Anti-Inflammatory and Anti-Oxidant Activity of Portulaca oleracea Extract on LPS-Induced Rat Lung Injury
Abstract
1. Introduction
2. Results
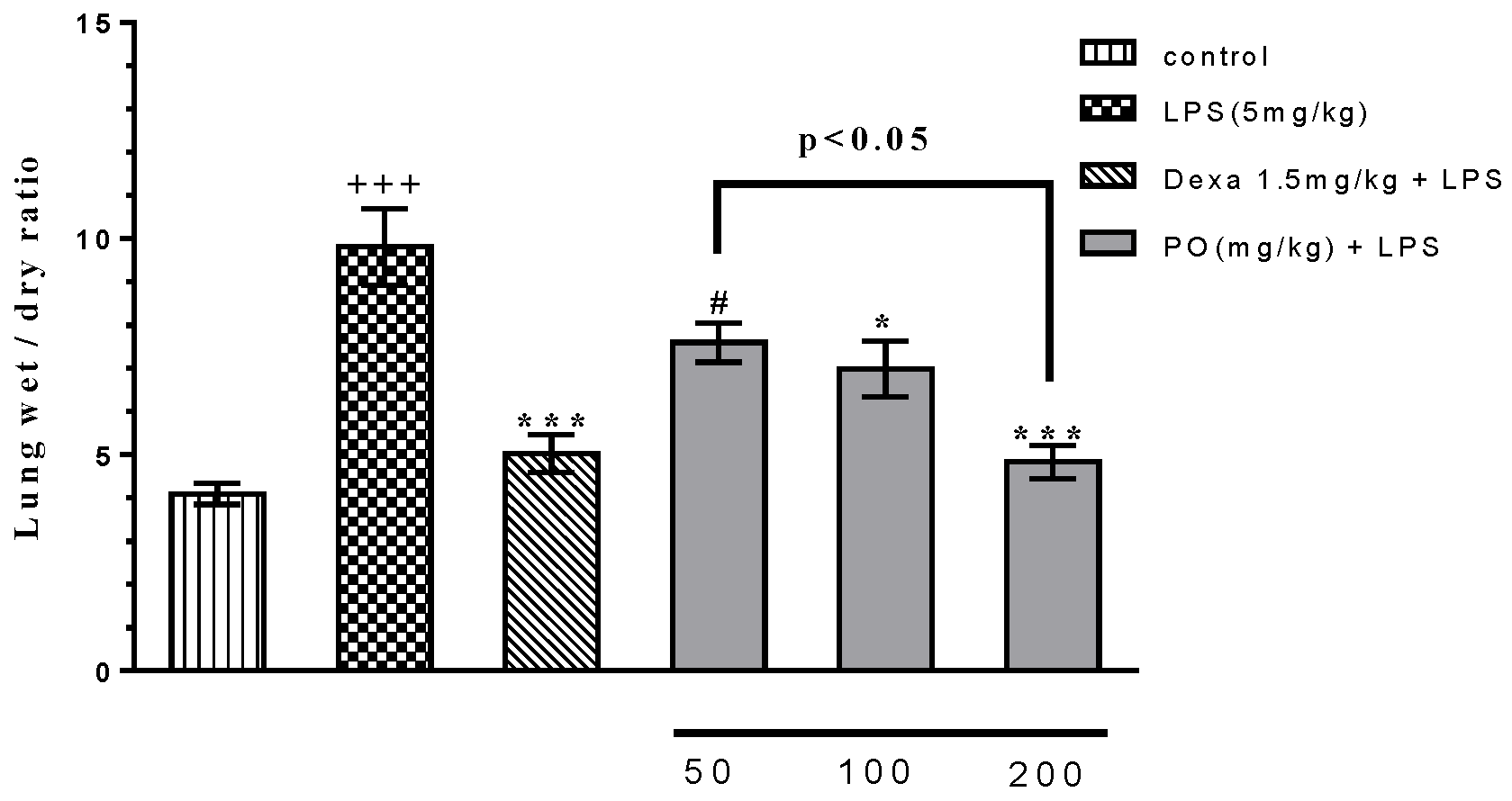
2.1. Effects of LPS and PO on Body and Absolute Organ Weights, and Lung Wet/Dry Ratio
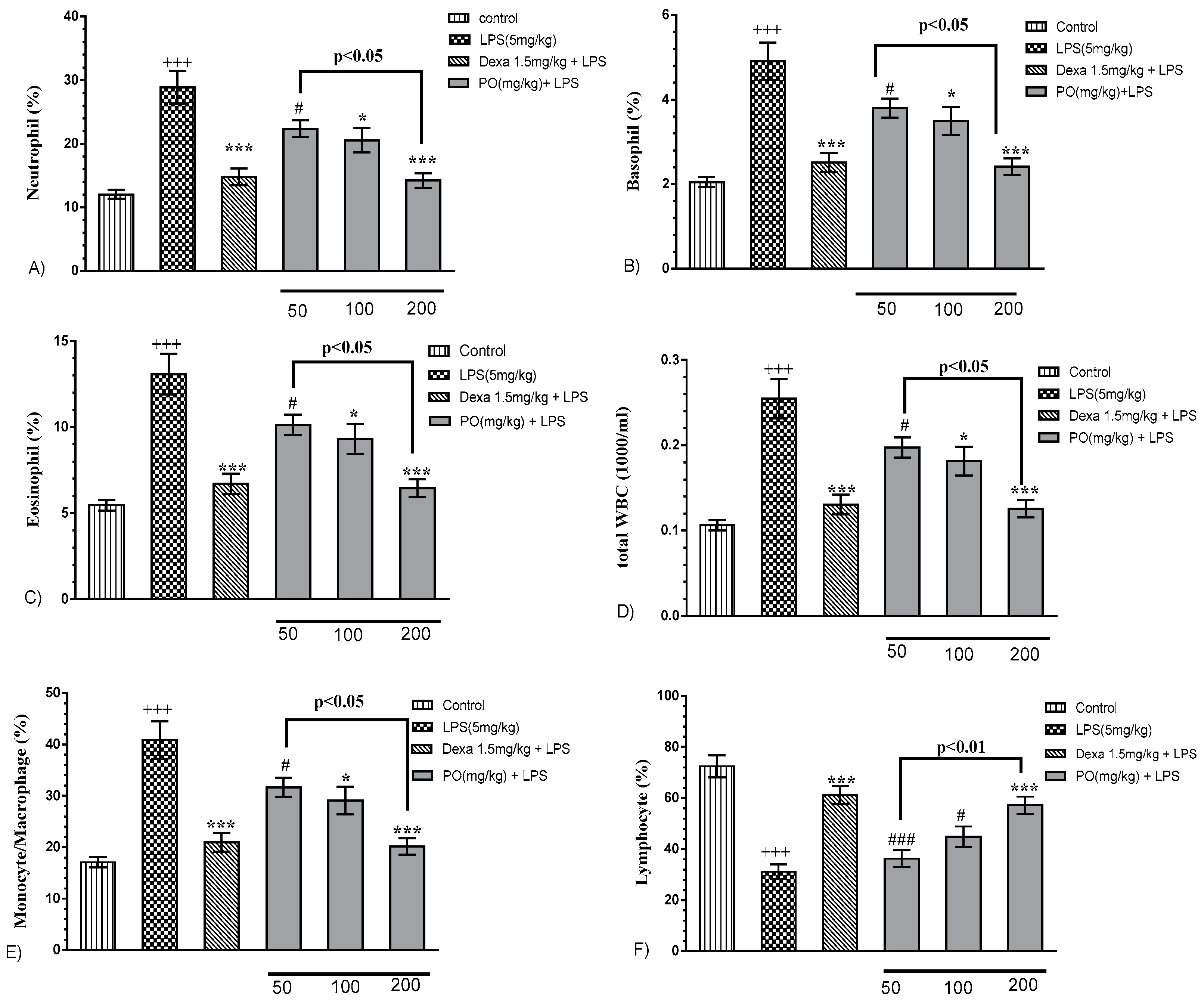
2.2. Effects of LPS and PO on Bronchoalveolar Lavage Fluid (BALF) Hematologic Indices
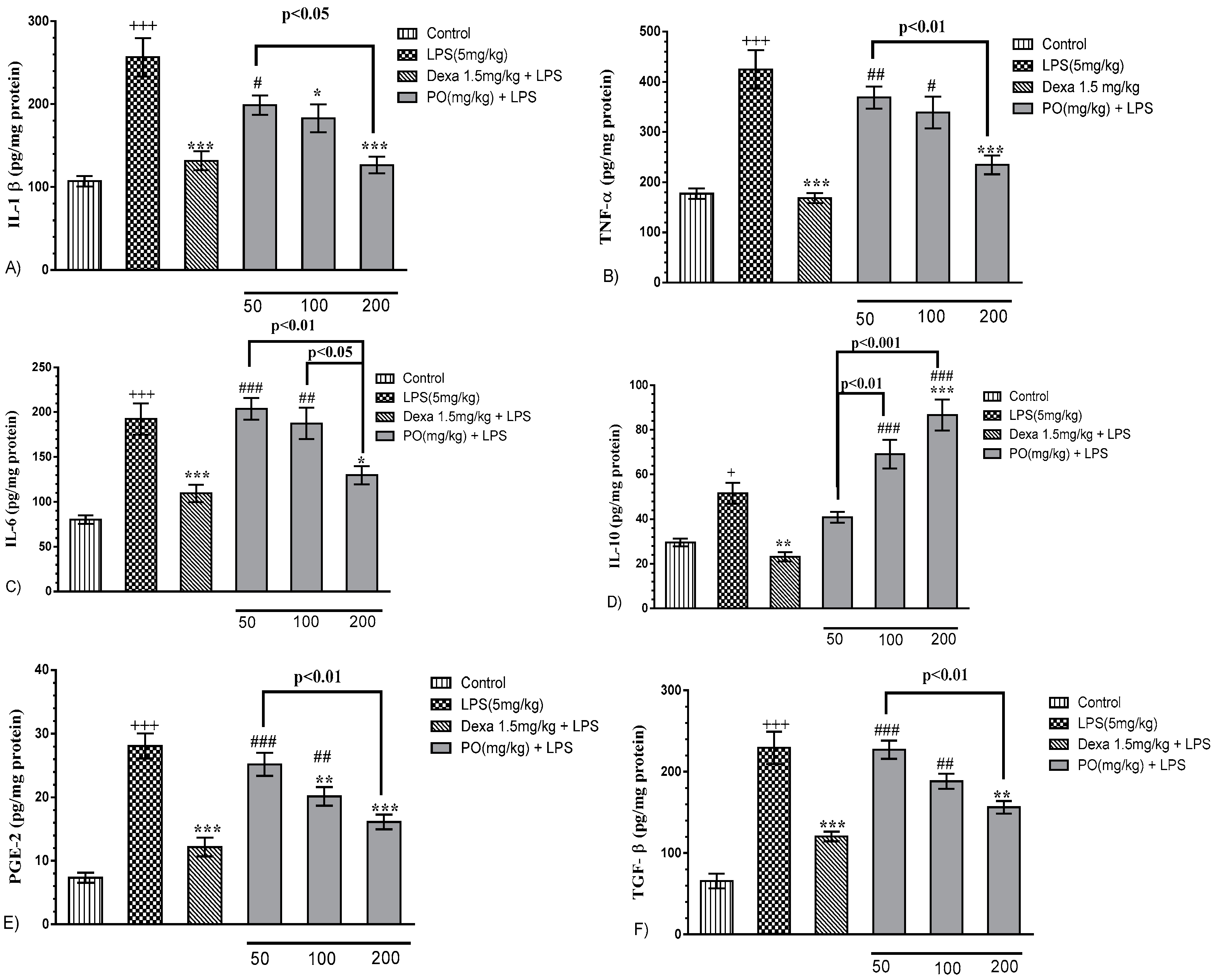
2.3. Effects of LPS and PO Extract on BALF Inflammatory Cytokines
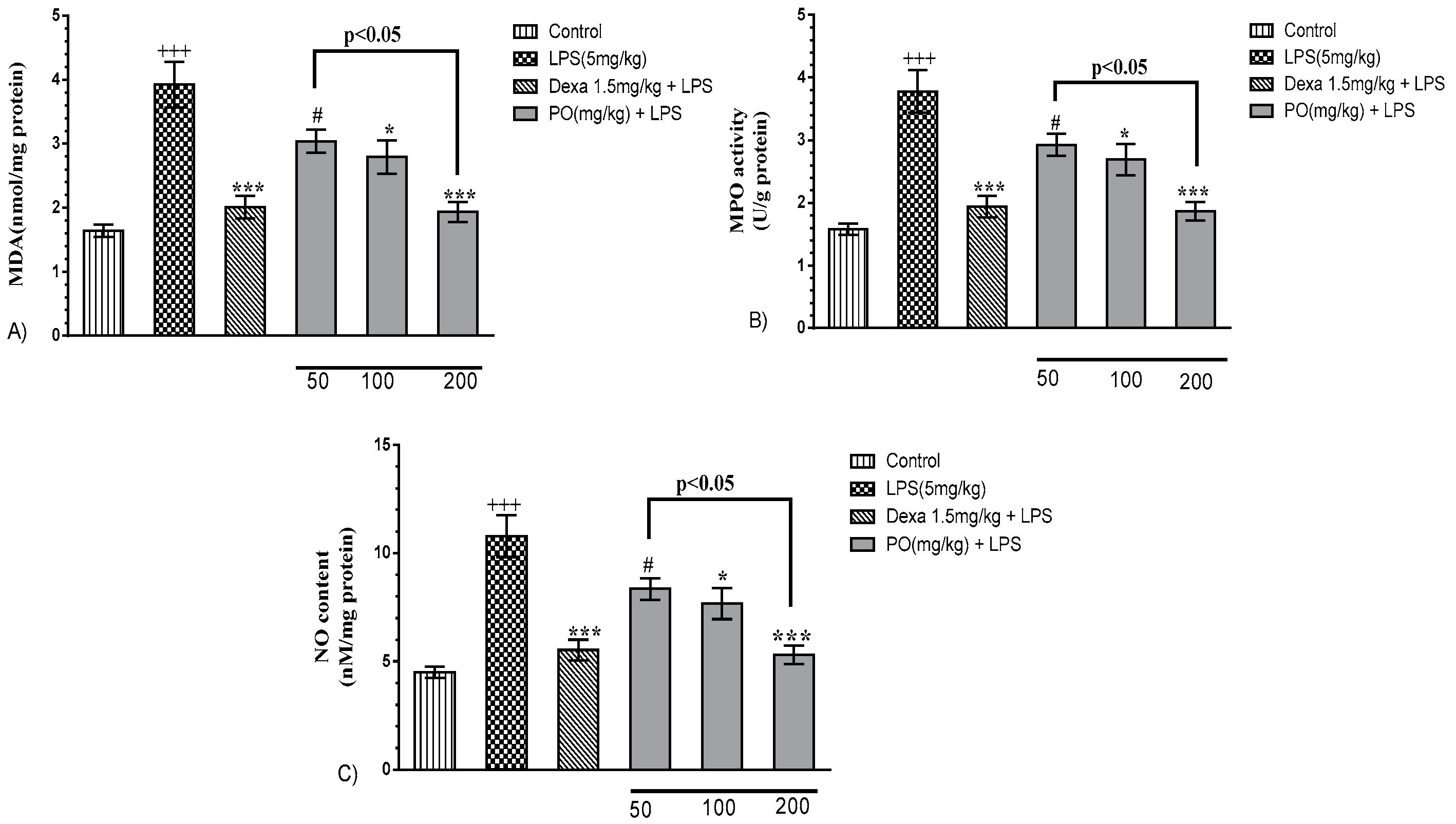
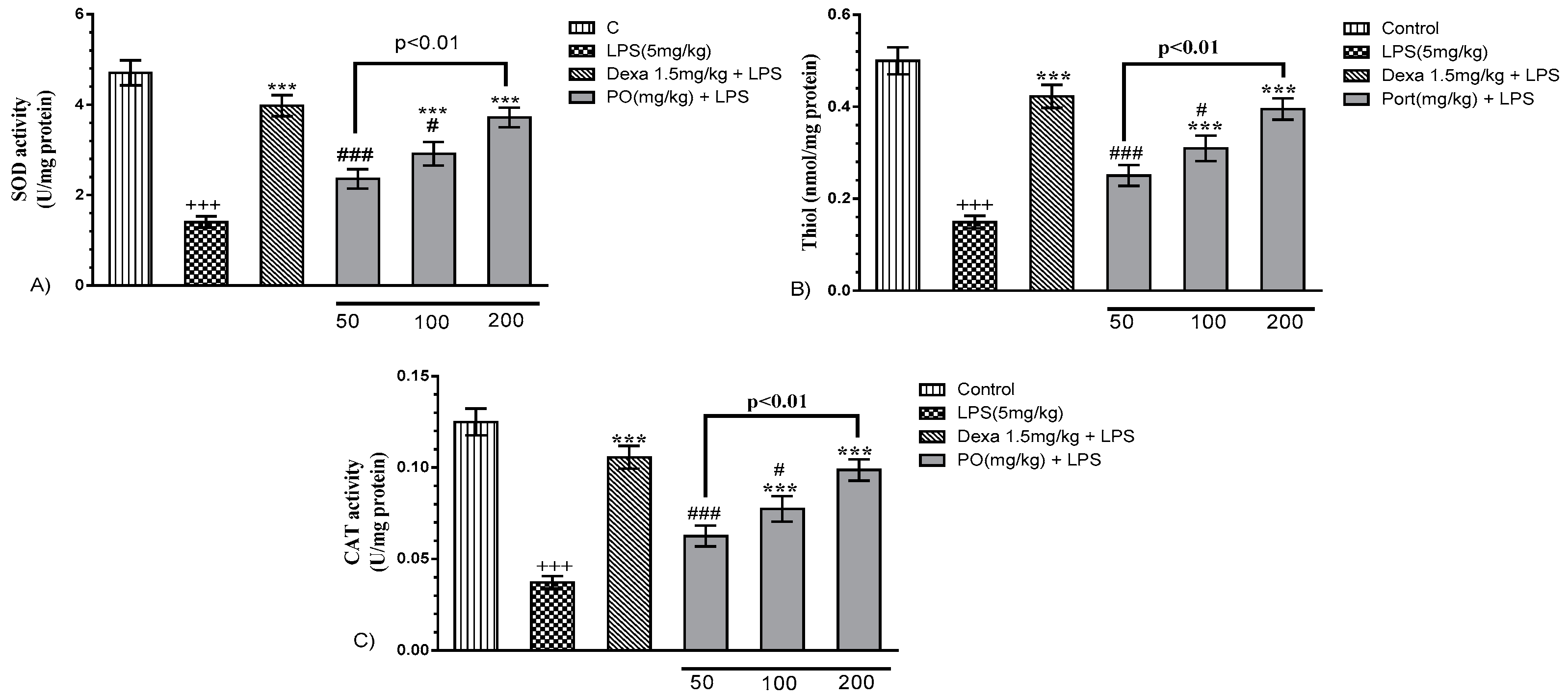
2.4. Impact of PO on the BALF Oxidant/Antioxidant Status
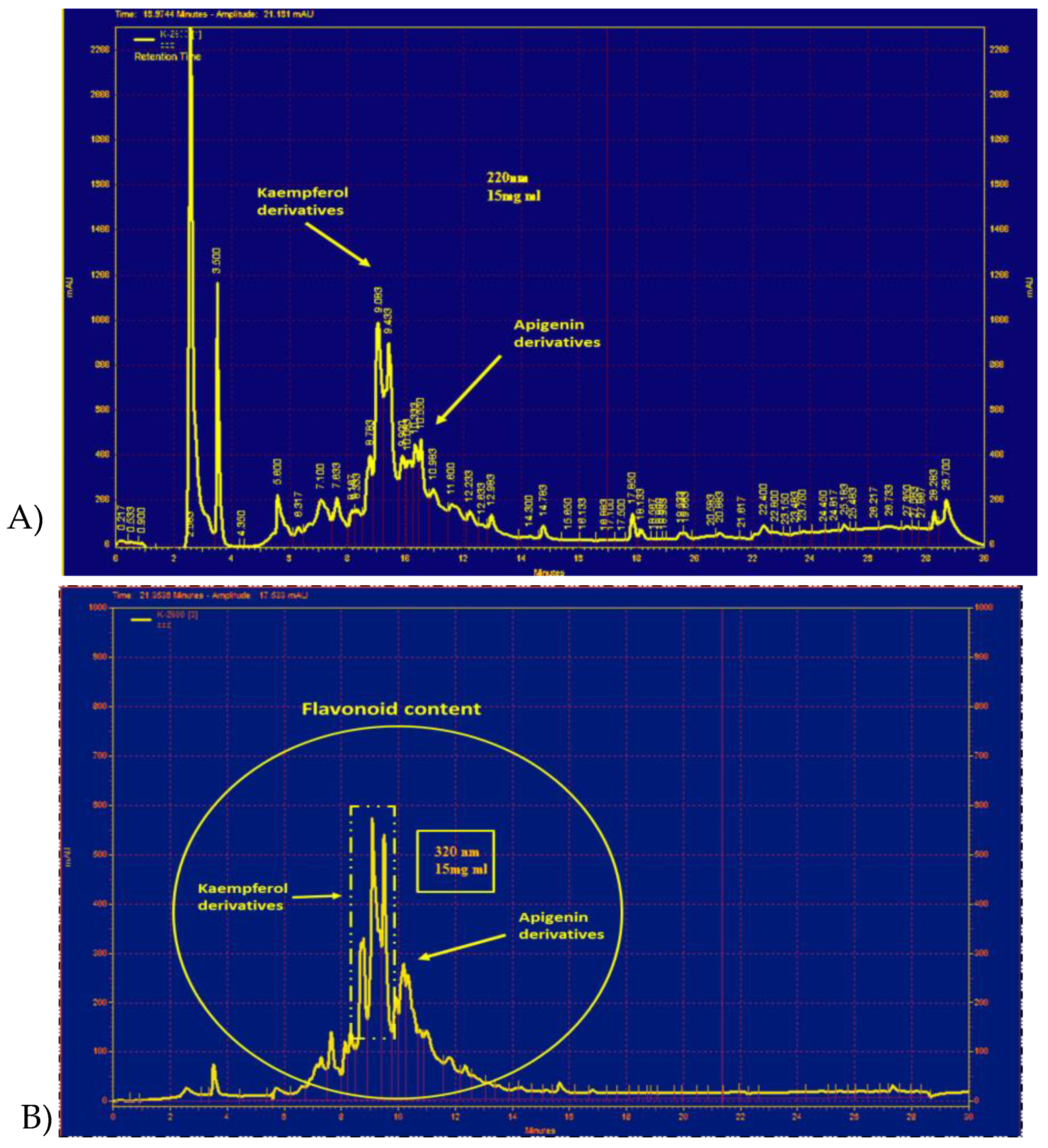
2.5. Characteristics of PO Hydro-Ethanolic Extract
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of PO Extract
4.3. Animals and Husbandry
4.4. Experimental Protocol
4.5. Absolute Organ Weight and Lung Wet/dry Weight Ratio
4.6. Broncho-Alveolar Lavage Fluid (BALF) Preparation
4.7. Measurement of Total and Differential White Blood Cells (WBC) in BALF
4.8. Oxidant-Antioxidant Assessment in BALF
4.9. Enzyme-Linked Immunosorbent Assay (ELISA) Assay
4.10. Myeloperoxidase Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Villar, J.; Sulemanji, D.; Kacmarek, R.M. The acute respiratory distress syndrome: Incidence and mortality, has it changed? Curr. Opin. Crit. Care 2014, 20, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zemans, R.L. The Acute Respiratory Distress Syndrome: Pathogenesis and Treatment. Ann. Rev. Pathol. Mech. Dis. 2011, 6, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Matthay, M.A. The Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Kosutova, P. Biomarkers in acute lung injury. Respir. Physiol. Neurobiol. 2015, 209, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Zambon, M.; Vincent, J.-L. Mortality rates for patients with acute lung injury/ARDS have decreased over time. CHEST J. 2008, 133, 1120–1127. [Google Scholar] [CrossRef]
- Kim, H.P.; Lim, H.; Kwon, Y.S. Therapeutic Potential of Medicinal Plants and Their Constituents on Lung Inflammatory Disorders. Biomol. Ther. 2017, 25, 91–104. [Google Scholar] [CrossRef]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 30, 1343–1352. [Google Scholar] [CrossRef]
- Elkhayat, E.S.; Ibrahim, S.R.; Aziz, M.A. Portulene, a new diterpene from Portulaca oleracea L. J. Asian Natl. Prod. Res. 2008, 10, 1039–1043. [Google Scholar] [CrossRef]
- Palaniswamy, U.R.; Bible, B.B.; McAvoy, R.J. Effect of nitrate: Ammonium nitrogen ratio on oxalate levels of purslane. Trends New Crops New Uses 2002, 11, 453–455. [Google Scholar]
