Deconstructing Wine Grape Cell Walls with Enzymes During Winemaking: New Insights from Glycan Microarray Technology
Abstract
:1. Introduction
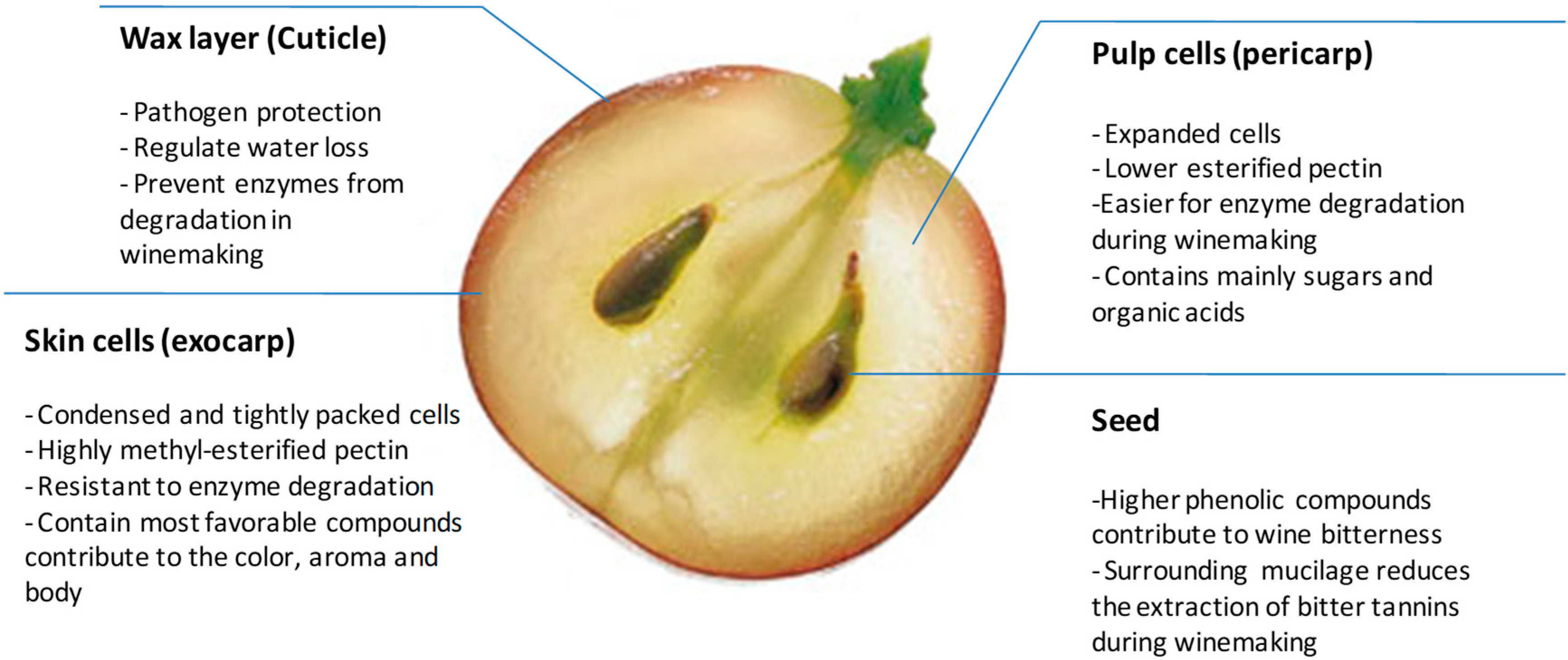
2. The Cell Wall Composition of the Grape Berry from Structure to Function
3. Grape Cell Wall Integrity Associated with Ripening and Berry Health
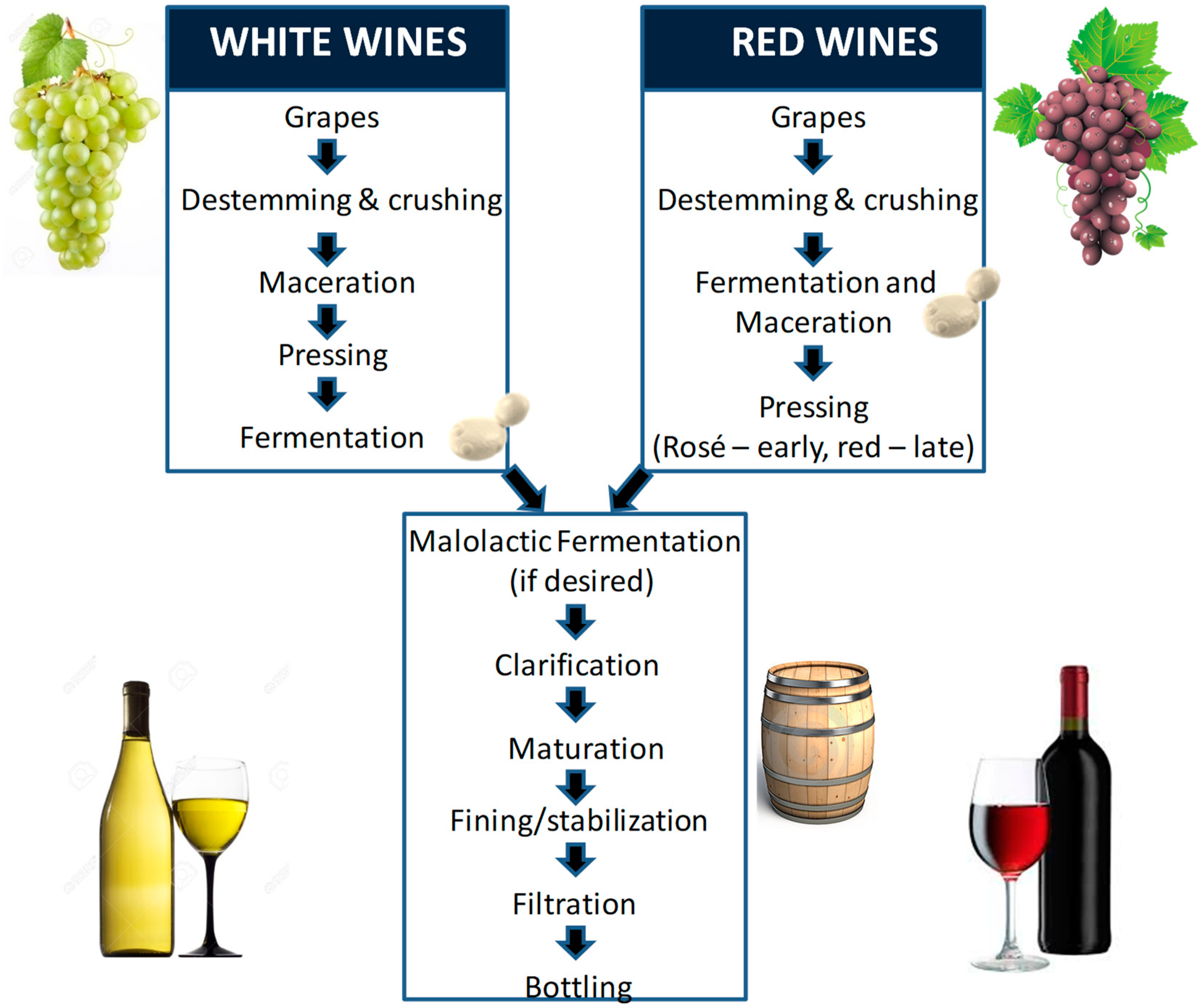
4. The Benefits and Drawbacks of Maceration in Winemaking
5. Polyphenol Extractability during Wine Fermentation, Interaction with Cell Wall Polymers and the Role of Maceration Enzymes
5.1. Extractability of Polyphenols from the Grape Berries during Crushing and Their Interactions with Cell Walls during Winemaking
5.2. The Effect of Maceration Enzymes on the Polyphenol-Cell Wall Polysaccharide Interactions
6. Developing Models of Wine Grape Berry Cell Wall Architecture from Enzyme and Glyco-Array Approaches
- A thorough study on Cabernet Sauvignon pectin polymers during winemaking yielded the first comprehensive wine grape berry cell wall model using recombinant enzymes. This new model replaces previous more ‘simplistic’ concepts in the literature [95]. Polygalacturonases and Pectinmethylesterases do NOT have a significant effect in Cabernet Sauvignon de-pectination/degradation—previous wine grape and enzyme models are therefore outdated [95]. RG-Lyases and Pectin Lyases are essential components for any commercial enzyme preparation to be effective in Cabernet Sauvignon winemaking releasing significant colour pigments and tannins into red wines [95].
- Overripe Pinotage grapes have a more de-pectinated cell wall composition and hence enzymes are more effective at early stages of ripening [96] and work in synergy [94]. Synergistic actions of purified enzymes help to achieve effective maceration, colour and tannin extraction and consistency of polyphenol levels in final Pinotage wines [19,95].
- Natural intra-vineyard wine grape cell wall variability occurs in a model Cabernet Sauvignon vineyard and effects colour and tannin extraction [19]. Commercial enzymes are able to reduce via de-pectination the natural intra-vineyard grape cell wall variability, which helps to achieve maceration that is more effective, colour and tannin extraction and consistency in wines from mixed harvests [19].
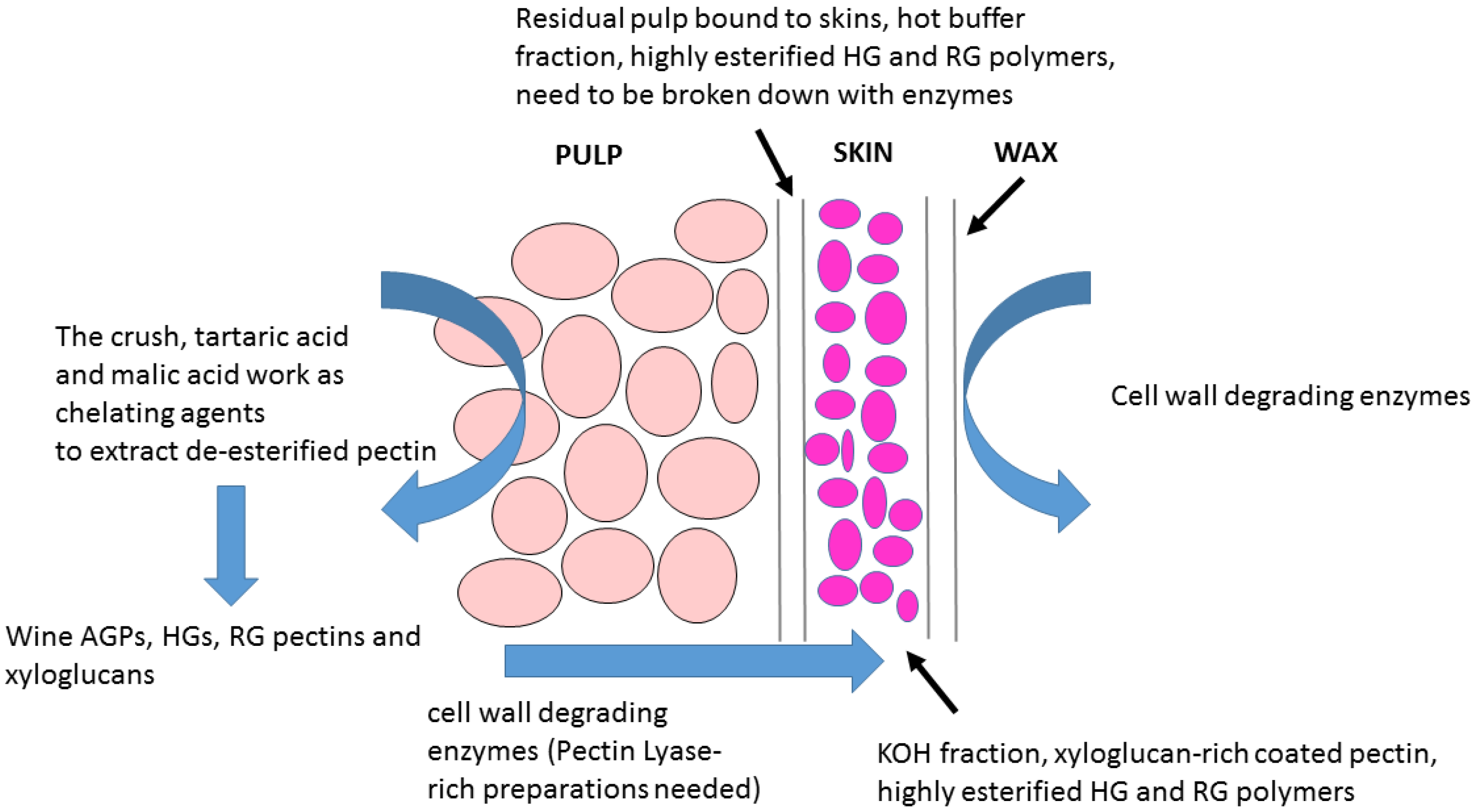
- Wine grape cell walls are composed of two major tissue layers (a) a pectin-rich tissue and (b) a pectin-coated hemicellulose-rich layer [28]. However, the RG-1-rich pectin coating-layer of Chardonnay wine grapes can be removed using a combination of hydrothermal pre-treatment and pectinase addition [97].
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morrison-Whittle, P.; Goddard, M.R. From vineyard to winery: A source map of microbial diversity driving wine fermentation. Environ. Microb. 2018, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, B.; Olmedo, P.; Ejsmentewicz, T.; Sepúlveda, P.; Balic, I.; Balladares, C.; Delgado-Rioseco, J.; Fuentealba, C.; Moreno, A.A.; Defilippi, B.G.; et al. Cell wall and metabolite composition of berries of Vitis vinifera (L.) cv. Thompson Seedless with different firmness. Food Chem. 2018, 268, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, D.M.; Lerouxel, O.; Neumetzler, L.; Yamauchi, K.; Reinecke, A.; Freshour, G.; Zabotina, O.A.; Hahn, M.G.; Burgert, I.; Pauly, M.; et al. Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 2008, 20, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Somerville, C.; Bauer, S.; Brininstool, G.; Facette, M.; Hamann, T.; Milne, J.; Osborne, E.; Paredez, A.; Persson, S.; Raab, T.; et al. Toward a systems approach to understanding plant cell walls. Science 2004, 306, 2206–2211. [Google Scholar] [CrossRef]
