Novel Hexadeca-Substituted Metal Free and Zinc(II) Phthalocyanines; Design, Synthesis and Photophysicochemical Properties
Abstract
:1. Introduction
2. Results and Discussion
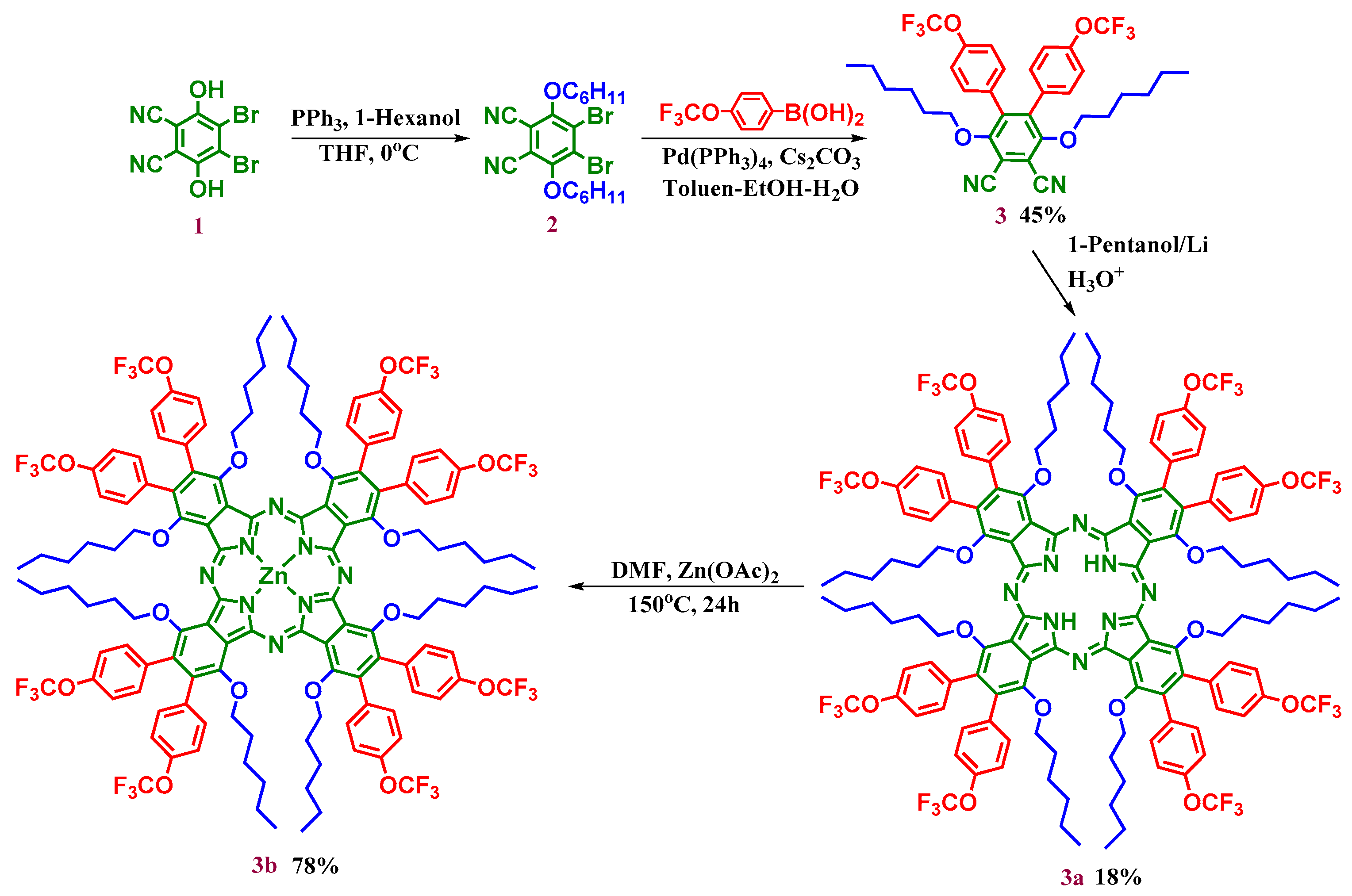
2.1. Synthesis and Characterization
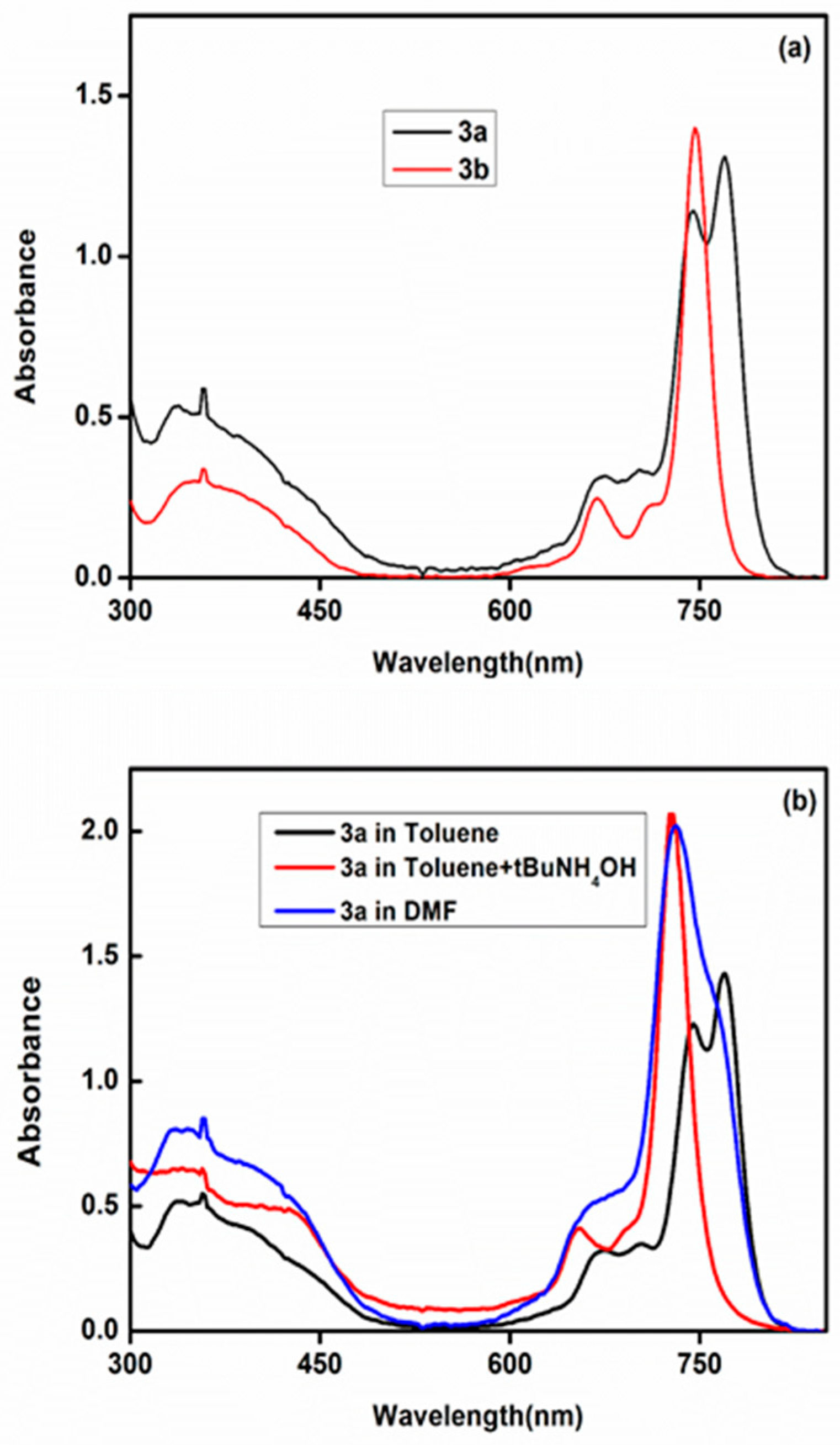
2.2. Electronic Absorption Spectra
2.3. Aggregation Studies
2.4. Fluorescence Studies
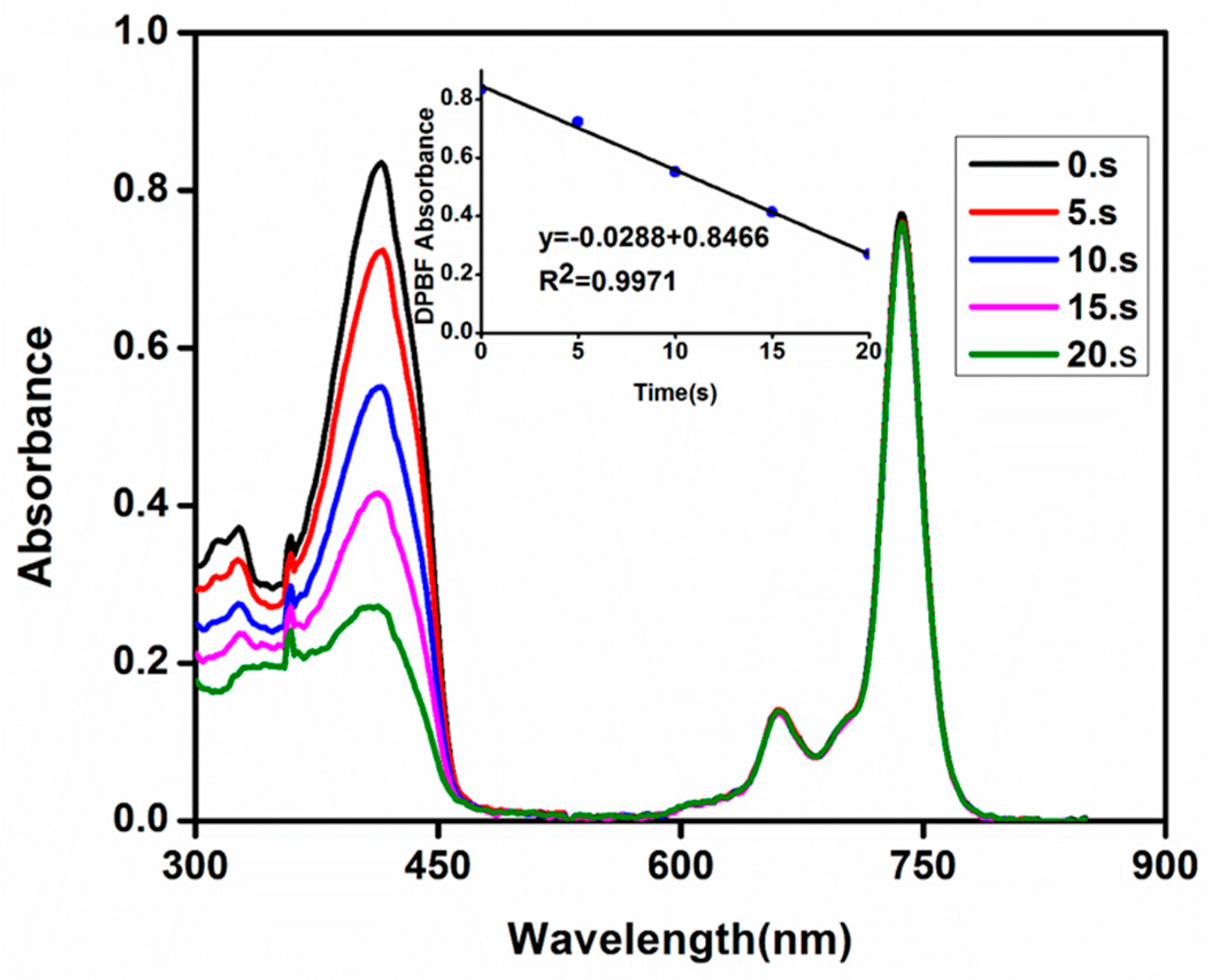
2.5. Singlet Oxygen Generation Measurements
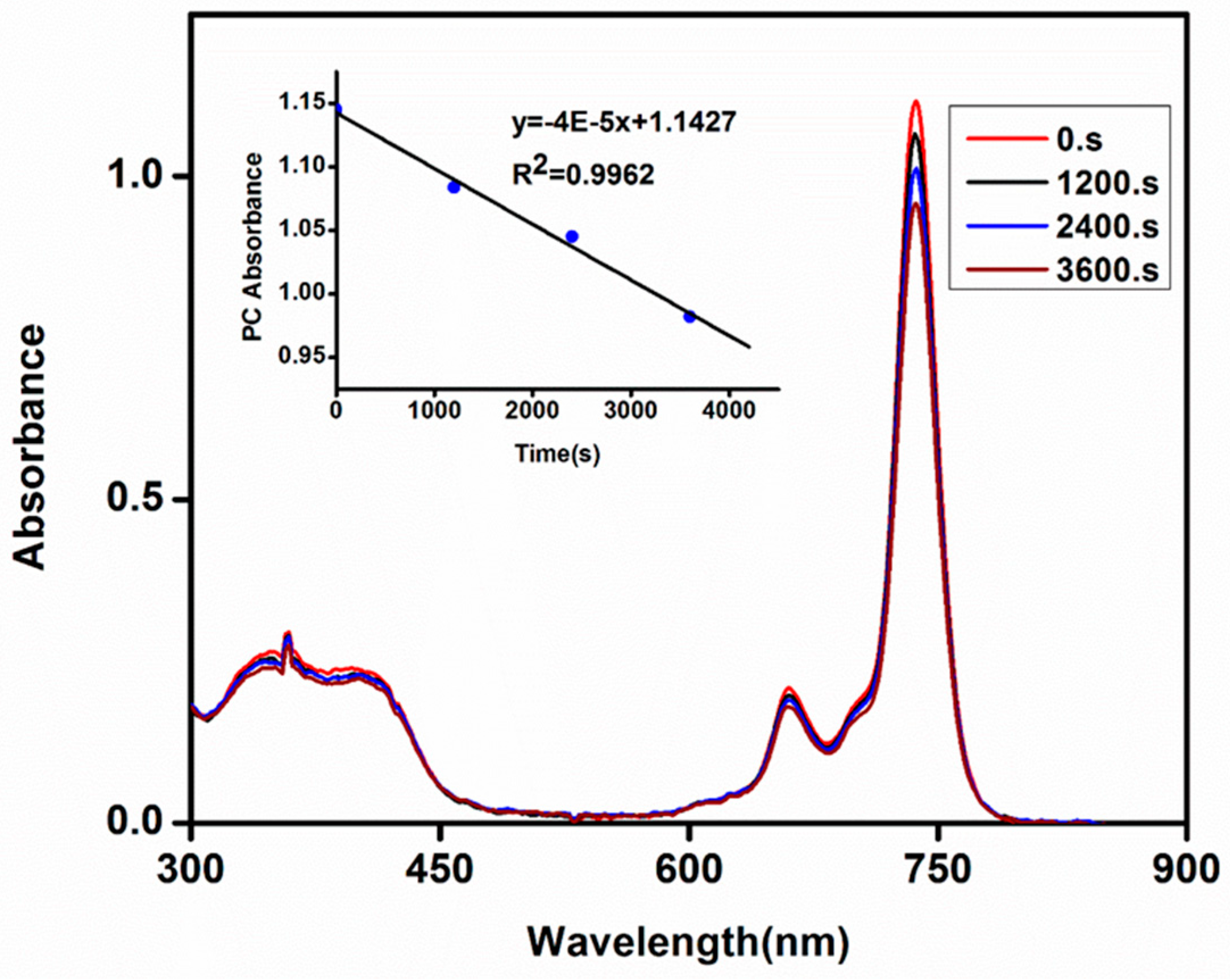
2.6. Photodegradation Studies
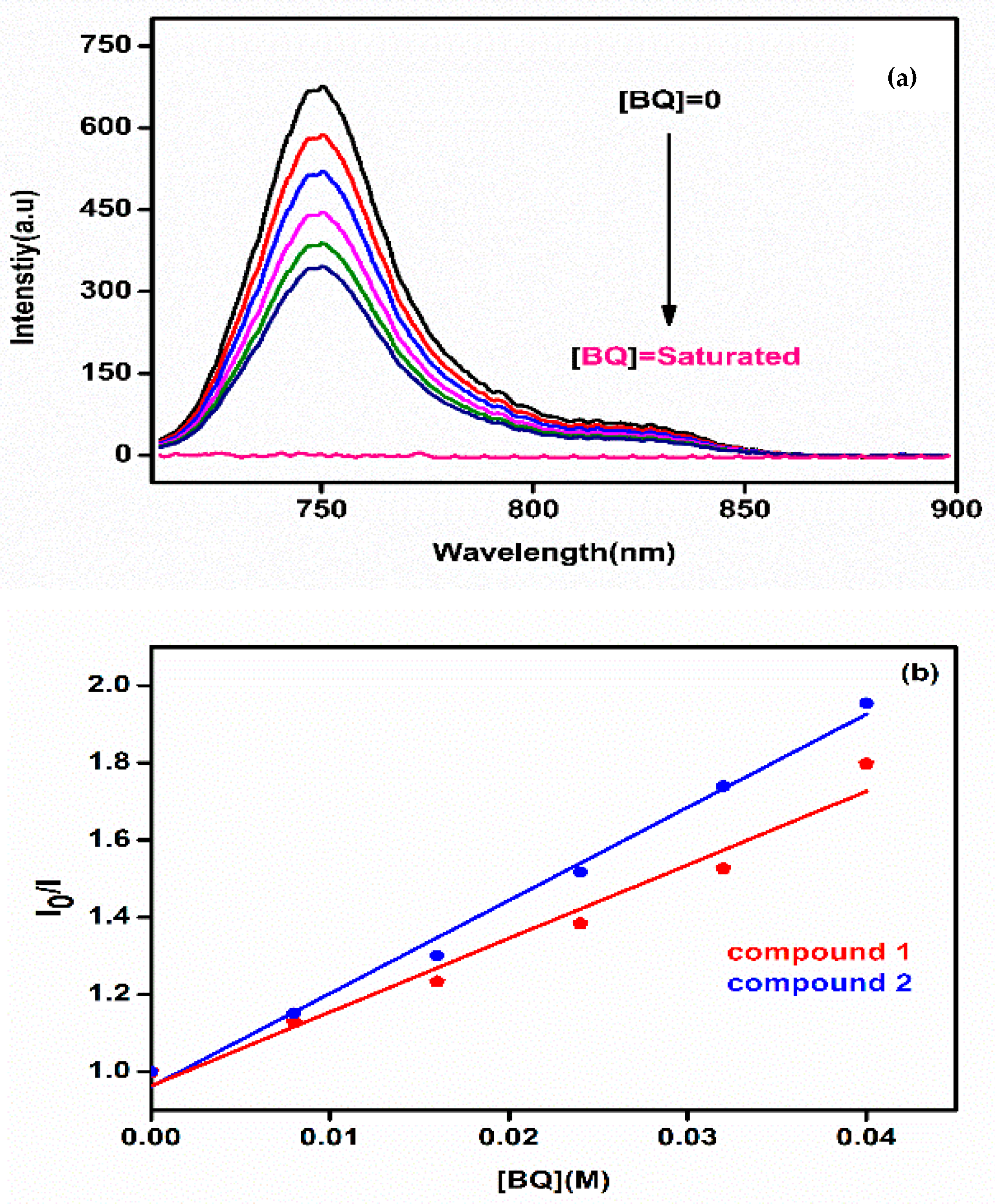
2.7. Fluorescence Quenching Studies by 1,4-Benzoquinone
3. Materials and Methods
3.1. Materials
3.2. Equipment
3.3. Photophysical and Photochemical Parameters
3.3.1. Fluorescence Quantum Yields and Lifetimes
3.3.2. Singlet Oxygen Quantum Yields
3.3.3. Photodegradation Quantum Yields
3.3.4. Fluorescence Quenching by 1,4-Benzoquinone (BQ)
3.4. Synthesis
3.4.1. 4,5-Bis(4-trifloromethoxyphenyl)-3,6-bis(hexyloxy)phthalonitrile (3)
3.4.2. 1,4,8,11,15,18,22,25-Octakis(hexyloxy)-2,3,9,10,16,17,23,24-octakis(4-trifloro methoxyphenyl) phthalocyanine (3a)
