The Protective Effect of Sheep Placental Extract on Concanavalin A-induced Liver Injury in Mice
Abstract
1. Introduction
2. Results and Discussion
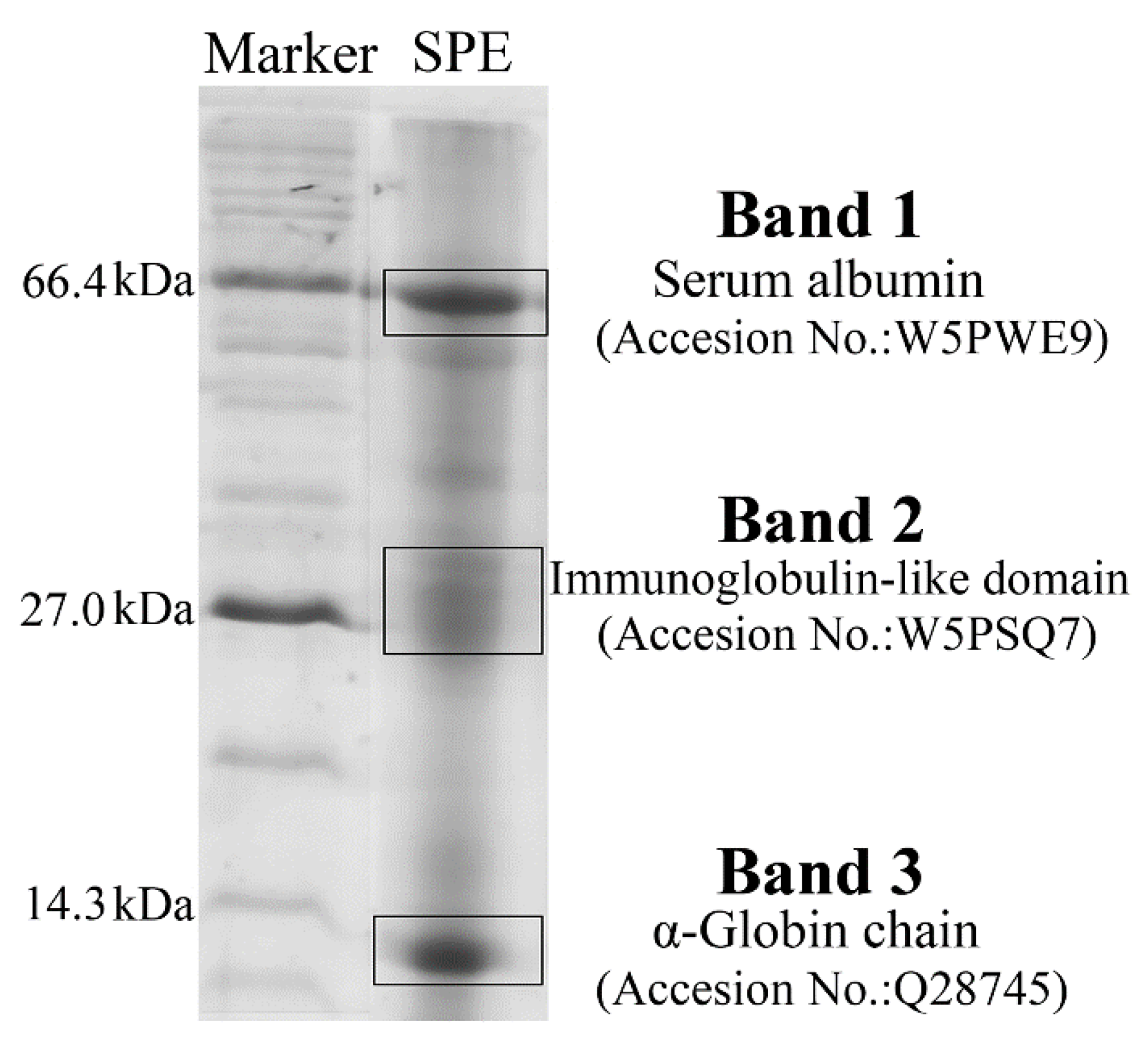
2.1. Composition Analysis of SPE
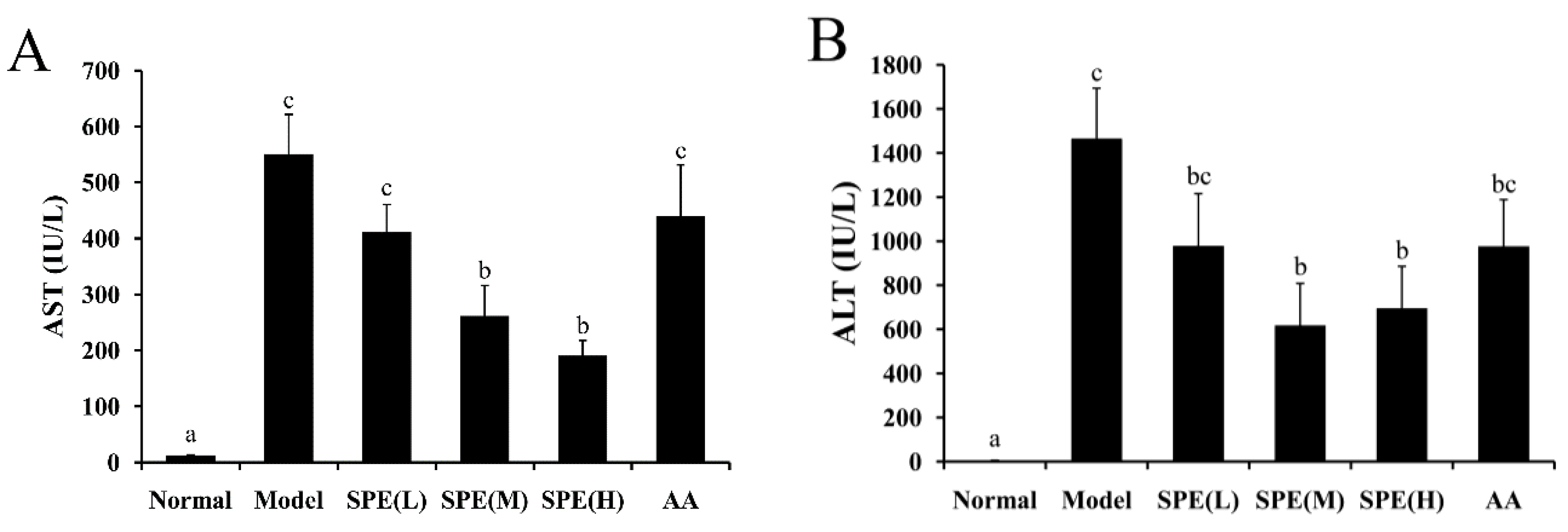
2.2. Serum Biochemical Markers
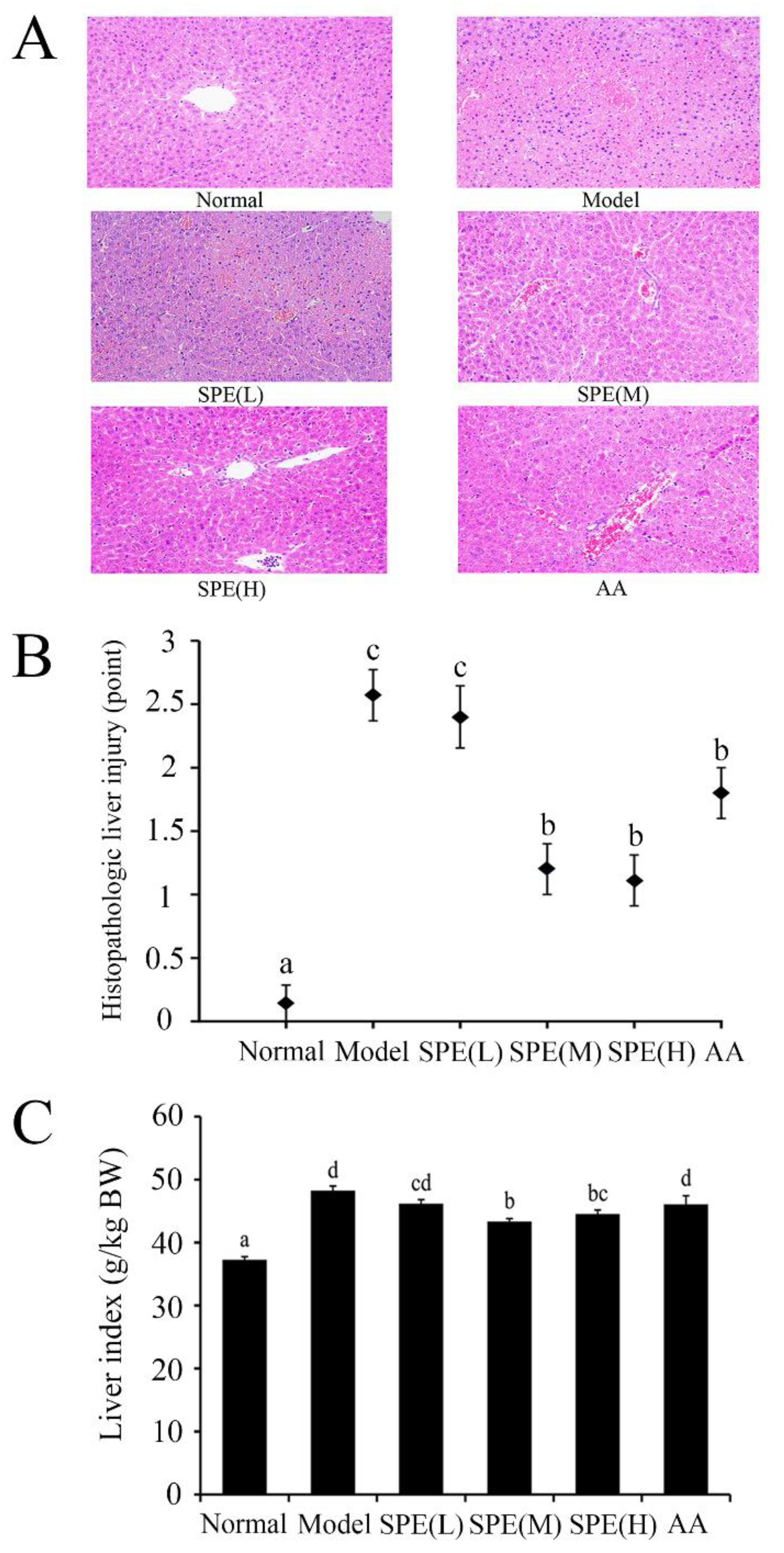
2.3. Histological Examination of the Liver
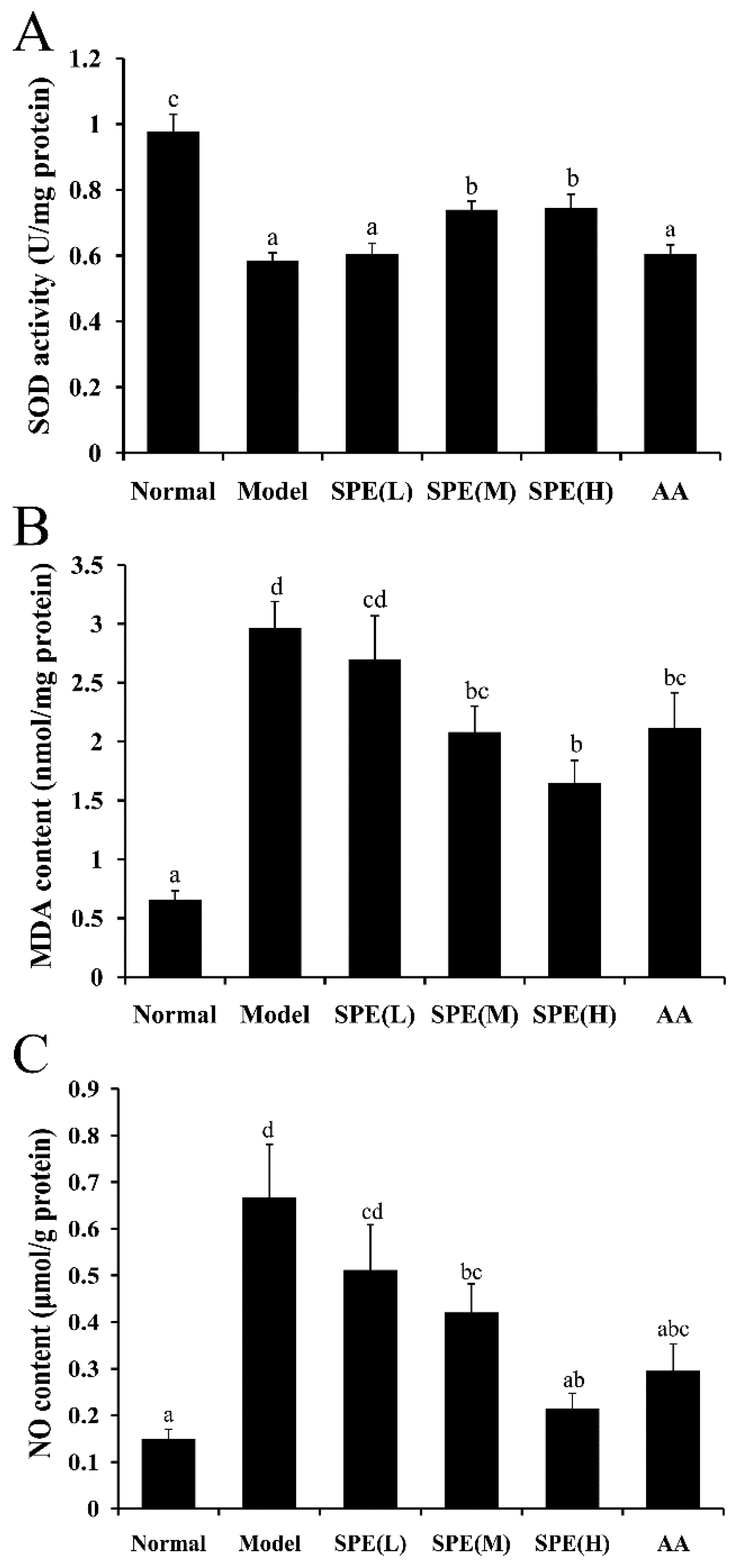
2.4. Superoxide Dismutase (SOD) Activity, Malondialdehyde (MDA) Content and NO Content in Liver Tissues
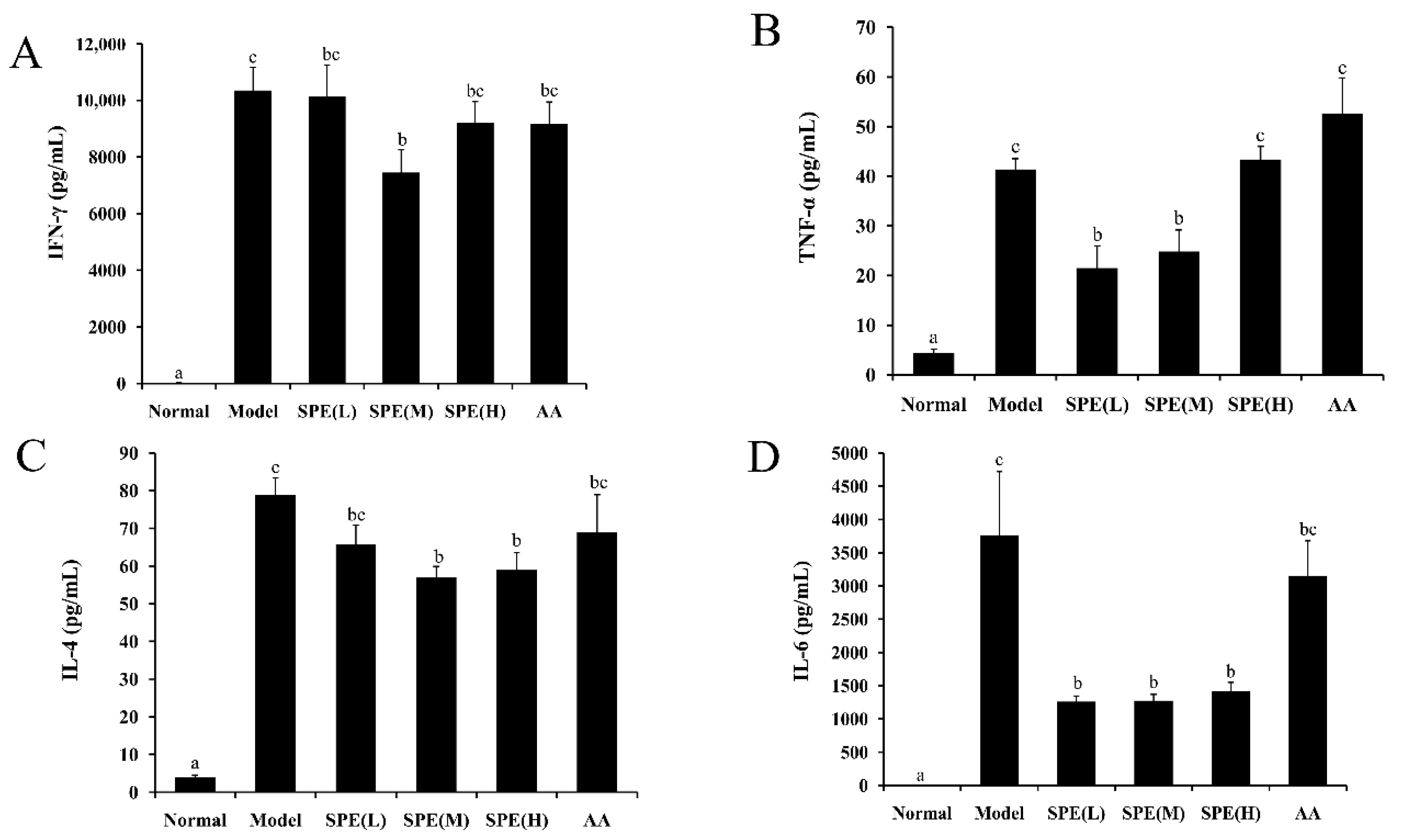
2.5. The Cytokine Content in Serum
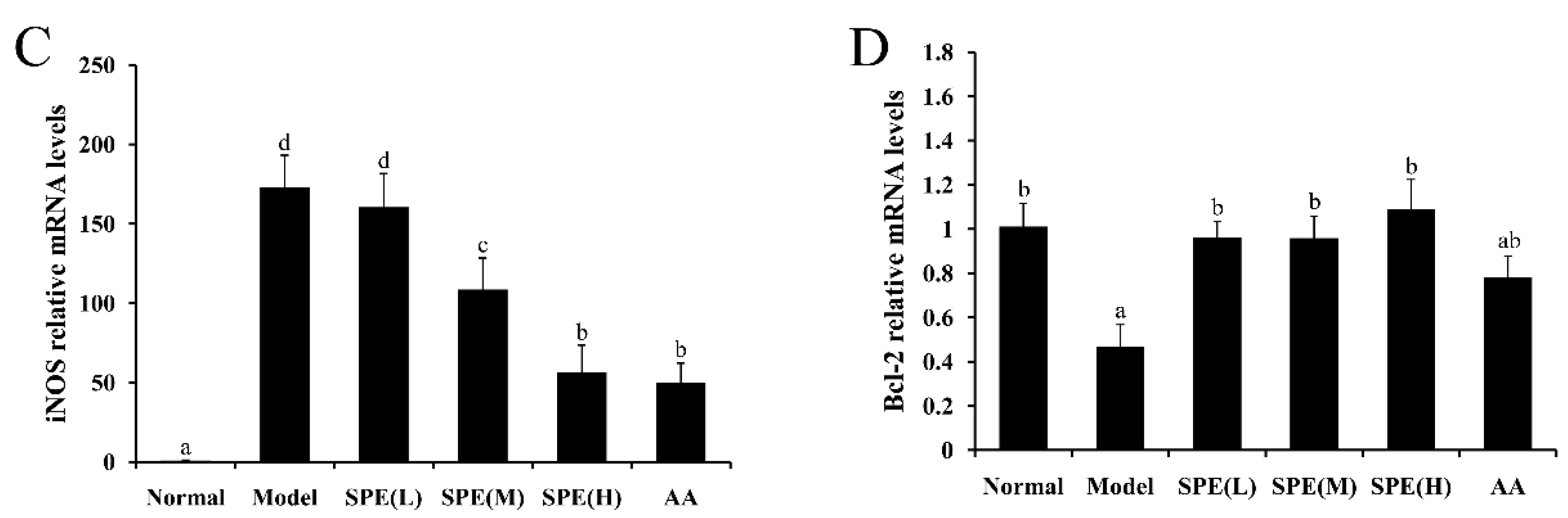
2.6. The Expression of mRNA in Liver Tissues
3. Materials and Methods
3.1. Materials
3.2. Extraction of Sheep Placenta
3.3. Composition Analysis of SPE
3.4. Animals and Experiment Design
3.5. Measurement of Biochemical Markers in Serum
3.6. Liver index and Histopathological Analysis
3.7. Measurements of SOD, MDA and NO in Liver Tissue
3.8. Serum Cytokine Measurement
3.9. Real-time PCR Analysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Donnelly, L.; Campling, G. Functions of the placenta. Anaesth. Intensive Care Med. 2014, 15, 136–139. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, G.Y.; Chang, W.C.; Hsu, C.T.; Chen, R.S. Wound healing effects of porcine placental extracts on rats with thermal injury. Br. J. Dermatol. 2003, 148, 236–245. [Google Scholar] [CrossRef]
- Kim, B.Y.; Park, H.R.; Shin, J.H.; Kim, S.W.; Kim, S.W. Human placental extract reduces allergic inflammation in a murine allergic rhinitis model. Laryngoscope 2014, 124, E399–E404. [Google Scholar] [CrossRef]