- Lee, A.S.; Kim, J.S.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Anti-TNF-α activity of Portulaca oleracea in vascular endothelial cells. Int. J. Mol. Sci. 2012, 13, 5628–5644. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, H.; Zhao, W.; Zhou, W.; Yuan, Q.; Yang, G. Effects of aqueous extract of Portulaca oleracea L. on oxidative stress and liver, spleen leptin, PARα and FAS mRNA expression in high-fat diet induced mice. Mol. Biol. Rep. 2012, 39, 7981–7988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ji, Y.; Qu, Z.; Xia, J.; Wang, L. Experimental studies on antibiotic functions of Portulaca oleracea L. in vitro. Chin. J. Microecol. 2002, 14, 277–280. [Google Scholar]
- Karimi, G.; Hosseinzadeh, H.; Ettehad, N. Evaluation of the gastric antiulcerogenic effects of Portulaca oleracea L. extracts in mice. Phytother. Res. 2004, 18, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Islam, M.; Kamil, M.; Radhakrishnan, R.; Zakaria, M.; Habibullah, M.; Attas, A. The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp. sativa (Haw.) Celak. J. Ethnopharmacol. 2000, 73, 445–451. [Google Scholar] [CrossRef]
- Rashed, A.; Afifi, F.; Disi, A. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L.(growing in Jordan) in Mus musculus JVI-1. J. Ethnopharmacol. 2003, 88, 131–136. [Google Scholar] [CrossRef]
- Xu, X.; Yu, L.; Chen, G. Determination of flavonoids in Portulaca oleracea L. by capillary electrophoresis with electrochemical detection. J. Pharm. Biomed. Anal. 2006, 41, 493–499. [Google Scholar] [CrossRef]
- Meng, Y.; Ying, Z.; Xiang, Z.; Hao, D.; Zhang, W.; Zheng, Y.; Gao, Y.; Ying, X. The anti-inflammation and pharmacokinetics of a novel alkaloid from Portulaca oleracea L. J. Pharm. Pharmacol. 2016, 68, 397–405. [Google Scholar] [CrossRef]
- Askari, V.R.; Rezaee, S.A.; Abnous, K.; Iranshahi, M.; Boskabady, M.H. The influence of hydro-ethanolic extract of Portulaca oleracea L. on Th1/Th2 balance in isolated human lymphocytes. J. Ethnopharmacol. 2016, 194, 1112–1121. [Google Scholar] [CrossRef]
- Kaveh, M.; Eidi, A.; Nemati, A.; Boskabady, M.H. Modulation of lung inflammation and immune markers in asthmatic rats treated by Portulaca oleracea. Avicenna J. Phytomed. 2017, 7, 409–416. [Google Scholar] [PubMed]
- Stearns-Kurosawa, D.J.; Osuchowski, M.F.; Valentine, C.; Kurosawa, S.; Remick, D.G. The Pathogenesis of Sepsis. Ann. Rev. Pathol. Mech. Dis. 2011, 6, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Fang, X.; Dolganov, G.; Fremont, R.D.; Bastarache, J.A.; Ware, L.B.; Matthay, M.A. Acute lung injury edema fluid decreases net fluid transport across human alveolar epithelial type II cells. J. Biol. Chem. 2007, 282, 24109–24119. