Epoxy–PCM Composites with Nanocarbons or Multidimensional Boron Nitride as Heat Flow Enhancers
Abstract
1. Introduction
2. Results and Discussion
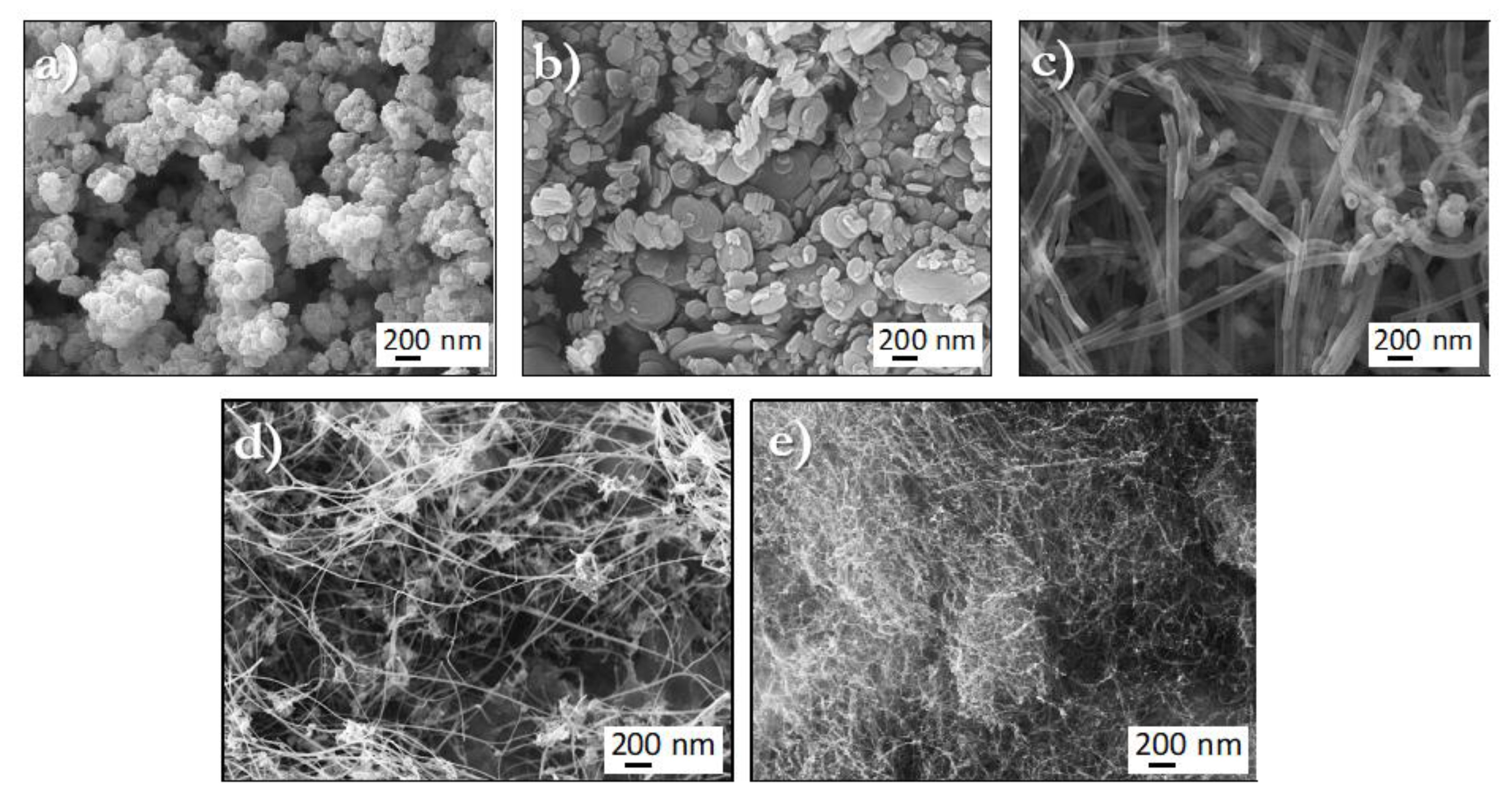
2.1. Microstructural Characterization
2.2. DSC Analysis
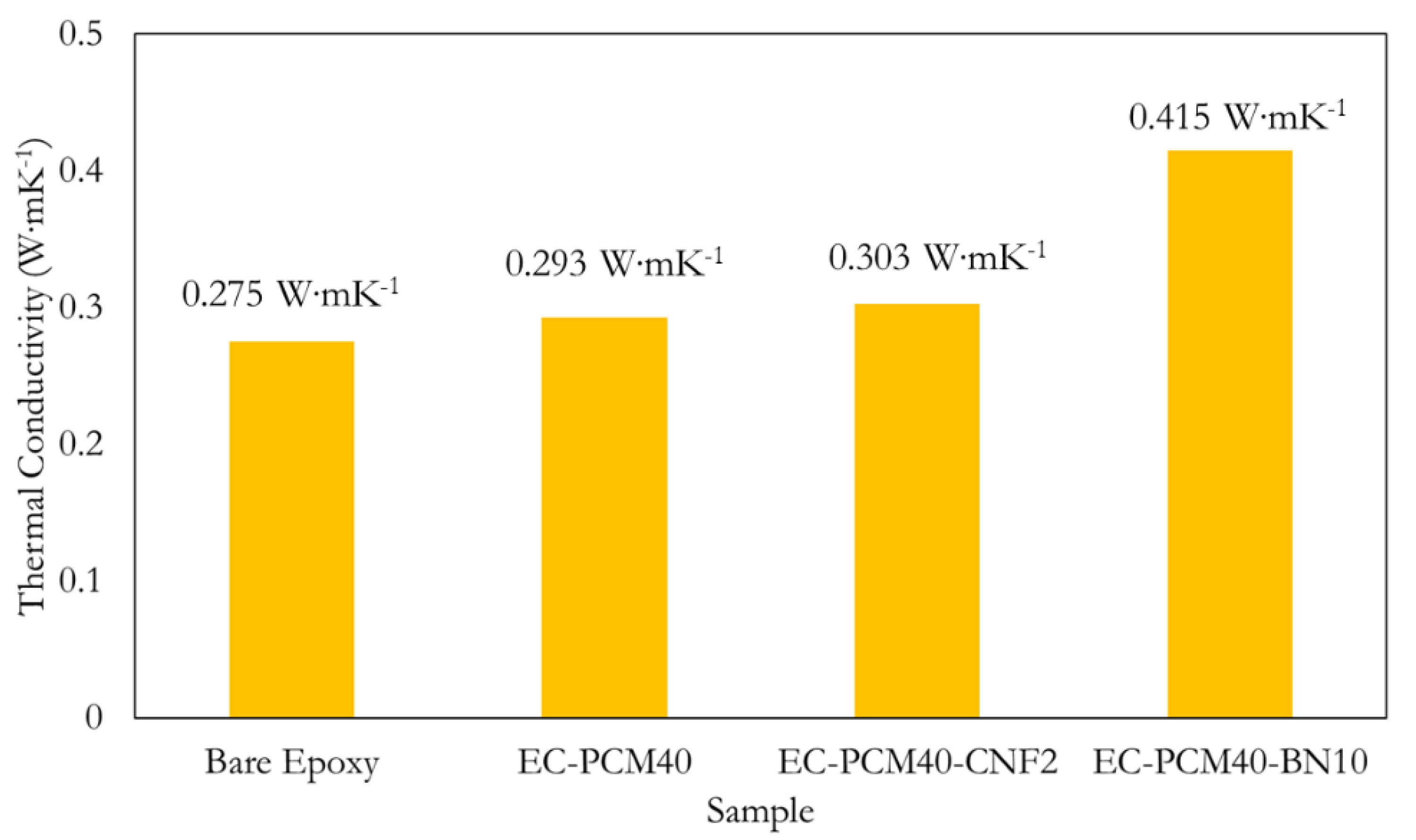
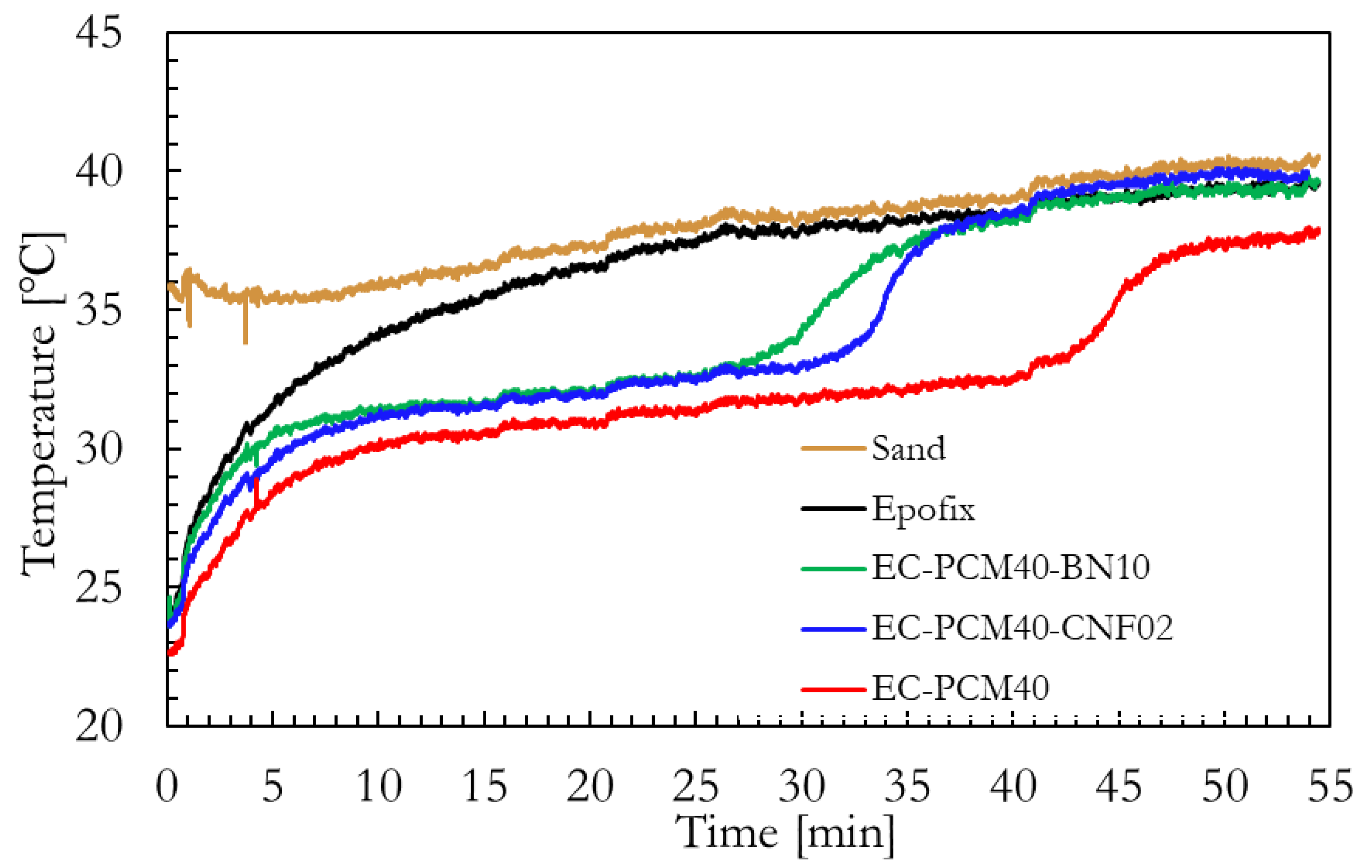
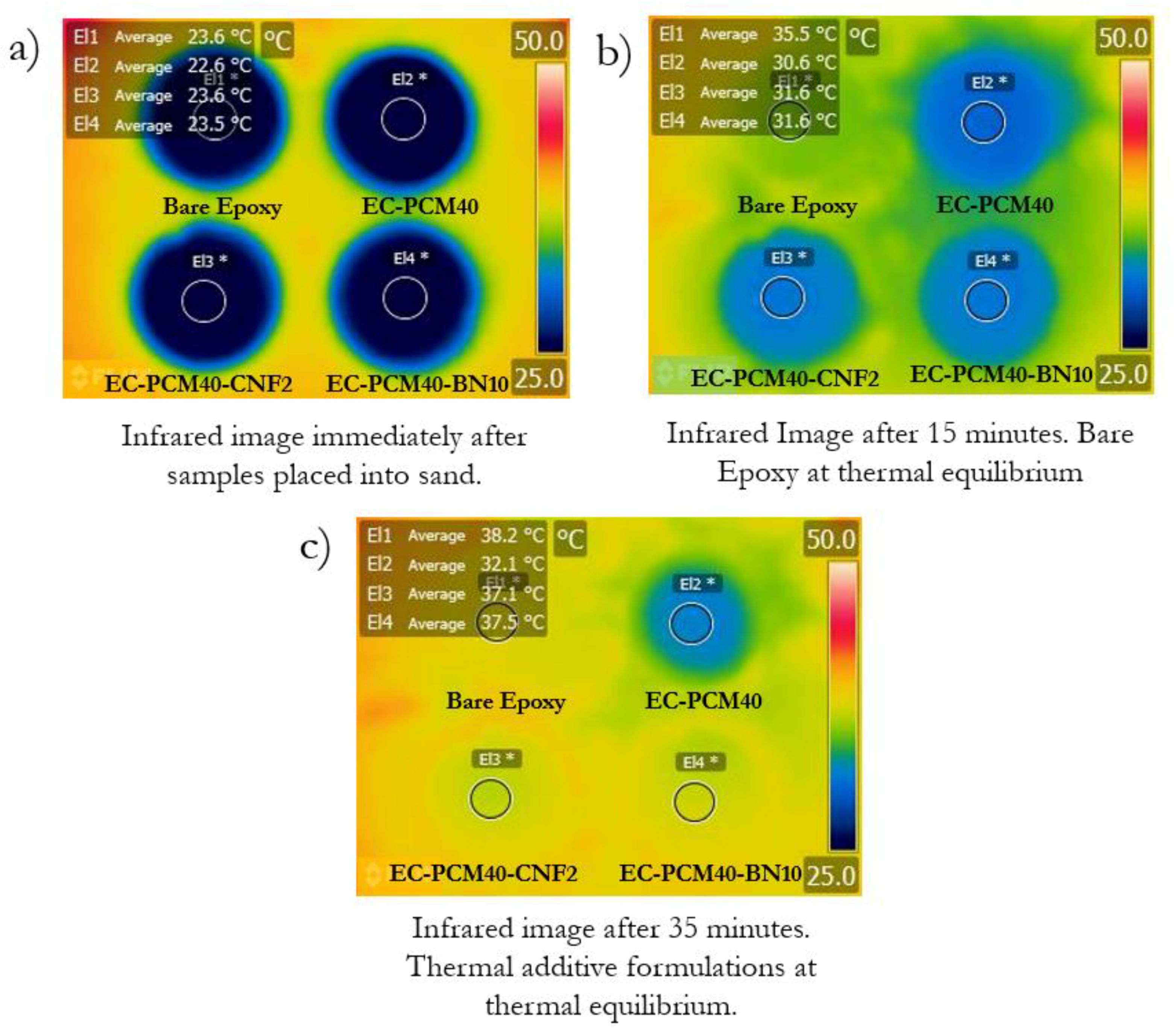
2.3. Thermal Conductivity and Thermal Energy Storage Characteristics
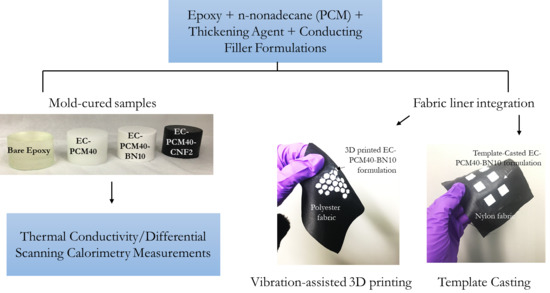
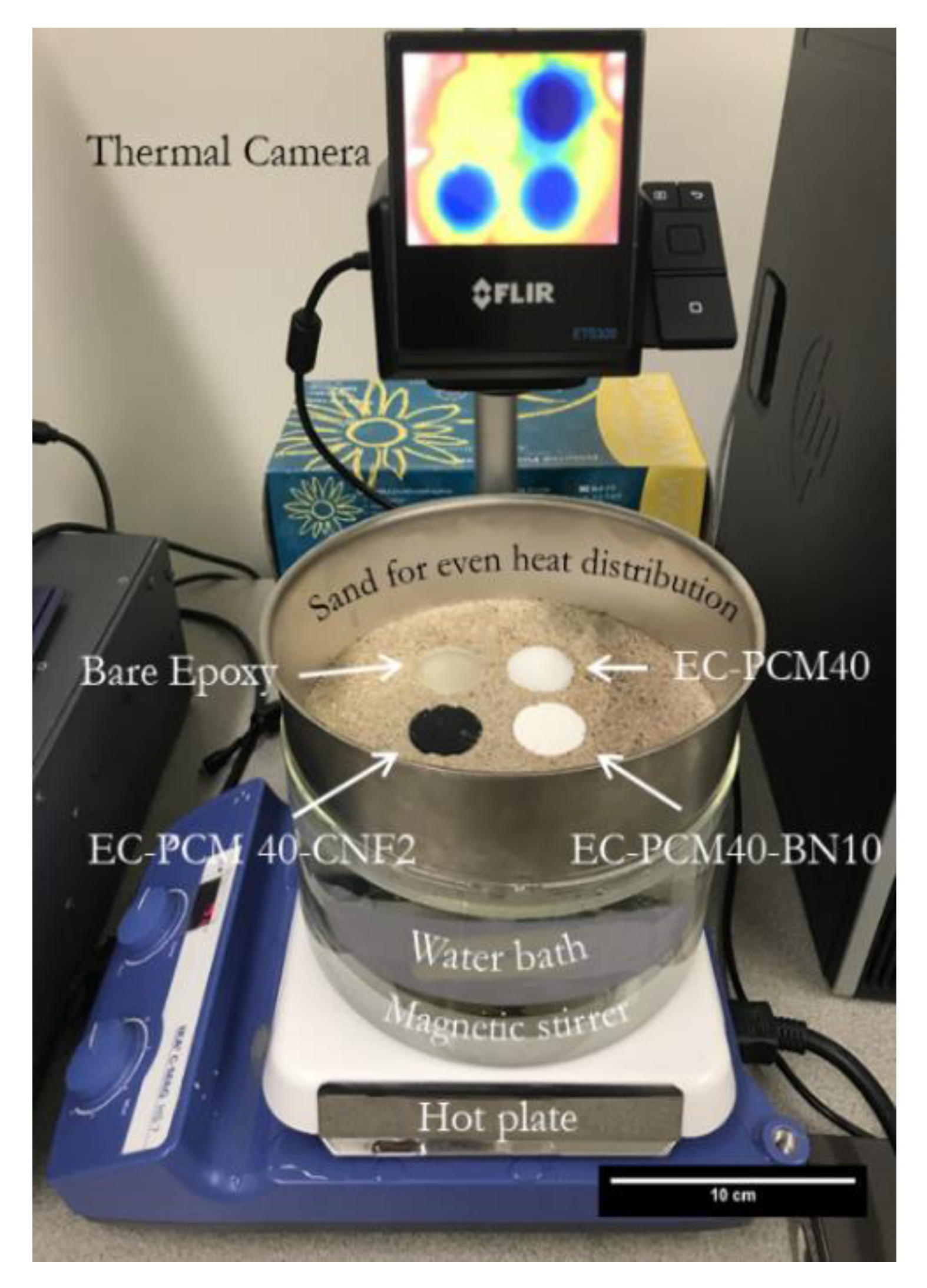
3. Materials and Methods
3.1. Precursor Materials
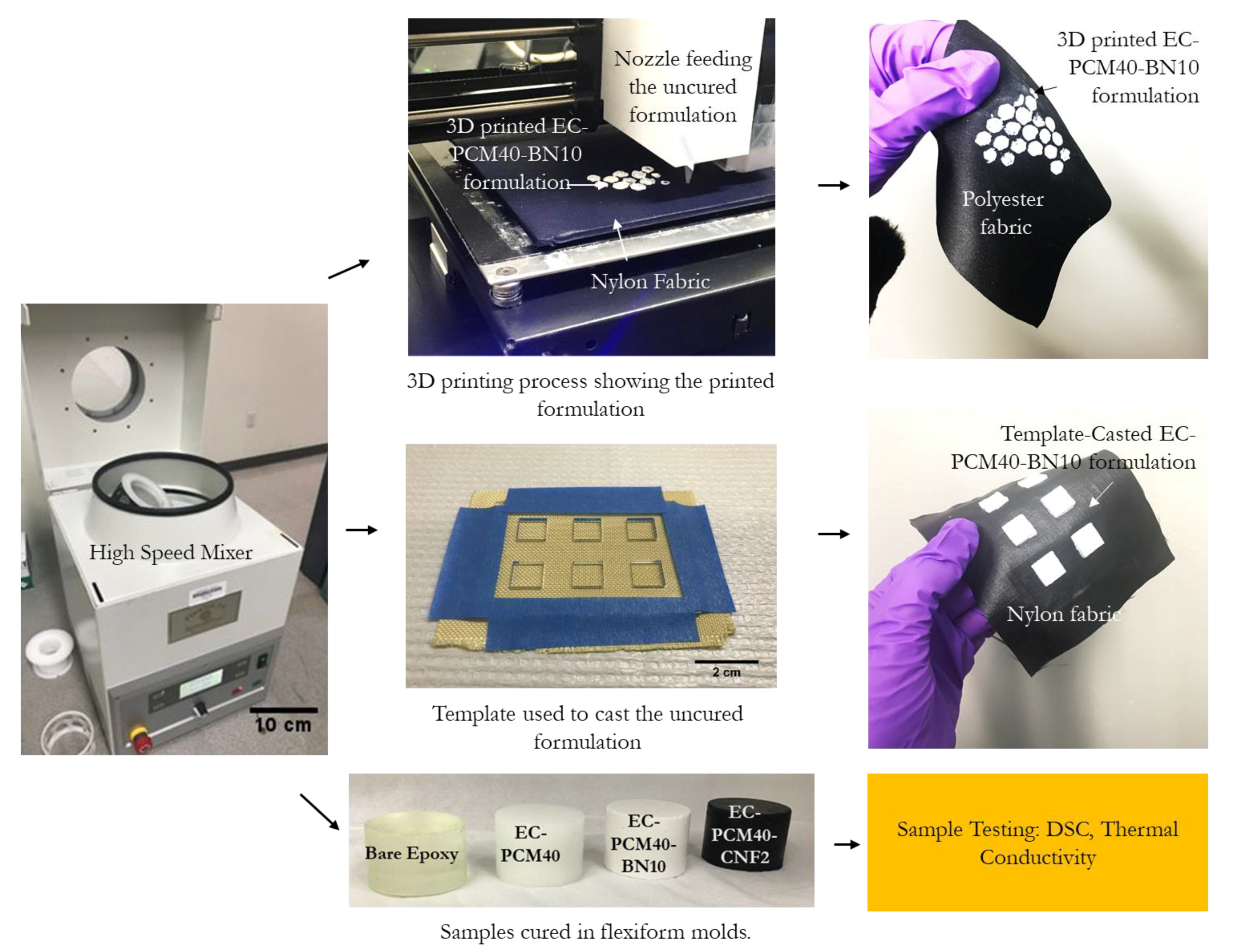
3.2. Fabrication of Epoxy–Carbopol–PCM Conducting Agent Composites
3.3. Characterization and Testing
3.4. D Printing Conditions
3.5. Template Casting
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Arce, M.; Alvarez Feijoo, M.; Suarez Garcia, A.; Luhrs, C. Novel Formulations of Phase Change Materials—Epoxy Composites for Thermal Energy Storage. Materials 2018, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Kim, S.; Drzal, L.T. High latent heat storage and high thermal conductive phase change materials using exfoliated graphite nanoplatelets. Solar Energy Materials and Solar Cells 2009, 93, 136–142. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Nutt, S. Carbon nanotube/paraffin/montmorillonite composite phase change material for thermal energy storage. Solar Energy 2017, 146, 1–7. [Google Scholar] [CrossRef]