3.4.3. 1,4,8,11,15,18,22,25-Octakis(hexyloxy)-2,3,9,10,16,17,23,24-octakis(4-trifloro methoxyphenyl) phthalocyaninato zinc(II) (3b)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Leznoff, C.C.; Lever, A.B.P. Phthalocyanines: Properties and Applications; Wiley-VCH: New York, NY, USA, 1989; Volume 3. [Google Scholar]
- Bouvet, M.; Gaudillat, P.; Suisse, J.M. Phthalocyanine-based hybrid materials for chemosensing. J. Porphyr. Phthalocyanines 2013, 17, 913–919. [Google Scholar] [CrossRef]
- Zhao, J.L.; Guo, S.H.; Qiu, J.; Gou, X.F.; Hua, C.W.; Chen, B. Iron(III) phthalocyanine-chloride-catalyzed synthesis of sulfones from sulfonylhydrazones. Tetrahedron Lett. 2016, 57, 2375–2378. [Google Scholar] [CrossRef]
- Sergeev, S.; Pouzet, E.; Debever, O.; Levin, J.; Gierschner, J.; Cornil, J.; Aspe, R.G.; Geerts, Y.H. Liquid crystalline octaalkoxycarbonyl phthalocyanines: Design, synthesis, electronic structure, self-aggregation and mesomorphism. Mater. Chem. 2007, 17, 1777–1784. [Google Scholar] [CrossRef]
- Al-Raqa, S.; Köksoy, B.; Durmuş, M. A novel lutetium(III) acetate phthalocyanine directly substituted with N.,N’-dimethylaminophenyl groups via CC bonds and its water-soluble derivative for photodynamic therapy. Tetrahedron Lett. 2017, 58, 685–689. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Wang, C.; Zhu, C.; Zhang, Z.; Xue, J. Preparation and in vitro photodynamic activity of glucosylated zinc(II) phthalocyanines as underlying targeting photosensitizers. Molecules 2017, 22, 845. [Google Scholar] [CrossRef] [PubMed]
- Tritsch, J.R.; Chan, W.L.; Wu, X.; Monahan, N.R.; Zhu, X.-Y. Harvesting singlet fission for solar energy conversion via triplet energy transfer. Nat. Comm. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Maksheed, S.; Al-Sawah, M.; Samuel, J.; Manaa, H. Synthesis, characterization and nonlinear optical properties of nonaggregating hexadeca-substituted phthalocyanines. Tetrahedron Lett. 2009, 50, 165–168. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Yan, X.; Wang, J.; Shi, J.; Yan, D. Phthalocyanine composites as high-mobility semiconductors for organic thin-film transistors. Adv. Mat. 2005, 17, 1191–1193. [Google Scholar] [CrossRef]
- Usol’tseva, N.V.; Smirnova, A.I.; Kazaka, A.V.; Giricheva, N.I.; Galanin, N.E.; Shaposhnikov, G.P.; Bodnarchuk, V.V.; Yablonskii, S.V. Mix-substituted phthalocyanines of “push–pull”-type and their metal complexes as prospective nanostructured materials for optoelectronics. Opt. Electron. Rev. 2017, 25, 127–136. [Google Scholar] [CrossRef]
