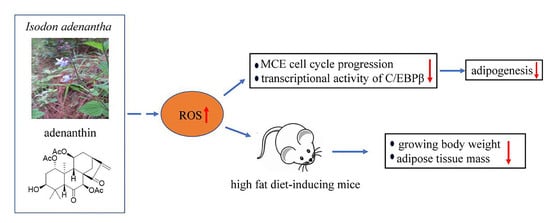
Adenanthin, a Natural ent-Kaurane Diterpenoid Isolated from the Herb Isodon adenantha Inhibits Adipogenesis and the Development of Obesity by Regulation of ROS
Abstract
1. Introduction
2. Results
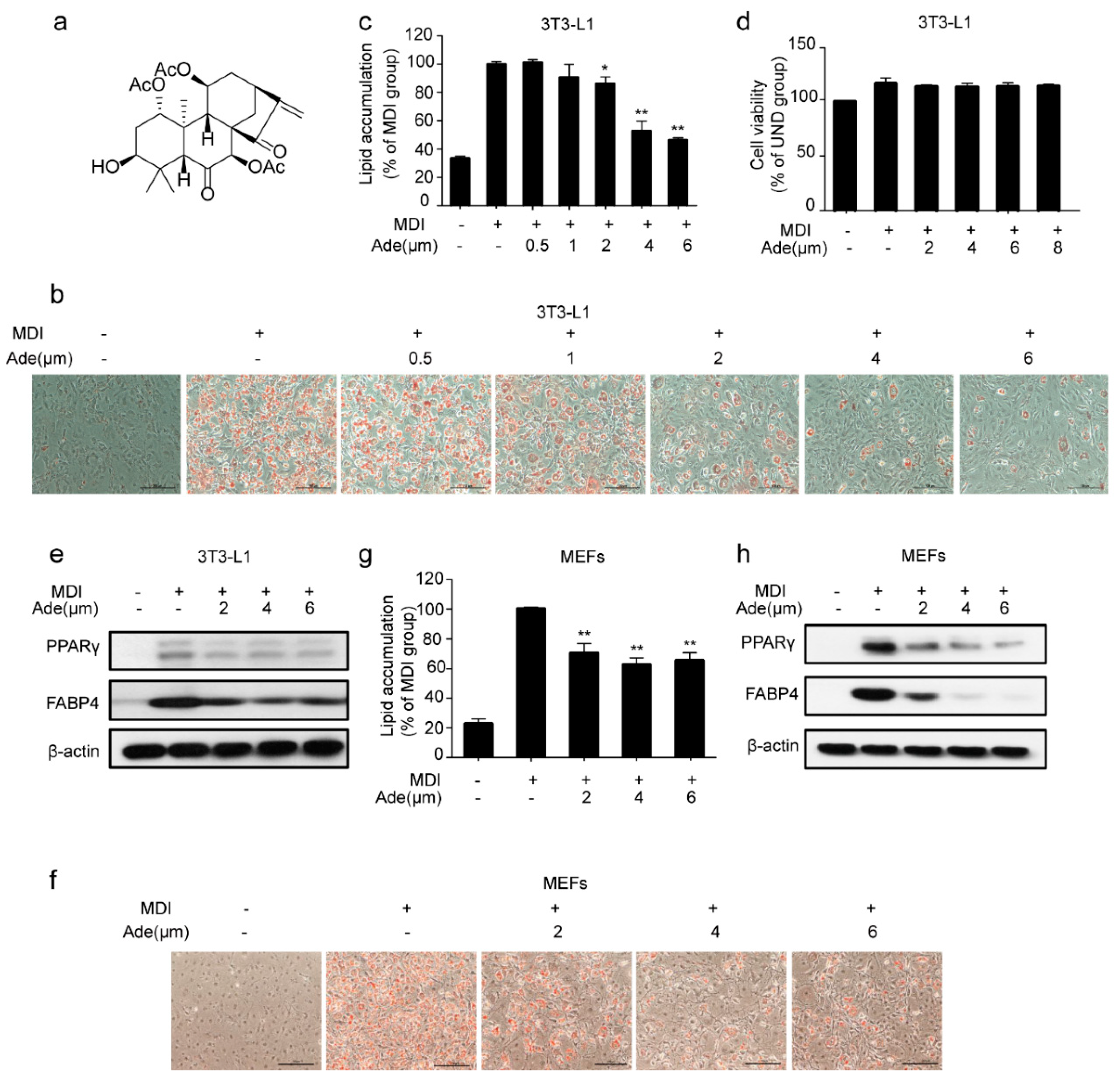
2.1. Adenanthin Inhibits Adipogenesis in 3T3-L1 Preadipocytes and MEFs
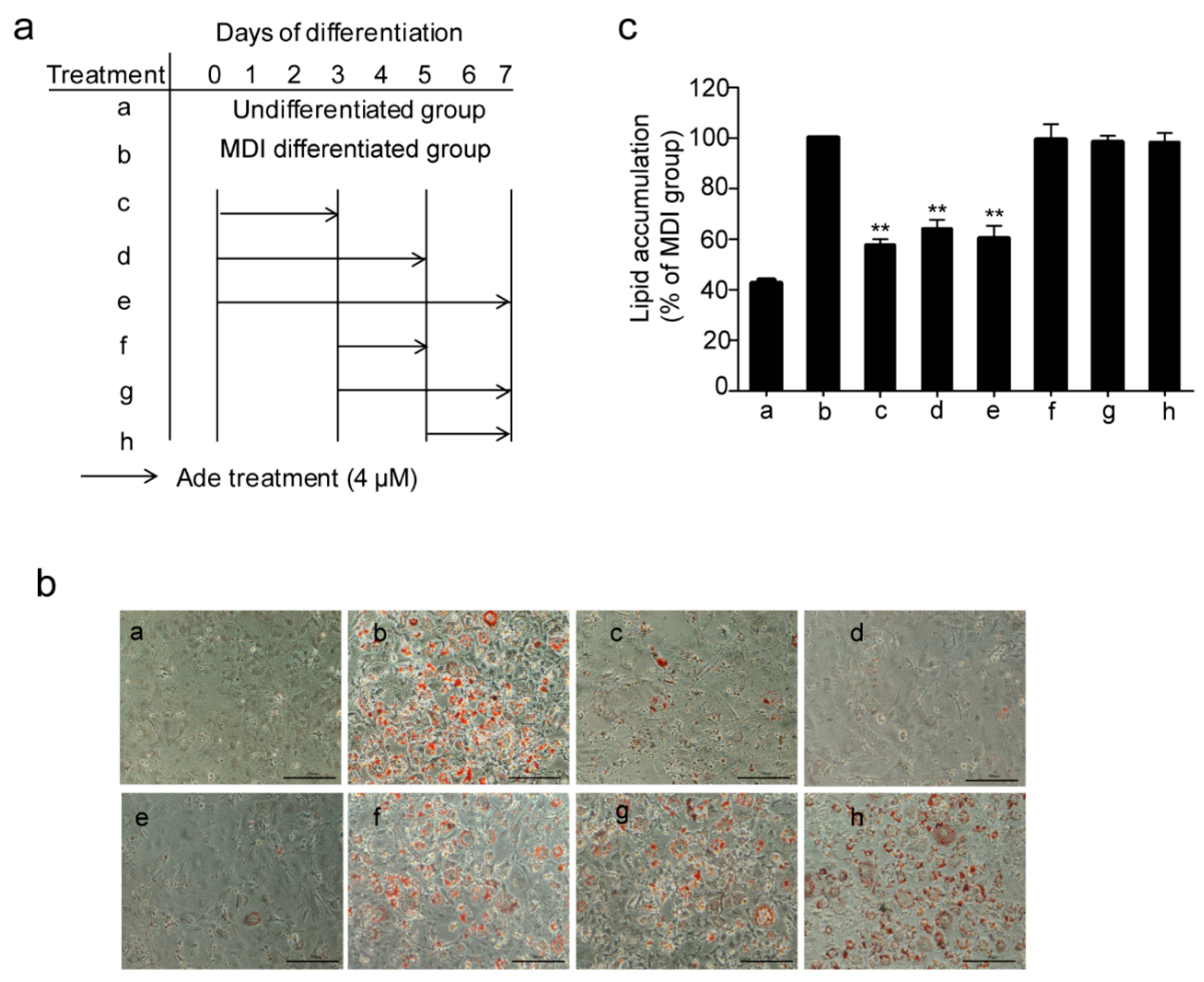
2.2. Adenanthin Functions in the Early Stage of Adipogenesis
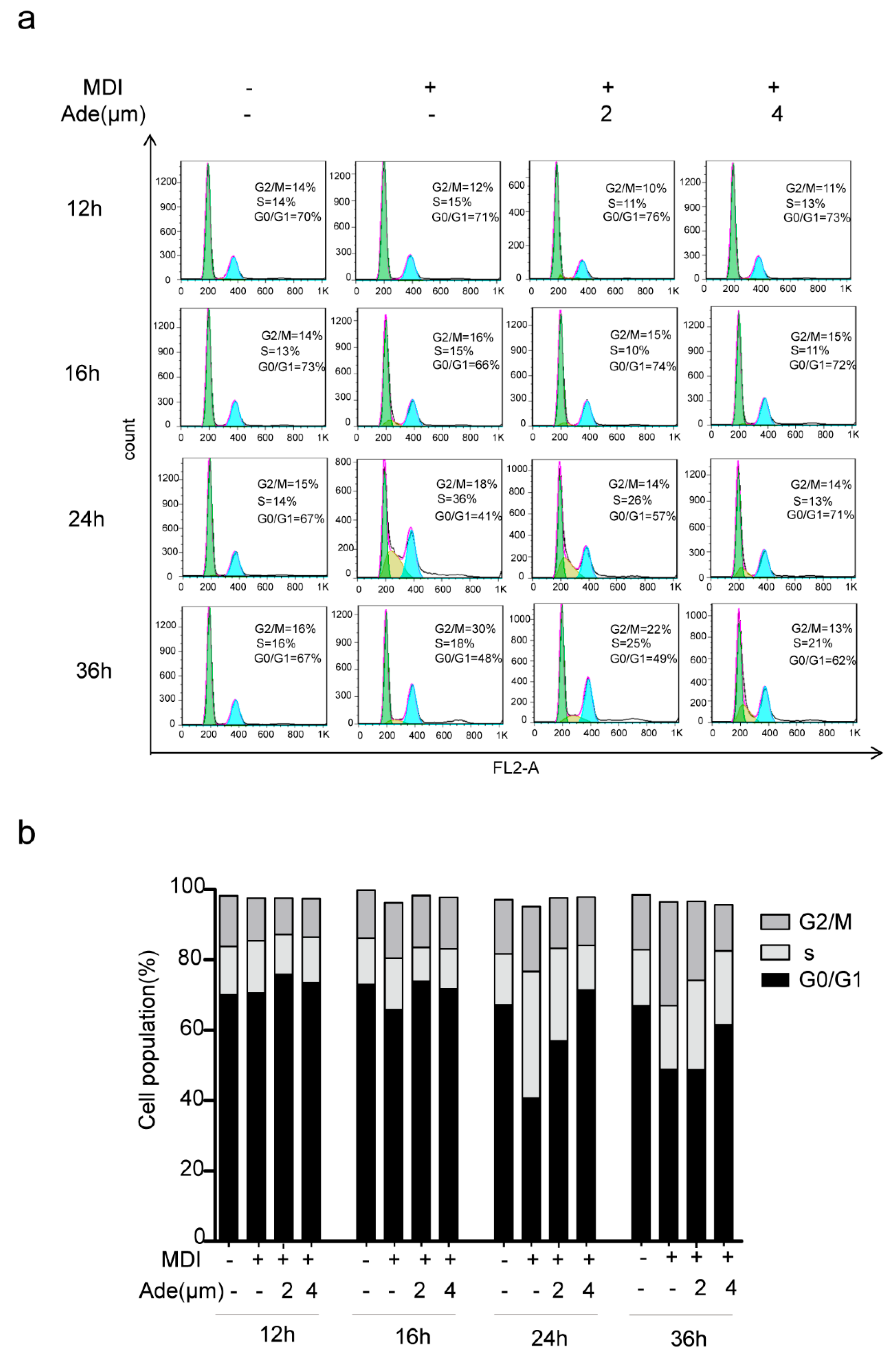
2.3. Adenanthin Suppresses Cell Cycle Progression during MCE
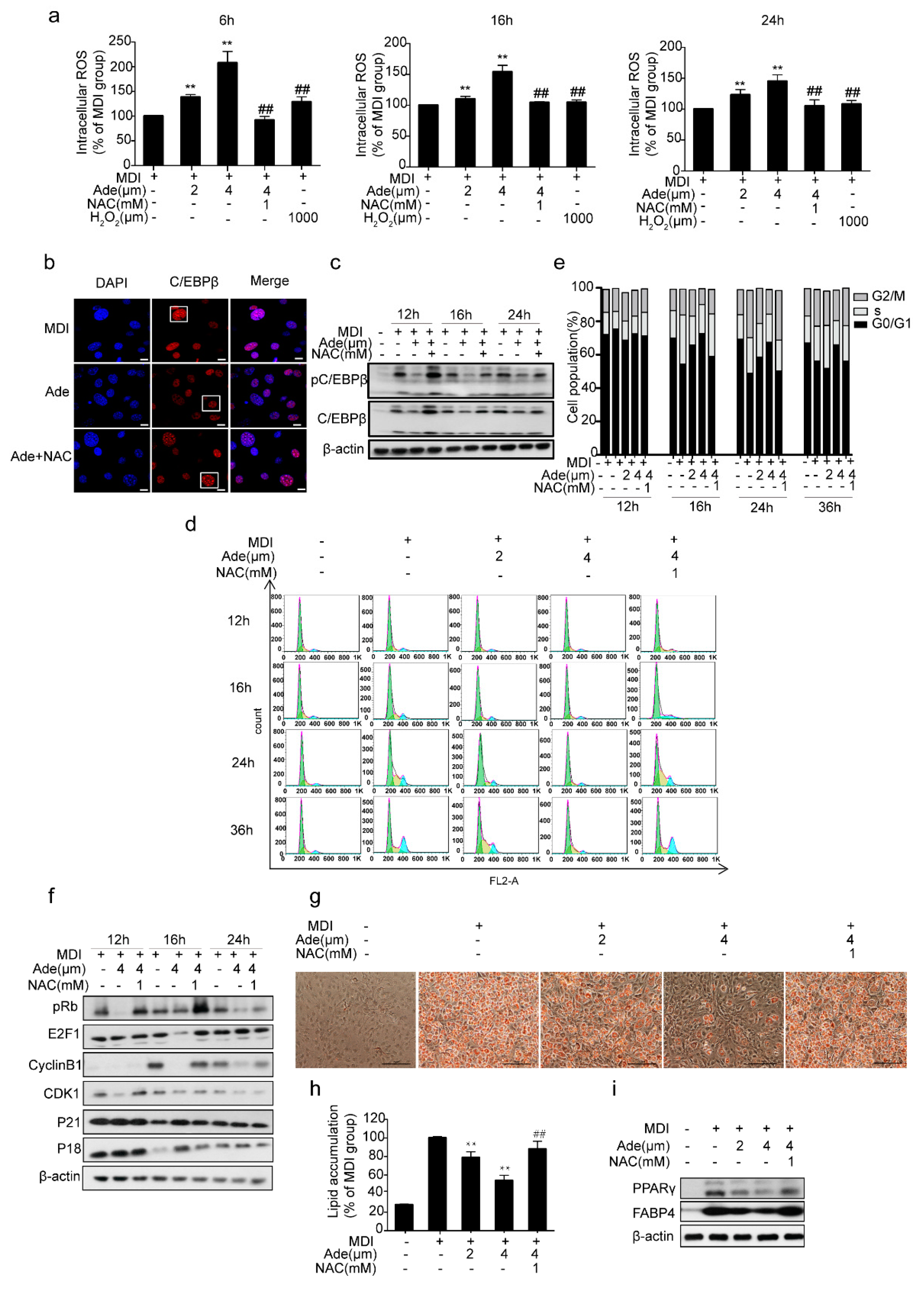
2.4. ROS Controls Adenanthin-Mediated Inhibition of MCE
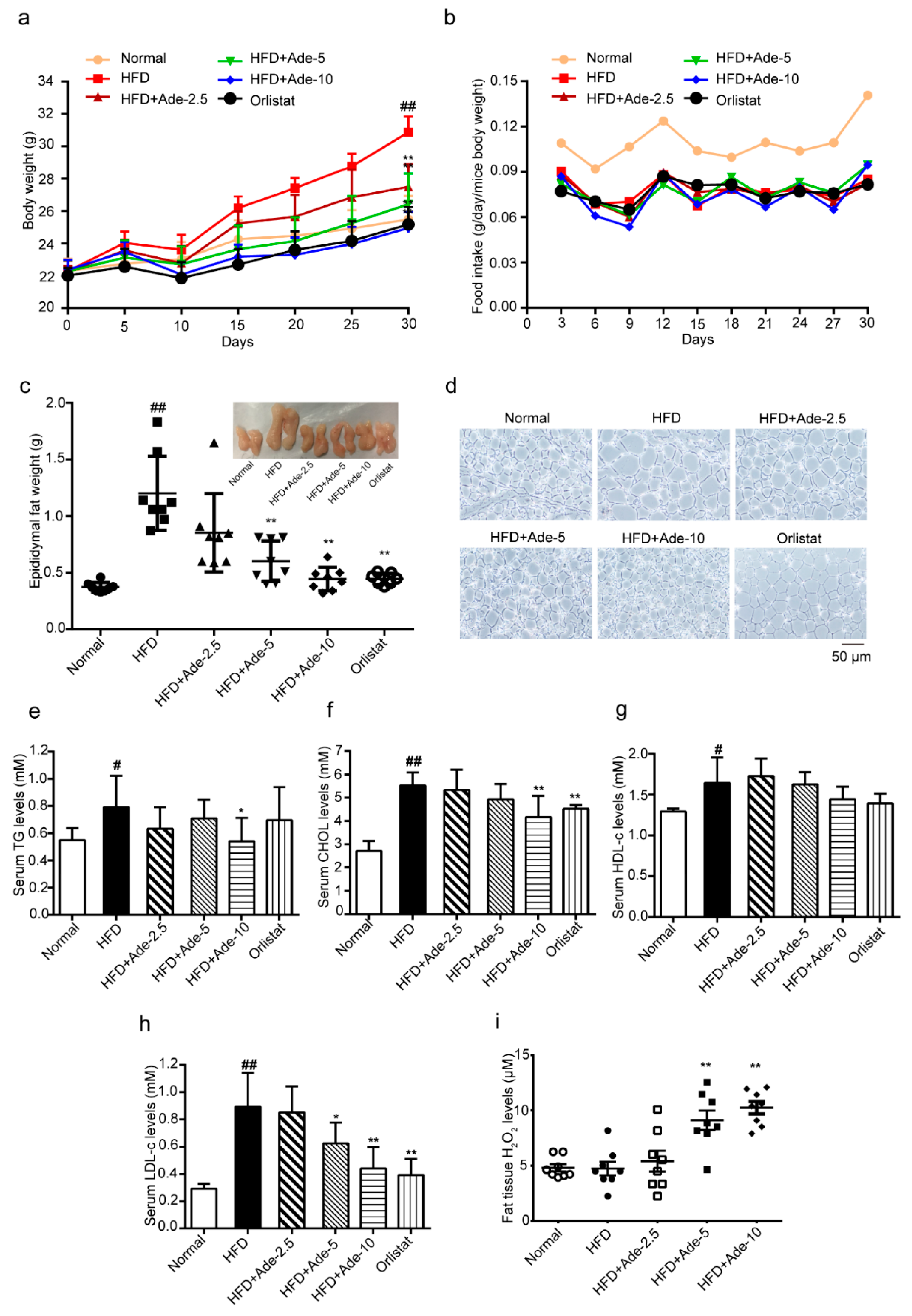
2.5. Adenanthin Delayed the HFD-Induced Development of Obesity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Extraction and Isolation of Adenanthin
4.3. 3T3-L1 Culture and Differentiation
4.4. Isolation, Culture and Differentiation of MEFs
4.5. Cell Viability Assay
4.6. Oil Red O Staining
4.7. Cell Cycle Analysis
4.8. Western Blotting Analysis
4.9. Intracellular ROS Measurement
4.10. Immunofluorescence Staining
4.11. Animal Study
4.12. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arner, P.; Kulyte, A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat. Rev. Endocrinol. 2015, 11, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. (Lond.) 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Haslam, D.W.; James, W.P.T. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Flier, J.S. Obesity and the regulation of energy balance. Cell 2001, 104, 531–543. [Google Scholar] [CrossRef]