- Nunan, K.J.; Sims, I.M.; Bacic, A.; Robinson, S.P.; Fincher, G.B. Changes in cell wall composition during ripening of grape berries. Plant Physiol. 1998, 118, 783–792. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Changes in skin cell wall composition during the maturation of four premium wine grape varieties. J. Sci. Food Agric. 2008, 88, 420–428. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; Pérez-Magariño, S.; González-Sanjosé, M.L. Comparative study of the use of maceration enzymes and cold pre-fermentative maceration on phenolic and anthocyanic composition and colour of a Mencía red wine. LWT-Food Sci. Technol. 2012, 48, 1–8. [Google Scholar] [CrossRef]
- Romero-Cascales, I.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. The effect of a commercial pectolytic enzyme on grape skin cell wall degradation and colour evolution during the maceration process. Food Chem. 2012, 130, 626–631. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Moller, I.; Sørensen, I.; Bernal, A.J.; Blaukopf, C.; Lee, K.; Øbro, J.; Pettolino, F.; Roberts, A.; Mikkelsen, J.D.; Knox, J.P.; et al. High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J. 2007, 50, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Bindon, K.A.; Madani, S.H.; Pendleton, P.; Smith, P.A.; Kennedy, J.A. Factors affecting skin tannin extractability in ripening grapes. J. Agric. Food Chem. 2014, 62, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Rid, M.; Markheiser, A.; Hoffmann, C.; Gross, J. Waxy bloom on grape berry surface is one important factor for oviposition of European grapevine moths. J. Pest Sci. 2018, 91, 1225–1239. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Lecas, M.; Brillouet, J.M. Cell wall composition of grape berry skins. Phytochemistry 1994, 35, 1241–1243. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Differences in morphology and composition of skin and pulp cell walls from grapes (Vitis vinifera L.): Technological implications. Eur. Food Res. Technol. 2008, 227, 223–231. [Google Scholar] [CrossRef]
- Arnous, A.; Meyer, A.S. Quantitative prediction of cell wall polysaccharide composition in grape (Vitis vinifera L.) and apple (Malus domestica) skins from acid hydrolysis monosaccharide profiles. J. Agric. Food Chem. 2009, 57, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Clarke, S.J.; Barril, C.; Rogiers, S.Y. Spatiotemporal changes in the accumulation of sugar and potassium within individual ‘Sauvignon Blanc’ (Vitis vinifera L.) berries. Vitis-J. Grape. Res. 2017, 56, 189–195. [Google Scholar] [CrossRef]
- Doco, T.; Williams, P.; Pauly, M.; O’Neill, M.A.; Pellerin, P. Polysaccharides from grape berry cell walls. Part II. Structural characterization of the xyloglucan polysaccharides. Carbohyd. Polym. 2003, 53, 253–261. [Google Scholar] [CrossRef]
- Gao, Y.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A.; Moore, J.P. Effect of commercial enzymes on berry cell wall deconstruction in the context of intravineyard ripeness variation under winemaking conditions. J. Agric. Food Chem. 2016, 64, 3862–3872. [Google Scholar] [CrossRef]
- Cadot, Y.; Miñana-Castelló, M.T.; Chevalier, M. Anatomical, histological, and histochemical changes in grape seeds from Vitis vinifera L. cv Cabernet franc during fruit development. J. Agric. Food Chem. 2006, 54, 9206–9215. [Google Scholar] [CrossRef]