- Nyokong, T. Effects of substituents on the photochemical and photophysical properties of main group metal phthalocyanines. Coord. Chem. Rev. 2007, 251, 1707–1722. [Google Scholar] [CrossRef]
- Okura, I. Photosensitization of Porphyrins and Phthalocyanines, 1st ed.; Gordon and Breach Publishers: Tokyo, Japan, 2001. [Google Scholar]
- Al-Raqa, S.Y. The synthesis and photophysical properties of novel, symmetrical, hexadecasubstituted Zn phthalocyanines and related unsymmetrical derivatives. Dyes Pigments 2008, 77, 259–265. [Google Scholar] [CrossRef]
- Atsay, A.; Nar, I.; Hamuryudan, E.; Koçak, M.B.; Gul, A. A honeycomb-like crystalline self-assembled hexadeca-substituted phthalocyanine. ChemistrySelect 2017, 2, 9233–9235. [Google Scholar] [CrossRef]
- Lyubimtsev, A.; Iqbal, Z.; Crucius, G.; Syrbu, S.; Taraymovich, E.S.; Ziegler, T.; Hanack, M. Aggregation behavior and UV-vis spectra of tetra- and octaglycosylated zinc phthalocyanines. J. Porphyr. Phthalocyanines 2011, 15, 39–46. [Google Scholar] [CrossRef]
- Khanh, B.V.; Phung, T.K. cis-Cyclooctene epoxidation catalyzed by bulk metallophthalocyanines, metallohexadecafluorophthalocyanines and hollow silica-supported metallohexadecafluorophthalocyanine. J. Ind. Eng. Chem. 2016, 40, 40–46. [Google Scholar]