- Hirsch, J.; Batchelor, B. Adipose tissue cellularity in human obesity. Endocrinol. Metab. Clin. N. Am. 1976, 5, 299–311. [Google Scholar] [CrossRef]
- Gaya, M.; Repetto, V.; Toneatto, J.; Anesini, C.; Piwien-Pilipuk, G.; Moreno, S. Antiadipogenic effect of carnosic acid, a natural compound present in Rosmarinus officinalis, is exerted through the C/EBPs and PPARgamma pathways at the onset of the differentiation program. Biochim. Biophys. Acta 2013, 1830, 3796–3806. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.H.; Ma, J.Z.; Yang, X.W.; Hu, Y.J.; Zhou, J.; Fu, L.C.; Tian, R.H.; Liu, S.; Xu, G.; Shen, X.L. A Triterpenoid Inhibited Hormone-Induced Adipocyte Differentiation and Alleviated Dexamethasone-Induced Insulin Resistance in 3T3-L1 adipocytes. Natur. Prod. Bioprosp. 2015, 5, 159–166. [Google Scholar] [CrossRef]
- Chen, C.C.; Chuang, W.T.; Lin, A.H.; Tsai, C.W.; Huang, C.S.; Chen, Y.T.; Chen, H.W.; Lii, C.K. Andrographolide inhibits adipogenesis of 3T3-L1 cells by suppressing C/EBPbeta expression and activation. Toxicol. Appl. Pharm. 2016, 307, 115–122. [Google Scholar] [CrossRef]
- Tian, L.; Song, Z.; Shao, W.; Du, W.W.; Zhao, L.R.; Zeng, K.; Yang, B.B.; Jin, T. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2017, 8, e2559. [Google Scholar] [CrossRef]
- Che, Y.; Wang, Q.; Xiao, R.; Zhang, J.; Zhang, Y.; Gu, W.; Rao, G.; Wang, C.; Kuang, H. Kudinoside-D, a triterpenoid saponin derived from Ilex kudingcha suppresses adipogenesis through modulation of the AMPK pathway in 3T3-L1 adipocytes. Fitoterapia 2018, 125, 208–216. [Google Scholar] [CrossRef]