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, V.B.; Shirazinia, R.; Fereydouni, N.; Zamani, P.; Darroudi, S.; Sahebkar, A.H.; Askari, V.R. Comparison of honey and dextrose solution on post-operative peritoneal adhesion in rat model. Biomed. Pharmacother. 2017, 92, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Y.; Dong, L.; Wang, Z.; Chen, L.; Liang, D.; Shi, D.; Shan, X.; Liang, G. Design, Synthesis, and Biological Evaluation of Novel Quinazoline Derivatives as Anti-inflammatory Agents against Lipopolysaccharide-induced Acute Lung Injury in Rats. Chem. Biol. Drug Des. 2015, 85, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Kohler, G.; Headley, S.; Tolley, E.; Stentz, F.; Postlethwaite, A. Inflammatory Cytokines in the BAL of Patients With ARDS: Persistent Elevation Over Time Predicts Poor Outcome. Chest 1995, 108, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Schurr, J.R.; Young, E.; Byrne, P.; Steele, C.; Shellito, J.E.; Kolls, J.K. Central role of toll-like receptor 4 signaling and host defense in experimental pneumonia caused by Gram-negative bacteria. Infect. Immunity 2005, 73, 532–545. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. Promising neuroprotective effects of β-caryophyllene against LPS-induced oligodendrocyte toxicity: A mechanistic study. Biochem. Pharmacol. 2018, 159, 154–171. [Google Scholar] [CrossRef]
- Szarka, R.J.; Wang, N.; Gordon, L.; Nation, P.; Smith, R.H. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J. Immunol. Methods 1997, 202, 49–57. [Google Scholar] [CrossRef]
- Thangavel, J.; Samanta, S.; Rajasingh, S.; Barani, B.; Xuan, Y.T.; Dawn, B.; Rajasingh, J. Epigenetic modifiers reduce inflammation and modulate macrophage phenotype during endotoxemia-induced acute lung injury. J. Cell Sci. 2015, 128, 3094–3105. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. Oxidants and redox signaling in acute lung injury. Compr. Physiol. 2011, 1, 1365–1381. [Google Scholar] [CrossRef] [PubMed]
- Brigham, K.L.; Meyrick, B. Endotoxin and lung injury. Am. Respir. Dis. 1986, 133, 913–927. [Google Scholar]
- Martin, M.A.; Silverman, H.J. Gram-negative sepsis and the adult respiratory distress syndrome. Clin. Infect. Dis. 1992, 14, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.C.; Ryan, J.L. Endotoxins and disease mechanisms. Ann. Rev. Med. 1987, 38, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.O. Molecular biology of macrophage activation: A pathway whereby psychosocial factors can potentially affect health. Psychosom. Med. 1994, 56, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.W.; Koon, C.M.; Wat, E.; Ko, C.H.; Chan, J.Y.; Yew, D.T.; Leung, P.C.; Chan, W.Y.; Lau, C.B.; Fung, K.P. A herbal formula containing roots of Salvia miltiorrhiza (Danshen) and Pueraria lobata (Gegen) inhibits inflammatory mediators in LPS-stimulated RAW 264.7 macrophages through inhibition of nuclear factor kappaB (NFkappaB) pathway. J. Ethnopharmacol. 