- Lee, K.K.; Choi, W.S.; Yum, K.S.; Song, S.W.; Ock, S.M.; Park, S.B.; Kim, M.J. Efficacy and safety of human placental extract solution on fatigue: A double-blind, randomized, placebo-controlled study. Evid.-Based Complement. Alternat. Med. 2012, 1, 130875–130881. [Google Scholar] [CrossRef]
- Liu, K.X.; Kato, Y.; Kaku, T.; Sugiyama, Y. Human placental extract stimulates liver regeneration in rats. Biol. Pharm. Bull. 1998, 21, 44–49. [Google Scholar] [CrossRef]
- Jung, J.; Lee, H.J.; Lee, J.M.; Na, K.H.; Hwang, S.G.; Kim, G.J. Placenta extract promote liver regeneration in CCl4-injured liver rat model. Inter. Immunopharmacol. 2011, 11, 976–984. [Google Scholar] [CrossRef]
- Park, H.J.; Suh, H.G.; Kim, J.H.; Jang, A.; Jung, H.J.; Lee, S.D.; Ha, W.T.; Lee, R.; Kim, J.H.; Kim, S.H.; et al. Immune modulation effect of pig placenta extracts in a mouse model: putative use as a functional food supplement. Korean. J. Food Sci. Anim. 2011, 31, 701–709. [Google Scholar] [CrossRef]
- Low, B.G.; Hansen, P.J.; Drost, M. Inhibition of in vitro lymphocyte proliferation by ovine placenta-conditioned culture medium. J. Reprod. Immunol. 1991, 19, 25–41. [Google Scholar] [CrossRef]
- Tiegs, G.; Hentschel, J.; Wendel, A. A T-cell-dependent experimental liver-injury in mice inducible by concanavalin-A. J. Clin. Investig. 1992, 90, 196–203. [Google Scholar] [CrossRef]
- Kokhdan, E.P.; Ahmadi, K.; Sadeghi, H.; Sadeghi, H.; Dadgary, F.; Danaei, N.; Aghamaali, M.R. Hepatoprotective effect of stachys pilifera ethanol extract in carbon tetrachloride-induce hepatotoxicity in rats. Pharm. Biol. 2017, 55, 1389–1393. [Google Scholar] [CrossRef]