- Vélez, C.; Khayet, M.; De Zárate, J.O. Temperature-dependent thermal properties of solid/liquid phase change even-numbered n-alkanes: N-Hexadecane, n-octadecane and n-eicosane. Appl. Energy 2015, 143, 383–394. [Google Scholar] [CrossRef]
- Liu, Z.; Chung, D.D. Boron nitride particle filled paraffin wax as a phase-change thermal interface material. J. Electron. Packag. 2006, 128, 319–323. [Google Scholar] [CrossRef]
- Vélez, C.; De Zárate, J.M.O.; Khayet, M. Thermal properties of n-pentadecane, n-heptadecane and n-nonadecane in the solid/liquid phase change region. Int. J. Therm. Sci. 2015, 94, 139–146. [Google Scholar] [CrossRef]
- Karaipekli, A.; Biçer, A.; Sarı, A.; Tyagi, V.V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Lin, C.; Rao, Z. Thermal conductivity enhancement of paraffin by adding boron nitride nanostructures: A molecular dynamics study. Appl. Therm. Eng. 2017, 110, 1411–1419. [Google Scholar] [CrossRef]
- Liu, L.; Su, D.; Tang, Y.; Fang, G. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2016, 62, 305–317. [Google Scholar] [CrossRef]
- Fan, L.; Khodadadi, J.M. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2011, 15, 24–46. [Google Scholar] [CrossRef]
- Qian, Z.; Shen, H.; Fang, X.; Fan, L.; Zhao, N.; Xu, J. Phase change materials of paraffin in h-BN porous scaffolds with enhanced thermal conductivity and form stability. Energy Build. 2018, 158, 1184–1188. [Google Scholar] [CrossRef]
