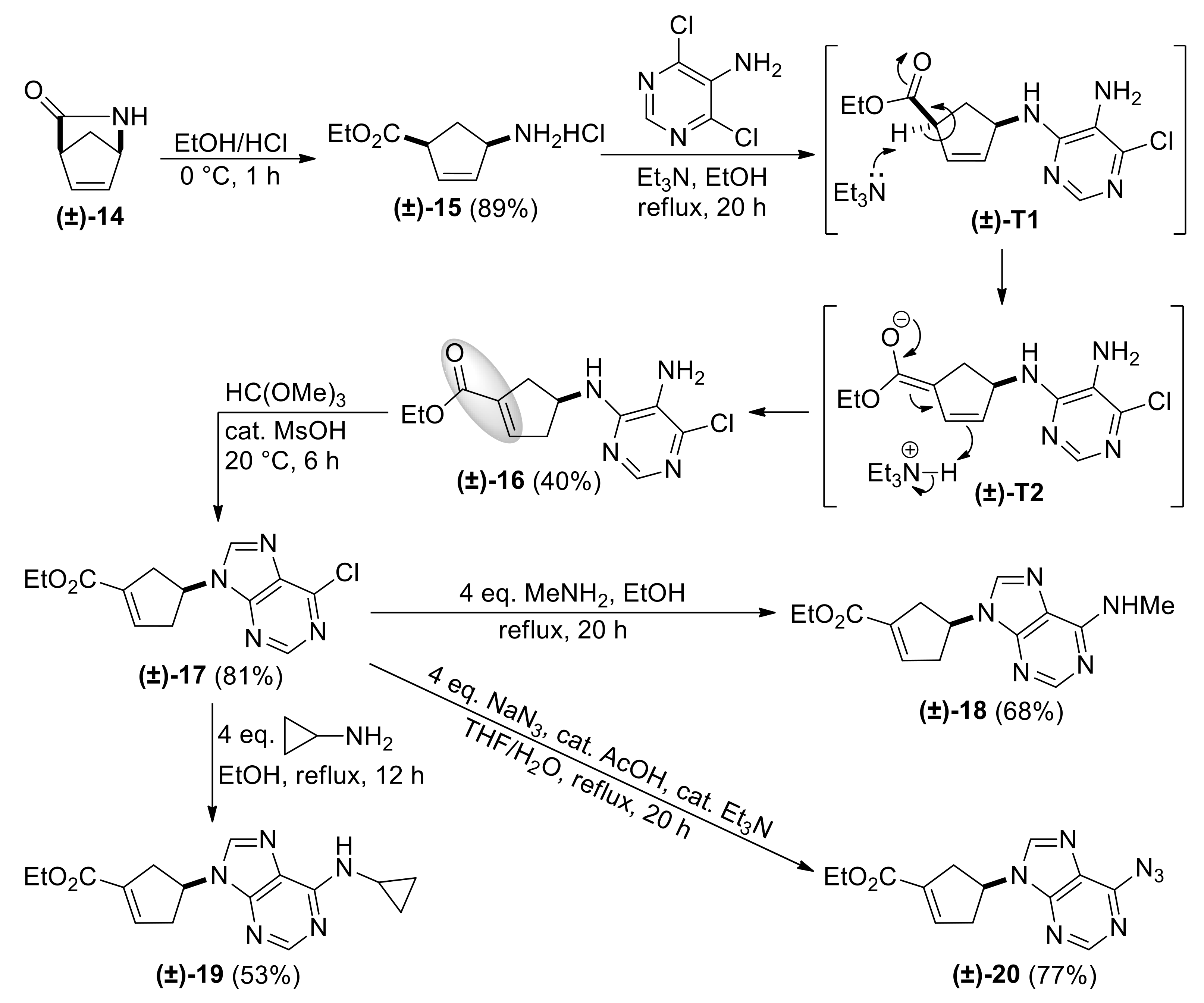
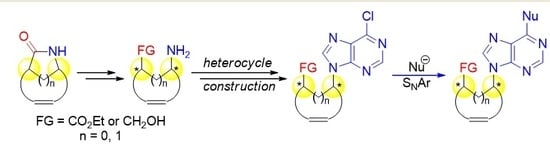
4.2. Synthesis of Methylamino Compound (±)-18
To a solution of 6-chloropurinyl nucleoside analogue (±)-17 (150 mg) in EtOH (10 mL), MeNH2 (4 eq.) was added. After heating under reflux for 20 h, the reaction mixture was concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (eluent: n-hexane-EtOAc 1:1).
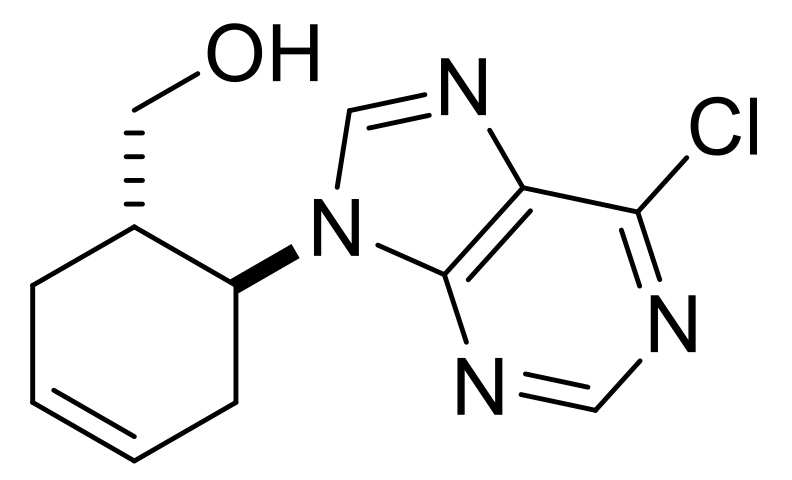
![Molecules 24 00161 i001 Molecules 24 00161 i001]() |
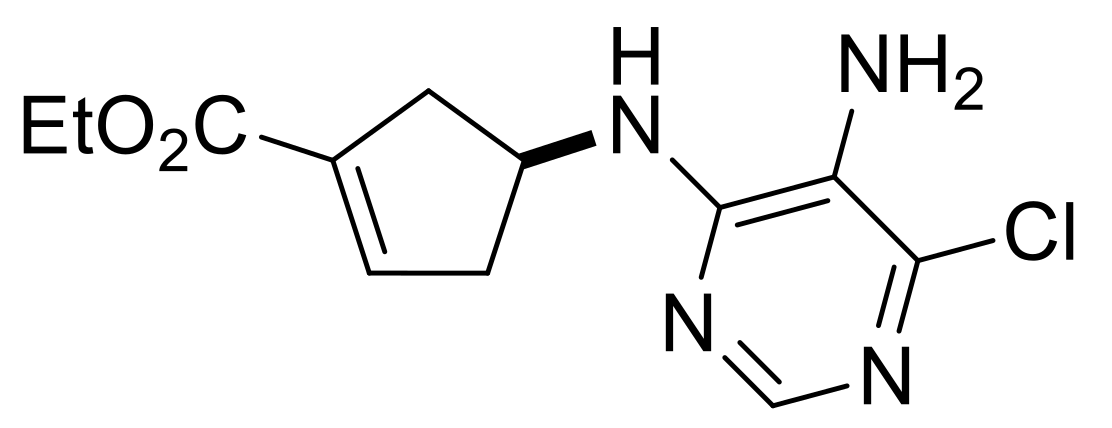
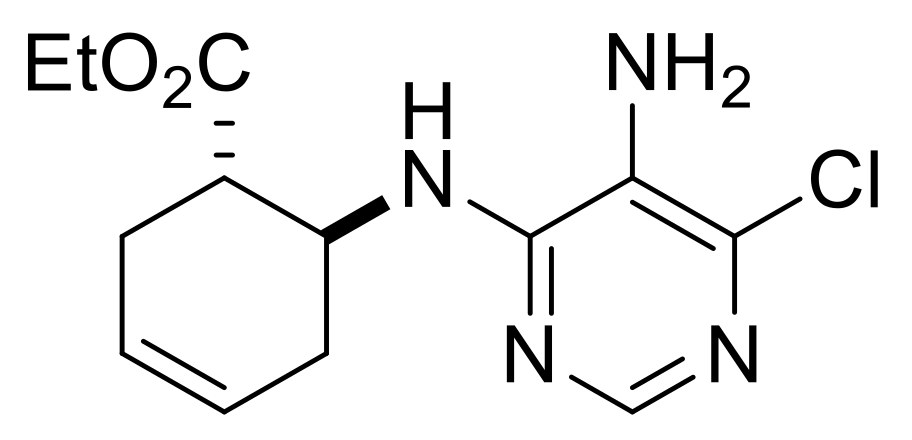
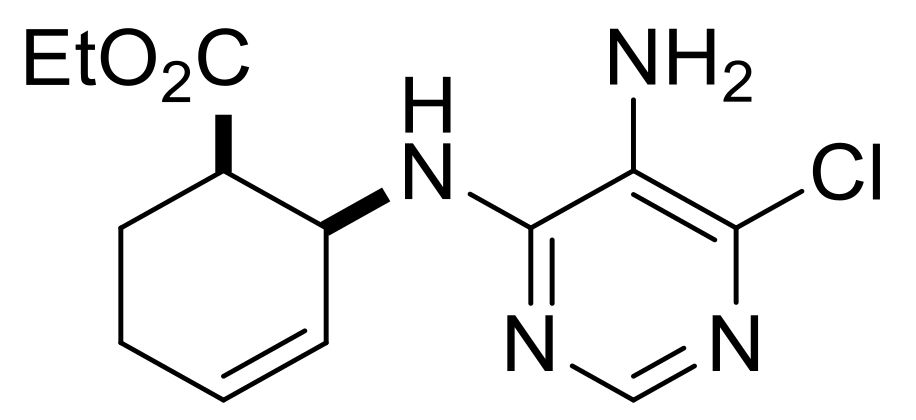
| Ethyl (S*)-4-((5-amino-6-chloropyrimidin-4-yl)amino)cyclopent-1-ene-1-carboxylate, (±)-16. |
Brownish white solid, m.p. 121–123 °C, 40%; 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 1.30 (t, 3H, CH3, J = 7.14 Hz), 2.43–2.59 (m, 2H, CH2), 3.00–3.16 (m, 2H, CH2), 3.44 (brs, 2H, NH2), 4.17–4.25 (m, 2H, OCH2), 4.79–4.86 (m, 1H, H-4), 5.08 (d, 1H, N-H, J = 5.76 Hz), 6.76-6.78 (m, 1H, H-2), 8.08 (s, 1H, Ar-H); 13C-NMR (DMSO, 100 MHz): δ (ppm) = 15.0, 39.3, 41.2, 51.3, 60.7, 124.6, 135.1, 137.7, 142.6, 146.4, 152.0, 164.9; MS (ES, pos) m/z = 283 (M + 1).
![Molecules 24 00161 i002 Molecules 24 00161 i002]() |
