Drug Deliv. Rev. 2016, 103, 144–156. [Google Scholar] [CrossRef]
- Simon, V.; Ho, D.D.; Abdool Karim, Q. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet 2006, 368, 489–504. [Google Scholar] [CrossRef]
- Halambage, U.D.; Wong, J.P.; Melancon, B.J.; Lindsley, C.W.; Aiken, C. Microplate-based assay for identifying small molecules that bind a specific intersubunit interface within the assembled HIV-1 capsid. Antimicrob. Agents Chemother. 2015, 59, 5190–5195. [Google Scholar] [CrossRef]
- Song, A.; Yu, H.; Wang, C.; Zhu, X.; Liu, K.; Ma, X. Novel dual small-molecule HIV inhibitors: Scaffolds and discovery strategies. Curr. Pharm. Des. 2015, 21, 950–962. [Google Scholar] [CrossRef]
- Grande, F.; Garofalo, A.; Neamati, N. Small molecules anti-HIV therapeutics targeting CXCR4. Curr. Pharm. Des. 2008, 14, 385–404. [Google Scholar]
- Hirata, K.; Ito, C.; Furukawa, H.; Itoigawa, M.; Cosentino, L.M.; Lee, K.H. Substituted 7H-pyrido[4,3-c]carbazoles with potent anti-HIV activity. Bioorg. Med. Chem. Lett. 1999, 9, 119–122. [Google Scholar] [CrossRef]
- Yan, H.; Mizutani, T.C.; Nomura, N.; Takakura, T.; Kitamura, Y.; Miura, H.; Nishizawa, M.; Tatsumi, M.; Yamamoto, N.; Sugiura, W. A novel small molecular weight compound with a carbazole structure that demonstrates potent human immunodeficiency virus type-1 integrase inhibitory activity. Antivir. Chem. Chemother. 2005, 16, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Munch, J.; Goerls, H.; Maier, A.; Fiebig, H.H.; Lin, W.H.; Hertweck, C. Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg. Med. Chem. Lett. 2010, 20, 6685–6687. [Google Scholar] [CrossRef]
- Xu, Z.; Baunach, M.; Ding, L.; Hertweck, C. Bacterial synthesis of diverse indole terpene alkaloids by an unparalleled cyclization sequence. Angew. Chem. 2012, 51, 10293–10297. [Google Scholar] [CrossRef]
- Rault, S.; Lancelot, J.C.; Caruso, A.; Lesnard, A.; Cresteil, N.; Aubert, G. Use of Carbazole-phenone Derivatives for Treating Cancer. Patent No. WO 2013/121385 A1, 22 August 2013. [Google Scholar]
- Crough, T.; Khanna, R. Immunobiology of human cytomegalovirus: From bench to bedside. Clin. Microbiol. Rev. 2009, 22, 76–98. [Google Scholar] [CrossRef]
- Staras, S.A.; Dollard, S.C.; Radford, K.W.; Flanders, W.D.; Pass, R.F.; Cannon, M.J. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis. 2006, 43, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A.; Boppana, S.B. Vaccination against the human cytomegalovirus. Vaccine 2018. [Google Scholar] [CrossRef]
- Fowler, K.B.; Boppana, S.B. Congenital cytomegalovirus (CMV) infection and hearing deficit. J. Clin. Virol. 2006, 35, 226–231. [Google Scholar] [CrossRef]
- Boppana, S.B.; Britt, W.J. Synopsis of Clinical Aspects of Human Cytomegalovirus Disease. In Cytomegaloviruses: From Molecular Pathogenesis to Intervention; Reddehase, M.J., Ed.; Caister Academic Press: Norfolk, UK, 2013; Volume II, pp. 1–25. [Google Scholar]
- Dollard, S.C.; Grosse, S.D.; Ross, D.S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.L.; King, C.C.; Kourtis, A.P. Cytomegalovirus infection in pregnancy. Birth Defects Res. 2017, 109, 336–346. [Google Scholar] [CrossRef]
- Erice, A.; Chou, S.; Biron, K.K.; Stanat, S.C.; Balfour, H.H., Jr.; Jordan, M.C. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N. Engl. J. Med. 1989, 320, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.; Marousek, G.; Guentzel, S.; Follansbee, S.E.; Poscher, M.E.; Lalezari, J.P.; Miner, R.C.; Drew, W.L. Evolution of mutations conferring multidrug resistance during prophylaxis and therapy for cytomegalovirus disease. J. Infect. Dis. 1997, 176, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.J.; Cockerill, S.; Baxter, R.; Bonser, R.W.; Gohil, K.; Gowrie, C.; Robinson, J.E.; Littler, E.; Parry, N.; Randall, R.; et al. Indolocarbazoles: Potent, selective inhibitors of human cytomegalovirus replication. Bioorg. Med. Chem. 1999, 7, 1067–1074. [Google Scholar] [CrossRef]
- Patzold, S.; Schneider, J.; Rudolph, C.; Marme, D.; Schachtele, C. Novel indolocarbazole protein kinase C inhibitors prevent reactivation of HIV-1 in latently infected cells. Antivir. Res. 1993, 22, 273–283. [Google Scholar] [CrossRef]
- Michelson, S.; Tardy-Panit, M.; Barzu, O. Properties of a human cytomegalovirus-induced protein kinase. Virology 1984, 134, 259–268. [Google Scholar] [CrossRef]
- Muganda, P.M.; Fischer, A.; Bernal, R.A. Identification of a casein kinase activity found elevated in human cytomegalovirus transformed cells. Biochem. Biophys. Res. Commun. 1995, 207, 740–746. [Google Scholar] [CrossRef]
- Chee, M.S.; Lawrence, G.L.; Barrell, B.G. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 1989, 70 (Pt 5), 1151–1160. [Google Scholar] [CrossRef]
- Michel, D.; Kramer, S.; Hohn, S.; Schaarschmidt, P.; Wunderlich, K.; Mertens, T. Amino acids of conserved kinase motifs of cytomegalovirus protein UL97 are essential for autophosphorylation. J. Virol. 1999, 73, 8898–8901. [Google Scholar]
- Puchades Renau, L.; Berenguer, M. Introduction to hepatitis C virus infection: Overview and history of hepatitis C virus therapies. Hemodial. Int. 2018, 22 (Suppl. 1), S8–S21. [Google Scholar] [CrossRef]
- Mohd Hanafiah, K.; Groeger, J.; Flaxman, A.D.; Wiersma, S.T. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013, 57, 1333–1342. [Google Scholar] [CrossRef]
- World Health Organization. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017; pp. 1–83. ISBN 978-92-4-156545-5. [Google Scholar]
- Hajarizadeh, B.; Grebely, J.; Dore, G.J. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 553–562. [Google Scholar] [CrossRef]
- Hauri, A.M.; Armstrong, G.L.; Hutin, Y.J. The global burden of disease attributable to contaminated injections given in health care settings. Int. J. STD AIDS 2004, 15, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Poynard, T.; Ghillani, P.; Charlotte, F.; Olivi, M.; Piette, J.C.; Opolon, P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999, 42, 2204–2212. [Google Scholar] [CrossRef]
- Hepmag.com. Hepatitis C Drug List. Available online: http://www.hepmag.com/pdfs/hepatitis_c_drug_list.pdf (accessed on 11 December 2018).