- Moore, J.P.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A. Pectic-β(1,4)-galactan, extensin and arabinogalactan-protein epitopes differentiate ripening stages in wine and table grape cell walls. Ann. Bot. 2014, 114, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Bapat, V.A.; Trivedi, P.K.; Ghosh, A.; Sane, V.A.; Ganapathi, T.R.; Nath, P. Ripening of fleshy fruit: Molecular insight and the role of ethylene. Biotechnol. Adv. 2010, 28, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Fasoli, M.; Dell’Anna, R.; Dal Santo, S.; Balestrini, R.; Sanson, A.; Pezzotti, M.; Monti, F.; Zenoni, S. Pectins, Hemicelluloses and celluloses show specific dynamics in the internal and external surfaces of grape berry skin during ripening. Plant Cell Physiol. 2016, 57, 1332–1349. [Google Scholar] [CrossRef] [PubMed]
- Franková, L.; Fry, S.C. Biochemistry and physiological roles of enzymes that ‘cut and paste’ plant cell-wall polysaccharides. J. Exp. Bot. 2013, 64, 3519–3550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, S.C. Primary cell wall metabolism: Tracking the careers of wall polymers in living plant cells. New Phytol. 2004, 161, 641–675. [Google Scholar] [CrossRef]
- Minic, Z.; Jouanin, L. Plant glycoside hydrolases involved in cell wall polysaccharide degradation. Plant Physiol. Biochem. 2006, 44, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Nunan, K.J.; Davies, C.; Robinson, S.P.; Fincher, G.B. Expression patterns of cell wall-modifying enzymes during grape berry development. Planta 2001, 214, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A.; Moore, J.P. Dissecting the polysaccharide-rich grape cell wall changes during winemaking using combined high-throughput and fractionation methods. Carbohydr. Polym. 2015, 133, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Yakushiji, H.; Sakurai, N.; Morinaga, K. Changes in cell-wall polysaccharides from the mesocarp of grape berries during veraison. Physiol. Plant. 2001, 111, 188–195. [Google Scholar] [CrossRef]
- Guillaumie, S.; Fouquet, R.; Kappel, C.; Camps, C.; Terrier, N.; Moncomble, D.; Dunlevy, J.D.; Davies, C.; Boss, P.K.; Delrot, S. Transcriptional analysis of late ripening stages of grapevine berry. BMC Plant Biol. 2011, 11. [Google Scholar] [CrossRef]
- Davidsson, P.R.; Kariola, T.; Niemi, O.; Tapio Palva, E. Pathogenicity of and plant immunity to soft rot pectobacteria. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Ulate, B.; Morales-Cruz, A.; Amrine, K.C.H.; Labavitch, J.M.; Powell, A.L.T.; Cantu, D. Genome-wide transcriptional profiling of Botrytis cinerea genes targeting plant cell walls during infections of different hosts. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. In Annual Review of Plant Biology; Annual Reviews: Palo Alto, CA, USA, 2009; Volume 60, pp. 379–407. [Google Scholar]
- Ma, H.; Xiang, G.; Li, Z.; Wang, Y.; Dou, M.; Su, L.; Yin, X.; Liu, R.; Wang, Y.; Xu, Y. Grapevine VpPR10.1 functions in resistance to Plasmopara viticola through triggering a cell death-like defence response by interacting with VpVDAC3. Plant Biotechnol. J. 2018, 16, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Gauthier, A.; Bézier, A.; Poinssot, B.; Joubert, J.M.; Pugin, A.; Heyraud, A.; Baillieul, F. Elicitor and resistance-inducing activities of β-1,4 cellodextrins in grapevine, comparison with β-1,3 glucans and α-1,4 oligogalacturonides. J. Exp. Bot. 2007, 58, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; De Lorenzo, G. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Gallego-Giraldo, L.; Posé, S.; Pattathil, S.; Peralta, A.G.; Hahn, M.G.; Ayre, B.G.; Sunuwar, J.; Hernandez, J.; Patel, M.; Shah, J.; et al. Elicitors and defense gene induction in plants with altered lignin compositions. New Phytol. 2018, 219, 1235–1251. [Google Scholar] [CrossRef] [PubMed]