- Wang, P.; Mariman, E.; Keijer, J.; Bouwman, F.; Noben, J.P.; Robben, J.; Renes, J. Profiling of the secreted proteins during 3T3-L1 adipocyte differentiation leads to the identification of novel adipokines. CMLS 2004, 61, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Reddy, S.T.; Vergnes, L.; Pervin, S.; Grijalva, V.; Stout, D.; David, J.; Li, X.; Tomasian, V.; Reid, C.B.; et al. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J. Lipid Res. 2014, 55, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.S.; Tsai, M.L.; Badmaev, V.; Jimenez, M.; Ho, C.T.; Pan, M.H. Xanthigen Suppresses Preadipocyte Differentiation and Adipogenesis through Down-regulation of PPAR gamma and C/EBPs and Modulation of SIRT-1, AMPK, and FoxO Pathways. J. Agric. Food Chem. 2012, 60, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Tang, Q.Q.; Vinson, C.; Lane, M.D. Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. USA 2004, 101, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Abella, A.; Dubus, P.; Malumbres, M.; Rane, S.G.; Kiyokawa, H.; Sicard, A.; Vignon, F.; Langin, D.; Barbacid, M.; Fajas, L. Cdk4 promotes adipogenesis through PPAR gamma activation. Cell Metab. 2005, 2, 239–249. [Google Scholar] [CrossRef]
- Yeh, W.C.; Cao, Z.D.; Classon, M.; Mcknight, S.L. Cascade Regulation of Terminal Adipocyte Differentiation by 3 Members of the C/Ebp Family of Leucine-Zipper Proteins. Genes Dev. 1995, 9, 168–181. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev. 1999, 13, 2231–2241. [Google Scholar] [CrossRef]