2013, 145, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Sun, S.; Zhong, W.; Soromou, L.W.; Zhou, X.; Wei, M.; Ren, Y.; Ding, Y. Zingerone attenuates lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2014, 19, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Do-Umehara, H.C.; Chen, C.; Urich, D.; Zhou, L.; Qiu, J.; Jang, S.; Zander, A.; Baker, M.A.; Eilers, M.; Sporn, P.H.S.; et al. Suppression of inflammation and acute lung injury by the transcription factor Miz1 via repression of C/EBP-δ. Nat. Immunol. 2013, 14, 461–469. [Google Scholar] [CrossRef]
- Kim, K.H.; Kwun, M.J.; Han, C.W.; Ha, K.-T.; Choi, J.-Y.; Joo, M. Suppression of lung inflammation in an LPS-induced acute lung injury model by the fruit hull of Gleditsia sinensis. BMC Complement. Altern. Med. 2014, 14, 402. [Google Scholar] [CrossRef]
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.P.; Mahyari, S.; Hassani, F.V.; Karimi, G. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef]
- Dan, Z. Study on Antimicrobial Effect of Flavonoids from Portulace oleracea L. J. Anhui Agric. Sci. 2006, 34, 7. [Google Scholar]
- Malek, F.; Boskabady, M.; Borushaki, M.; Tohidi, M. Bronchodilatory effect of Portulaca oleracea in airways of asthmatic patients. J. Ethnopharmacol. 2004, 93, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Hozayen, W.; Bastawy, M.; Elshafeey, H. Effects of aqueous purslane (portulaca oleracea) extract and fish oil on gentamicin nephrotoxicity in albino rats. Nat. Sci. 2011, 9, 47–62. [Google Scholar]
- Wang, W.-Y.; Gu, L.-M.; Dong, L.-W.; Wang, X.-L.; Ling, C.-Q.; Li, M. Protective effect of Portulaca oleracea extracts on hypoxic nerve tissue and its mechanism. Asia Pac. J. Clin. Nutr. 2007, 16, 227–233. [Google Scholar] [PubMed]
- Parry, O.; Marks, J.; Okwuasaba, F. The skeletal muscle relaxant action of Portulaca oleracea: Role of potassium ions. J. Ethnopharmacol. 1993, 40, 187–194. [Google Scholar] [CrossRef]
- Eidi, A.; Mortazavi, P.; Moghadam, J.Z.; Mardani, P.M. Hepatoprotective effects of Portulaca oleracea extract against CCl4-induced damage in rats. Pharm. Biol. 2015, 53, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, A.; Vijayakumar, M.; Rao Ch, V. Antiulcerogenic Effect Of Ethanolic Extract Of Portulaca oleracea Experimental Study. Pharmacol. Online 2010, 1, 417–432. [Google Scholar]