- Bethke, G.; Grundman, R.E.; Sreekanta, S.; Truman, W.; Katagiri, F.; Glazebrook, J. Arabidopsis PECTIN METHYLESTERASEs contribute to immunity against Pseudomonas syringae. Plant Physiol. 2014, 164, 1093–1107. [Google Scholar] [CrossRef]
- Joubert, D.A.; de Lorenzo, G.; Vivier, M.A. Regulation of the grapevine polygalacturonase-inhibiting protein encoding gene: Expression pattern, induction profile and promoter analysis. J. Plant Res. 2013, 126, 267–281. [Google Scholar] [CrossRef]
- Nguema-Ona, E.; Moore, J.P.; Fagerström, A.D.; Fangel, J.U.; Willats, W.G.T.; Hugo, A.; Vivier, M.A. Overexpression of the grapevine PGIP1 in tobacco results in compositional changes in the leaf arabinoxyloglucan network in the absence of fungal infection. BMC Plant Biol. 2013, 13. [Google Scholar] [CrossRef]
- Bramley, R.G.V. Understanding variability in winegrape production systems 2. Within vineyard variation in quality over several vintages. Aust. J. Grape Wine Res. 2005, 11, 33–42. [Google Scholar] [CrossRef]
- Zhang, P.; Barlow, S.; Krstic, M.; Herderich, M.; Fuentes, S.; Howell, K. Within-vineyard, within-vine, and within-bunch variability of the rotundone concentration in berries of Vitis vinifera L. cv. shiraz. J. Agric. Food Chem. 2015, 63, 4276–4283. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudo, J.L.; du Toit, W. Cold maceration application in red wine production and its effects on phenolic compounds: A review. LWT 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Geffroy, O.; Lopez, R.; Feilhes, C.; Violleau, F.; Kleiber, D.; Favarel, J.L.; Ferreira, V. Modulating analytical characteristics of thermovinified Carignan musts and the volatile composition of the resulting wines through the heating temperature. Food Chem. 2018, 257, 7–14. [Google Scholar] [CrossRef]
- Cholet, C.; Delsart, C.; Petrel, M.; Gontier, E.; Grimi, N.; L’Hyvernay, A.; Ghidossi, R.; Vorobiev, E.; Mietton-Peuchot, M.; Gény, L. Structural and biochemical changes induced by pulsed electric field treatments on Cabernet Sauvignon grape berry skins: Impact on cell wall total tannins and polysaccharides. J. Agric. Food Chem. 2014, 62, 2925–2934. [Google Scholar] [CrossRef] [PubMed]
- Carranza-Concha, J.; Del Mar Camacho, M.; Martínez-Navarrete, N. Effects of blanching on grapes (Vitis vinifera) and changes during storage in syrup. J. Food Proc. Preserv. 2012, 36, 11–20. [Google Scholar] [CrossRef]
- Benucci, I.; Río Segade, S.; Cerreti, M.; Giacosa, S.; Paissoni, M.A.; Liburdi, K.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Gerbi, V.; Esti, M.; et al. Application of enzyme preparations for extraction of berry skin phenolics in withered winegrapes. Food Chem. 2017, 237, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Ortín, A.B.; Martínez-Cutillas, A.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. Improving colour extraction and stability in red wines: The use of maceration enzymes and enological tannins. Int. J. Food Sci. Technol. 2005, 40, 867–878. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Williams, P.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M.; Doco, T. Effect of winemaking techniques on polysaccharide composition of Cabernet Sauvignon, Syrah and Monastrell red wines. Aust. J. Grape Wine Res. 2014, 20, 62–71. [Google Scholar] [CrossRef]
- Castro-López, L.D.R.; Gómez-Plaza, E.; Ortega-Regules, A.; Lozada, D.; Bautista-Ortín, A.B. Role of cell wall deconstructing enzymes in the proanthocyanidin-cell wall adsorption-desorption phenomena. Food Chem. 2016, 196, 526–532. [Google Scholar] [CrossRef]