- Kriegerbrauer, H.I.; Kather, H. Antagonistic Effects of Different Members of the Fibroblast and Platelet-Derived Growth-Factor Families on Adipose Conversion and Nadph-Dependent H2o2 Generation in 3t3 L1-Cells. Biochem. J. 1995, 307, 549–556. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.W. Reactive Oxygen Species Facilitate Adipocyte Differentiation by Accelerating Mitotic Clonal Expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Xiao, L.; Mizuno, H.; Kato, S.; Aoshima, H.; Taira, H.; Kokubo, K.; Miwa, N. Novel polyhydroxylated fullerene suppresses intracellular oxidative stress together with repression of intracellular lipid accumulation during the differentiation of OP9 preadipocytes into adipocytes. Free Radic. Res. 2010, 44, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Pessler-Cohen, D.; Pekala, P.H.; Kovsan, J.; Bloch-Damti, A.; Rudich, A.; Bashan, N. GLUT4 repression in response to oxidative stress is associated with reciprocal alterations in C/EBP alpha and delta isoforms in 3T3-L1 adipocytes. Arch. Physiol. Biochem. 2006, 112, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Carriere, A.; Fernandez, Y.; Rigoulet, M.; Penicaud, L.; Casteilla, L. Inhibition of preadipocyte proliferation by mitochondrial reactive oxygen species. FEBS Lett. 2003, 550, 163–167. [Google Scholar] [CrossRef]
- Castro, J.P.; Grune, T.; Speckmann, B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol. Chem. 2016, 397, 709–724. [Google Scholar] [CrossRef]
- Xu, Y.L.; Sun, H.D.; Wang, D.Z.; Iwashita, T.; Komura, H.; Kozuka, M.; Naya, K.; Kubo, I. Structure of Adenanthin. Tetrahedron Lett. 1987, 28, 499–502. [Google Scholar] [CrossRef]