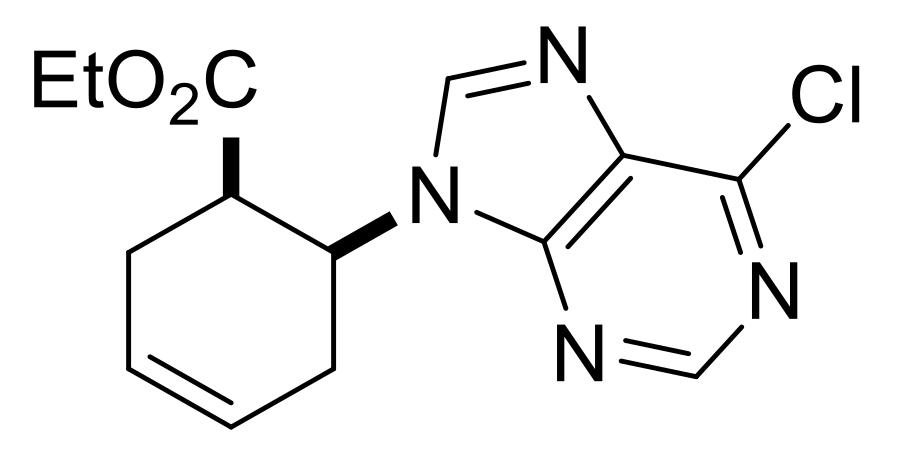
| Ethyl (S*)-4-(6-chloro-9H-purin-9-yl)cyclopent-1-ene-1-carboxylate, (±)-17. |
Yellowish white solid, m.p. 83–85 °C, 81%; 1H-NMR (DMSO, 400 MHz): δ = 1.20 (t, 3H, CH3, J = 7.08 Hz), 2.93–3.05 (m, 2H, CH2), 3.06–3.20 (m, 2H, CH2), 4.09–4.17 (m, 2H, OCH2), 5.38–5.47 (m, 1H, H-4), 6.70–6.81 (m, 1H, H-2), 8.69 (s, 1H, Ar-H), 8.74 (s, 1H, Ar-H); 13C-NMR (CDCl3, 100 MHz): δ (ppm) = 14.6, 39.2, 40.9, 54.2, 61.2, 132.2, 135.5, 140.1, 143.5, 151.6, 151.8, 152.3, 164.2; MS (ES, pos) m/z = 293 (M + 1).
![Molecules 24 00161 i003 Molecules 24 00161 i003]() |
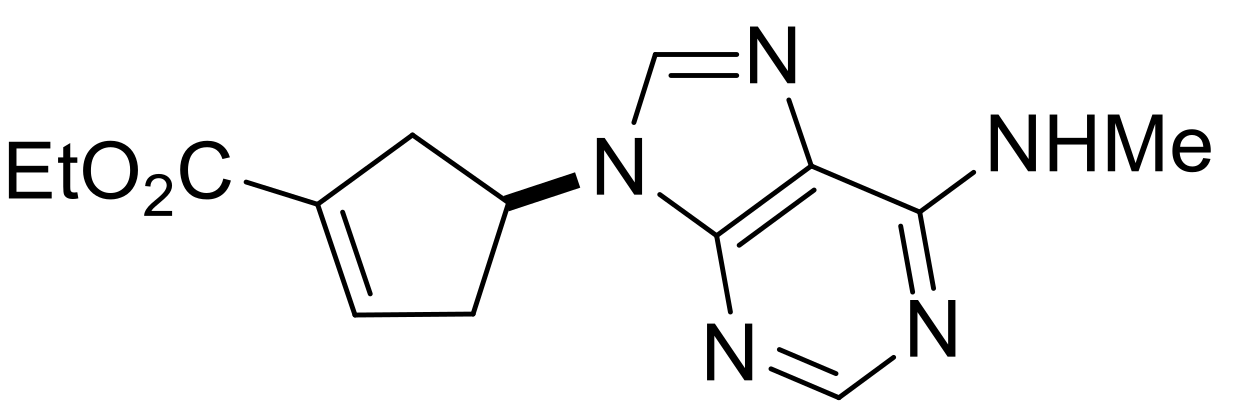
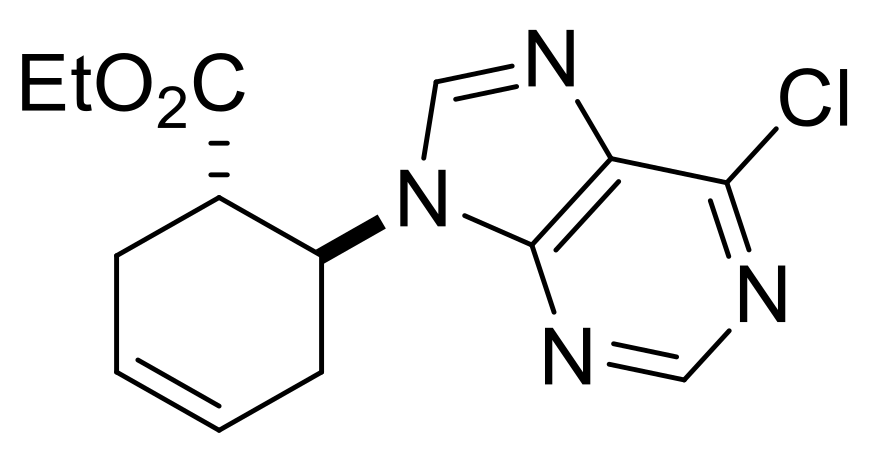
| Ethyl (S*)-4-(6-(methylamino)-9H-purin-9-yl)cyclopent-1-ene-1-carboxylate, (±)-18. |
Yellow oil, 68%; 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 1.32 (t, 3H, CH3, J = 7.12 Hz), 2.86–3.03 (m, 2H, CH2), 3.10–3.29 (m, 5H, NCH3 and CH2), 4.20–4.31 (m, 2H, OCH2), 5.41–5.50 (m, 1H, H-4), 6.16 (brs, 1H, N-H), 6.86–6.89 (m, 1H, H-2), 7.75 (s, 1H, Ar-H), 8.41 (s, 1H, Ar-H); 13C-NMR (CDCl3, 100 MHz): δ (ppm) = 14.6, 39.3, 41.1, 50.6, 58.9, 61.1, 120.4, 135.5, 136.1, 137.7, 140.5, 153.4, 155.9, 164.5; MS (ES, pos) m/z = 288 (M + 1).