- Ducasse, M.A.; Canal-Llauberes, R.M.; de Lumley, M.; Williams, P.; Souquet, J.M.; Fulcrand, H.; Doco, T.; Cheynier, V. Effect of macerating enzyme treatment on the polyphenol and polysaccharide composition of red wines. Food Chem. 2010, 118, 369–376. [Google Scholar] [CrossRef]
- Revilla, I.; González-SanJosé, M.L. Methanol release during fermentation of red grapes treated with pectolytic enzymes. Food Chem. 1998, 63, 307–312. [Google Scholar] [CrossRef]
- Romero-Cascales, I.; Fernández-Fernández, J.I.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. Characterisation of the main enzymatic activities present in six commercial macerating enzymes and their effects on extracting colour during winemaking of Monastrell grapes. Int. J. Food Sci. Technol. 2008, 43, 1295–1305. [Google Scholar] [CrossRef]
- Revilla, I.; González-SanJosé, M.L. Multivariate evaluation of changes induced in red wine characteristics by the use of extracting agents. J. Agric. Food Chem. 2002, 50, 4525–4530. [Google Scholar] [CrossRef] [PubMed]
- Wightman, J.D.; Price, S.F.; Watson, B.T.; Wrolstad, R.E. Some effects of processing enzymes on anthocyanins and phenolics in Pinot noir and Cabernet Sauvignon wines. AM J. Enol. Vitic. 1997, 48, 39–48. [Google Scholar]
- Parley, A.; Vanhanen, L.; Heatherbell, D. Effects of pre-fermentation enzyme maceration on extraction and colour stability in Pinot Noir wine. Aust. J. Grape Wine Res. 2001, 7, 146–152. [Google Scholar] [CrossRef]
- Fia, G.; Canuti, V.; Rosi, I. Evaluation of potential side activities of commercial enzyme preparations used in winemaking. Int. J. Food Sci. Technol. 2014, 49, 1902–1911. [Google Scholar] [CrossRef]
- Guérin, L.; Sutter, D.H.; Demois, A.; Chereau, M.; Trandafir, G. Determination of activity profiles of the main commercial enzyme preparations used in winemaking. Am. J. Enol. Vitic. 2009, 60, 322–331. [Google Scholar]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M. The composition of cell walls from grape marcs is affected by grape origin and enological technique. Food Chem. 2015, 167, 370–377. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Williams, P.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M.; Doco, T. Oligosaccharides of Cabernet Sauvignon, Syrah and Monastrell red wines. Food Chem. 2015, 179, 311–317. [Google Scholar] [CrossRef]
- Yacco, R.S.; Watrelot, A.A.; Kennedy, J.A. Red wine tannin structure-activity relationships during fermentation and maceration. J. Agric. Food Chem. 2016, 64, 860–869. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Schulz, D.L.; Kennedy, J.A. Wine polysaccharides influence tannin-protein interactions. Food Hydrocoll. 2017, 63, 571–579. [Google Scholar] [CrossRef]
- Rustioni, L.; Fiori, S.; Failla, O. Evaluation of tannins interactions in grape (Vitis vinifera L.) skins. Food Chem. 2014, 159, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Ortín, A.B.; Molero, N.; Marín, F.; Ruiz-García, Y.; Gómez-Plaza, E. Reactivity of pure and commercial grape skin tannins with cell wall material. Eur. Food Res. Technol. 2014, 240, 645–654. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Ruiz-García, Y.; Marín, F.; Molero, N.; Apolinar-Valiente, R.; Gómez-Plaza, E. Remarkable proanthocyanidin adsorption properties of Monastrell pomace cell wall material highlight its potential use as an alternative fining agent in red wine production. J. Agric. Food Chem. 2015, 63, 620–633. [Google Scholar] [CrossRef]
