A New Polyoxygenated Flavonol Gossypetin-3-O-β-d-Robinobioside from Caesalpinia gilliesii (Hook.) D. Dietr. and In Vivo Hepatoprotective, Anti-Inflammatory, and Anti-Ulcer Activities of the Leaf Methanol Extract
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Composition
2.2. Hepatoprotective Activity
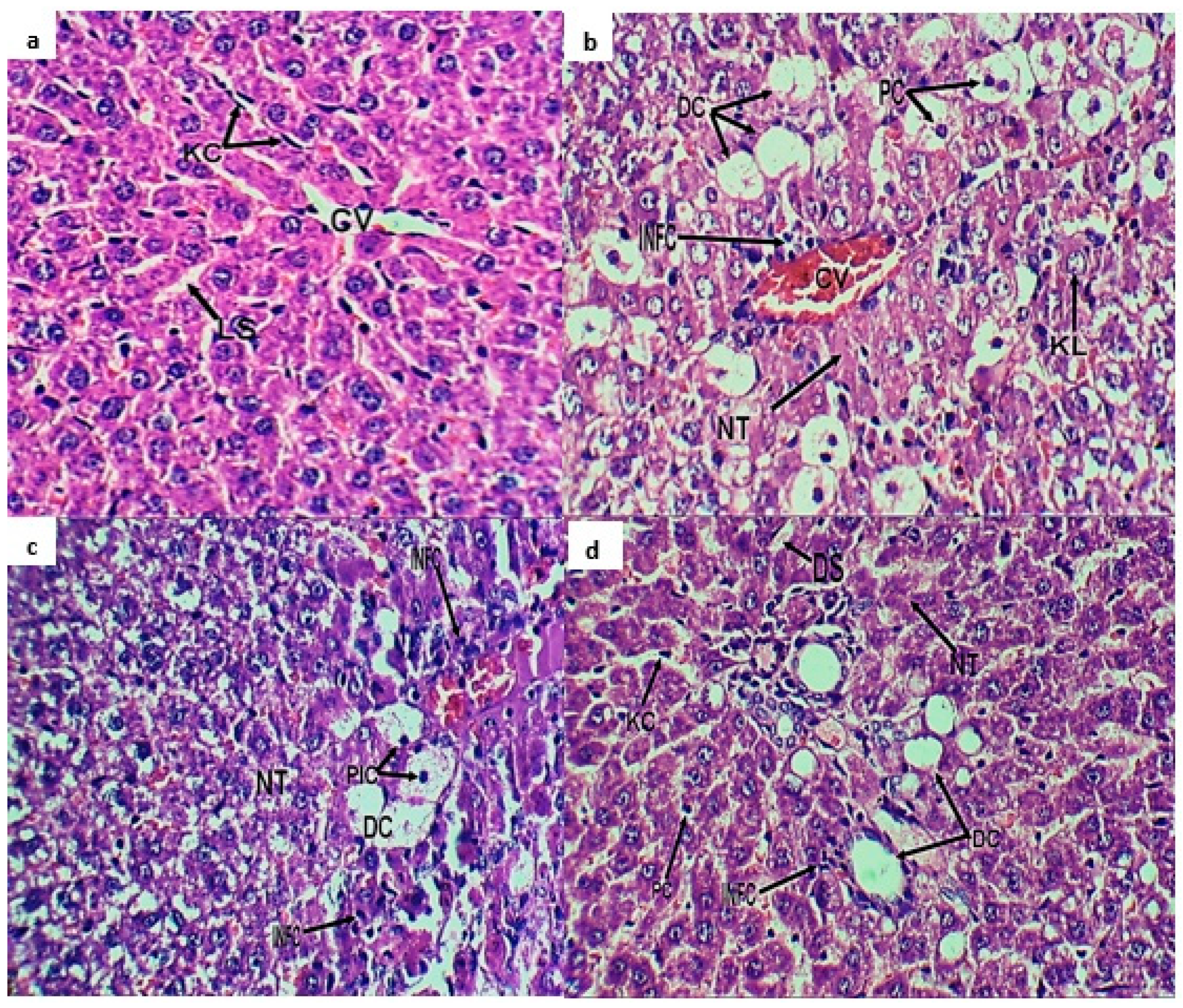
2.3. Histopathological Investigation
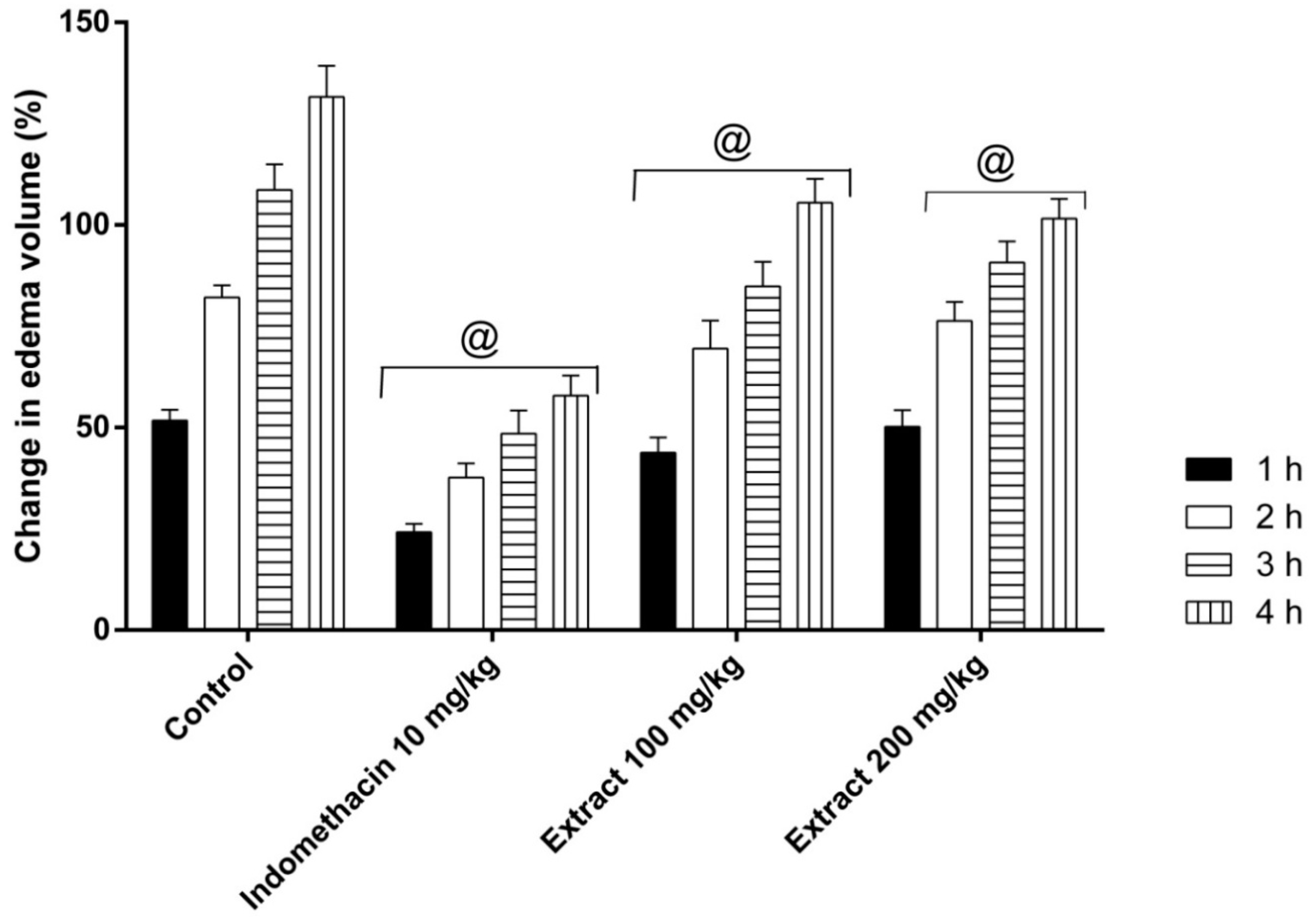
2.4. Anti-Inflammatory Activity: Carrageenan-Induced Edema Assay
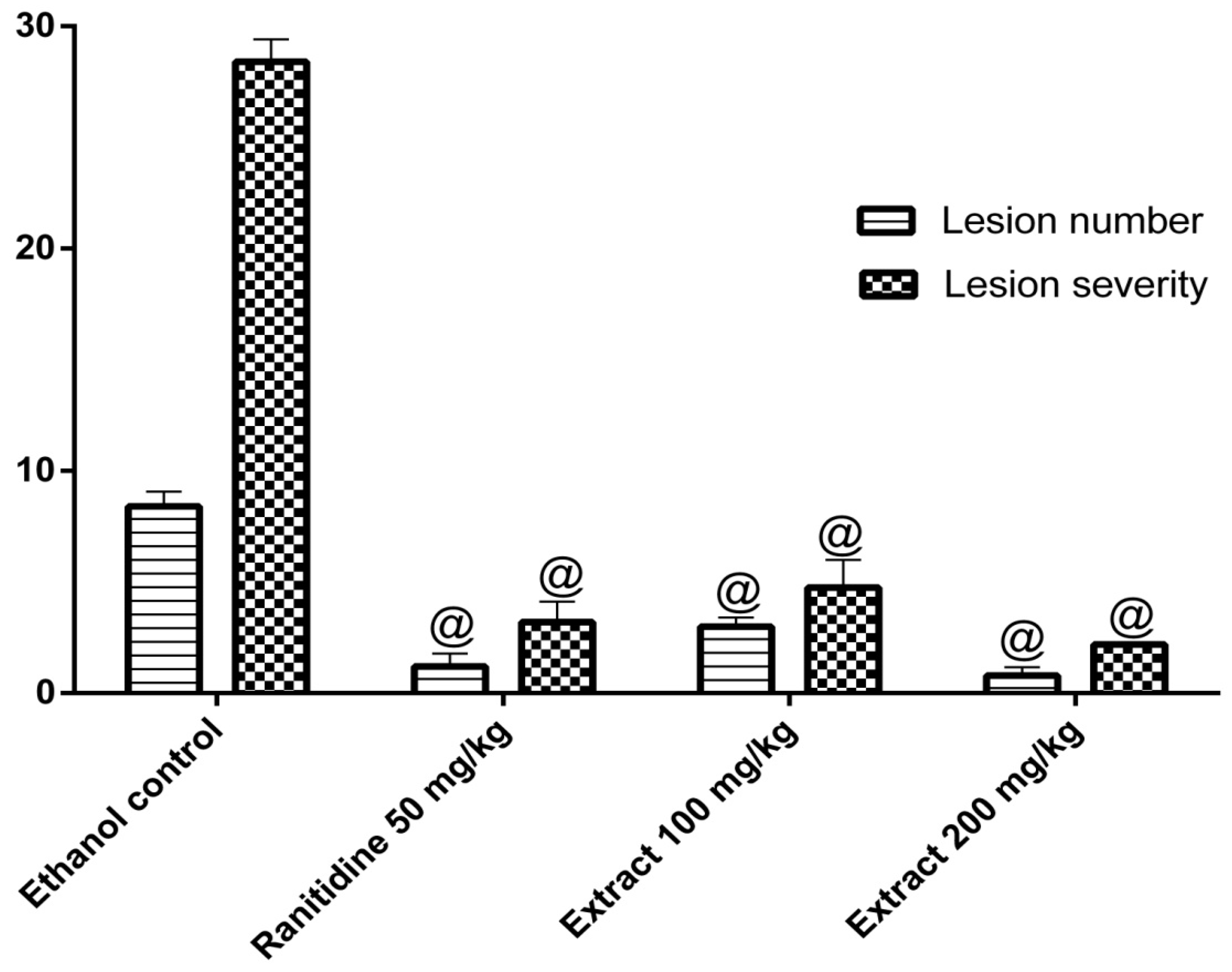
2.5. Anti-Ulcer Activity
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. Experimental Analysis
4.4. Biological Experiments
4.4.1. Drugs and Chemicals
4.4.2. Animals
4.4.3. Hepatoprotective Activity
4.4.4. Histopathological Investigation
4.4.5. Anti-Inflammatory Activity: Carrageenan-Induced Paw Edema
4.4.6. Anti-Ulcer Activity: Ethanol-Induced Gastric Ulcer Model
5. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kaplowitz, N. Idiosyncratic drug hepatotoxicity. Nat. Rev. Drug Discov. 2005, 4, 489. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Sachdeva, T.; Bafna, P. Drug-induced hepatotoxicity: A review. J. Appl. Pharm. Sci. 2012, 2, 233–243. [Google Scholar] [CrossRef]
- Rubenstein, J.H.; Laine, L. Systematic review: The hepatotoxicity of non-steroidal anti-inflammatory drugs. Aliment. Pharmacol. Ther. 2004, 20, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Schellack, N.; Fourie, J. A review of nonsteroidal anti-inflammatory drugs. SA Pharm. J. 2015, 82, 8–18. [Google Scholar]