- Teng, D.; Fang, Y.; Song, X.; Gao, Y. Optimization of enzymatic hydrolysis parameters for antioxidant capacity of peptide from goat placenta. Food Bioprod. Process. 2011, 89, 202–208. [Google Scholar] [CrossRef]
- Park, S.Y.; Phark, S.; Lee, M.; Lim, J.Y.; Sul, D. Anti-oxidative and anti-inflammatory activities of placental extracts in benzo a pyrene-exposed rats. Placenta 2010, 31, 873–879. [Google Scholar] [CrossRef]
- Chakraborty, P.D.; Bhattacharyya, D. Isolation of fibronectin type III like peptide from human placental extract used as wound healer. J. Chromatogr. B 2005, 818, 67–73. [Google Scholar] [CrossRef]
- Wolf, H.K.; Zarnegar, R.; Oliver, L.; Michalopoulos, G.K. Hepatocyte growth-factor in human placenta and trophoblastic disease. Am. J. Pathol. 1991, 138, 1035–1043. [Google Scholar]
- Saito, S.; Sakakura, S.; Enomoto, M.; Ichijo, M.; Matsumoto, K.; Nakamura, T. Hepatocyte growth-factor promotes the growth of cytotrophoblasts by the paracrine mechanism. J. Biochem. 1995, 117, 671–676. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Yang, J.; Lin, F.; Sun, Y.; Li, T.; Wu, C. Hepatoprotective effect of apple polyphenols against concanavalin A-induced immunological liver injury in mice. Chem.-Biol. Interact. 2016, 258, 159–165. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; You, S.; Liu, T.; Xu, F.; Ji, T.; Gu, Z. Hepatoprotective effects of nicotiflorin from nymphaea candida against concanavalin A-induced and D-galactosamine-induced liver injury in mice. Int. J. Mol. Sci. 2017, 18, 587. [Google Scholar] [CrossRef]