![Molecules 24 00161 i004 Molecules 24 00161 i004]() |
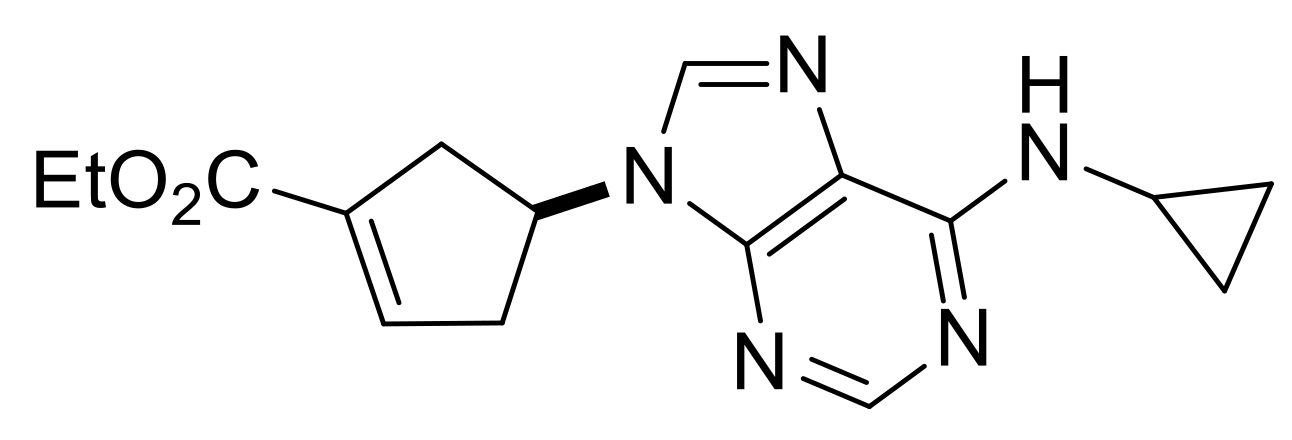
| Ethyl (S*)-4-(6-(cyclopropylamino)-9H-purin-9-yl)cyclopent-1-ene-1-carboxylate, (±)-19. |
Yellow oil, 53%; 1H-NMR (CDCl3, 500 MHz): δ (ppm) = 0.64–0.69 (m, 2H, CH2), 0.90–0.97 (m, 2H, CH2), 1.32 (t, 3H, CH3, J = 7.13 Hz), 2.86–3.08 (m, 3H, CH2), 3.18–3.32 (m, 2H, CH2, CH), 4.21–4.28 (m, 2H, OCH2), 5.35–5.43 (m, 1H, H-4), 6.03 (brs, 1H, N-H), 6.86-6.91 (m, 1H, H-2), 7.75 (s, 1H, Ar-H), 8.48 (s, 1H, Ar-H); 13C-NMR (CDCl3, 126 MHz): δ (ppm) = 7.4, 14.3, 23.7, 38.9, 40.8, 52.9, 60.7, 119.9, 135.1, 137.6, 140.2, 153.2, 155.8, 164.1; MS (ES, pos) m/z = 314 (M + 1).
![Molecules 24 00161 i005 Molecules 24 00161 i005]() |
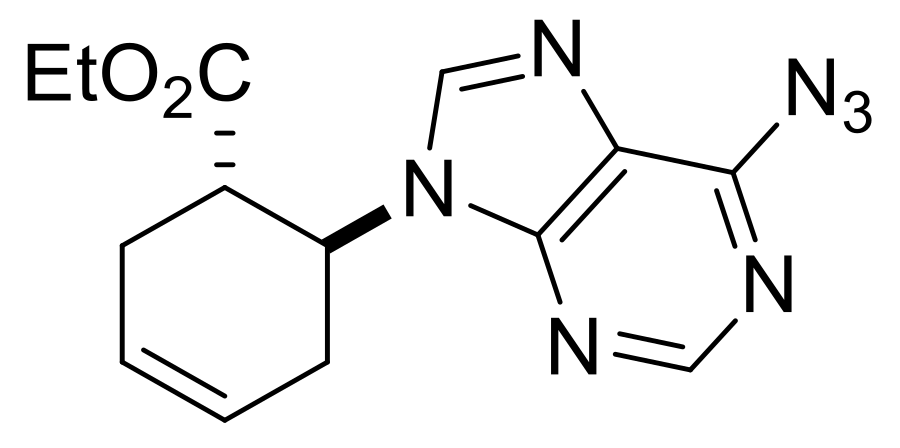
| Ethyl (S*)-4-(6-azido-9H-purin-9-yl)cyclopent-1-ene-1-carboxylate, (±)-20. |
White solid, m.p. 145–147 °C, 68%; 1H-NMR (DMSO, 400 MHz): δ (ppm) = 1.22 (t, 3H, CH3, J = 7.08 Hz), 2.97–3.09 (m, 2H, CH2), 3.22–3.28 (m, 2H, CH2), 4.12–4.23 (m, 2H, OCH2), 5.53–5.64 (m, 1H, H-4), 6.86–6.89 (m, 1H, H-2), 8.66 (s, 1H, Ar-H), 10.07 (s, 1H, Ar-H); 13C-NMR (DMSO, 100 MHz): δ (ppm) = 15.0, 34.9, 39.2, 55.1, 61.0, 121.2, 134.5, 136.3, 141.8, 142.8, 143.8, 146.3, 164.5; MS (ES, pos) m/z = 300 (M + 1).