- Thapa, S.; Chukwu, S.; Khaliq, A.; Weiss, L. Fabrication and analysis of small-scale thermal energy storage with conductivity enhancement. Energy Convers. Manag. 2014, 79, 161–170. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J.; Yan, J. Enhanced thermal conductivity and thermal performance of form-stable composite phase change materials by using β-Aluminum nitride. Appl. Energy 2009, 86, 1196–1200. [Google Scholar] [CrossRef]
- Chintakrinda, K.; Weinstein, R.D.; Fleischer, A.S. A direct comparison of three different material enhancement methods on the transient thermal response of paraffin phase change material exposed to high heat fluxes. Int. J. Therm. Sci. 2011, 50, 1639–1647. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, S.; Wei, X.; Liu, Z.; Guo, Q.; Shi, J.; Liu, L. Heat transfer enhancement of paraffin wax using compressed expanded natural graphite for thermal energy storage. Carbon 2010, 48, 300–304. [Google Scholar] [CrossRef]
- Elgafy, A.; Lafdi, K. Effect of carbon nanofiber additives on thermal behavior of phase change materials. Carbon 2005, 43, 3067–3074. [Google Scholar] [CrossRef]
- Jeong, S.G.; Chung, O.; Yu, S.; Kim, S.; Kim, S. Improvement of the thermal properties of Bio-based PCM using exfoliated graphite nanoplatelets. Sol. Energy Mater. Sol. Cells 2013, 117, 87–92. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P. Morphologies and thermal characterization of paraffin/carbon foam composite phase change material. Sol. Energy Mater. Sol. Cells 2013, 117, 451–461. [Google Scholar] [CrossRef]