- Mangano, K.; Cavalli, E.; Mammana, S.; Basile, M.S.; Caltabiano, R.; Pesce, A.; Puleo, S.; Atanasov, A.G.; Magro, G.; Nicoletti, F.; et al. Involvement of the Nrf2/HO-1/CO axis and therapeutic intervention with the CO-releasing molecule CORM-A1, in a murine model of autoimmune hepatitis. J. Cell. Physiol. 2018, 233, 4156–4165. [Google Scholar] [CrossRef]
- Tiberio, G.A.M.; Tiberio, L.; Benetti, A.; Cervi, E.; Montani, N.; Dreano, M.; Garotta, G.; Cerea, K.; Steimberg, N.; Pandolfo, G.; et al. IL-6 promotes compensatory liver regeneration in cirrhotic rat after partial hepatectomy. Cytokine 2008, 42, 372–378. [Google Scholar] [CrossRef]
- Ksontini, R.; Colagiovanni, D.B.; Josephs, M.D.; Edwards, C.K.; Tannahill, C.L.; Solorzano, C.C.; Norman, J.; Denham, W.; Clare-Salzler, M.; MacKay, S.L.D.; et al. Disparate roles for TNF-alpha and Fas ligand in concanavalin A-induced hepatitis. J. Immunol. 1998, 160, 4082–4089. [Google Scholar]
- Küsters, S.; Tiegs, G.; Alexopoulou, L.; Pasparakis, M.; Douni, E.; Künstle, G.; Bluethmann, H.; Wendel, A.; Pfizenmaier, K.; Kollias, G.; et al. In vivo evidence for a functional role of both tumor necrosis factor (TNF) receptors and transmembrane TNF in experimental hepatitis. Eur. J. Immunol. 1997, 27, 2870–2875. [Google Scholar] [CrossRef]
- Jaruga, B.; Hong, F.; Sun, R.; Radaeva, S.; Gao, B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J. Immunol. 2003, 171, 3233–3244. [Google Scholar] [CrossRef]