![Molecules 24 00161 i006 Molecules 24 00161 i006]() |
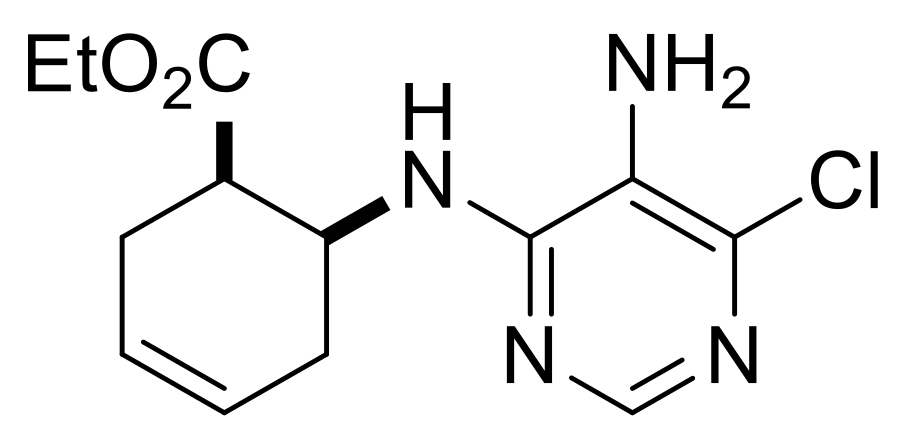
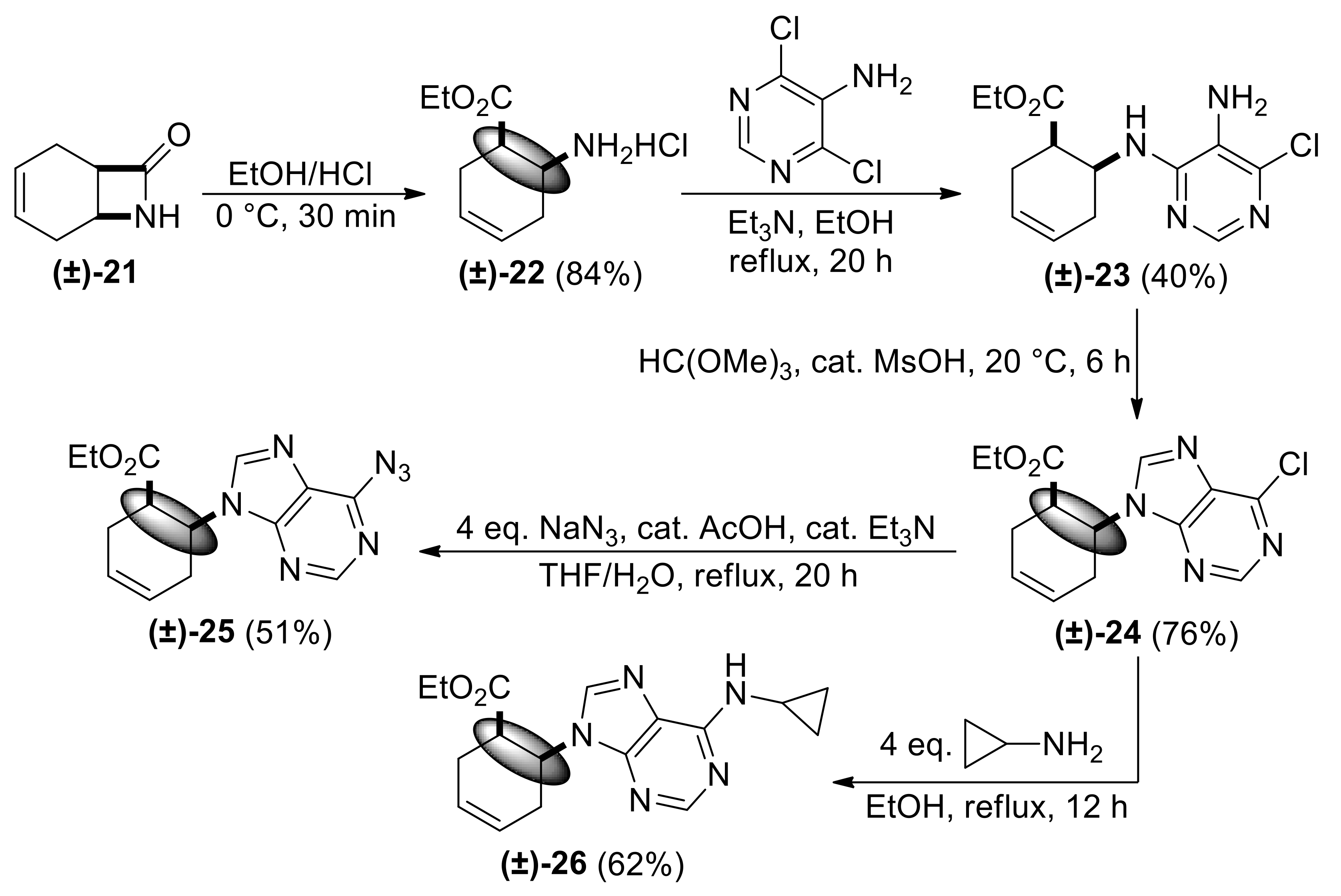
| Ethyl (1R*,6S*)-6-((5-amino-6-chloropyrimidin-4-yl)amino)cyclohex-3-ene-1-carboxylate, (±)-23. |
White solid, m.p. 120–121 °C, 42%; 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 1.28 (t, 3H, CH3, J = 7.12 Hz), 2.26–2.38 (m, 1H, CH2), 2.42–2.56 (m, 2H, CH2), 2.62–2.73 (m, 1H, CH2), 2.95–3.03 (m, 1H, H-1), 3.39 (brs, 2H, NH2), 4.11–4.24 (m, 2H, OCH2), 4.74–4.85 (m, 1H, H-6), 5.66–5.87 (m, 3H, H-3, H-4, N-H), 8.08 (s, 1H, Ar-H); 13C-NMR (DMSO, 100 MHz): δ (ppm) = 14.8, 25.5, 30.4, 41.4, 47.1, 60.6, 124.6, 125.4, 125.9, 138.0, 146.1, 152.2, 173.4; MS (ES, pos) m/z = 297 (M + 1), 299 (M + 3).
![Molecules 24 00161 i007 Molecules 24 00161 i007]() |
| Ethyl (1R*,6S*)-6-(6-chloro-9H-purin-9-yl)cyclohex-3-ene-1-carboxylate, (±)-24. |
Yellow oil, 76%. 1H-NMR (DMSO, 400 MHz): δ (ppm) = 0.98 (t, 3H, CH3, J = 7.12 Hz), 2.31–2.52 (m, 2H, CH2), 2.74–2.84 (m, 2H, CH2), 3.28–3.33 (m, 1H, H-1), 3.83–3.90 (m, 2H, OCH2), 5.25–5.32 (m, 1H, H-6), 5.85–5.89 (m, 2H, H-3, H-4), 8.56 (s, 1H, Ar-H), 8.78 (s, 1H, Ar-H). 13C-NMR (DMSO, 100 MHz): δ (ppm) = 14.5, 25.4, 29.3, 42.0, 51.2, 61.2, 125.3, 126.4, 131.2, 146.6, 149.9, 152.2, 153.0, 172.4; MS (ES, pos) m/z = 307 (M + 1).
![Molecules 24 00161 i008 Molecules 24 00161 i008]() |
| Ethyl (1R*,6S*)-6-(6-azido-9H-purin-9-yl)cyclohex-3-ene-1-carboxylate, (±)-25. |
White solid, m.p. 130–132 °C, 51%; 1H-NMR (DMSO, 400 MHz): δ (ppm) = 1.03 (t, 3H, CH3, J = 7.10 Hz), 2.34–2.65 (m, 2H, CH2), 2.78–3.00 (m, 2H, CH2), 3.31–3.43 (m, 1H, H-1), 3.82–4.00 (m, 2H, OCH2), 5.40-5.48 (m, 1H, H-6), 8.54 (s, 1H, Ar-H), 10.13 (s, 1H, Ar-H); 13C-NMR (DMSO, 100 MHz): δ (ppm) = 14.6, 25.4, 29.7, 42.3, 51.5, 61.3, 125.3, 126.4, 129.4, 130.4, 135.3, 142.8, 147.9, 170.5; MS (ES, pos) m/z = 314 (M + 1).
![Molecules 24 00161 i009 Molecules 24 00161 i009]() |
| Ethyl (1R*,6S*)-6-(6-(cyclopropylamino)-9H-purin-9-yl)cyclohex-3-ene-1-carboxylate, (±)-26. |
White solid, m.p. 128–129 °C, 62%; 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 0.61–0.65 (m, 2H, CH2), 0.88–0.93 (m, 2H, CH2), 1.20 (t, 3H, CH3), 2.48–2.55 (m, 2H, CH2), 2.70–2.74 (m, 2H, CH2), 2.99–3.04 (m, 1H, H-1), 3.14–3.20 (m, 1H, CH), 3.97–4.08 (m, 2H, OCH2), 5.30–5.38 (m, 1H, H-6), 5.91–5.98 (m, 2H, H-3, H-4), 6.04 (brs, 1H, N-H), 7.98 (s, 1H, Ar-H), 8.44 (s, 1H, Ar-H); 13C-NMR (CDCl3, 126 MHz): δ (ppm) = 7.4, 13.9, 23.7, 25.2, 29.8, 42.0, 49.2, 61.0, 119.3, 124.8, 125.9, 139.0, 148.6, 153.0, 155.7, 172.1; MS (ES, pos) m/z = 328 (M + 1).