- Van Wyk, B.-E.; Wink, M. Phytomedicines, Herbal Drugs, and Poisons; University of Chicago Press: Chicago, IL, USA, 2015. [Google Scholar]
- Mabberley, D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classifications and Uses; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Saeed, M.A.; Sabir, A. Antibacterial activity of Caesalpinia bonducella seeds. Fitoterapia 2001, 72, 807–809. [Google Scholar] [CrossRef]
- Nakamura, E.S.; Kurosaki, F.; Arisawa, M.; Mukainaka, T.; Okuda, M.; Tokuda, H.; Nishino, H.; Pastore, F. Cancer chemopreventive effects of constituents of Caesalpinia ferrea and related compounds. Cancer Lett. 2002, 177, 119–124. [Google Scholar] [CrossRef]
- Saenjum, C.; Chaiyasut, C.; Kadchumsang, S.; Chansakaow, S.; Suttajit, M. Antioxidant activity and protective effects on DNA damage of Caesalpinia sappan L. extract. J. Med. Plants Res. 2010, 4, 1594–1608. [Google Scholar]
- Shukla, S.; Mehta, A.; John, J.; Singh, S.; Mehta, P.; Vyas, S.P. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem. Toxicol. 2009, 47, 1848–1851. [Google Scholar] [CrossRef]
- Osman, S.M.; El-Haddad, A.E.; El-Raey, M.A.; El-Khalik, S.M.A.; Koheil, M.A.; Wink, M. A new octadecenoic acid derivative from Caesalpinia gilliesii flowers with potent hepatoprotective activity. Pharmacog. Mag. 2016, 12, S332. [Google Scholar]
- Ulubelen, A.; McCaughey, W.; Cole, J. Proteinaceous antitumor substances from plants III. Caesalpinia gilliesii (Leguminosae). J. Pharm. Sci. 1967, 56, 914–916. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.; Thomas, M. The Systematic Identification of Flavonoids; Springer: New York, NY, USA, 1970. [Google Scholar]