- Liu, C.X.; Yin, Q.Q.; Zhou, H.C.; Wu, Y.L.; Pu, J.X.; Xia, L.; Liu, W.; Huang, X.; Jiang, T.; Wu, M.X.; et al. Adenanthin targets peroxiredoxin I and II to induce differentiation of leukemic cells. Nat. Chem. Biol. 2012, 8, 486–493. [Google Scholar] [CrossRef]
- Yin, Q.Q.; Liu, C.X.; Wu, Y.L.; Wu, S.F.; Wang, Y.; Zhang, X.; Hu, X.J.; Pu, J.X.; Lu, Y.; Zhou, H.C.; et al. Preventive and Therapeutic Effects of Adenanthin on Experimental Autoimmune Encephalomyelitis by Inhibiting NF-kappa B Signaling. J. Immunol. 2013, 191, 2115–2125. [Google Scholar] [CrossRef]
- Hou, J.K.; Huang, Y.; He, W.; Yan, Z.W.; Fan, L.; Liu, M.H.; Xiao, W.L.; Sun, H.D.; Chen, G.Q. Adenanthin targets peroxiredoxin I/II to kill hepatocellular carcinoma cells. Cell Death Dis. 2014, 5. [Google Scholar] [CrossRef]
- Xiong, W.Y.; Jordens, I.; Gonzalez, E.; McGraw, T.E. GLUT4 Is Sorted to Vesicles Whose Accumulation Beneath and Insertion into the Plasma Membrane Are Differentially Regulated by Insulin and Selectively Affected by Insulin Resistance. Mol. Biol. Cell 2010, 21, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Bajor, M.; Zych, A.O.; O’Leary, P.C.; Czekalska, A.; Gallagher, W.M.; Golab, J.; Zagozdzon, R. Adenanthin, a new peroxiredoxin inhibitor, induces a switch between estrogen receptor alpha-mediated and Src/Akt-driven signaling in breast cancer cells. Cancer Res. 2015, 75. [Google Scholar] [CrossRef]