- Sarrazin, C.; Zeuzem, S. Resistance to direct antiviral agents in patients with hepatitis C virus infection. Gastroenterology 2010, 138, 447–462. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Qing, J.; Huang, M.; Wu, M.; Gao, F.; Li, D.; Hong, Z.; Kong, L.; Huang, W.; et al. Discovery of Novel Hepatitis C Virus NS5B Polymerase Inhibitors by Combining Random Forest, Multiple e-Pharmacophore Modeling and Docking. PLoS ONE 2016, 11, e0148181. [Google Scholar] [CrossRef] [PubMed]
- Gopalsamy, A.; Lim, K.; Ciszewski, G.; Park, K.; Ellingboe, J.W.; Bloom, J.; Insaf, S.; Upeslacis, J.; Mansour, T.S.; Krishnamurthy, G.; et al. Discovery of pyrano[3,4-b]indoles as potent and selective HCV NS5B polymerase inhibitors. J. Med. Chem. 2004, 47, 6603–6608. [Google Scholar] [CrossRef]
- Murakami, Y.; Noguchi, K.; Yamagoe, S.; Suzuki, T.; Wakita, T.; Fukazawa, H. Identification of bisindolylmaleimides and indolocarbazoles as inhibitors of HCV replication by tube-capture-RT-PCR. Antivir. Res. 2009, 83, 112–117. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, O.Q.; Graf, P.; Kisicki, J.C.; Schran, H. Dose- and time-dependent pharmacokinetics of midostaurin in patients with diabetes mellitus. J. Clin. Pharmacol. 2008, 48, 763–775. [Google Scholar] [CrossRef]
- Zhu, G.; Conner, S.; Zhou, X.; Shih, C.; Brooks, H.B.; Considine, E.; Dempsey, J.A.; Ogg, C.; Patel, B.; Schultz, R.M.; et al. Synthesis of quinolinyl/isoquinolinyl[a]pyrrolo [3,4-c] carbazoles as cyclin D1/CDK4 inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 1231–1235. [Google Scholar] [CrossRef]