- Babaei, H.; Keblinski, P.; Khodadadi, J.M. Thermal conductivity enhancement of paraffins by increasing the alignment of molecules through adding CNT/graphene. Int. J. Heat Mass Transf. 2013, 58, 209–216. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, C.; Hu, S.; Yu, X. The experimental exploration of carbon nanofiber and carbon nanotube additives on thermal behavior of phase change materials. Sol. Energy Mater. Sol. Cells 2011, 95, 1208–1212. [Google Scholar] [CrossRef]
- Nan, C.W.; Birringer, R.; Clarke, D.R.; Gleiter, H. Effective thermal conductivity of particulate composites with interfacial thermal resistance. J. Appl. Phys. 1997, 81, 6692–6699. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Xin, Z. Thermal properties of paraffin based composites containing multi-walled carbon nanotubes. Thermochim. Acta 2009, 488, 39–42. [Google Scholar] [CrossRef]
- Kim, D.; Kirakosyan, A.; Lee, J.W.; Jeong, J.R.; Choi, J. Flexible h-BN foam sheets for multifunctional electronic packaging materials with ultrahigh thermostability. Soft Matter 2018, 14, 4204–4212. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron nitride nanotubes and nanosheets. Acs Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Golberg, D.; Bando, Y.; Tang, C.C.; Zhi, C.Y. Boron nitride nanotubes. Adv. Mater. 2007, 19, 2413–2432. [Google Scholar] [CrossRef]