- Nicoletti, F.; Zaccone, P.; Xiang, M.; Magro, G.; Mauro, M.D.; Marco, R.D.; Garotta, G.; Meroni, P. Essential pathogenetic role for interferon (IFN-)gamma in concanavalin A-induced T cell-dependent hepatitis: exacerbation by exogenous IFN-gamma and prevention by IFN-gamma receptor-immunoglobulin fusion protein. Cytokine. 2000, 12, 315–323. [Google Scholar] [CrossRef]
- Yan, S.; Wang, L.; Liu, N.; Wang, Y.; Chu, Y. Critical role of interleukin-17/interleukin-17 receptor axis in mediating Con A-induced hepatitis. Immunol. Cell Biol. 2012, 90, 421–428. [Google Scholar] [CrossRef]
- Heymann, F.; Hamesch, K.; Weiskirchen, R.; Tacke, F. The concanavalin A model of acute hepatitis in mice. Lab. Anim. 2015, 49, 12–20. [Google Scholar] [CrossRef]
- Ye, T.; Wang, T.; Yang, X.; Fan, X.; Wen, M.; Shen, Y.; Xi, X.; Men, R.; Yang, L. Comparison of concanavalin a-induced murine autoimmune hepatitis models. Cell. Physiol. Biochem. 2018, 46, 1241–1251. [Google Scholar] [CrossRef]
- Yuan, X.; Li, Y.; Pan, X.; Peng, X.; Song, G.; Jiang, W.; Gao, Q.; Li, M. IL-38 alleviates concanavalin A-induced liver injury in mice. Inter. Immunopharmacol. 2016, 40, 452–457. [Google Scholar] [CrossRef]
- Nicoletti, F.; Beltrami, B.; Raschi, E.; Marco, R.D.; Margo, G.; Grasso, S.; Bendtzen, K.; Fiorelli, G.; Meroni, P.L. Protection from concanavalin A (Con A)-induced T cell-dependent hepatic lesions and modulation of cytokine release in mice by sodium fusidate. Clin. Exp. Immunol. 1997, 110, 479–484. [Google Scholar] [CrossRef]