- Kang, I.J.; Wang, L.W.; Hsu, S.J.; Lee, C.C.; Lee, Y.C.; Wu, Y.S.; Yueh, A.; Wang, J.C.; Hsu, T.A.; Chao, Y.S.; et al. Design and efficient synthesis of novel arylthiourea derivatives as potent hepatitis C virus inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 6063–6068. [Google Scholar] [CrossRef]
- Singh, S.; Kulshrestha, P.; Agarwal, M. Review on Herpes simplex virus: Causes, available drugs and future prospects. Int. J. Pharm. Bio Sci. 2015, 6, 1076–1086. [Google Scholar]
- Koelle, D.M.; Corey, L. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin. Microbiol. Rev. 2003, 16, 96–113. [Google Scholar] [CrossRef]
- Azwa, A.; Barton, S.E. Aspects of herpes simplex virus: A clinical review. J. Fam. Plan. Reprod. Health Care 2009, 35, 237–242. [Google Scholar] [CrossRef][Green Version]
- Lafferty, W.E.; Downey, L.; Celum, C.; Wald, A. Herpes simplex virus type 1 as a cause of genital herpes: Impact on surveillance and prevention. J. Infect. Dis. 2000, 181, 1454–1457. [Google Scholar] [CrossRef]
- Strutt, M.; Bailey, J.; Tenant-Flowers, M.; Graham, D.; Zuckerman, M. Ethnic variation in type of genital herpes simplex virus infection in a South London genitourinary medicine clinic. J. Med. Virol. 2003, 69, 108–110. [Google Scholar] [CrossRef]
- Scoular, A.; Norrie, J.; Gillespie, G.; Mir, N.; Carman, W.F. Longitudinal study of genital infection by herpes simplex virus type 1 in Western Scotland over 15 years. Bmj 2002, 324, 1366–1367. [Google Scholar] [CrossRef]