![Molecules 24 00161 i010 Molecules 24 00161 i010]() |
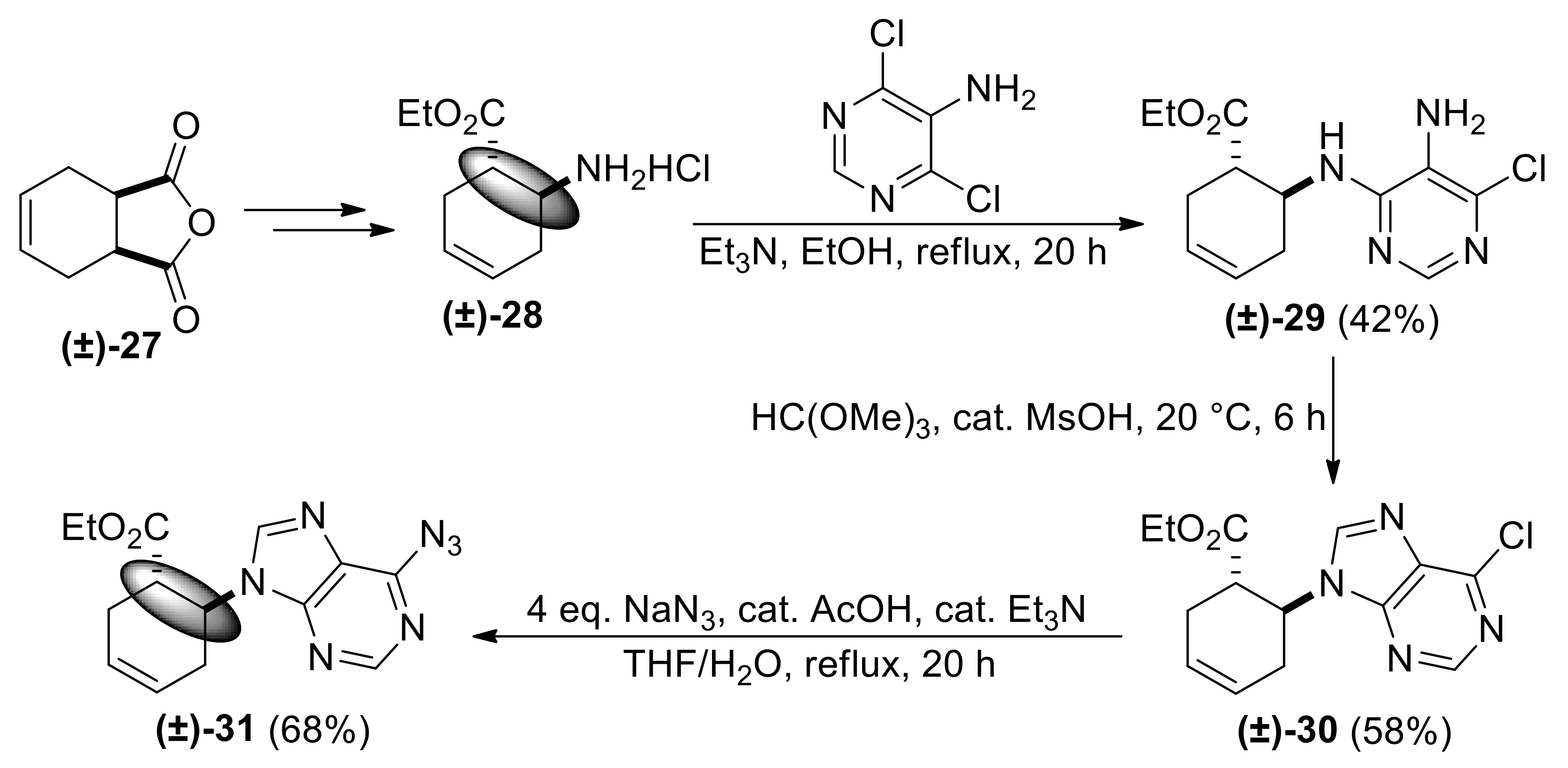
| Ethyl (1S*,6S*)-6-((5-amino-6-chloropyrimidin-4-yl)amino)cyclohex-3-ene-1-carboxylate, (±)-29. |
White solid, m.p. 120–121 °C, 42%; 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 1.19 (t, 3H, CH3, J = 7.12 Hz), 2.03–2.12 (m, 1H, CH2), 2.34–2.43 (m, 1H, CH2), 2.56–2.75 (m, 2H, CH2), 2.83–2.92 (m, 1H, H-1), 3.47 (brs, 2H, NH2), 4.06–4.16 (m, 2H, OCH2), 4.62–4.69 (m, 1H, H-6), 5.13 (d, 1H, N-H, J = 8.12 Hz), 5.66–5.78 (m, 2H, H-3, H-4), 8.09 (s, 1H, Ar-H); 13C-NMR (CDCl3, 100 MHz): δ (ppm) = 14.5, 27.5, 31.9, 45.2, 48.5, 61.3, 122.1, 124.2, 125.1, 143.7, 150.1, 154.8, 174.1; MS (ES, pos) m/z = 297 (M + 1).
![Molecules 24 00161 i011 Molecules 24 00161 i011]() |
| Ethyl (1S*,6S*)-6-(6-chloro-9H-purin-9-yl)cyclohex-3-ene-1-carboxylate, (±)-30. |
Colorless oil, 58%; 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 0.96 (t, 3H, CH3, J = 7.12 Hz), 2.53–2.68 (m, 3H, CH2), 3.01–3.07 (m, 1H, CH2), 3.56–3.66 (m, 1H, H-1), 3.86–3.96 (m, 2H, OCH2), 4.94–5.03 (m, 1H, H-6), 5.78−5.89 (m, 2H, H-3, H-4), 8.17 (s, 1H, Ar-H), 8.76 (s, 1H, Ar-H); 13C-NMR (CDCl3, 100 MHz): δ (ppm) = 14.2, 29.0, 30.7, 44.1, 54.5, 61.4, 124.4, 125.6, 132.2, 145.3, 151.5, 151.9, 152.1, 173.0; MS (ES, pos) m/z = 307 (M + 1).
![Molecules 24 00161 i012 Molecules 24 00161 i012]() |
| Ethyl (1S*,6S*)-6-(6-azido-9H-purin-9-yl)cyclohex-3-ene-1-carboxylate, (±)-31. |
White solid, m.p. 140–141 °C, 68%; 1H-NMR (DMSO, 400 MHz): δ (ppm) = 0.74 (t, 3H, CH3, J = 7.08 Hz), 2.46–2.57 (m, 3H, CH2), 2.86–3.00 (m, 1H, CH2), 3.60–3.66 (m, 1H, H-1), 3.69–3.76 (m, 2H, OCH2), 5.08–5.15 (m, 1H, H-6), 5.80–5.92 (m, 2H, H-3, H-4), 8.79 (s, Ar-H), 10.13 (s, 1H, Ar-H). 13C-NMR (DMSO, 100 MHz): δ (ppm) = 14.3, 29.3, 31.9, 44.7, 54.3, 61.1, 125.1, 125.9, 136.5, 139.3, 142.9, 144.6, 146.3, 173.1. MS (ES, pos) m/z = 314 (M + 1).