- Günther, S.; Fagone, P.; Jalce, G.; Atanasov, A.G.; Guignabert, C.; Nicoletti, F. Role of MIF and D-DT in immune-inflammatory, autoimmune, and chronic respiratory diseases: from pathogenic factors to therapeutic targets. Drug Discov. Today. Available online: https://www.sciencedirect.com/science/article/pii/S1359644618303295 (accessed on 13 November 2018). [CrossRef]
- Shao, X.; Qian, Y.; Xu, C.; Hong, B.; Xu, W.; Shen, L.; Jin, C.; Wu, Z.; Tong, X.; Yao, H. The protective effect of intrasplenic transplantation of Ad-IL-18BP/IL-4 gene-modified fetal hepatocytes on ConA-induced hepatitis in mice. PLoS ONE 2013, 8, e58836. [Google Scholar] [CrossRef]
- Marco, R.D.; Xiang, M.; Zaccone, P.; Leonardi, C.; Franco, S.; Meroni, P.; Nicolettl, F. Concanavalin A-induced hepatitis in mice is prevented by interleukin (IL)-10 and exacerbated by endogenous IL-10 deficiency. Autoimmunity 1999, 31, 75–83. [Google Scholar] [CrossRef]
- Yuan, Y.; Gong, X.; Zhang, L.; Jiang, R.; Yang, J.; Wang, B.; Wan, J. Chlorogenic acid ameliorated concanavalin A-induced hepatitis by suppression of Toll-like receptor 4 signaling in mice. Int. Immunopharmacol. 2017, 44, 97–104. [Google Scholar] [CrossRef]
- Tu, C.T.; Han, B.; Liu, H.C.; Zhang, S.C. Curcumin protects mice against concanavalin A-induced hepatitis by inhibiting intrahepatic intercellular adhesion molecule-1 (ICAM-1) and CXCL10 expression. Mol. Cell. Biochem. 2011, 358, 53–60. [Google Scholar] [CrossRef]