- Agrawal, R.; Nieto, A.; Chen, H.; Mora, M.; Agarwal, A. Nanoscale damping characteristics of boron nitride nanotubes and carbon nanotubes reinforced polymer composites. Acs Appl. Mater. Interfaces 2013, 5, 12052–12057. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Terao, T.; Tang, C.; Kuwahara, H.; Golberg, D. Towards thermoconductive, electrically insulating polymeric composites with boron nitride nanotubes as fillers. Adv. Funct. Mater. 2009, 19, 1857–1862. [Google Scholar] [CrossRef]
- Cho, H.B.; Tokoi, Y.; Tanaka, S.; Suematsu, H.; Suzuki, T.; Jiang, W.; Niihara, K.; Nakayama, T. Modification of BN nanosheets and their thermal conducting properties in nanocomposite film with polysiloxane according to the orientation of BN. Compos. Sci. Technol. 2011, 71, 1046–1052. [Google Scholar] [CrossRef]
- Li, T.L.; Hsu, S.L.C. Enhanced thermal conductivity of polyimide films via a hybrid of micro-and nano-sized boron nitride. J. Phys. Chem. B 2010, 114, 6825–6829. [Google Scholar] [CrossRef]
- Yung, K.C.; Liem, H. Enhanced thermal conductivity of boron nitride epoxy-matrix composite through multi-modal particle size mixing. J. Appl. Polym. Sci. 2007, 106, 3587–3591. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C.; Jiang, P.; Golberg, D.; Bando, Y.; Tanaka, T. Polyhedral oligosilsesquioxane-modified boron nitride nanotube based epoxy nanocomposites: An ideal dielectric material with high thermal conductivity. Adv. Funct. Mater. 2013, 23, 1824–1831. [Google Scholar] [CrossRef]