![Molecules 24 00161 i013 Molecules 24 00161 i013]() |
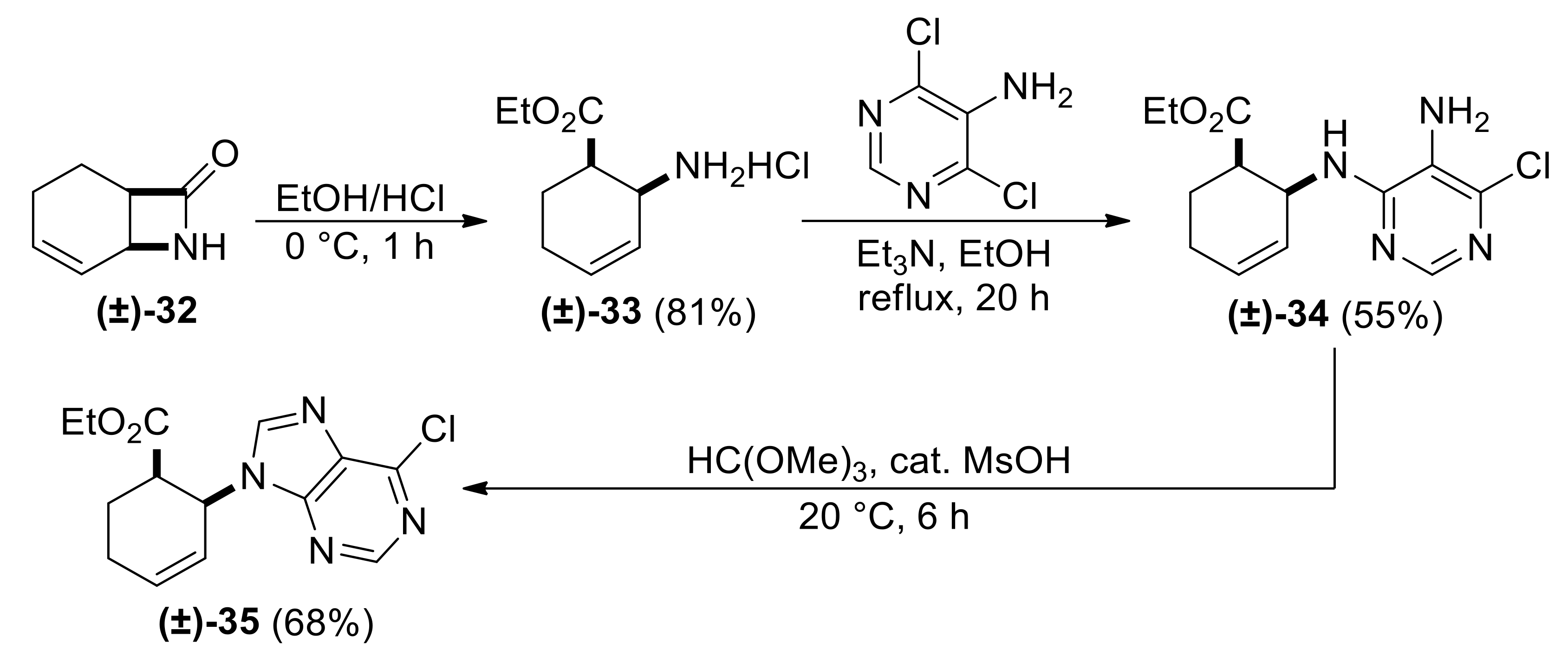
| Ethyl (1R*,2S*)-2-((5-amino-6-chloropyrimidin-4-yl)amino)cyclohex-3-ene-1-carboxylate, (±)-34. |
Brown oil, 55%; 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 1.20 (t, 3H, CH3, J = 7.14 Hz), 1.99–2.19 (m, 4H, CH2), 2.99–3.05 (m, 1H, H-1), 4.00–4.17 (m, 2H, OCH2), 5.23–5.26 (m, 1H, H-2), 5.59 (d, 1H, N-H, J = 9.04 Hz), 5.70–5.78 (m, 1H, H-4), 5.87–5.92 (m, 1H, H-3), 8.06 (s, 1H, Ar-H), 13C-NMR (CDCl3, 100 MHz): δ (ppm) = 14.5, 22.7, 23.6, 43.4, 46.9, 61.1, 122.4, 127.5, 130.0, 143.4, 149.7, 154.6, 174.2; MS (ES, pos) m/z = 297 (M + 1).
![Molecules 24 00161 i014 Molecules 24 00161 i014]() |
| Ethyl (1R*,2S*)-2-(6-chloro-9H-purin-9-yl)cyclohex-3-ene-1-carboxylate, (±)-35. |
Brown solid, m.p. 119–121 °C, 68%; 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 1.03 (t, 3H, CH3, J = 7.14 Hz), 2.00–2.08 (m, 2H, CH2), 2.27–2.33 (m, 1H, CH2), 2.41–2.46 (m, 1H, CH2), 3.12–3.20 (m, 1H, H-1), 3.72–3.87 (m, 2H, OCH2), 5.67–5.70 (m, 1H, H-2), 5.83–5.90 (m, 1H, H-4), 6.28–6.33 (m, 1H, H-3), 8.23 (s, 1H, Ar-H), 8.73 (s, 1H, Ar-H); 13C-NMR (DMSO, 100 MHz): δ (ppm) = 14.3, 20.3, 24.4, 44.1, 50.1, 61.0, 123.1, 134.8, 147.5, 147.8, 149.9, 152.3, 152.9, 172.4; MS (ES, pos) m/z = 307 (M + 1), 309 (M + 3).
![Molecules 24 00161 i015 Molecules 24 00161 i015]() |