- Sass, G.; Koerber, K.; Bang, R.; Guehring, H.; Tiegs, G. Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J. Clin. Investig. 2001, 107, 439–447. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, J.; Yu, F.; Cheng, J.; Li, H.; Guo, C.; Fan, X. Ghrelin reduces liver impairment in a model of concanavalin A-induced acute hepatitis in mice. Drug Des. Dev. Ther. 2015, 9, 5385–5396. [Google Scholar] [CrossRef]
- Fayad, R.; Sennello, J.A.; Kim, S.H.; Pini, M.; Dinarello, C.A.; Fantuzzi, G. Induction of thymocyte apoptosis by systemic administration of concanavalin A in mice: role of TNF-alpha, IFN-gamma and glucocorticoids. Eur. J. Immunol. 2015, 35, 2304–2312. [Google Scholar] [CrossRef]
- Shin, K.S.; Lee, H.J.; Jung, J.; Cha, D.H.; Kim, G.J. Culture and in vitro hepatogenic differentiation of placenta-derived stem cells, using placental extract as an alternative to serum. Cell Proliferation 2010, 43, 435–444. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Wu, J.; Yang, T.; Wang, C.; Liu, Q.; Yao, J.; Sun, H.; Kaku, T.I.; Liu, K.X. Laennec protects murine from concanavalin A-Induced liver injury through inhibition of inflammatory reactions and hepatocyte apoptosis. Biol. Pharm. Bull. 2008, 31, 2040–2044. [Google Scholar] [CrossRef]
- Xue, J.; Chen, F.; Wang, J.; Wu, S.; Zheng, M.; Zhu, H.; Liu, Y.; He, J.; Chen, Z. Emodin protects against concanavalin A-induced hepatitis in mice through inhibiting activation of the p38 MAPK-NF-κB signaling pathway. Cell. Physiol. Biochem. 2015, 35, 1557–1570. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data usng real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds not are available from the authors. |

| Category | Content (g/100 g) |
|---|---|
| Water | 4.07 ± 0.31 |
| Crude protein | 84.95 ± 0.81 |