- Lane, M.D.; Tang, Q.Q.; Jiang, M.S. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem. Biophys. Res. Commun. 1999, 266, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Tang, Q.Q.; Li, X.; Lane, M.D. Effect of phosphorylation and S-S bond-induced dimerization on DNA binding and transcriptional activation by C/EBPbeta. Proc. Natl. Acad. Sci. USA 2007, 104, 1800–1804. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Gronborg, M.; Huang, H.; Kim, J.W.; Otto, T.C.; Pandey, A.; Lane, M.D. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9766–9771. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Gao, Z.W.; Chen, Y.; Guan, M.X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 2017, 8, 439–445. [Google Scholar] [CrossRef]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The Role of Reactive Oxygen Species in Mesenchymal Stem Cell Adipogenic and Osteogenic Differentiation: A Review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Song, L.Q.; Li, Y. Controlling Redox Status for Stem Cell Survival, Expansion, and Differentiation. Oxid Med. Cell Longev. 2015. [Google Scholar] [CrossRef]
- Wang, W.M.; Zhang, Y.; Lu, W.Y.; Liu, K.Y. Mitochondrial Reactive Oxygen Species Regulate Adipocyte Differentiation of Mesenchymal Stem Cells in Hematopoietic Stress Induced by Arabinosylcytosine. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef]
- Lee, H.; Li, H.; Kweon, M.; Choi, Y.; Kim, M.J.; Ryu, J.H. Isobavachalcone from Angelica keiskei Inhibits Adipogenesis and Prevents Lipid Accumulation. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Yen, C.C.; Fan, C.T.; Chuang, W.T.; Huang, C.S.; Chen, H.W.; Lii, C.K. 14-Deoxy-11,12-didehydroandrographolide suppresses adipogenesis of 3 T3-L1 preadipocytes by inhibiting CCAAT/enhancer-binding protein beta activation and AMPK-mediated mitotic clonal expansion. Toxicol. Appl. Pharm. 2018, 359, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Jeon, M.; Kim, Y.S. Methyl Gallate, a potent antioxidant inhibits mouse and human adipocyte differentiation and oxidative stress in adipocytes through impairment of mitotic clonal expansion. Biofactors 2016, 42, 716–726. [Google Scholar] [CrossRef]
- Sun, Q.; Qi, W.; Yang, J.J.; Yoon, K.S.; Clark, J.M.; Park, Y. Fipronil promotes adipogenesis via AMPKalpha-mediated pathway in 3T3-L1 adipocytes. Food Chem. Toxicol. 2016, 92, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.E.; Qian, X.; Popescu, N.C.; Lowy, D.R. Isolation of Mouse Embryo Fibroblasts. Bio Protoc. 2013, 3, 18. [Google Scholar] [CrossRef]
- Xu, Y.; Niu, Y.; Gao, Y.; Wang, F.; Qin, W.; Lu, Y.; Hu, J.; Peng, L.; Liu, J.; Xiong, W. Borapetoside E, a Clerodane Diterpenoid Extracted from Tinospora crispa, Improves Hyperglycemia and Hyperlipidemia in High-Fat-Diet-Induced Type 2 Diabetes Mice. J. Nat. Prod. 2017, 80, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Adenanthin is available from the authors. |
| Initial Weight (g) | Final Weight (g) | Weight Gain (g) | |
|---|---|---|---|
| Normal | 22.29 ± 0.28 | 25.49 ± 0.51 | 3.20 ± 0.70 |
| HFD (2% DMSO) | 22.29 ± 0.23 | 30.86 ± 0.35 ## | 8.58 ± 0.43 ## |
| HFD + Ade (2.5 mg/kg) | 22.26 ± 0.12 | 27.50 ± 0.49 ** | 5.24 ± 0.47 ** |
| HFD + Ade (5 mg/kg) | 22.28 ± 0.24 | 26.43 ± 0.67 ** | 4.15 ± 0.57 ** |