- Doi, Y.; Ninomiya, T.; Hata, J.; Yonemoto, K.; Tanizaki, Y.; Arima, H.; Liu, Y.; Rahman, M.; Iida, M.; Kiyohara, Y. Seroprevalence of herpes simplex virus 1 and 2 in a population-based cohort in Japan. J. Epidemiol. 2009, 19, 56–62. [Google Scholar] [CrossRef]
- Taylor, G.S.; Long, H.M.; Brooks, J.M.; Rickinson, A.B.; Hislop, A.D. The immunology of Epstein-Barr virus-induced disease. Annu. Rev. Immunol. 2015, 33, 787–821. [Google Scholar] [CrossRef]
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Khan, G.; Hashim, M.J. Global burden of deaths from Epstein-Barr virus attributable malignancies 1990–2010. Infect. Agents Cancer 2014, 9, 38. [Google Scholar] [CrossRef]
- Young, L.S.; Yap, L.F.; Murray, P.G. Epstein-Barr virus: More than 50 years old and still providing surprises. Nat. Rev. Cancer 2016, 16, 789–802. [Google Scholar] [CrossRef]
- Stanfield, B.A.; Luftig, M.A. Recent advances in understanding Epstein-Barr virus. F1000Research 2017, 6, 386. [Google Scholar] [CrossRef]
- Knubel, G.; Larsen, L.K.; Moore, R.E.; Levine, I.A.; Patterson, G.M. Cytotoxic, antiviral indolocarbazoles from a blue-green alga belonging to the Nostocaceae. J. Antibiot. 1990, 43, 1236–1239. [Google Scholar] [CrossRef]
- Ito, C.; Itoigawa, M.; Sato, A.; Hasan, C.M.; Rashid, M.A.; Tokuda, H.; Mukainaka, T.; Nishino, H.; Furukawa, H. Chemical constituents of Glycosmis arborea: Three new carbazole alkaloids and their biological activity. J. Nat. Prod. 2004, 67, 1488–1491. [Google Scholar] [CrossRef]
- Satterwhite, C.L.; Torrone, E.; Meites, E.; Dunne, E.F.; Mahajan, R.; Ocfemia, M.C.; Su, J.; Xu, F.; Weinstock, H. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sex. Transm. Dis. 2013, 40, 187–193. [Google Scholar] [CrossRef]