- Springer, L.F.; Sacks, G.L. Protein-precipitable tannin in wines from Vitis vinifera and interspecific hybrid grapes (Vitis ssp.): Differences in concentration, extractability, and cell wall binding. J. Agric. Food Chem. 2014, 62, 7515–7523. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material: Part I. Effect of some environmental parameters. BBA-Gen. Subj. 2004, 1672, 192–202. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of polyphenols to plant cell wall analogues-Part 1: Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]
- Bindon, K.A.; Bacic, A.; Kennedy, J.A. Tissue-specific and developmental modifications of grape cell walls influence the adsorption of proanthocyanidins. J. Agric. Food Chem. 2012, 60, 9249–9260. [Google Scholar] [CrossRef]
- Riou, V.; Vernhet, A.; Doco, T.; Moutounet, M. Aggregation of grape seed tannins in model wine-Effect of wine polysaccharides. Food Hydrocoll. 2002, 16, 17–23. [Google Scholar] [CrossRef]
- Bindon, K.A.; Smith, P.A.; Kennedy, J.A. Interaction between Grape-Derived Proanthocyanidins and Cell Wall Material. 1. Effect on proanthocyanidin composition and molecular mass. J. Agric. Food Chem. 2010, 58, 2520–2528. [Google Scholar] [CrossRef]
- Hazak, J.C.; Harbertson, J.F.; Adams, D.O.; Lin, C.H.; Ro, B.H. The phenolic components of grape berries in relation to wine composition. In Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2005; Volume 689, pp. 189–196. [Google Scholar]
- Hanlin, R.L.; Hrmova, M.; Harbertson, J.F.; Downey, M.O. Review: Condensed tannin and grape cell wall interactions and their impact on tannin extractability into wine. Aust. J. Grape Wine Res. 2010, 16, 173–188. [Google Scholar] [CrossRef]
- Fournand, D.; Vicens, A.; Sidhoum, L.; Souquet, J.M.; Moutounet, M.; Cheynier, V. Accumulation and extractability of grape skin tannins and anthocyanins at different advanced physiological stages. J. Agric. Food Chem. 2006, 54, 7331–7338. [Google Scholar] [CrossRef] [PubMed]
- Río Segade, S.; Soto Vázquez, E.; Díaz Losada, E. Influence of ripeness grade on accumulation and extractability of grape skin anthocyanins in different cultivars. J. Food Compos. Anal. 2008, 21, 599–607. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Abdallah, R.B.; Castro-López, L.R.; Jiménez-Martínez, M.D.; Gómez-Plaza, E. Technological implications of modifying the extent of cell wall-proanthocyanidin interactions using enzymes. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- González-Neves, G.; Gil, G.; Barreiro, L. Influence of grape variety on the extraction of anthocyanins during the fermentation on skins. Eur. Food Res. Technol. 2008, 226, 1349–1355. [Google Scholar] [CrossRef]
- Romero-Cascales, I.; Ortega-Regules, A.; López-Roca, J.M.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Differences in anthocyanin extractability from grapes to wines according to variety. Am. J. Enol. Vitic. 2005, 56, 212–219. [Google Scholar]
- Saint-Cricq, N.; Vivas, N.; Glories, Y. Maturité phénolique: Définition et contrôle. Rev. Fr. Oenol. 1998, 173, 22–25. [Google Scholar]
- Ortega-Regules, A.; Romero-Cascales, I.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. A first approach towards the relationship between grape skin cell-wall composition and anthocyanin extractability. Anal. Chim. Acta 2006, 563, 26–32. [Google Scholar] [CrossRef]
