Optimization of the Steam Explosion Pretreatment Effect on Total Flavonoids Content and Antioxidative Activity of Seabuckthom Pomace by Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Steam Explosion Conditions for SP
2.1.1. Statistical Analysis and Model Fitting
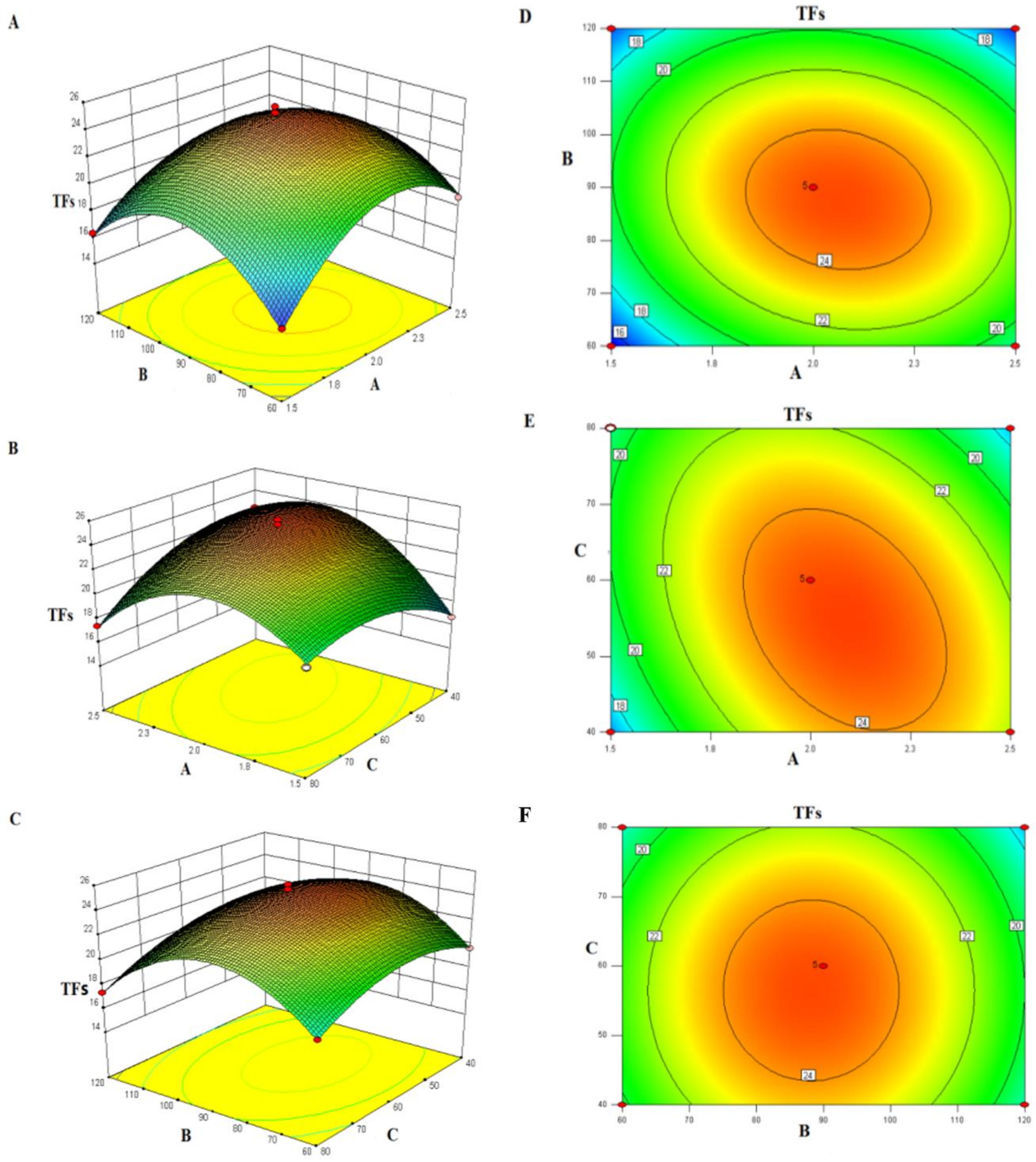
2.1.2. Optimization of Steam Explosion Pretreatment Conditions
2.2. Microstructure Changes of SP after Steam Explosion under the SEM
2.3. The Effect of Steam Explosion on TFs Content and Antioxidation of SP
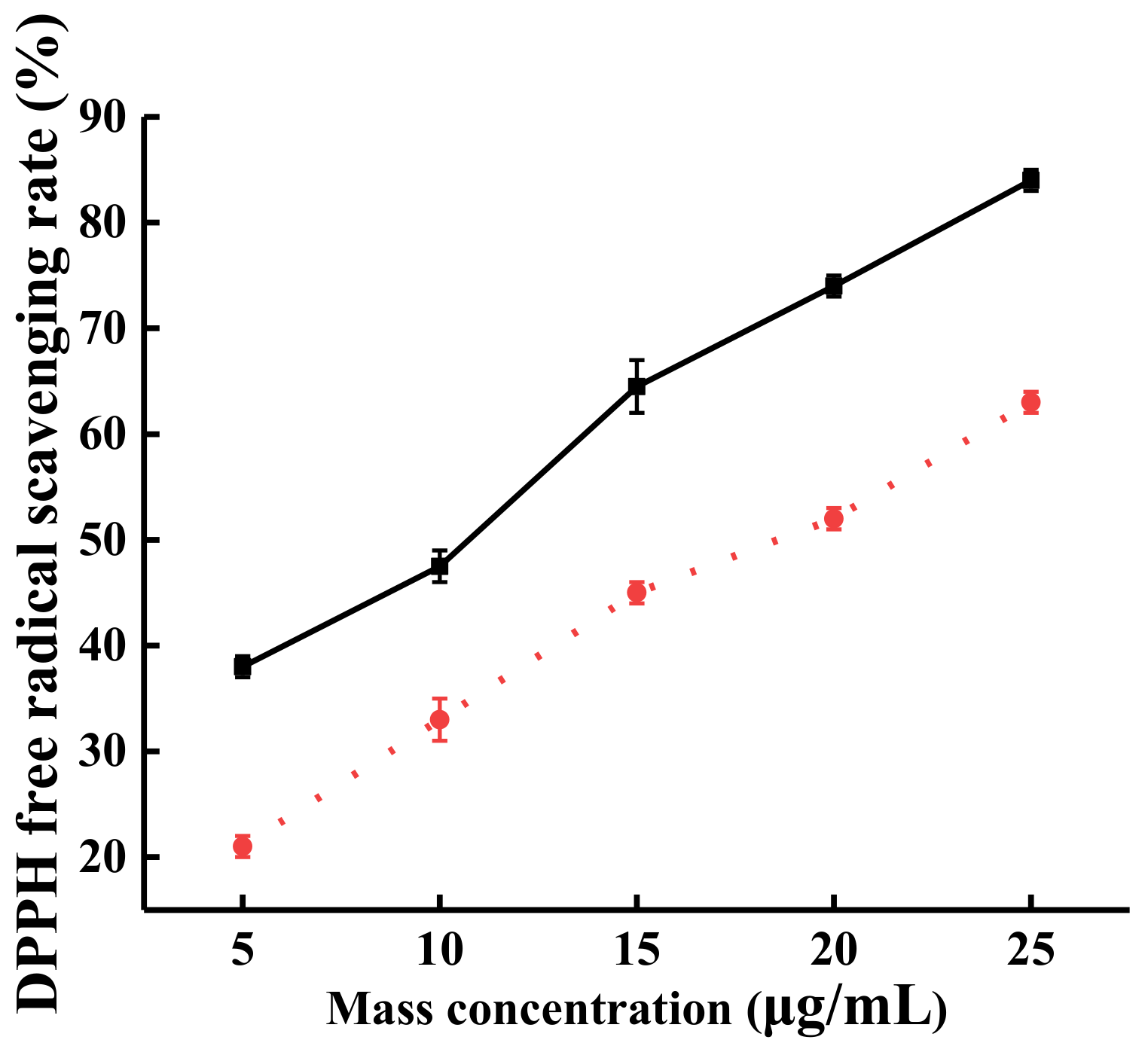
2.3.1. The Effect of Steam Explosion on the Ability of TFs to Scavenge DPPH Free Radical
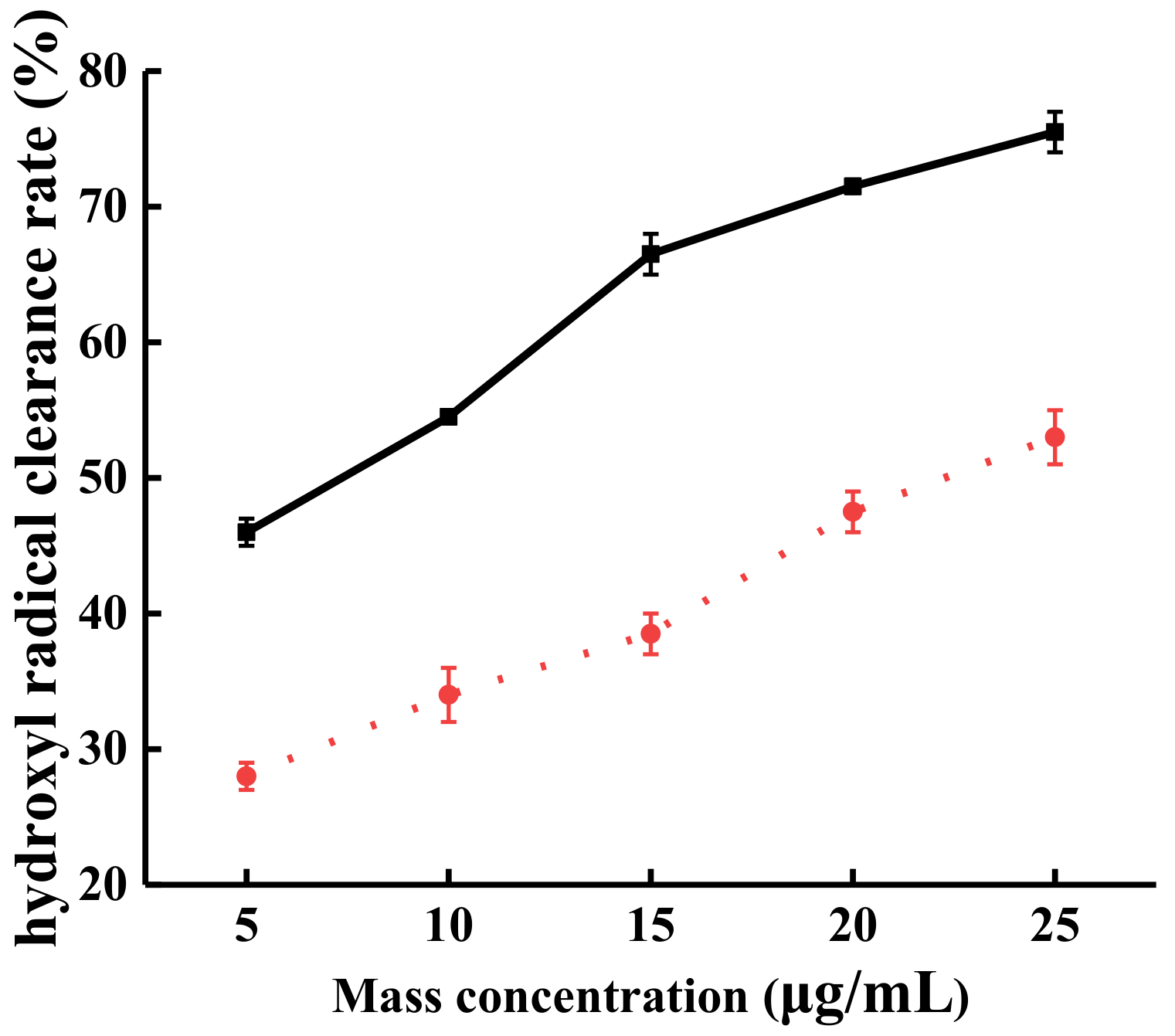
2.3.2. The Effect of Steam Explosion on the Ability of TFs to Scavenge·OH Free Radical
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Pretreatment of SP with Steam Explosion
3.4. Response Surface Experimental Design
3.5. Microstructure Observation of SP
3.6. Preparation of SP Extract
3.7. TFs Content Measurement in Seabuckthom Fruit
3.8. Measurement of DPPH Free Radical Scavenging Ability
3.9. Measurement of ·OH Free Radical Scavenging Ability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dulf, F.V. Fatty acids in berry lipids of six sea buckthorn (Hippophae rhamnoides L. subspecies carpatica) cultivars grown in Romania. Chem. Cent. J. 2012, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S.; Negi, P.S.; Ramteke, R.S. Antioxidant and antibacterial activities of aqueous extract of Seabuckthorn (Hippophae rhamnoides L.) seeds. Fitoterapia 2007, 78, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.C.; Hsu, Y.W.; Tsai, C.F.; Lu, F.J.; Chou, M.C.; Chen, W.K. The in vitro and in vivo antioxidant properties of sea buckthorn (Hippophae rhamnoides L.) seed oil. Food Chem. 2011, 125, 652–659. [Google Scholar] [CrossRef]