- Mana, H.; Al Mulla, A.; Makhseed, S.; Al-Sawah, M.; Samuel, J. Fluorescence and nonlinear optical properties of non-aggregating hexadeca-substituted phthalocyanine. J. Opt. Mat. 2009, 32, 108–114. [Google Scholar] [CrossRef]
- Gaffo, L.; Zucolotto, V.; Cordeiro, M.R.; Moreira, W.C.; Oliveira, O.N.; Cerdeira, F.; Brasil, M.J.S.P. Structural aspects of Langmuir–Blodgett and cast films of zinc phthalocyanine and zinc hexadecafluorophthalocyanine. Thin Solid Films 2007, 515, 7307–7312. [Google Scholar] [CrossRef]
- Kurt, O.; Ozcesmeci, I.; Sesalan, B.S.; Kocak, M.B. The synthesis and investigation of binding properties of a new water soluble hexadeca zinc(II) phthalocyanine with bovine serum albumin and DNA. New J. Chem. 2015, 39, 5767–5775. [Google Scholar] [CrossRef]
- Atsay, A.; Gül, A.; Koçak, M.B. A new hexadeca substituted non-aggregating zinc phthalocyanine. Dyes Pigments 2014, 100, 177–183. [Google Scholar] [CrossRef]
- Al-Raqa, S.Y.; Messali, M.; Al-Refae, S.; Ghanem, B.; Moussa, Z.; Ahmed, S.; El-Khouly, M.; Fukuzumi, S. Synthesis, electrochemical, and photophysical studies of hexadecachlorinatedphthalocyaninato zinc(II). Dyes Pigments 2011, 91, 231–236. [Google Scholar] [CrossRef]