| HFD + Ade (10 mg/kg) | 22.38 ± 0.22 | 24.95 ± 0.36 ** | 2.58 ± 0.49 ** |
| HFD + Orlistat (60 mg/kg) | 22.19 ± 0.17 | 25.17 ± 0.41 ** | 2.98 ± 0.28 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Li, X.; Tian, W.; Lu, Y.; Xu, Y.; Wang, F.; Qin, W.; Ma, X.; Puno, P.-T.; Xiong, W. Adenanthin, a Natural ent-Kaurane Diterpenoid Isolated from the Herb Isodon adenantha Inhibits Adipogenesis and the Development of Obesity by Regulation of ROS. Molecules 2019, 24, 158. https://doi.org/10.3390/molecules24010158
Hu J, Li X, Tian W, Lu Y, Xu Y, Wang F, Qin W, Ma X, Puno P-T, Xiong W. Adenanthin, a Natural ent-Kaurane Diterpenoid Isolated from the Herb Isodon adenantha Inhibits Adipogenesis and the Development of Obesity by Regulation of ROS. Molecules. 2019; 24(1):158. https://doi.org/10.3390/molecules24010158
Chicago/Turabian StyleHu, Jing, Xingren Li, Weifeng Tian, Yanting Lu, Yuhui Xu, Fang Wang, Wanying Qin, Xiuli Ma, Pema-Tenzin Puno, and Wenyong Xiong. 2019. "Adenanthin, a Natural ent-Kaurane Diterpenoid Isolated from the Herb Isodon adenantha Inhibits Adipogenesis and the Development of Obesity by Regulation of ROS" Molecules 24, no. 1: 158. https://doi.org/10.3390/molecules24010158
APA StyleHu, J., Li, X., Tian, W., Lu, Y., Xu, Y., Wang, F., Qin, W., Ma, X., Puno, P.-T., & Xiong, W. (2019). Adenanthin, a Natural ent-Kaurane Diterpenoid Isolated from the Herb Isodon adenantha Inhibits Adipogenesis and the Development of Obesity by Regulation of ROS. Molecules, 24(1), 158. https://doi.org/10.3390/molecules24010158