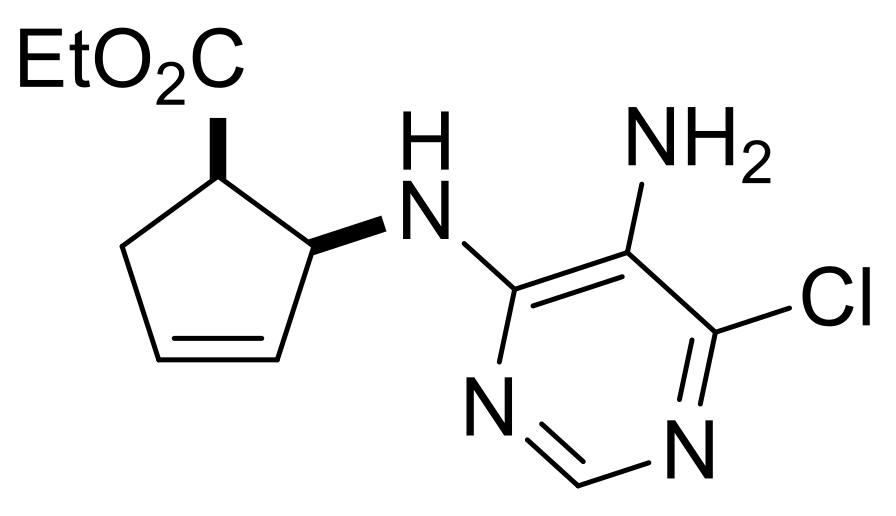
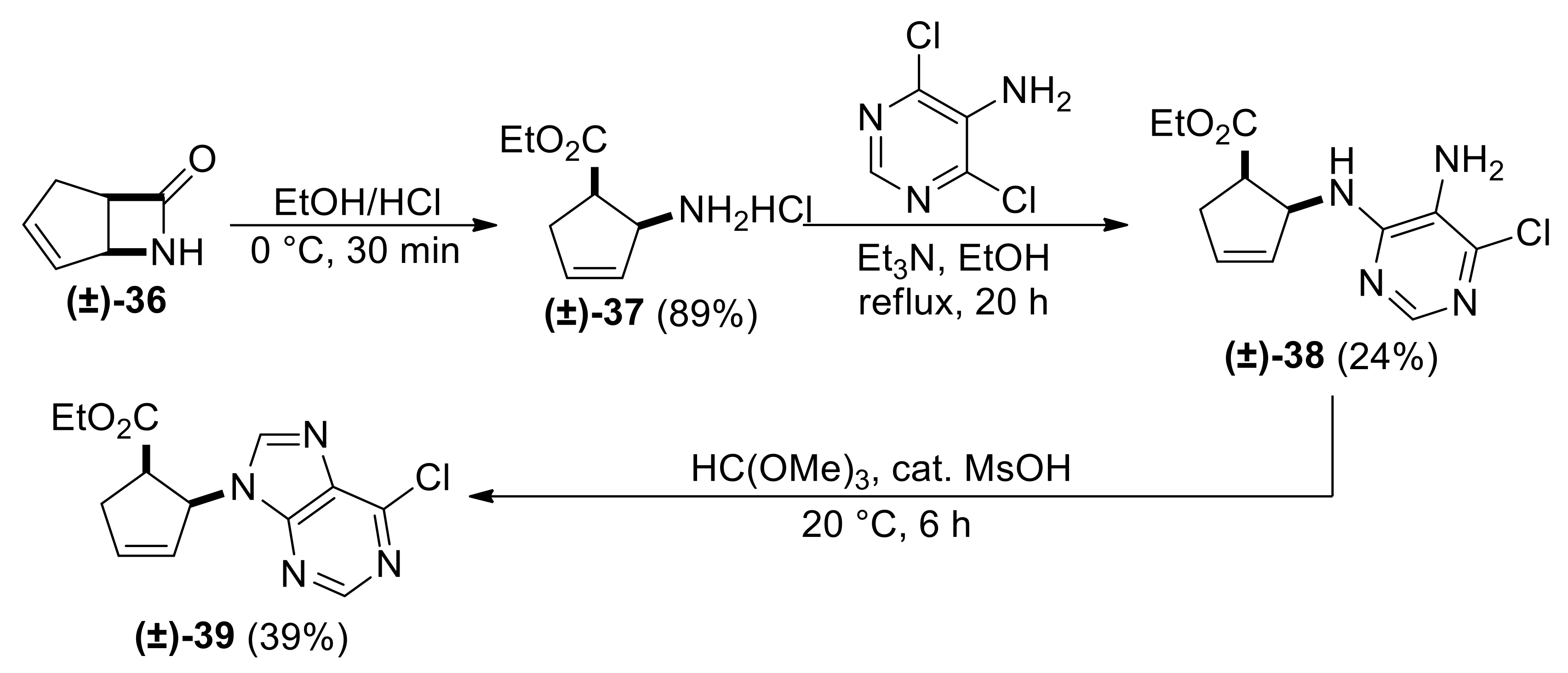
| Ethyl (1R*,2S*)-2-((5-amino-6-chloropyrimidin-4-yl)amino)cyclopent-3-ene-1-carboxylate, (±)-38. |
Brownish white solid, m.p. 104–106 °C, 34%; 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 1.04 (t, 3H, CH3, J = 7.12 Hz), 2.56–2.67 (m, 1H, CH2), 2.83–2.90 (m, 1H, CH2), 3.44–3.54 (m, 1H, H-1), 3.57 (brs, 2H, NH2), 3.85–4.02 (m, 2H, OCH2), 5.34 (d, 1H, N-H, J = 8.76 Hz), 5.69–5.76 (m, 2H, H-2, H-4), 5.98–6.01 (m, 1H, H-3), 8.06 (s, 1H, Ar-H); 13C-NMR (CDCl3, 100 MHz): δ (ppm) = 14.2, 35.4, 46.4, 58.2, 61.1, 122.6, 130.2, 134.0, 143.0, 149.3, 154.1, 174.0; MS (ES, pos) m/z = 283 (M + 1), 285 (M + 3).
![Molecules 24 00161 i016 Molecules 24 00161 i016]() |
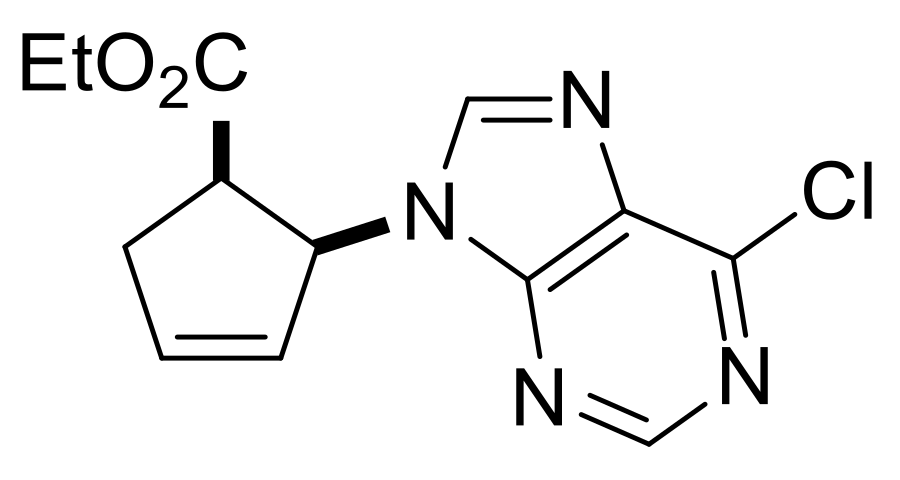
| Ethyl (1R*,2S*)-2-(6-chloro-9H-purin-9-yl)cyclopent-3-ene-1-carboxylate, (±)-39. |
Yellowish white solid, m.p. 118–119 °C, 39%; 1H-NMR (CDCl3, 400 MHz): δ (ppm) = 0.72 (t, 3H, CH3, J = 7.14 Hz), 2.76–2.85 (m, 1H, CH2), 3.14–3.23 (m, 1H, CH2), 3.49–3.56 (m, 1H, H-1), 3.65–3.77 (m, 2H, OCH2), 5.86–5.89 (m, 1H, H-2), 5.15–5.17 (m, 1H, H-4), 6.44–6.47 (m, 1H, H-3), 7.99–8.01 (m, 1H, Ar-H), 8.78–8.81 (m, 1H, Ar-H); 13C-NMR (CDCl3, 100 MHz): δ (ppm) = 13.8, 34.9, 47.2, 60.9, 61.4, 126.8, 134.7, 138.7, 150.0, 152.2, 152.3, 154.2, 170.9; MS (ES, pos) m/z = 293 (M + 1), 295 (M + 3).