- Markham, K.R.; Chari, V.M. Carbon-13 NMR Spectroscopy of Flavonoids, in the Flavonoids; Springer: New York, NY, USA, 1982; pp. 19–134. [Google Scholar]
- Harbone, J.; Mabry, T. The Flavonoids: Advances in Research; Chapman and Hall: New York, NY, USA, 1982. [Google Scholar]
- Agrawal, P. Flavonoid glycosides. In Carbon-13 NMR of Flavonoids; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Nawwar, M.A.; Buddrus, J. A gossypetin glucuronide sulphate from the leaves of Malva sylvestris. Phytochemistry 1981, 20, 2446–2448. [Google Scholar] [CrossRef]
- Bennini, B.; Chulia, A.J.; Kaouadji, M. Flavonol glycosides from Erica cinerea. J. Nat. Prod. 1994, 57, 178–180. [Google Scholar] [CrossRef]
- Harborne, J. Phytochemical Methods; Chapman and Hall: New York, NY, USA, 1984; Volume 3, pp. 100–117. [Google Scholar]
- Teles, Y.C.; Ribeiro-Filho, J.; Bozza, P.T.; Agra, M.d.F.; Siheri, W.; Igoli, J.O.; Gray, A.I.; Souza, M.d.F.V.d. Phenolic constituents from Wissadula periplocifolia (L.) C. Presl. and anti-inflammatory activity of 7, 4′-di-O-methylisoscutellarein. Nat. Prod. Res. 2016, 30, 1880–1884. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.A.; El-Mesallamy, A.M.; Souleman, A.M.; Mousa, M.A. Cytotoxic activity of bioactive compound from Caesalpinia ferrea Martius, Fabaceae. Int. J. Pharmacog. Phytochem. Res. 2016, 8, 2080–2084. [Google Scholar]
- N’guessan, J.; Bidié, A.; Lenta, B.; Weniger, B.; Andre, P.; Guédé-Guina, F. In vitro assays for bioactivity-guided isolation of antisalmonella and antioxidant compounds in Thonningia sanguinea flowers. Afr. J. Biotechnol. 2007, 6, 1685–1689. [Google Scholar]