- Skaaby, S.; Kofoed, K. Anogenital warts in Danish men who have sex with men. Int. J. STD AIDS 2011, 22, 214–217. [Google Scholar] [CrossRef]
- Boda, D.; Docea, A.O.; Calina, D.; Ilie, M.A.; Caruntu, C.; Zurac, S.; Neagu, M.; Constantin, C.; Branisteanu, D.E.; Voiculescu, V.; et al. Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int. J. Oncol. 2018, 52, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Steben, M.; Duarte-Franco, E. Human papillomavirus infection: Epidemiology and pathophysiology. Gynecol. Oncol. 2007, 107, S2–S5. [Google Scholar] [CrossRef]
- Stokley, S.; Jeyarajah, J.; Yankey, D.; Cano, M.; Gee, J.; Roark, J.; Curtis, R.C.; Markowitz, L. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 620–624. [Google Scholar] [PubMed]
- Daniyal, M.; Akhtar, N.; Ahmad, S.; Fatima, U.; Akram, M.; Asif, H.M. Update knowledge on cervical cancer incidence and prevalence in Asia. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 3617–3620. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Solomon, D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch. Pathol. Lab. Med. 2003, 127, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006, 24 (Suppl. 3), S11–S25. [Google Scholar] [CrossRef] [PubMed]
- Cutts, F.T.; Franceschi, S.; Goldie, S.; Castellsague, X.; de Sanjose, S.; Garnett, G.; Edmunds, W.J.; Claeys, P.; Goldenthal, K.L.; Harper, D.M.; et al. Human papillomavirus and HPV vaccines: A review. Bull. World Health Organ. 2007, 85, 719–726. [Google Scholar] [CrossRef]