- Cook, M.J.; Heeney, M.J. Phthalocyaninodehydroannulenes. Chem. Eur. J. 2000, 6, 3958–3967. [Google Scholar] [CrossRef]
- Catalán, J.; Díaz, C.; López, V.; Pérez, P.; De Paz, J.-L.G.; Rodríguez, J.G. A generalized solvent basicity scale: The solvatochromism of 5-nitroindoline and its homomorph 1-methyl-5-nitroindoline. Liebigs. Ann. 1996, 1785–1794. [Google Scholar] [CrossRef]
- Hans, E.; Roeland, J.M.N. Molecular materials based on crown ether functionalized phthalocyanines. J. Porphyr. Phthalocyanines 2000, 4, 454–459. [Google Scholar]
- Can, O.S.; Kaya, E.N.; Durmuş, M.; Bulut, M. High photosensitized singlet oxygen generating zinc(II) and indium(III) acetate phthalocyanines containing 6,8-di-tert-butyl-3-(p-oxyphenyl)coumarin groups. J. Photochem. Photobiol. A 2016, 317, 56–67. [Google Scholar]
- Zorlu, Y.; Dumoulin, F.; Durmuş, M.; Ahsen, V. Comparative studies of photophysical and photochemical properties of solketal substituted platinum(II) and zinc(II) phthalocyanine sets. Tetrahedron 2010, 66, 3248–3258. [Google Scholar] [CrossRef]