- Su, D.; Jia, Y.; Alva, G.; Tang, F.; Fang, G. Preparation and thermal properties of n–octadecane/stearic acid eutectic mixtures with hexagonal boron nitride as phase change materials for thermal energy storage. Energy Build. 2016, 131, 35–41. [Google Scholar] [CrossRef]
- Fang, X.; Fan, L.W.; Ding, Q.; Yao, X.L.; Wu, Y.Y.; Hou, J.F.; Wang, X.; Yu, Z.T.; Cheng, G.H.; Hu, Y.C. Thermal energy storage performance of paraffin-based composite phase change materials filled with hexagonal boron nitride nanosheets. Energy Convers. Manag. 2014, 80, 103–109. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, L.; Luo, W.; Wan, J.; Dai, J.; Han, X.; Fu, K.; Henderson, D.; Yang, B.; Hu, L. Thermally conductive, dielectric PCM–boron nitride nanosheet composites for efficient electronic system thermal management. Nanoscale 2016, 8, 19326–19333. [Google Scholar] [CrossRef] [PubMed]
- de Zárate, J.M.O.; Hita, J.L.; Khayet, M.; Legido, J.L. Measurement of the thermal conductivity of clays used in pelotherapy by the multi-current hot-wire technique. Appl. Clay Sci. 2010, 50, 423–426. [Google Scholar]
- Shaikh, S.; Lafdi, K.; Hallinan, K. Carbon nanoadditives to enhance latent energy storage of phase change materials. J. Appl. Phys. 2008, 103, 094302. [Google Scholar] [CrossRef]
- Epofix Cold-Setting Embedding Resin. Available online: https://www.emsdiasum.com/microscopy/technical/datasheet/1232.aspx (accessed on 15 May 2019).