- Gudmundsson, K.S.; Boggs, S.D.; Sebahar, P.R.; Richardson, L.D.; Spaltenstein, A.; Golden, P.; Sethna, P.B.; Brown, K.W.; Moniri, K.; Harvey, R.; et al. Tetrahydrocarbazole amides with potent activity against human papillomaviruses. Bioorg. Med. Chem. Lett. 2009, 19, 4110–4114. [Google Scholar] [CrossRef]

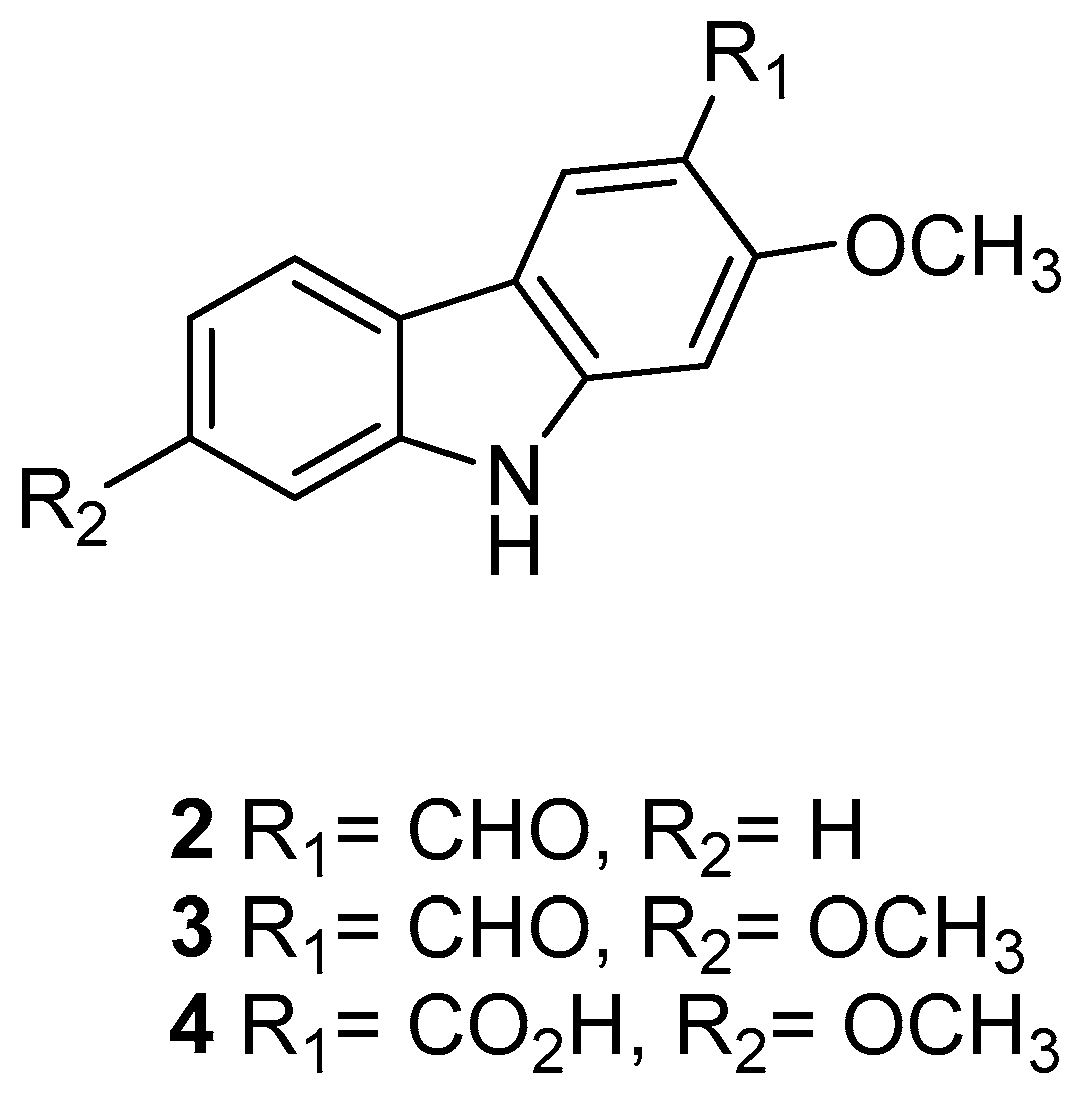
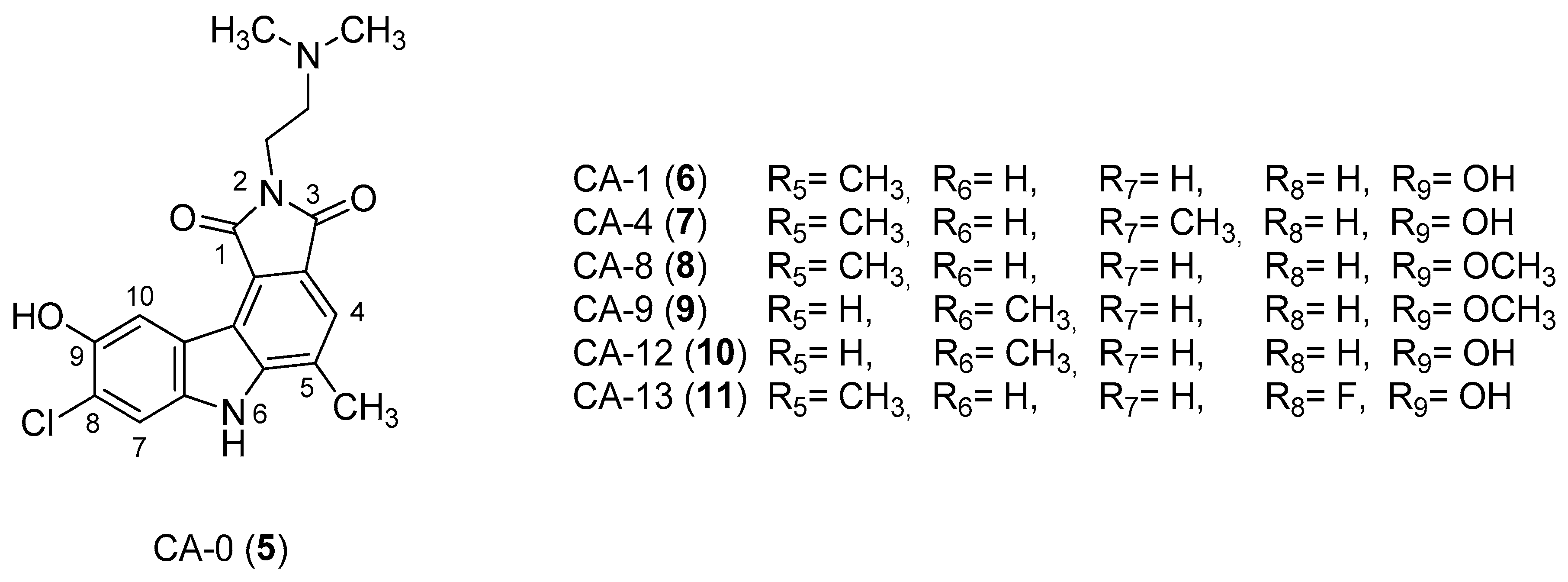
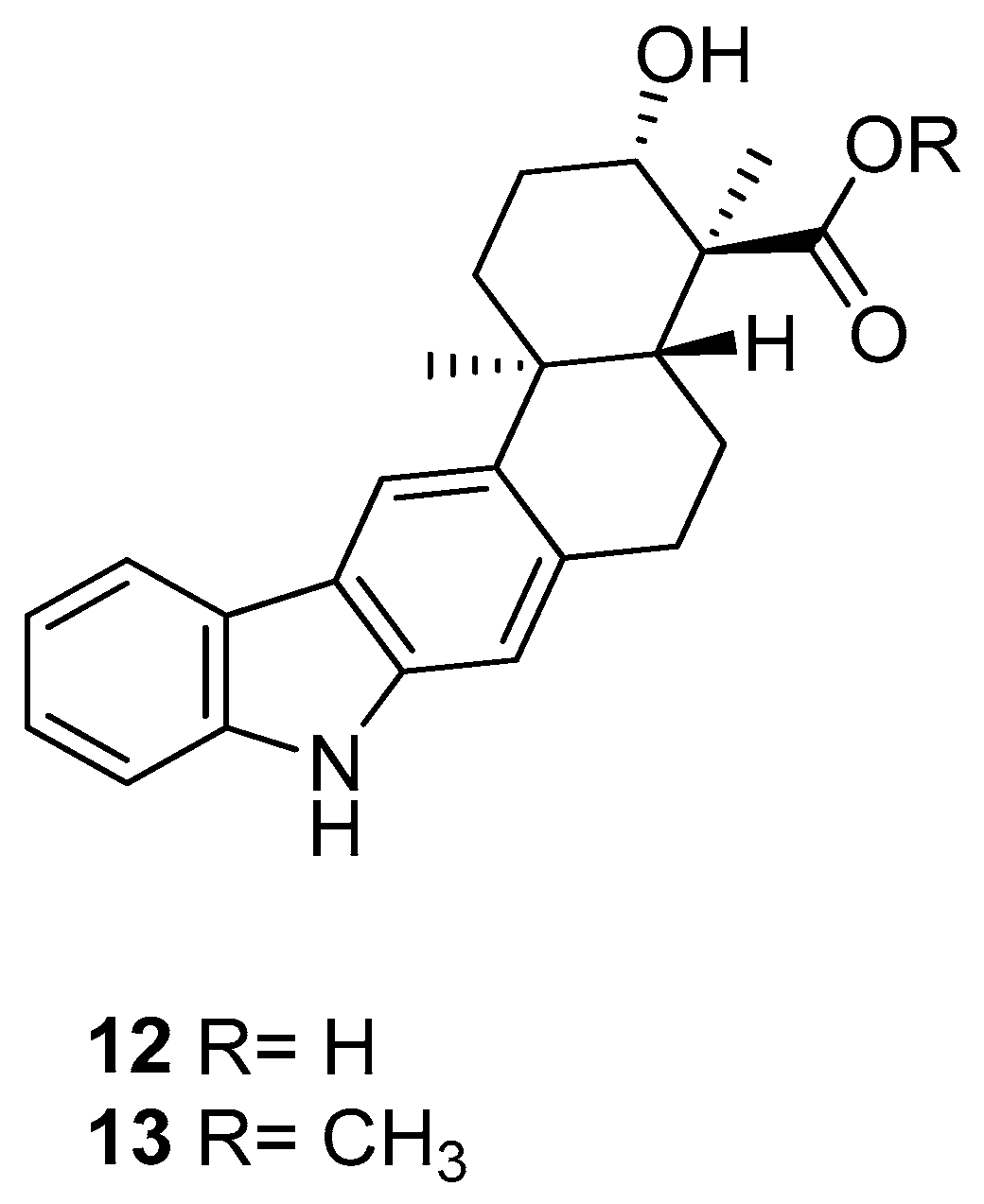
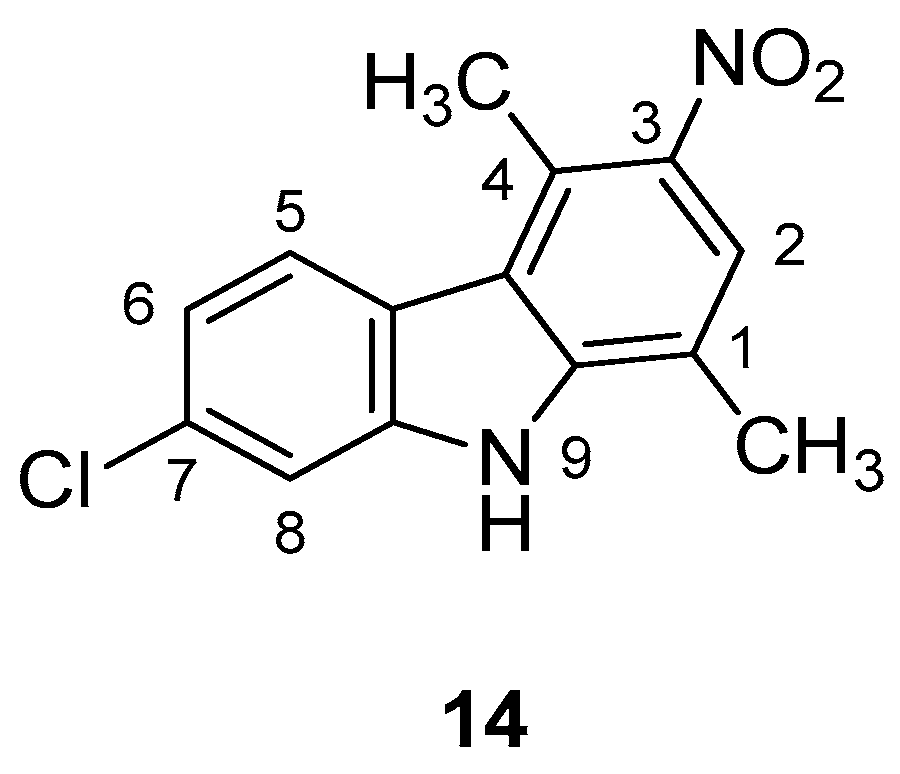
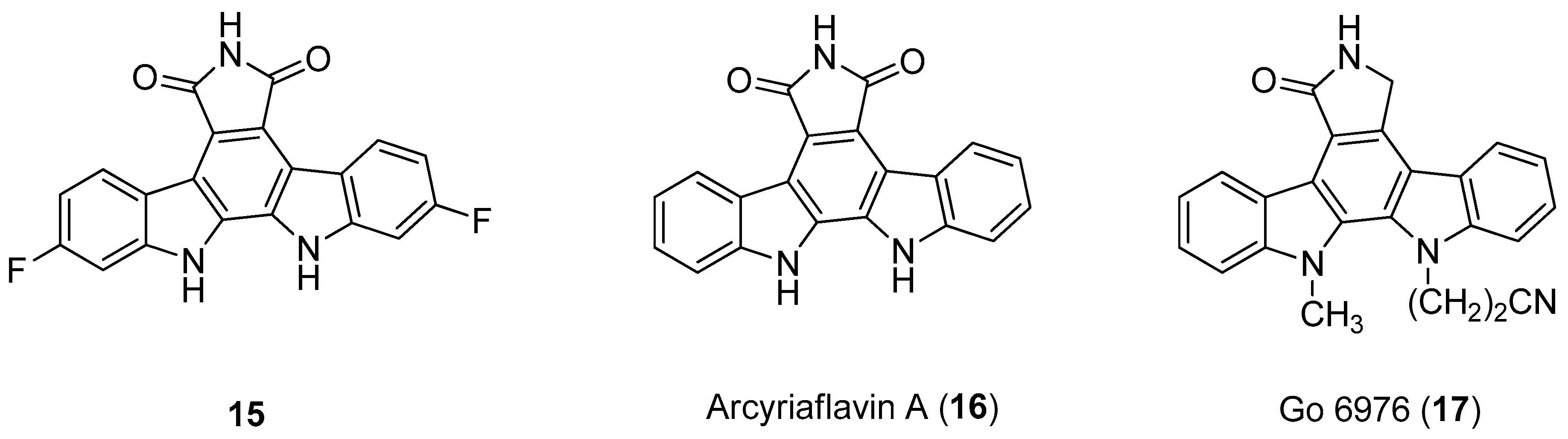
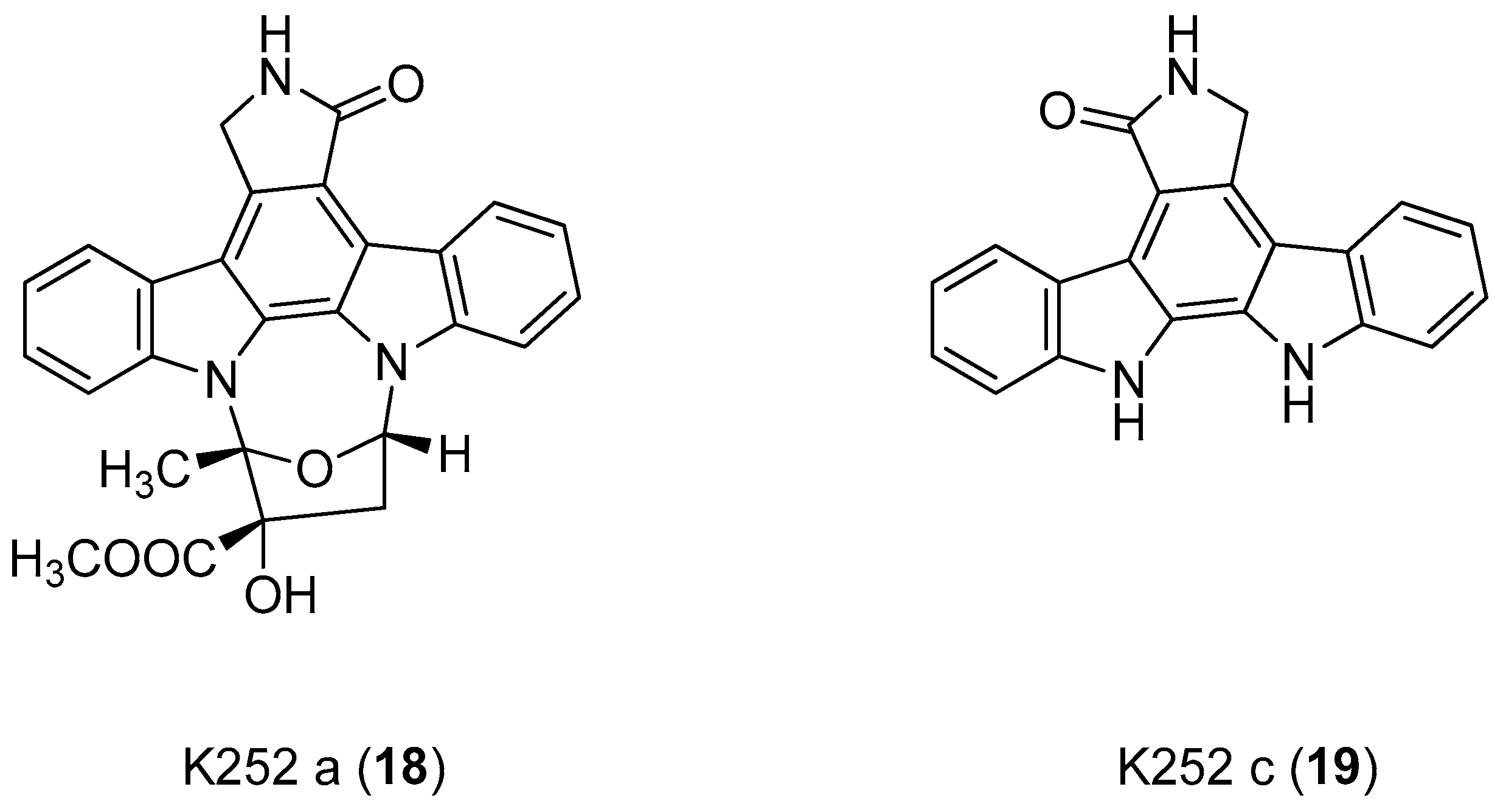
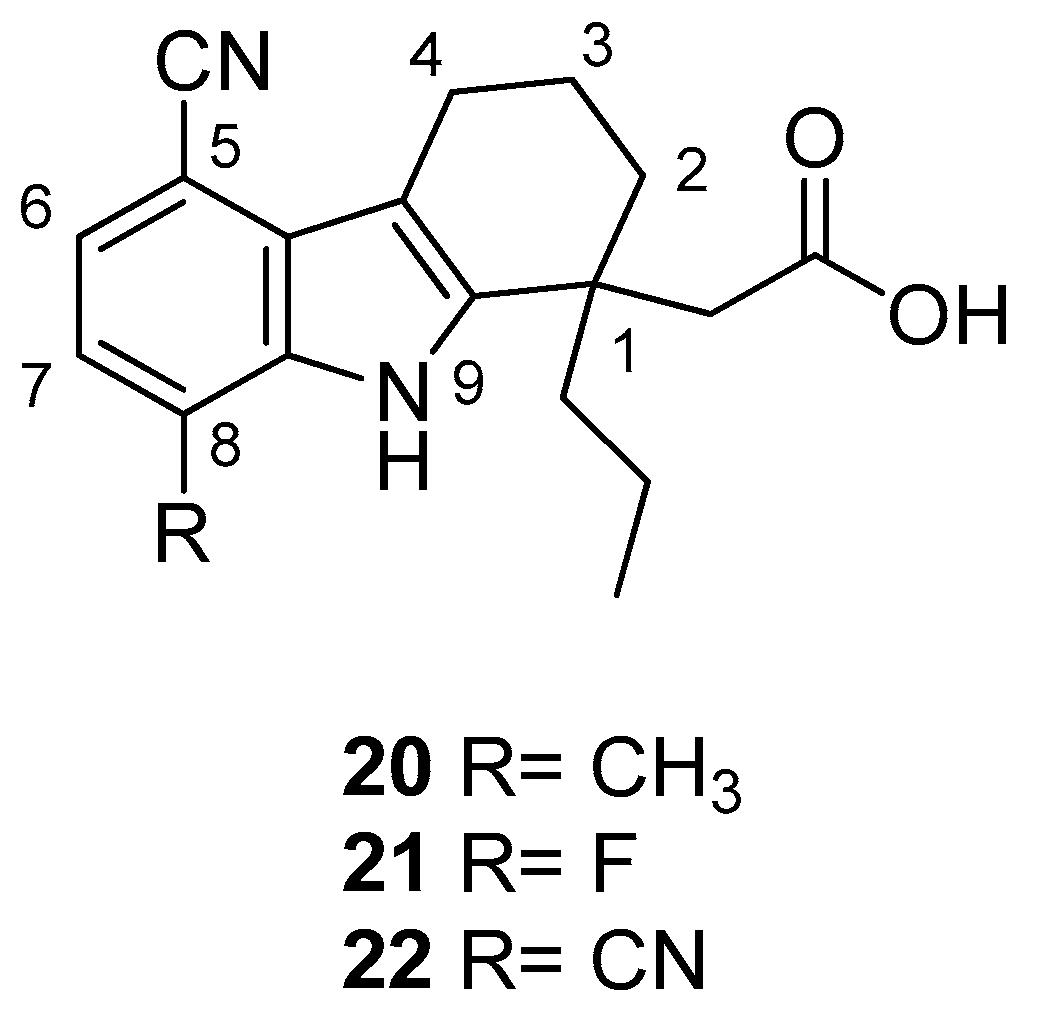
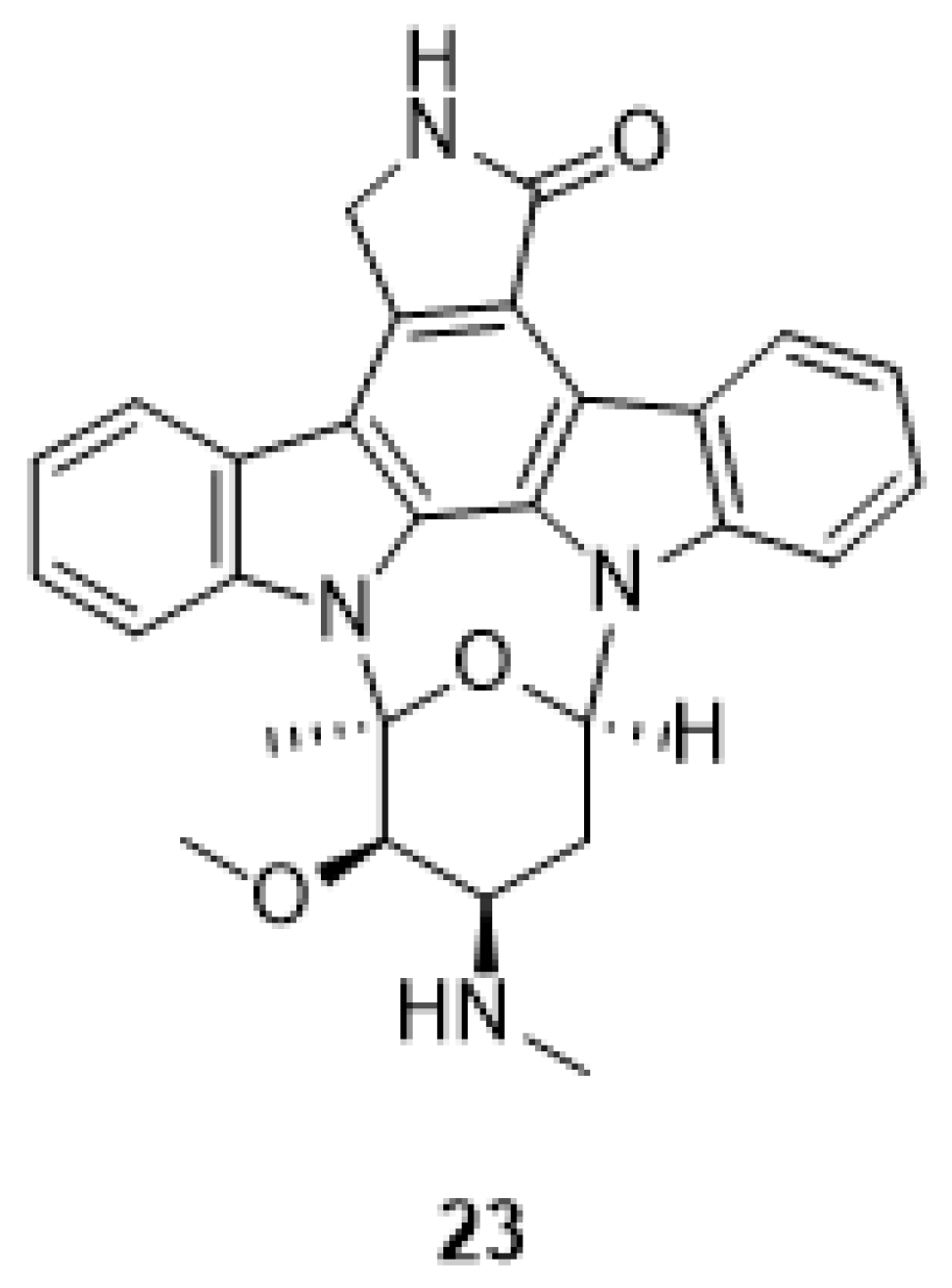
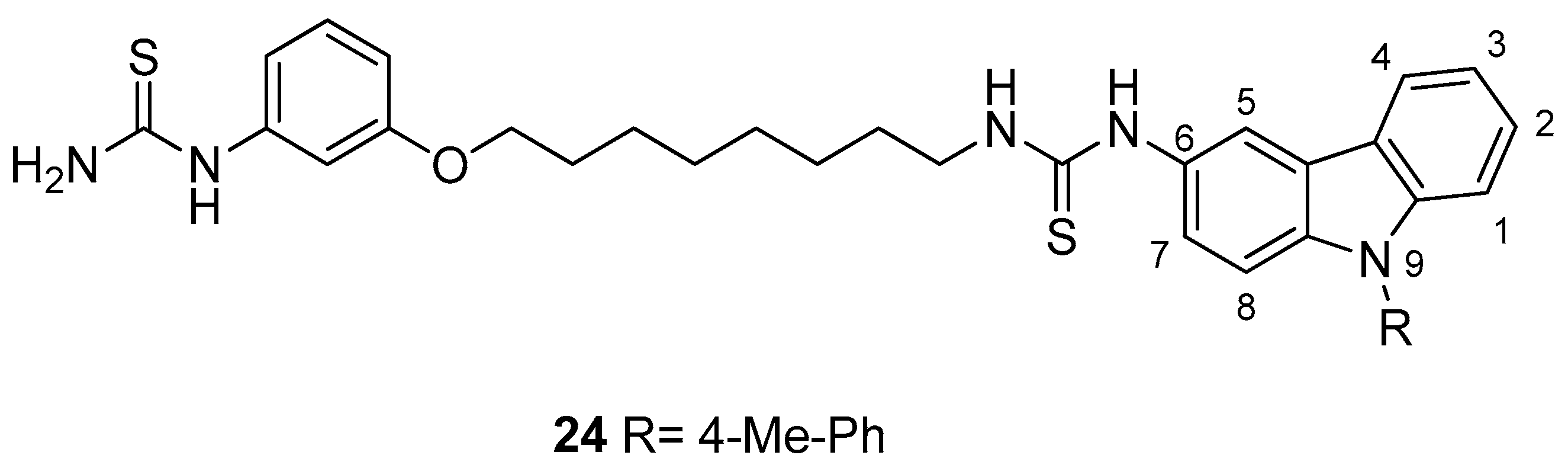
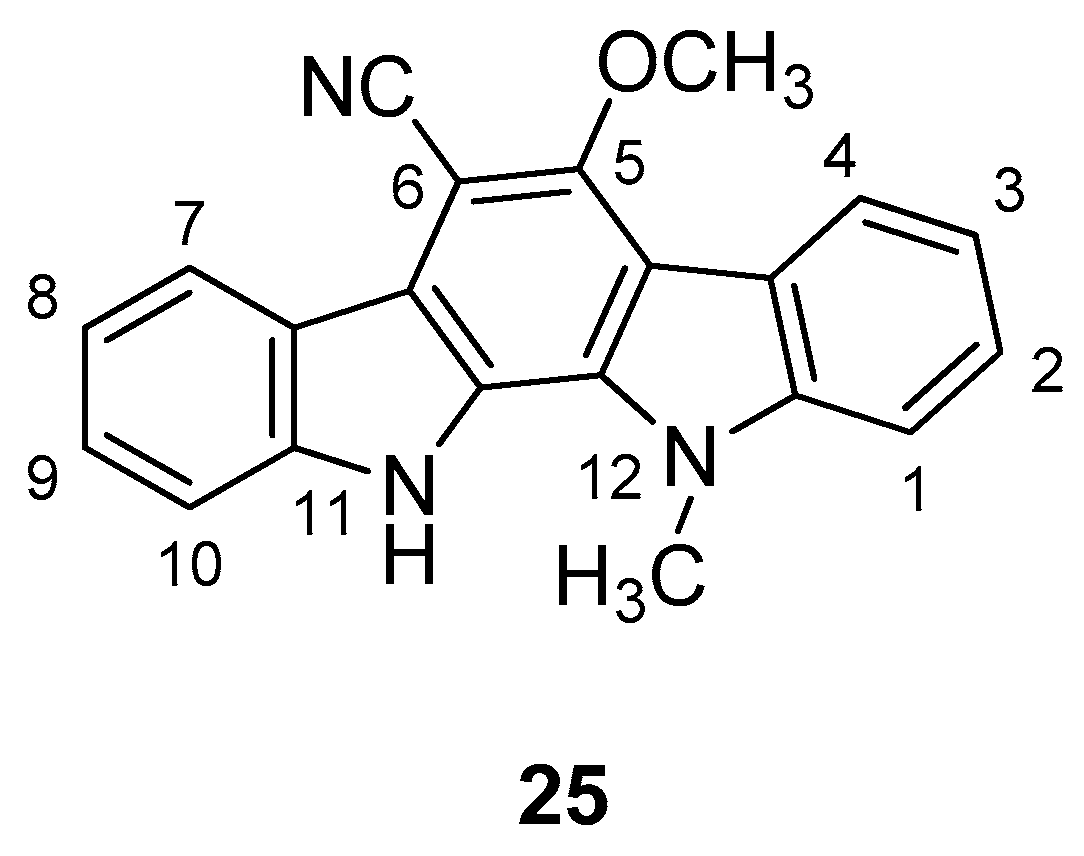
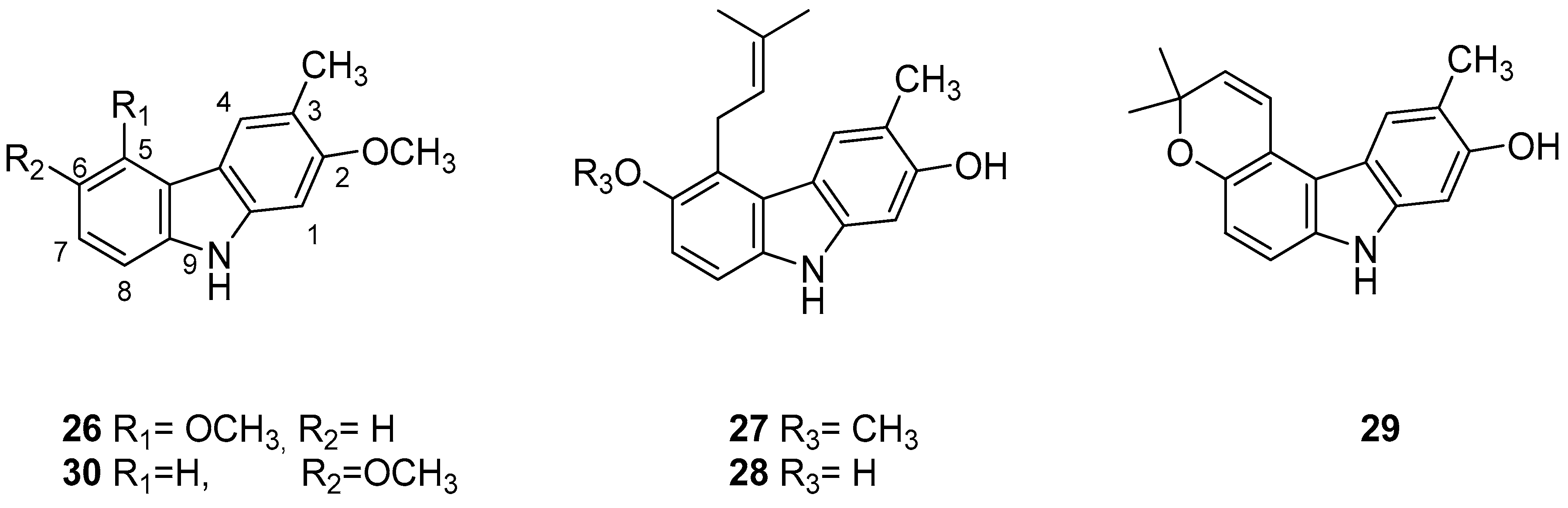
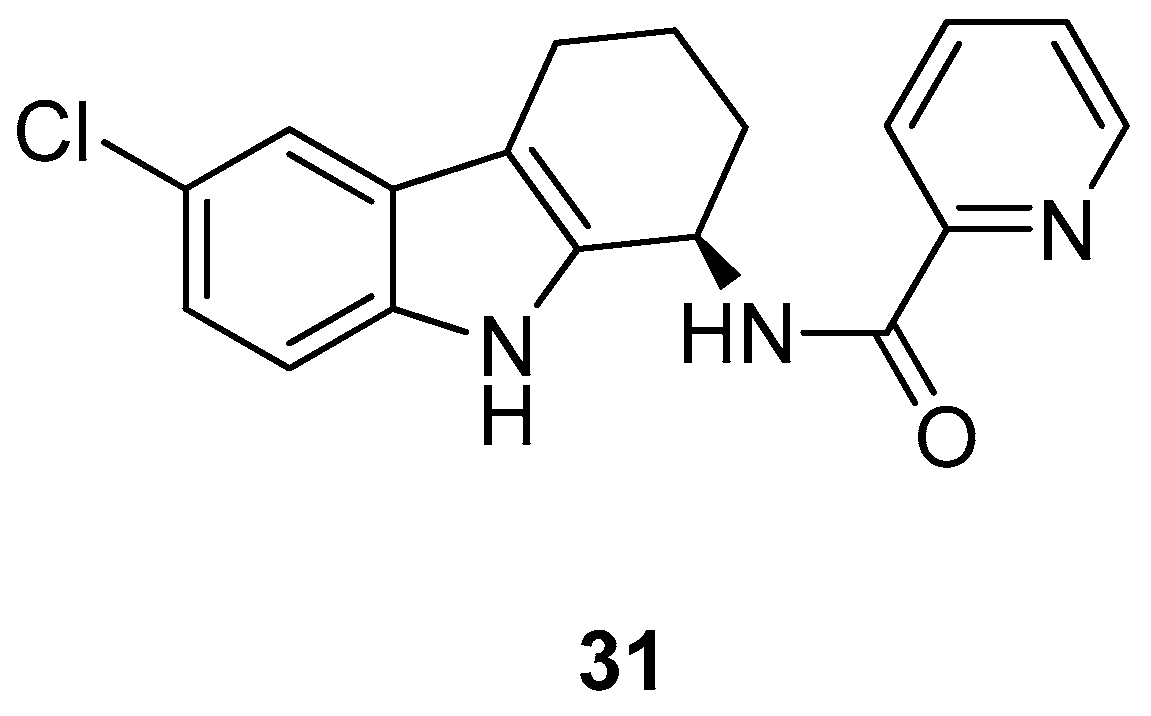
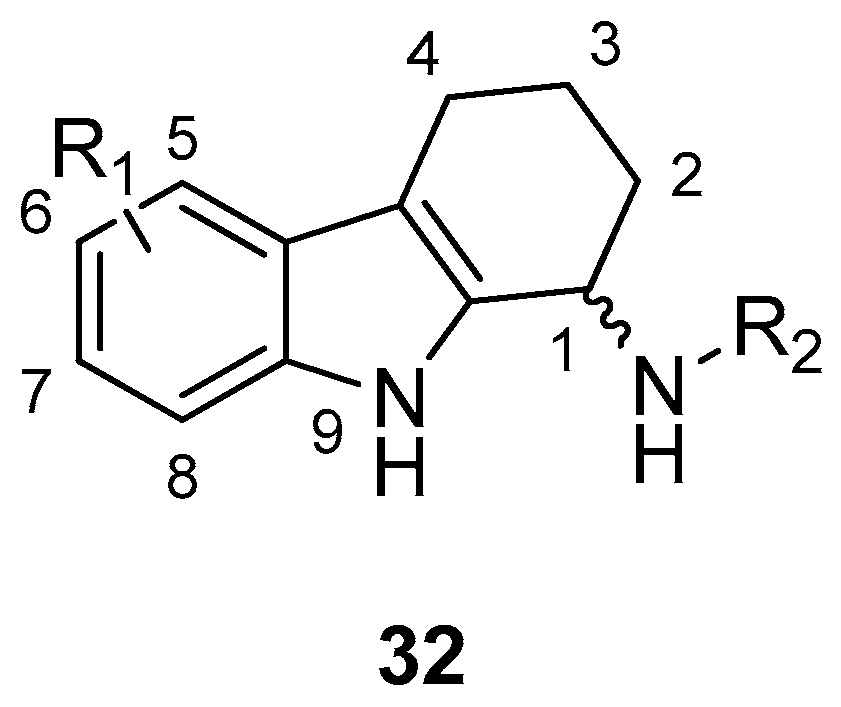
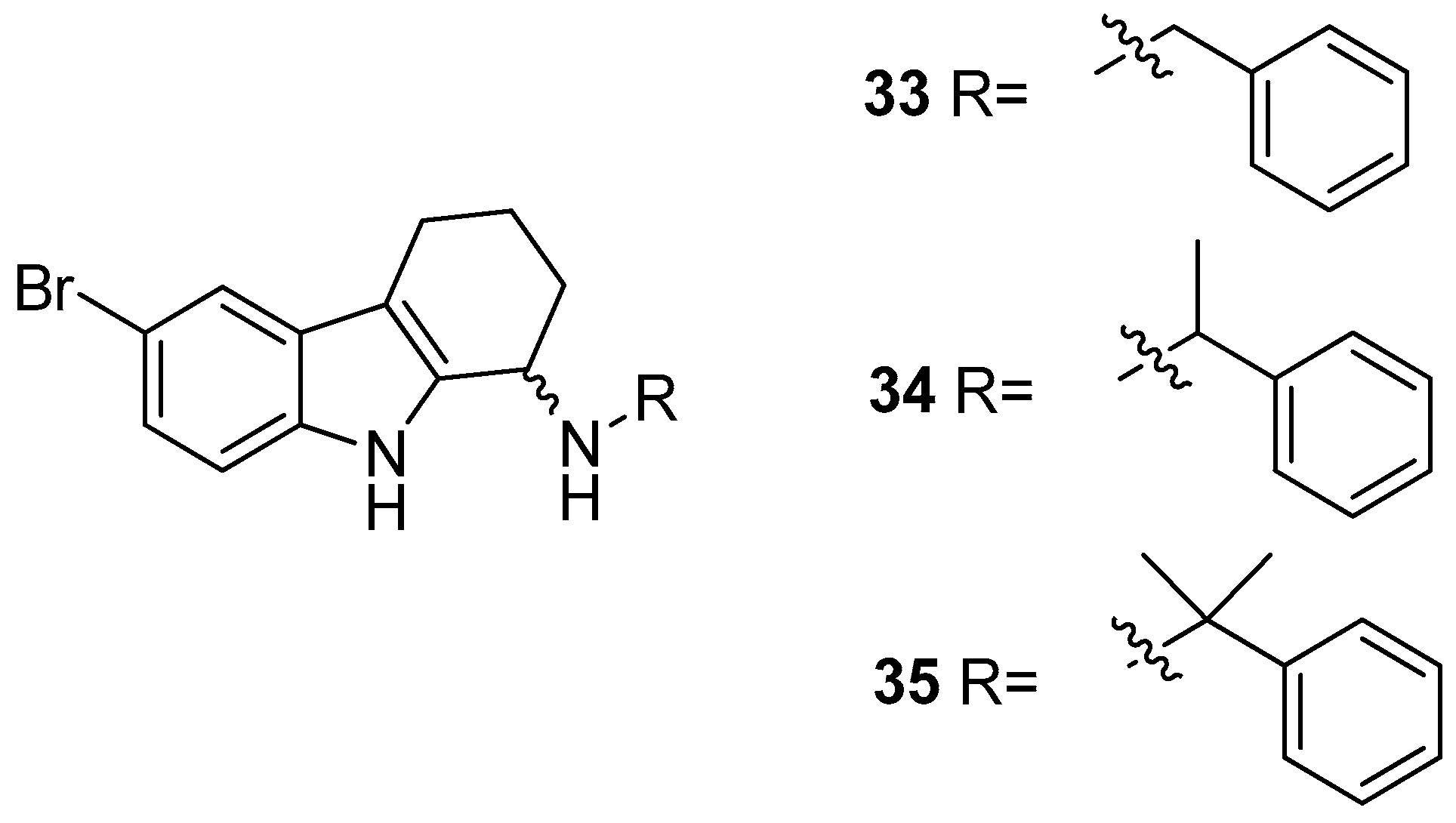
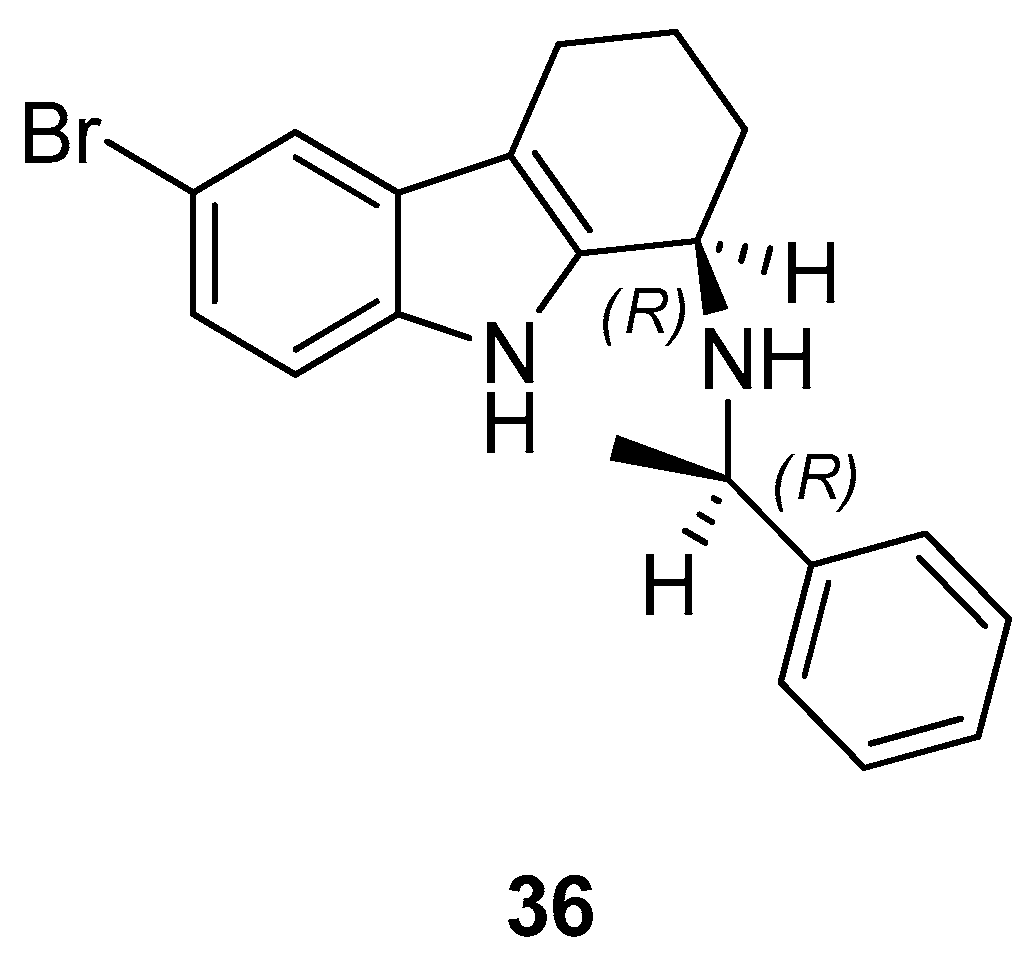
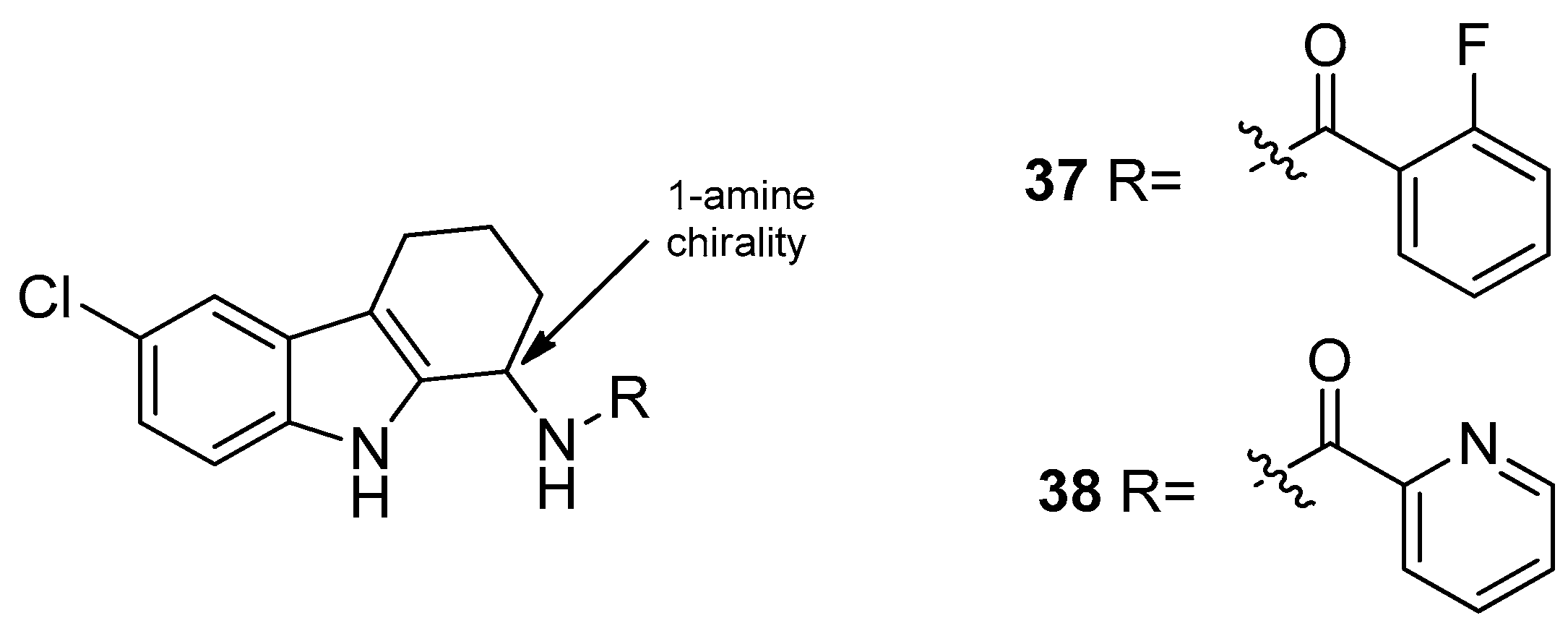
| Human Immunodeficiency Virus (HIV) | |
 | Hirata et al. 1999 [43] |
 | Kongkathip et al. 2005 [14] |
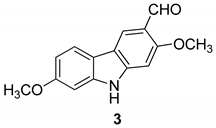 | Kongkathip et al. 2005 [14] |
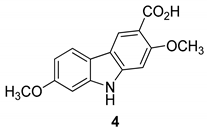 | Kongkathip et al. 2005 [14] |
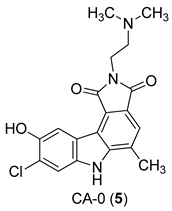 | Yan et al. 2005 [44] |
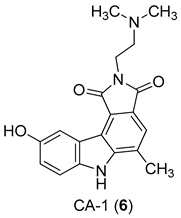 | Yan et al. 2005 [44] |
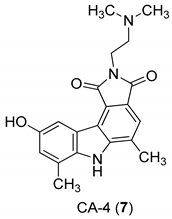 | Yan et al. 2005 [44] |
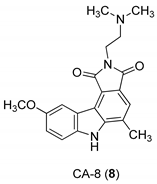 | Yan et al. 2005 [44] |
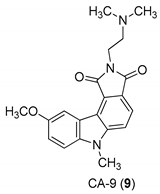 | Yan et al. 2005 [44] |