- Xue, Y.; Miao, Q.; Zhao, A.; Zheng, Y.; Zhang, Y.; Wang, P. Effects of sea buckthorn (Hippophaë rhamnoides) juice and l-quebrachitol on type 2 diabetes mellitus in db/db mice. J. Funct. Foods. 2015, 16, 223–233. [Google Scholar] [CrossRef]
- Larmo, P.S.; Kangas, A.J.; Soininen, P.; Lehtonen, H.M.; Suomela, J.P.; Yang, B. Effects of sea buckthorn and bilberry on serum metabolites differ according to baseline metabolic profiles in overweight women: A randomized crossover trial. Am. J. Clin. Nutr. 2013, 98, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Sayegh, M.; Miglio, C.; Ray, S. Potential cardiovascular implications of Sea Buckthorn berry consumption in humans. Int. J. Food Sci. Nutr. 2014, 65, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-J.; Kaur, M.; Dhillon, R.S.; Tappia, P.S.; Dhalla, N.S. Health benefits of sea buckthorn for the prevention of cardiovascular diseases. J. Funct. Foods. 2011, 3, 2–12. [Google Scholar] [CrossRef]
- Hou, D.; Wang, D.; Ma, X. Effects of TFs of sea buckthorn (Hippophae rhamnoides L.) on cytotoxicity of NK92-MI cells. Int. J. Immunopathol. Pharmacol. 2017, 6, 5–11. [Google Scholar]
- Jiang, F.; Guan, H.; Liu, D. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food Funct. 2017, 8, 1313–1322. [Google Scholar] [CrossRef]
- Gong, L.-X.; Huang, L.-L.; Zhang, Y. Effect of steam explosion treatment on barley bran phenolic compounds and antioxidant capacity. J. Agric. Food Chem. 2012, 60, 7177–7184. [Google Scholar] [CrossRef]
- Yu, Z.-D.; Zhang, B.-L.; Yu, F.-Q. A real explosion: The requirement of steam explosion pretreatment. Bioresour. Technol. 2012, 121, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-L.; Ke, G.-Z.; Wu, J.-H. Modification of wool fiber using steam explosion. Eur. Polym. J. 2006, 42, 2168–2173. [Google Scholar] [CrossRef]