- Segade, S.R.; Giacosa, S.; Gerbi, V.; Rolle, L. Berry skin thickness as main texture parameter to predict anthocyanin extractability in winegrapes. LWT-Food Sci. Technol. 2011, 44, 392–398. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Influence of the physiological stage and the content of soluble solids on the anthocyanin extractability of Vitis vinifera L. cv. Tempranillo grapes. Anal. Chim. Acta 2012, 732, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Relationship between skin cell wall composition and anthocyanin extractability of Vitis vinifera L. cv. Tempranillo at different grape ripeness degree. Food Chem. 2014, 146, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Kontoudakis, N.; González, E.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Influence of Grape maturity and maceration length on color, polyphenolic composition, and polysaccharide content of Cabernet Sauvignon and Tempranillo Wines. J. Agric. Food Chem. 2012, 60, 7988–8001. [Google Scholar] [CrossRef]
- Carn, F.; Guyot, S.; Baron, A.; Pérez, J.; Buhler, E.; Zanchi, D. Structural properties of colloidal complexes between condensed tannins and polysaccharide hyaluronan. Biomacromolecules 2012, 13, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Keegstra, K.; Talmadge, K.W.; Bauer, W.D.; Albersheim, P. The structure of plant cell walls III. A model of the walls of suspension-cultured Sycamore cells based on the interconnections of the macromolecular components. Plant Physiol. 1973, 51, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- Sørensen, I.; Willats, W.G. Screening and characterization of plant cell walls using carbohydrate microarrays. Methods Mol. Biol. 2011, 715, 115–121. [Google Scholar] [CrossRef]
- York, W.S.; Darvill, A.G.; McNeil, M.; Stevenson, T.T.; Albersheim, P. Isolation and characterization of plant cell walls and cell wall components. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1986; Volume 118, pp. 3–40. [Google Scholar]
- Moore, J.P.; Nguema-Ona, E.; Fangel, J.U.; Willats, W.G.T.; Hugo, A.; Vivier, M.A. Profiling the main cell wall polysaccharides of grapevine leaves using high-throughput and fractionation methods. Carbohydr. Polym. 2014, 99, 190–198. [Google Scholar] [CrossRef]
- Moore, J.P.; Zhang, S.L.; Nieuwoudt, H.; Divol, B.; Trygg, J.; Bauer, F.F. A multivariate approach using attenuated total reflectance mid-infrared spectroscopy to measure the surface mannoproteins and β-Glucans of yeast cell walls during wine fermentations. J. Agric. Food Chem. 2015, 63, 10054–10063. [Google Scholar] [CrossRef]
- Lerouxel, O.; Siang Choo, T.; Séveno, M.; Usadel, B.; Faye, L.; Lerouge, P.; Pauly, M. Rapid structural phenotyping of plant cell wall mutants by enzymatic oligosaccharide fingerprinting. Plant Physiol. 2002, 130, 1754–1763. [Google Scholar] [CrossRef]
- Zietsman, A.J.J.; Moore, J.P.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A. Profiling the hydrolysis of isolated grape berry skin cell walls by purified enzymes. J. Agric. Food Chem. 2015, 63, 8267–8274. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A.; Moore, J.P. Dissecting the polysaccharide-rich grape cell wall matrix using recombinant pectinases during winemaking. Carbohydr. Polym. 2016, 152, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Zietsman, A.J.J.; Moore, J.P.; Fangel, J.U.; Willats, W.G.T.; Trygg, J.; Vivier, M.A. Following the compositional changes of fresh grape skin cell walls during the fermentation process in the presence and absence of maceration enzymes. J. Agric. Food Chem. 2015, 63, 2798–2810. [Google Scholar] [CrossRef] [PubMed]
- Zietsman, A.J.J.; Moore, J.P.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A. Combining hydrothermal pretreatment with enzymes de-pectinates and exposes the inner most xyloglucan-rich hemicellulose layers of wine grape pomace. Food Chem. 2017, 232, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Bañuelos, G.; Buica, A.; Schückel, J.; Zietsman, A.J.J.; Willats, W.G.T.; Moore, J.P.; Du Toit, W.J. Investigating the relationship between grape cell wall polysaccharide composition and the extractability of phenolic compounds into Shiraz wines. Part I: Vintage and ripeness effects. Food Chem. 2018. [Google Scholar] [CrossRef]