- Panda, S.; Chowdary, P. Synthesis of novel indolyl-pyrimidine antiinflammatory, antioxidant and antibacterial agents. Ind. J. Pharm. Sci. 2008, 70, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Duwiejua, M.; Zeitlin, I.; Waterman, P.; Chapman, J.; Mhango, G.; Provan, G. Anti-inflammatory activity of resins from some species of the plant family Burseraceae. Planta Med. 1993, 59, 12–16. [Google Scholar] [CrossRef]
- Waldum, H.; Arnestad, J.; Brenna, E.; Eide, I.; Syversen, U.; Sandvik, A. Marked increase in gastric acid secretory capacity after omeprazole treatment. Gut 1996, 39, 649–653. [Google Scholar] [CrossRef]
- Hussaini, J.; Nazmul, M.; Abdullah, M.; Ismail, S. Recombinant Clone ABA392 Protects laboratory animals from Pasteurella multocida serotype B. J. Vet. Adv. 2012, 2, 114–119. [Google Scholar]
- Wei, X.-H.; Yang, S.-J.; Liang, N.; Hu, D.-Y.; Jin, L.-H.; Xue, W.; Yang, S. Chemical constituents of Caesalpinia decapetala (Roth) Alston. Molecules 2013, 18, 1325–1336. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.F.; Zhang, M.L.; Huo, C.H.; Dong, M.; Shi, Q.W.; Kiyota, H. Chemical constituents of plants from the genus Caesalpinia. Chem. Biodiv. 2011, 8, 1370–1399. [Google Scholar] [CrossRef]
- Ogunlana, O.O.; Ogunlana, O. Antiplasmodial flavonoid from young twigs and leaves of Caesalpinia bonduc (Linn) Roxb. J. Chem. Pharm. Res. 2015, 7, 931–937. [Google Scholar]