- Chen, G.-Z.; Chen, H.-Z. Extraction and deglycosylation of flavonoids from sumac fruits using steam explosion. Food Chem. 2010, 126, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, R.-J.; Zhang, Y.-Q. Sustainable and practical utilization of feather keratin by an innovative physicochemical pretreatment: High density steam flash-explosion. Green Chem. 2012, 14, 3352–3360. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.-G.; Yuan, F. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015, 185, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mu, T.; Sun, H.; Zhang, M.; Chen, J. Optimisation of aqueous two-phase extraction of anthocyanins from purple sweet potatoes by response surface methodology. Food Chem. 2013, 141, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, L.; Tang, J.; Yang, Z. Optimization of arsenic extraction in rice samples by Plackett-Burman design and response surface methodology. Food Chem. 2016, 204, 283–288. [Google Scholar] [CrossRef]
- Kobayashi, F.; Take, H.; Nakamura, Y. Methane production from steam exploded bamboo. J. Biosci. Bioeng. 2004, 97, 426–428. [Google Scholar] [CrossRef]
- Wardhani, D.H.; Vázquez, J.A.; Pandiella, S.S. Optimisation of antioxidants extraction from soybeans fermented by Aspergillus oryzae. Food Chem. 2010, 118, 731–739. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Zhang, H.; Wang, L.; Guo, X.-N.; Wang, X.-G.; Yao, H.-Y. Isolation and identification of antioxidative peptides from rice endosperm protein enzymatic hydrolysate by consecutive chromatography and MALDI-TOF/TOF MS/MS. Food Chem. 2010, 119, 226–234. [Google Scholar] [CrossRef]