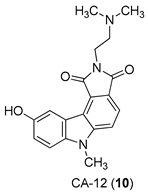 | Yan et al. 2005 [44] |
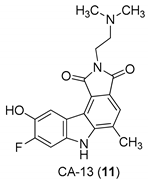 | Yan et al. 2005 [44] |
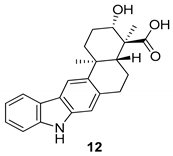 | Ding et al. 2010 [45] Xu et al. 2012 [46] |
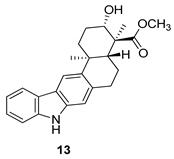 | Ding et al. 2010 [45] |
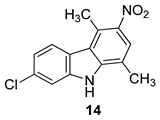 | Saturnino et al. 2018 [5] |
| Human Cytomegalovirus (HCMV) | |
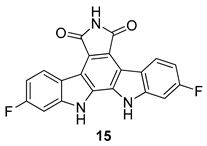 | Slater et al. 1999 [58] |
 | Slater et al. 1999 [58] Murakami et al. 2009 [74] Zhu et al. 2003 [76] |
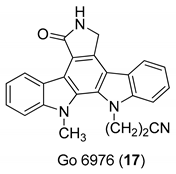 | Zimmermann et al. 2000 [16] Slater et al. 1999 [58] Patzold et al. 1993 [59] |
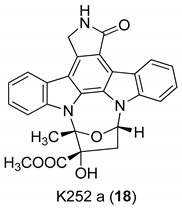 | Zimmermann et al. 2000 [16] |
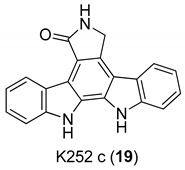 | Zimmermann et al. 2000 [16] |
| Chronic Hepatitis C Virus (HCV) | |
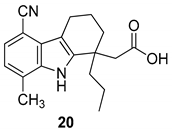 | Gopalsamy et al. 2006 [18] |
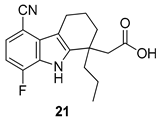 | Gopalsamy et al. 2006 [18] |
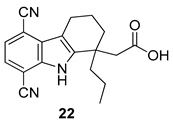 | Gopalsamy et al. 2006 [18] |
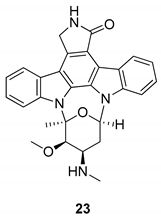 | Murakami et al. 2009 [74] Wang et al. 2008 [75] |
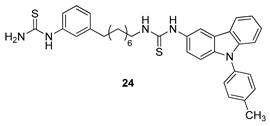 | Kang et al. 2009 [77] |
| Herpes Simplex Virus (HSV) | |
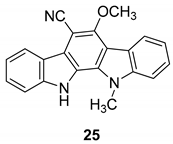 | Knübel et al. 1990 [89] |
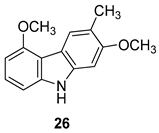 | Ito et al. 2004 [90] |
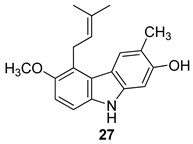 | Ito et al. 2004 [90] |
 | Ito et al. 2004 [90] |
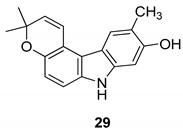 | Ito et al. 2004 [90] |
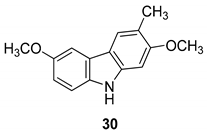 | Ito et al. 2004 [90] |