- Hanumantappa, B.N.; Ramesh, L.; Umesh, M. Evaluation of Potential Antifertility activity of Total Flavonoids, Isolated from Portulaca oleracea L on female albino rats. Int. J. PharmTech Res. 2014, 6, 783–793. [Google Scholar]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Askari, V.R.; Alavinezhad, A.; Boskabady, M.H. The impact of “Ramadan fasting period” on total and differential white blood cells, haematological indices, inflammatory biomarker, respiratory symptoms and pulmonary function tests of healthy and asthmatic patients. Allergol. Immunopathol. 2016, 44, 359–367. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Rezaee, S.A.; Boskabady, M.H. Auraptene regulates Th1/Th2/TReg balances, NF-κB nuclear localization and nitric oxide production in normal and Th2 provoked situations in human isolated lymphocytes. Phytomedicine 2018, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- Chuang, K.-H.; Peng, Y.-C.; Chien, H.-Y.; Lu, M.-L.; Du, H.-I.; Wu, Y.-L. Attenuation of LPS-Induced Lung Inflammation by Glucosamine in Rats. Am. J. Respir. Cell Mol. Biol. 2013, 49, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Luo, H.; Wang, N.; Li, J.; Xu, S.; Chen, K.; Feng, J.; Wu, L.; Li, S.; Liu, T.; et al. Portulaca Extract Attenuates Development of Dextran Sulfate Sodium Induced Colitis in Mice through Activation of PPARγ. PPAR Res. 2018, 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, X.; Tang, G.; Liu, H.; Zhang, Y.; Zhang, B.; Zhao, X.; Wang, W. Ethanol extract of Portulaca oleracea L. reduced the carbon tetrachloride induced liver injury in mice involving enhancement of NF-kappaB activity. Am. J. Transl. Res. 2014, 6, 746–755. [Google Scholar] [PubMed]
- Valenzuela, R.; Illesca, P.; Echeverria, F.; Espinosa, A.; Rincon-Cervera, M.A.; Ortiz, M.; Hernandez-Rodas, M.C.; Valenzuela, A.; Videla, L.A. Molecular adaptations underlying the beneficial effects of hydroxytyrosol in the pathogenic alterations induced by a high-fat diet in mouse liver: PPAR-alpha and Nrf2 activation, and NF-kappaB down-regulation. Food Funct. 2017, 8, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rodas, M.C.; Valenzuela, R.; Echeverria, F.; Rincon-Cervera, M.A.; Espinosa, A.; Illesca, P.; Munoz, P.; Corbari, A.; Romero, N.; Gonzalez-Manan, D.; et al. Supplementation with Docosahexaenoic Acid and Extra Virgin Olive Oil Prevents Liver Steatosis Induced by a High-Fat Diet in Mice through PPAR-alpha and Nrf2 Upregulation with Concomitant SREBP-1c and NF-kB Downregulation. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.R.; Wurfel, M.M. A TRIFfic Perspective on Acute Lung Injury. Cell 2008, 133, 208–210. [Google Scholar] [CrossRef]