- Gunduz, I.E.; McClain, M.S.; Cattani, P.; Chiu, G.C.; Rhoads, J.F.; Son, S.F. 3D printing of extremely viscous materials using ultrasonic vibrations. Addit. Manuf. 2018, 22, 98–103. [Google Scholar] [CrossRef]
- McClain, M.S.; Gunduz, I.E.; Son, S.F. Additive manufacturing of ammonium perchlorate composite propellant with high solids loadings. Proc. Combust. Inst. 2019, 37, 3135–3142. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
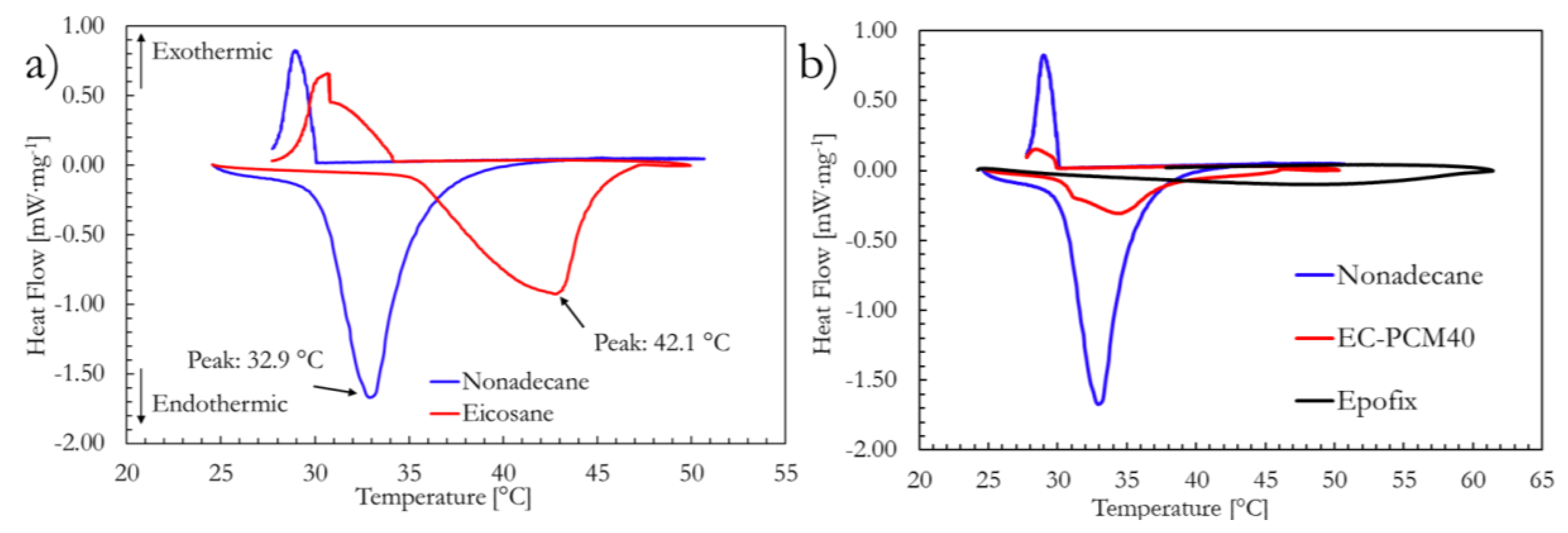
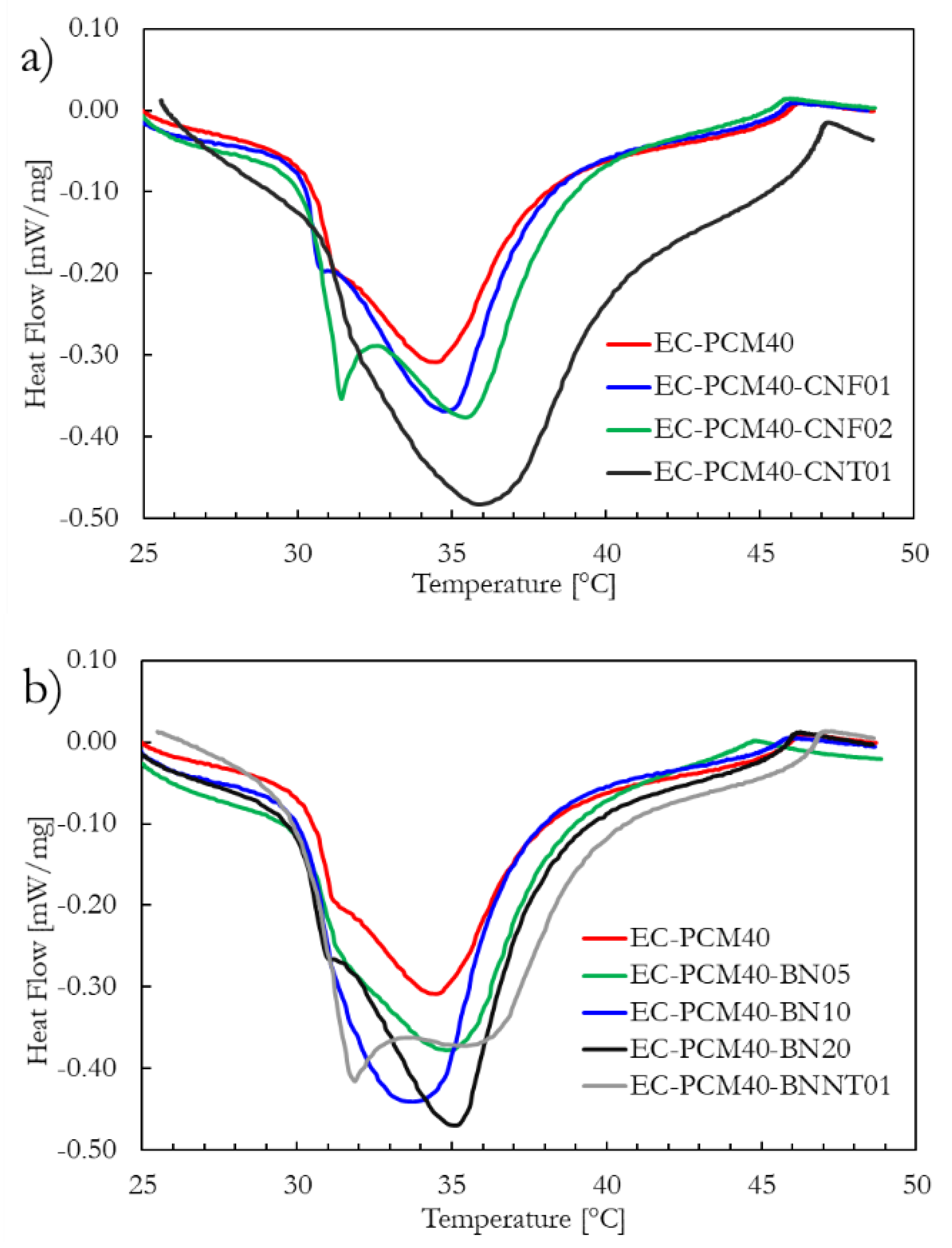

| Sample | Latent Heat (J∙g−1) | Transition Temperature (°C) |
|---|---|---|
| Nonadecane (C-19) | 160.2 | 32.9 |
| EC-PCM40 | 27.2 | 34.4 |
| EC-PCM40-CNF01 | 27.1 | 34.6 |
| EC-PCM40-CNF02 | 39.6 | 35.4 |
| EC-PCM40-CNT01 | 74.6 | 35.8 |
| EC-PCM40-BN05 | 32.2 | 34.8 |
| EC-PCM40-BN10 | 41.6 | 33.7 |
| EC-PCM40-BN20 | 42.6 | 35.1 |
| EC-PCM40-BNNT01 | 59.5 | 35.5 |
| Matrix Material | Filler | Filler Fraction | Thermal Conductivity | Ref |
|---|---|---|---|---|
| Paraffin | h-BN | 10 wt.% | ~0.2 W∙mK−1 | 14 |
| Paraffin | Porous h-BN scaffolds prepared by dispersion in polyvinylpyrrolidone (PVP), Na2SiO3, followed by freeze drying and calcination | 10 wt.% | 0.45 W∙mK−1 | 14 |
| Poly (vinyl butyral) (PVB) | BNNT | 18 wt.% | ~1.81 W∙mK−1 | 30 |
| Poly (siloxane) | BN | 30 vol.% | ~0.28 W∙mK−1 | 31 |
| Poly (imide) | BN micro and nanopowders | 10 wt.% | ~0.26 W∙mK−1 | 32 |
| Poly (imide) | BN micro and nanopowders | 30 wt.% | ~1.2 W∙mK−1 | 32 |
| Paraffin | h-BN nanosheets | 10 wt.% | ~0.48 W∙mK−1 | 37 |