- Durmuş, M. Photochemical and Photophysical Characterization. In Photosensitizers in Medicine, Environment, and Security; Nyokong, T., Ahsen, V., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Smith, K. The Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Eds.; Academic Press: New York, NY, USA, 2010. [Google Scholar]
- Dutt, G.B.; Periasamy, N. Electron-transfer distance in intermolecular diffusion-limited reactions. J. Chem. Soc. Faraday Trans. 1991, 87, 3815–3820. [Google Scholar] [CrossRef]
- Forgues, F.S.; Lavabre, D. Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products. J. Chem. Educ. 1999, 76, 1260–1264. [Google Scholar] [CrossRef]
- Maree, D.; Nyokong, T.; Suhling, K.; Phillips, D. Effects of axial ligands on the photophysical properties of silicon octaphenoxyphthalocyanine. J. Porphyr. Phthalocyanines 2002, 6, 373–376. [Google Scholar] [CrossRef]
- Spiller, W.; Kliesch, H.; Wöhrle, D.; Hackbarth, S.; Roder, B.; Schnurpfeil, G. Singlet oxygen quantum yields of different photosensitizers in polar solvents and micellar solutions. J. Porphyr. Phthalocyanines 1998, 2, 145–158. [Google Scholar] [CrossRef]
- Rose, J. Advanced Physico-Chemical Experiments; Sir Isaac Pitman & Sons Ltd.: Billerica, MA, USA, 1964. [Google Scholar]