- Reutershan, J.; Morris, M.A.; Burcin, T.L.; Smith, D.F.; Chang, D.; Saprito, M.S.; Ley, K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J. Clin. Investig. 2006, 116, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Verin, A.D.; Black, S.M.; Catravas, J.D. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem. Pharmacol. 2009, 77, 1763–1772. [Google Scholar] [CrossRef]
- Feng, G.; Sun, B.; Li, T.Z. Daidzein attenuates lipopolysaccharide-induced acute lung injury via toll-like receptor 4/NF-kappaB pathway. Int. Immunopharmacol. 2015, 26, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mallampalli, R.K. The acute respiratory distress syndrome: From mechanism to translation. J. Immunol. 2015, 194, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, V.B.; Askari, V.R.; Shirazinia, R.; Soheili-Far, S.; Askari, N.; Rahmanian-Devin, P.; Sanei-Far, Z.; Mousavi, S.H.; Ghodsi, R. Protective effects of hydro-ethanolic extract of Terminalia chebula on primary microglia cells and their polarization (M1/M2 balance). Mult. Scler. Relat. Disord. 2018, 25, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Askari, V.R.; Rahimi, V.B.; Zamani, P.; Fereydouni, N.; Rahmanian-Devin, P.; Sahebkar, A.H.; Rakhshandeh, H. Evaluation of the effects of Iranian propolis on the severity of post operational-induced peritoneal adhesion in rats. Biomed. Pharmacother. 2018, 99, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Askari, V.R.; Fereydouni, N.; Baradaran Rahimi, V.; Askari, N.; Sahebkar, A.H.; Rahmanian-Devin, P.; Samzadeh-Kermani, A. β-Amyrin, the cannabinoid receptors agonist, abrogates mice brain microglial cells inflammation induced by lipopolysaccharide/interferon-γ and regulates Mφ1/Mφ2 balances. Biomed. Pharmacother. 2018, 101, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Till, G.O.; Hatherill, J.R.; Tourtellotte, W.W.; Lutz, M.J.; Ward, P.A. Lipid peroxidation and acute lung injury after thermal trauma to skin. Evidence of a role for hydroxyl radical. Am. J. Pathol. 1985, 119, 376–384. [Google Scholar] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 31. [Google Scholar] [CrossRef]
- Kristof, A.S.; Goldberg, P.; Laubach, V.; Hussain, S.N.A. Role of Inducible Nitric Oxide Synthase in Endotoxin-induced Acute Lung Injury. Am. J. Respir. Crit. Care Med. 1998, 158, 1883–1889. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.-F.; Teixeira, M.; Valentine, J.S. Superoxide Dismutases and Superoxide Reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Brigham, K.L. Role of Free Radicals in Lung Injury. Chest 1986, 89, 859–863. [Google Scholar] [CrossRef]