- Kaushik, N.; Rao, P.S.; Mishra, H.N. Process optimization for thermal-assisted high pressure processing of mango (Mangifera indica L.) pulp using response surface methodology. LWT-Food Sci. Technol. 2016, 69, 372–381. [Google Scholar] [CrossRef]
- Xu, B.-J.; Chang, S.-K. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. Food Sci. 2007, 72, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Heimler, D.; Vignolini, P.; Dini, M.-G. Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.-L.; Dai, D.-H.; Hu, W.-L. Antimicrobial and antioxidant activities of the essential oil from onion (Allium cepa L.). Food Control 2013, 30, 48–53. [Google Scholar] [CrossRef]
- Zhong, K.; Lin, W.-J.; Wang, Q. Extraction and radicals scavenging activity of polysaccharides with microwave extraction from mung bean hulls. Int. J. Biol. Macromol. 2012, 51, 612–617. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Runs | A Steam Pressure (MPa) | B Duration (s) | C Particle Size (mesh) | Y TFs (mg CAE/g) |
|---|---|---|---|---|
| 1 | 2.5 | 60 | 60 | 18.75 |
| 2 | 2.5 | 120 | 60 | 15.62 |
| 3 | 2.0 | 120 | 40 | 18.65 |
| 4 | 2.0 | 90 | 60 | 24.24 |
| 5 | 2.0 | 90 | 60 | 24.51 |
| 6 | 2.0 | 90 | 60 | 25.52 |
| 7 | 2.0 | 120 | 80 | 17.36 |
| 8 | 2.0 | 60 | 40 | 19.69 |
| 9 | 1.5 | 90 | 80 | 18.89 |
| 10 | 2.0 | 90 | 60 | 24.34 |
| 11 | 2.5 | 90 | 80 | 17.44 |
| 12 | 1.5 | 120 | 60 | 16.39 |
| 13 | 1.5 | 60 | 60 | 15.34 |
| 14 | 2.0 | 90 | 60 | 25.09 |
| 15 | 2.5 | 90 | 40 | 22.54 |
| 16 | 2.0 | 60 | 80 | 18.46 |
| 17 | 1.5 | 90 | 60 | 16.67 |
| Source | Sum of Squares | df | Mean Square | Coefficient | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Model | 203.99 | 9 | 22.67 | 24.74 | 98.76 | <0.0001 |
| A | 6.23 | 1 | 6.23 | 0.88 | 27.15 | 0.0012 |
| B | 2.23 | 1 | 2.23 | −0.53 | 9.7 | 0.0170 |
| C | 3.65 | 1 | 3.65 | −0.68 | 15.88 | 0.0053 |