- Garrido-Bañuelos, G.; Buica, A.; Schückel, J.; Zietsman, A.J.J.; Willats, W.G.T.; Moore, J.P.; Du Toit, W.J. Investigating the relationship between cell wall polysaccharide composition and the extractability of grape phenolic compounds into Shiraz wines. Part II: Extractability during fermentation into wines made from grapes of different ripeness levels. Food Chem. 2018. [Google Scholar] [CrossRef]
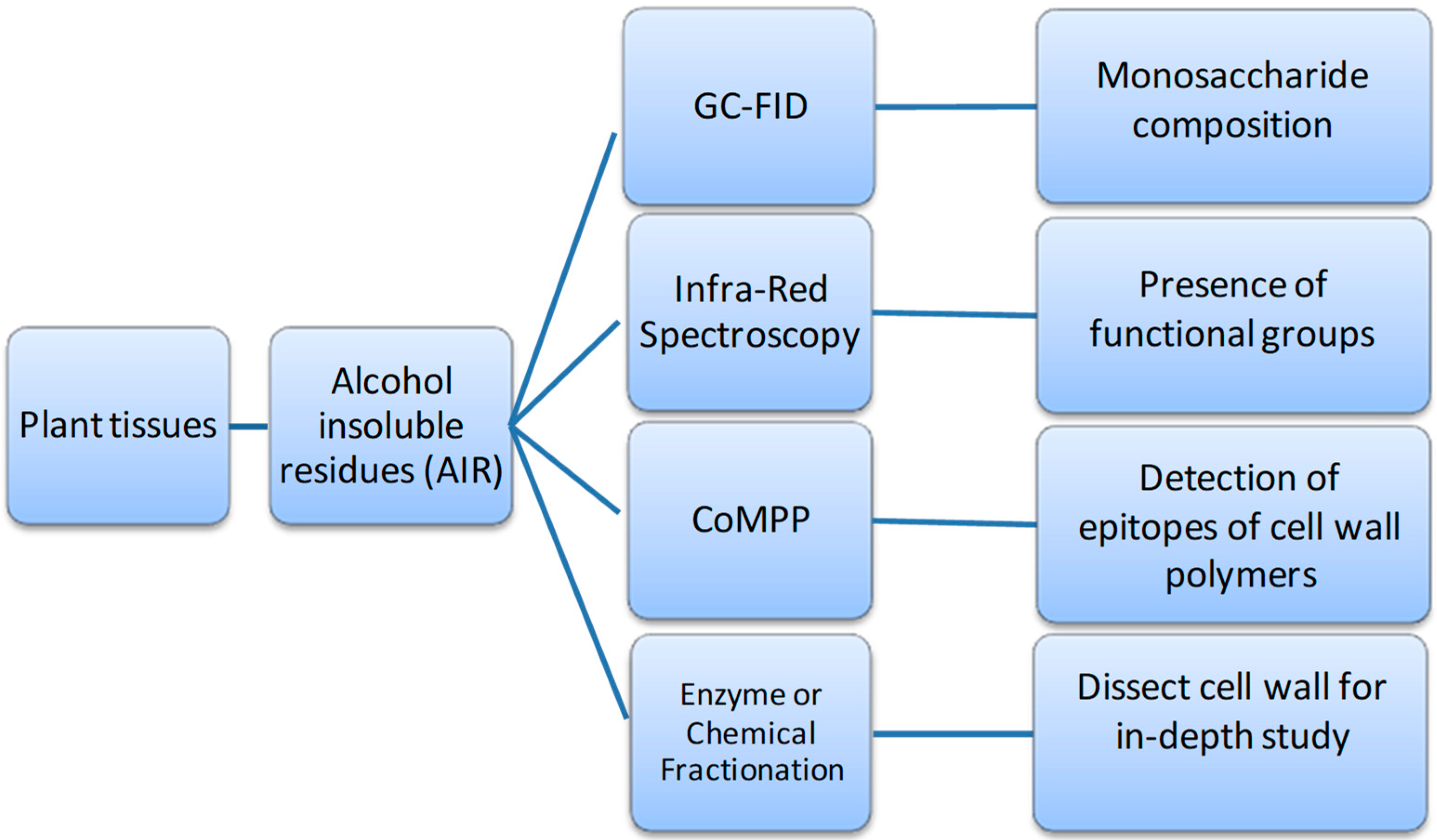
| Category | mAbs/CBMs | Epitope Recognition | Reference |
|---|---|---|---|
| HG | JIM5 | HG with a low DE (mAb JIM5) | Clausen et al., 2003 |
| JIM7 | HG with a high DE (mAb JIM7) | Clausen et al., 2003 | |
| LM18 | HG Partially methylesterified (mAb LM18) | Verhertbrugger et al., 2009 | |
| LM19 | HG Partially methylesterified (mAb LM19) | Verhertbrugger et al., 2009 | |
| LM20 | HG Partially methylesterified (mAb LM20) | Verhertbrugger et al., 2009 | |
| 2F4 | HG Ca2+ crosslinked (mAb 2F4) | Ralet et al., 2010 | |
| LM8 | Xylogalacturonan (mAb LM8) | Ralet et al., 2010 | |
| RGI | INRA-RU1 | Backbone of rhamnogalacturonan I (mAb INRA-RU1) | Ralet et al., 2010 |
| INRA-RU2 | Backbone of rhamnogalacturonan I (mAb INRA-RU2) | ||
| RGI side chains | LM5 | (1→4)-β-d-galactan (mAb LM5) | Jones et al., 1997 |
| LM6 | (1→5)-α-l-arabinan (mAb LM6) | Willats et al., 1998 | |
| LM13 | Linearised (1→5)-α-l-arabinan (mAb LM13) | Verhertbrugger et al., 2009 | |
| Mannan | LM21 | (1→4)-β-d-(galacto)(gluco)mannan (mAb LM21) | Marcus et al., 2009 |
| LM22 | (1→4)-β-d-(gluco)mannan (mAb LM22) | ||
| Glucan, xyloglucan | BS-400-2 | (1→3)-β-d-glucan (mAb BS-400-2) | Meikle et al., 1991 |
| LM15 | Xyloglucan (XXXG motif) (mAb LM15) | Marcus et al., 2008 | |
| LM25 | Xyloglucan/unsibstituted β-d-glucan (mAb LM25) | Pedersen et al., 2012 | |
| Xylan/cellulose | LM10 | (1→4)-β-d-xylan (mAb LM10) | McCartney et al., 2005 |
| LM11 | (1→4)-β-d-xylan/arabinoxylan (mAb LM11) | ||
| CBM3a | Celulose (crystalline) (CBM3a) | Tormo et al., 1996 | |
| Extensins | LM1 | Extensin (mAb LM1) | Smallwood et al., 1995 |
| JIM11 | Extensin (mAb JIM11) | ||
| JIM20 | Extensin (mAb JIM20) | ||
| AGP | JIM8 | AGP (mAb JIM8) | McCabe et al., 1997 |
| JIM13 | AGP (mAb JIM13) | Knox et al., 1991 | |
| LM14 | AGP (mAb LM14) | Moller et al., 2008 | |
| LM2 | AGP, β-linked GlcA (mAb LM2) | Smallwood et al., 1996 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zietsman, A.J.J.; Vivier, M.A.; Moore, J.P. Deconstructing Wine Grape Cell Walls with Enzymes During Winemaking: New Insights from Glycan Microarray Technology. Molecules 2019, 24, 165. https://doi.org/10.3390/molecules24010165
Gao Y, Zietsman AJJ, Vivier MA, Moore JP. Deconstructing Wine Grape Cell Walls with Enzymes During Winemaking: New Insights from Glycan Microarray Technology. Molecules. 2019; 24(1):165. https://doi.org/10.3390/molecules24010165
Chicago/Turabian StyleGao, Yu, Anscha J. J. Zietsman, Melané A. Vivier, and John P. Moore. 2019. "Deconstructing Wine Grape Cell Walls with Enzymes During Winemaking: New Insights from Glycan Microarray Technology" Molecules 24, no. 1: 165. https://doi.org/10.3390/molecules24010165
APA StyleGao, Y., Zietsman, A. J. J., Vivier, M. A., & Moore, J. P. (2019). Deconstructing Wine Grape Cell Walls with Enzymes During Winemaking: New Insights from Glycan Microarray Technology. Molecules, 24(1), 165. https://doi.org/10.3390/molecules24010165