- He, G.; Dong, C.; Luan, Z.; McAllan, B.M.; Xu, T.; Zhao, L.; Qiao, J. Oxygen free radical involvement in acute lung injury induced by H5N1 virus in mice. Influ. Other Respir. Viruses 2013, 7, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Junod, A.F. Oxygen free radicals and lungs. Intensiv. Care Med. 1989, 15 (Suppl. 1), S21–S23. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Liu, Y.; Xia, Y.; Tang, T. Analysis of Flavonoids in Portulaca oleracea L. by UV-Vis Spectrophotometry with Comparative Study on Different Extraction Technologies. Food Anal. Methods 2010, 3, 90–97. [Google Scholar] [CrossRef]
- Hwang, J.; Hwang, H.; Lee, H.W.; Suk, K. Microglia signaling as a target of donepezil. Neuropharmacology 2010, 58. [Google Scholar] [CrossRef] [PubMed]
- Karimi, G.; Aghasizadeh, M.; Razavi, M.; Taghiabadi, E. Protective effects of aqueous and ethanolic extracts of Nigella sativa L. and Portulaca oleracea L. on free radical induced hemolysis of RBCs. Daru 2011, 19, 295–300. [Google Scholar] [PubMed]
- Feizpour, A.; Boskabady, M.H.; Ghorbani, A. Adipose-Derived Stromal Cell Therapy Affects Lung Inflammation and Tracheal Responsiveness in Guinea Pig Model of COPD. PLoS ONE 2014, 9, e108974. [Google Scholar] [CrossRef]
- Kaveh, M.; Eidi, A.; Nemati, A.; Boskabady, M.H. The Extract of Portulaca oleracea and its Constituent, Alpha Linolenic Acid Affects Serum Oxidant Levels and Inflammatory Cells in Sensitized Rats. Iran. J. Allergy Asthma Immunol. 2017, 16, 256–270. [Google Scholar] [PubMed]
- Bahramsoltani, R.; Farzaei, M.H.; Abdolghaffari, A.H.; Rahimi, R.; Samadi, N.; Heidari, M.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M.; et al. Evaluation of phytochemicals, antioxidant and burn wound healing activities of Cucurbita moschata Duchesne fruit peel. Iran. J. Basic Med. Sci. 2017, 20, 798–805. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Askari, V.R.; Emami, S.A.; Tayarani-Najaran, Z. Anti-melanogenic activity of Viola odorata different extracts on B16F10 murine melanoma cells. Iran. J. Basic Med. Sci. 2017, 20, 242–249. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Askari, V.R.; Mehrdad, A.; Sadeghnia, H.R. Boswellia serrata has promising impact on glutamate and quinolinic acid-induced toxicity on oligodendroglia cells: In vitro study. Acta Pol. Pharm. 2017, 74, 1803–1811. [Google Scholar]
- Maione, F.; Paschalidis, N.; Mascolo, N.; Dufton, N.; Perretti, M.; D’Acquisto, F. Interleukin 17 sustains rather than induces inflammation. Biochem. Pharmacol. 2009, 77, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.J.; Bond, R.A.; Spina, D.; Ahluwalia, A.; Alexander, S.P.; Giembycz, M.A.; Gilchrist, A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; et al. Experimental design and analysis and their reporting: New guidance for publication in BJP. Br. J. Pharmacol. 2015, 172, 3461–3471. [Google Scholar] [CrossRef] [PubMed]