| Total fat | 2.08 ± 0.04 |
| Ash | 5.53 ± 0.09 |
| Total carbohydrate | 2.06 ± 0.03 |
| Amino Acids | Content (g/100 g) | Amino Acids | Content (g/100 g) |
|---|---|---|---|
| Aspartic acid | 8.00 | Isoleucine | 2.74 |
| Threonine | 4.01 | Leucine | 6.88 |
| Serine | 4.07 | Tyrosine | 2.72 |
| Glutamic acid | 12.51 | Phenylalanine | 3.97 |
| Proline | 3.78 | Histidine | 2.32 |
| Glycine | 3.78 | Lysine | 6.90 |
| Alanine | 4.17 | Arginine | 4.79 |
| Valine | 4.14 | Cystine | 1.69 |
| Methionine | 1.56 | Tryptophan | 1.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Luo, S.; Yang, J.; Ren, F.; Zhao, Y.; Luo, H.; Ge, K.; Zhang, H. The Protective Effect of Sheep Placental Extract on Concanavalin A-induced Liver Injury in Mice. Molecules 2019, 24, 28. https://doi.org/10.3390/molecules24010028
Liu J, Luo S, Yang J, Ren F, Zhao Y, Luo H, Ge K, Zhang H. The Protective Effect of Sheep Placental Extract on Concanavalin A-induced Liver Injury in Mice. Molecules. 2019; 24(1):28. https://doi.org/10.3390/molecules24010028
Chicago/Turabian StyleLiu, Jingwen, Suting Luo, Jun Yang, Fazheng Ren, Yu Zhao, Hailing Luo, Keshan Ge, and Hao Zhang. 2019. "The Protective Effect of Sheep Placental Extract on Concanavalin A-induced Liver Injury in Mice" Molecules 24, no. 1: 28. https://doi.org/10.3390/molecules24010028
APA StyleLiu, J., Luo, S., Yang, J., Ren, F., Zhao, Y., Luo, H., Ge, K., & Zhang, H. (2019). The Protective Effect of Sheep Placental Extract on Concanavalin A-induced Liver Injury in Mice. Molecules, 24(1), 28. https://doi.org/10.3390/molecules24010028