| AB | 4.37 | 1 | 4.37 | −1.05 | 19.03 | 0.0033 |
| AC | 13.40 | 1 | 13.40 | −1.83 | 58.37 | 0.0001 |
| BC | 9.000 × 10−4 | 1 | 9.000 × 10−4 | −0.015 | 3.922 × 10−3 | 0.9518 |
| A2 | 65.20 | 1 | 65.20 | −3.94 | 284.08 | <0.0001 |
| B2 | 77.13 | 1 | 77.13 | −4.28 | 336.08 | <0.0001 |
| C2 | 15.52 | 1 | 15.52 | −1.92 | 67.63 | <0.0001 |
| Residual | 1.61 | 7 | 0.23 | |||
| Lack of fit | 0.41 | 3 | 0.14 | 0.46 | 0.7247 | |
| Pure error | 1.19 | 4 | 0.30 | |||
| Cor total | 205.60 | 16 | ||||
| R2 | 0.992 | |||||
| AdjR2 | 0.9821 | |||||
| C.V. % | 2.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, J.; Liu, H.; Sun, N.; Liu, S.; Chen, P. Optimization of the Steam Explosion Pretreatment Effect on Total Flavonoids Content and Antioxidative Activity of Seabuckthom Pomace by Response Surface Methodology. Molecules 2019, 24, 60. https://doi.org/10.3390/molecules24010060
Tu J, Liu H, Sun N, Liu S, Chen P. Optimization of the Steam Explosion Pretreatment Effect on Total Flavonoids Content and Antioxidative Activity of Seabuckthom Pomace by Response Surface Methodology. Molecules. 2019; 24(1):60. https://doi.org/10.3390/molecules24010060
Chicago/Turabian StyleTu, Jianqiu, Huiping Liu, Naxin Sun, Shaojuan Liu, and Pei Chen. 2019. "Optimization of the Steam Explosion Pretreatment Effect on Total Flavonoids Content and Antioxidative Activity of Seabuckthom Pomace by Response Surface Methodology" Molecules 24, no. 1: 60. https://doi.org/10.3390/molecules24010060
APA StyleTu, J., Liu, H., Sun, N., Liu, S., & Chen, P. (2019). Optimization of the Steam Explosion Pretreatment Effect on Total Flavonoids Content and Antioxidative Activity of Seabuckthom Pomace by Response Surface Methodology. Molecules, 24(1), 60. https://doi.org/10.3390/molecules24010060