| Paraffin–epoxy | BN particles | 10 wt.% | ~0.415 W∙mK−1 | This work |
| Sample | Filler | Epoxy (Resin + Hardener) (wt.%) | Carbopol (wt.%) | PCM (n-nonadecane) (wt.%) | Filler (wt.%) |
|---|---|---|---|---|---|
| Bare epoxy | None | 100 | 0 | 0 | 0 |
| EC-PCM40 | None | 55 | 5 | 40 | 0 |
| EC-PCM40-CNF01 | CNF | 54 | 5 | 40 | 1 |
| EC-PCM40-CNF02 | CNF | 53 | 5 | 40 | 2 |
| EC-PCM40-CNT01 | CNT | 54 | 5 | 40 | 1 |
| EC-PCM40-BN05 | BN | 50 | 5 | 40 | 5 |
| EC-PCM40-BN10 | BN | 45 | 5 | 40 | 10 |
| EC-PCM40-BN20 | BN | 35 | 5 | 40 | 20 |
| EC-PCM40-BNNT01 | BNNT | 54 | 5 | 40 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, R.; Hanna, J.; Gunduz, I.E.; Luhrs, C.C. Epoxy–PCM Composites with Nanocarbons or Multidimensional Boron Nitride as Heat Flow Enhancers. Molecules 2019, 24, 1883. https://doi.org/10.3390/molecules24101883
Agrawal R, Hanna J, Gunduz IE, Luhrs CC. Epoxy–PCM Composites with Nanocarbons or Multidimensional Boron Nitride as Heat Flow Enhancers. Molecules. 2019; 24(10):1883. https://doi.org/10.3390/molecules24101883
Chicago/Turabian StyleAgrawal, Richa, Joshua Hanna, I. Emre Gunduz, and Claudia C. Luhrs. 2019. "Epoxy–PCM Composites with Nanocarbons or Multidimensional Boron Nitride as Heat Flow Enhancers" Molecules 24, no. 10: 1883. https://doi.org/10.3390/molecules24101883
APA StyleAgrawal, R., Hanna, J., Gunduz, I. E., & Luhrs, C. C. (2019). Epoxy–PCM Composites with Nanocarbons or Multidimensional Boron Nitride as Heat Flow Enhancers. Molecules, 24(10), 1883. https://doi.org/10.3390/molecules24101883