![Molecules 24 00161 i017 Molecules 24 00161 i017]() |
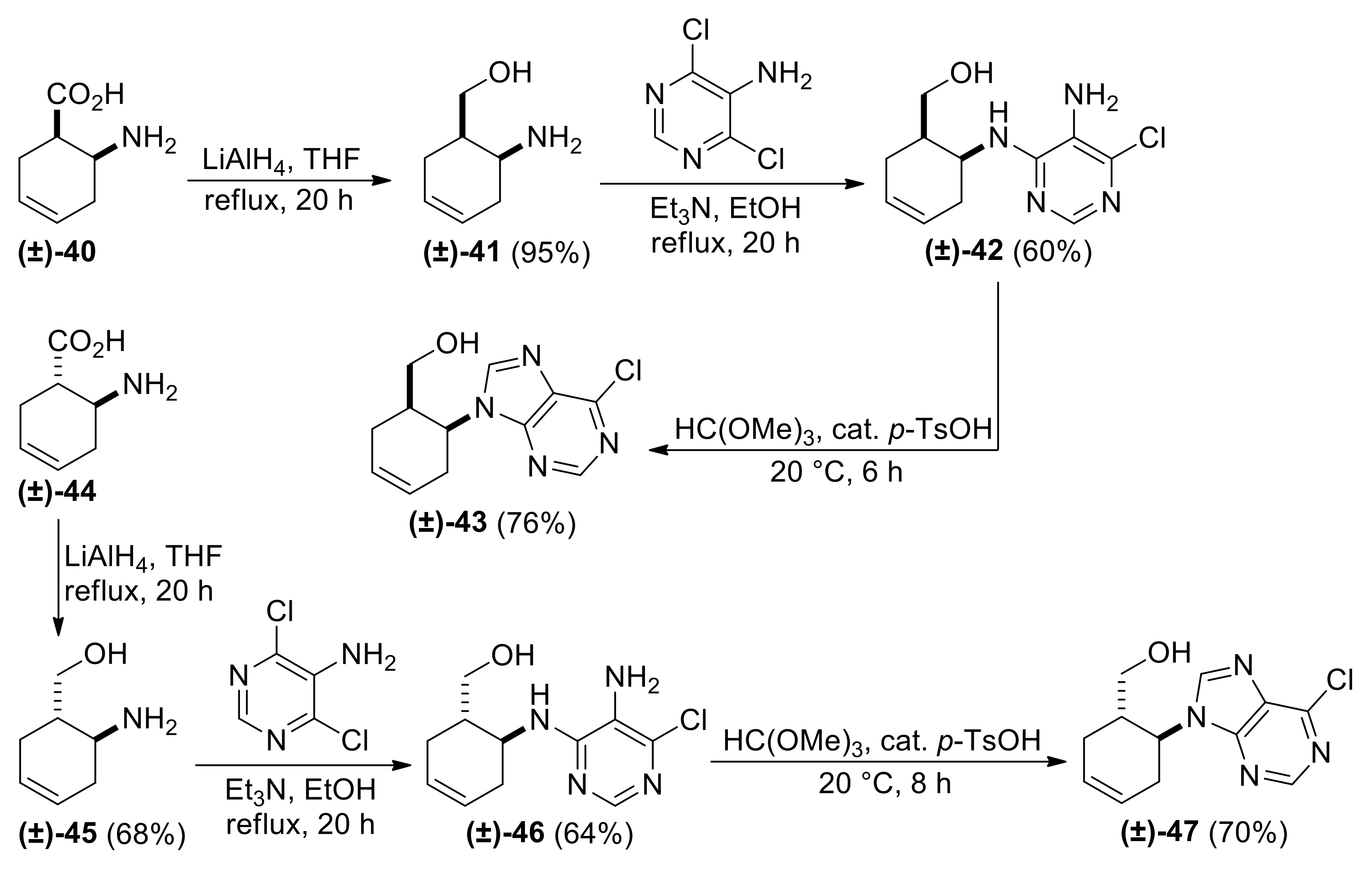
| ((1R*,6S*)-6-((5-Amino-6-chloropyrimidin-4-yl)amino)cyclohex-3-en-1-yl)methanol, (±)-42. |
White solid, m.p. 186–188 °C, 60%; 1H-NMR (DMSO, 400 MHz): δ (ppm) = 1.96–2.12 (m, 4H, CH2), 2.21–2.30 (m, 1H, H-1), 3.24–3.32 (m, 1H, OCH2), 3.40-3.49 (m, 1H, OCH2), 4.43–4.48 (m, 2H, H-6 and O-H), 5.16 (brs, 2H, N-H), 5.59–5.70 (m, 2H, H-3, H-4), 6.24 (d, 1H, N-H, J = 7.56 Hz), 7.68 (s, 1H, Ar-H); 13C-NMR (DMSO, 100 MHz): δ (ppm) = 25.9, 30.1, 39.8, 47.2, 61.4, 124.5, 125.6, 126.6, 138.0, 146.4, 152.6; MS (ES, pos) m/z = 255 (M + 1), 257 (M + 3).
![Molecules 24 00161 i018 Molecules 24 00161 i018]() |
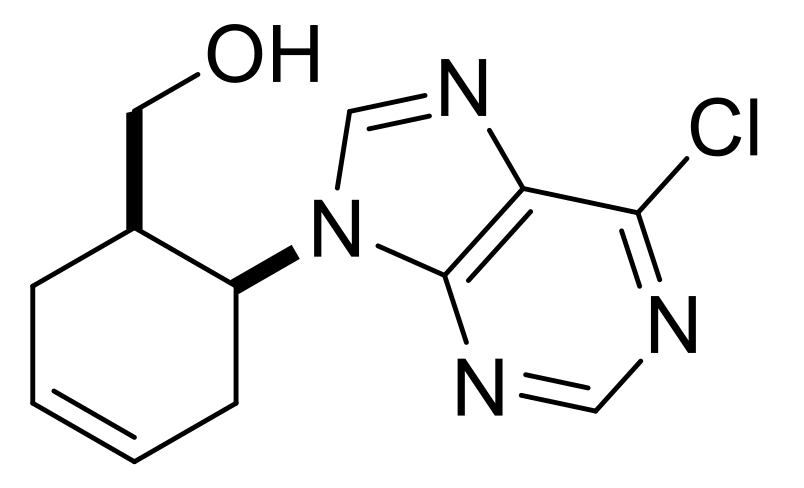
| ((1R*,6S*)-6-(6-Chloro-9H-purin-9-yl)cyclohex-3-en-1-yl)methanol, (±)-43. |
White solid, m.p. 109–111 °C, 76%, 1H-NMR (CDCl3, 500 MHz): δ (ppm) = 1.36–1.47 (m, 1H, CH2), 2.05–2.15 (m, 1H, CH2), 2.34–2.44 (m, 1H, H-1), 2.50–2.59 (m, 1H, CH2), 2.73–2.81 (m, 1H, OCH2), 2.93–3.03 (m, 1H, CH2), 3.44–3.53 (m, 1H, OCH2), 4.65 (brs, 1H, OH), 5.31–5.37 (m, 1H, H-6), 5.98–6.06 (m, 2H, H-3, H-4), 8.33 (s, 1H, Ar-H), 8.76 (s, 1H, Ar-H); 13C-NMR (CDCl3, 126 MHz): 22.6, 31.0, 39.7, 48.6, 62.1, 124.7, 127.8, 131.1, 144.6, 151.6, 151.7, 152.5, MS (ES, pos) m/z = 265 (M + 1), 267 (M + 3).
![Molecules 24 00161 i019 Molecules 24 00161 i019]() |
| ((1S*,6S*)-6-((5-Amino-6-chloropyrimidin-4-yl)amino)cyclohex-3-en-1-yl)methanol, (±)-46. |
White solid, m.p. 164–167 °C, 64%; 1H-NMR (DMSO, 400 MHz): δ (ppm) = 1.70–1.84 (m, 1H, CH2), 1.89–2.05 (m, 2H, CH2), 2.14–2.25 (m, 1H, CH2), 2.26–2.36 (m, 1H, H-1), 3.26–3.43 (m, 2H, OCH2), 4.05–4.15 (m, 1H, H-6), 4.36 (t, 1H, O-H, J = 5.32 Hz), 4.99 (brs, 2H, N-H), 5.51-5.67 (m, 2H, H-3, H-4), 6.50 (d, 1H, N-H, J = 7.96 Hz), 7.64 (s, 1H, Ar-H), 13C-NMR (DMSO, 100 MHz): δ (ppm) = 28.7, 32.3, 41.3, 48.3, 62.9, 124.2, 125.5, 127.3, 137.7, 146.4, 152.7, MS (ES, pos) m/z = 255 (M + 1), 257 (M + 3).
![Molecules 24 00161 i020 Molecules 24 00161 i020]() |
| ((1S*,6S*)-6-(6-Chloro-9H-purin-9-yl)cyclohex-3-en-1-yl)methanol, (±)-47. |
White solid, m.p. 160–162 °C, 70%; 1H-NMR (DMSO, 500 MHz): δ (ppm) = 2.13–2.29 (m, 2H, CH2), 2.42–2.49 (m, 1H, CH2), 2.53–2.62 (m, 1H, H-1), 2.83–2.95 (m, 1H, CH2), 2.98–3.05 (m, 1H, OCH2), 3.11–3.17 (m, 1H, OCH2), 4.69–4.78 (m, 1H, H-6), 5.68–5.85 (m, 2H, H-3, H-4), 8.73–8.78 (m, 2H, Ar-H), 13C-NMR (DMSO, 126 MHz): 28.5, 31.3, 39.0, 54.1, 61.7, 124.6, 127.2, 131.6, 147.6, 149.4, 151.7, 152.4; MS (ES, pos) m/z = 265 (M + 1), 267 (M + 3).