- George, C.H.; Stanford, S.C.; Alexander, S.; Cirino, G.; Docherty, J.R.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Updating the guidelines for data transparency in the British Journal of Pharmacology—Data sharing and the use of scatter plots instead of bar charts. Br. J. Pharmacol. 2017, 174, 2801–2804. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Roberts, R.E.; Broughton, B.R.S.; Sobey, C.G.; George, C.H.; Stanford, S.C.; Cirino, G.; Docherty, J.R.; Giembycz, M.A.; Hoyer, D.; et al. Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. Br. J. Pharmacol. 2018, 175, 407–411. [Google Scholar] [CrossRef] [PubMed]
| C | LPS | Dexa | PO 50 mg/kg | PO 100 mg/kg | PO 200 mg/kg | |
|---|---|---|---|---|---|---|
| Body weight (g) | 220 ± 31 | 218 ± 22 | 208 ± 19 | 215 ± 21 | 223 ± 22 | 231 ± 19 |
| Lung (g) | 1.05 ± 0.08 *** | 2.78 ± 0.15 | 1.28 ± 0.07 *** | 1.87 ± 0.14 ** | 1.61 ± 0.09 *** | 1.23 ± 0.11 *** |
| Liver (g) | 5.41 ± 1.21 ** | 7.94 ± 1.24 | 5.77 ± 1.06 ** | 8.01 ± 1.28 | 7.23 ± 1.18 | 5.69 ± 1.13 ** |
| Heart (g) | 0.86 ± 0.08 *** | 1.21 ± 0.09 | 0.89 ± 0.12 *** | 1.17 ± 0.07 | 1.05 ± 0.17 | 0.83 ± 0.12 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baradaran Rahimi, V.; Rakhshandeh, H.; Raucci, F.; Buono, B.; Shirazinia, R.; Samzadeh Kermani, A.; Maione, F.; Mascolo, N.; Askari, V.R. Anti-Inflammatory and Anti-Oxidant Activity of Portulaca oleracea Extract on LPS-Induced Rat Lung Injury. Molecules 2019, 24, 139. https://doi.org/10.3390/molecules24010139
Baradaran Rahimi V, Rakhshandeh H, Raucci F, Buono B, Shirazinia R, Samzadeh Kermani A, Maione F, Mascolo N, Askari VR. Anti-Inflammatory and Anti-Oxidant Activity of Portulaca oleracea Extract on LPS-Induced Rat Lung Injury. Molecules. 2019; 24(1):139. https://doi.org/10.3390/molecules24010139
Chicago/Turabian StyleBaradaran Rahimi, Vafa, Hassan Rakhshandeh, Federica Raucci, Benedetta Buono, Reza Shirazinia, Alireza Samzadeh Kermani, Francesco Maione, Nicola Mascolo, and Vahid Reza Askari. 2019. "Anti-Inflammatory and Anti-Oxidant Activity of Portulaca oleracea Extract on LPS-Induced Rat Lung Injury" Molecules 24, no. 1: 139. https://doi.org/10.3390/molecules24010139
APA StyleBaradaran Rahimi, V., Rakhshandeh, H., Raucci, F., Buono, B., Shirazinia, R., Samzadeh Kermani, A., Maione, F., Mascolo, N., & Askari, V. R. (2019). Anti-Inflammatory and Anti-Oxidant Activity of Portulaca oleracea Extract on LPS-Induced Rat Lung Injury. Molecules, 24(1), 139. https://doi.org/10.3390/molecules24010139