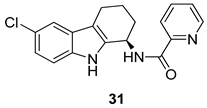 | Harvey et al. 2009 [2] |
| Human Papilloma Virus | |
 | Gudmundsson et al. 2009 [17] |
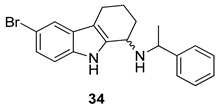 | Gudmundsson et al. 2009 [17] |
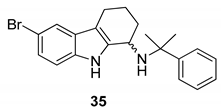 | Gudmundsson et al. 2009 [17] |
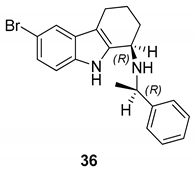 | Gudmundsson et al. 2009 [17] |
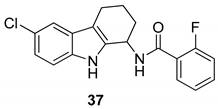 | Gudmundsson et al. 2009 [100] |
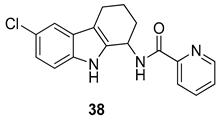 | Gudmundsson et al. 2009 [100] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, A.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Mauro, M.V.; Bruno, R.; Aquaro, S.; Sinicropi, M.S. Carbazole Derivatives as Antiviral Agents: An Overview. Molecules 2019, 24, 1912. https://doi.org/10.3390/molecules24101912
Caruso A, Ceramella J, Iacopetta D, Saturnino C, Mauro MV, Bruno R, Aquaro S, Sinicropi MS. Carbazole Derivatives as Antiviral Agents: An Overview. Molecules. 2019; 24(10):1912. https://doi.org/10.3390/molecules24101912
Chicago/Turabian StyleCaruso, Anna, Jessica Ceramella, Domenico Iacopetta, Carmela Saturnino, Maria Vittoria Mauro, Rosalinda Bruno, Stefano Aquaro, and Maria Stefania Sinicropi. 2019. "Carbazole Derivatives as Antiviral Agents: An Overview" Molecules 24, no. 10: 1912. https://doi.org/10.3390/molecules24101912
APA StyleCaruso, A., Ceramella, J., Iacopetta, D., Saturnino, C., Mauro, M. V., Bruno, R., Aquaro, S., & Sinicropi, M. S. (2019). Carbazole Derivatives as Antiviral Agents: An Overview. Molecules, 24(10), 1912. https://doi.org/10.3390/molecules24101912