Sample Availability: Samples of the compounds 1, 2, 3, 3a and 3b are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awaji, A.I.; Köksoy, B.; Durmuş, M.; Aljuhani, A.; Alraqa, S.Y. Novel Hexadeca-Substituted Metal Free and Zinc(II) Phthalocyanines; Design, Synthesis and Photophysicochemical Properties. Molecules 2019, 24, 77. https://doi.org/10.3390/molecules24010077
Awaji AI, Köksoy B, Durmuş M, Aljuhani A, Alraqa SY. Novel Hexadeca-Substituted Metal Free and Zinc(II) Phthalocyanines; Design, Synthesis and Photophysicochemical Properties. Molecules. 2019; 24(1):77. https://doi.org/10.3390/molecules24010077
Chicago/Turabian StyleAwaji, Ayoub I., Baybars Köksoy, Mahmut Durmuş, Ateyatallah Aljuhani, and Shaya Y. Alraqa. 2019. "Novel Hexadeca-Substituted Metal Free and Zinc(II) Phthalocyanines; Design, Synthesis and Photophysicochemical Properties" Molecules 24, no. 1: 77. https://doi.org/10.3390/molecules24010077
APA StyleAwaji, A. I., Köksoy, B., Durmuş, M., Aljuhani, A., & Alraqa, S. Y. (2019). Novel Hexadeca-Substituted Metal Free and Zinc(II) Phthalocyanines; Design, Synthesis and Photophysicochemical Properties. Molecules, 24(1), 77. https://doi.org/10.3390/molecules24010077