- Osman, S.M.; Khalek, S.M.A.; Koheil, M.A.; El-Haddad, A.E.; Wink, M. A new steroidal compound (β-sitosterol-3-O-butyl) isolated from Caesalpinia gilliesii flowers. IJARNP 2015, 8, 14–19. [Google Scholar]
- Sobeh, M.; Esmat, A.; Petruk, G.; Abdelfattah, M.A.O.; Dmirieh, M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Phenolic compounds from Syzygium jambos (Myrtaceae) exhibit distinct antioxidant and hepatoprotective activities in vivo. J. Funct. Food 2018, 41, 223–231. [Google Scholar] [CrossRef]
- Sarkar, R.; Hazra, B.; Mandal, N. Hepatoprotective potential of Caesalpinia crista against iron-overload-induced liver toxicity in mice. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Zanin, J.L.B.; De Carvalho, B.A.; Salles Martineli, P.; Dos Santos, M.H.; Lago, J.H.G.; Sartorelli, P.; Viegas, C.; Soares, M.G. The genus Caesalpinia L. (Caesalpiniaceae): Phytochemical and pharmacological characteristics. Molecules 2012, 17, 7887–7902. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Petruk, G.; Rezq, S.; Ashour, M.L.; Youssef, F.S.; El-Shazly, A.M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Syzygium aqueum: A polyphenol- rich leaf extract exhibits antioxidant, hepatoprotective, pain-killing and anti-inflammatory activities in animal models. Front Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Tapas, A.R.; Sakarkar, D.; Kakde, R. Flavonoids as nutraceuticals: A review. Trop J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World. J. 2013. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.-H.; Tzeng, Y.-M. Anti-inflammatory activities of flavonoids isolated from Caesalpinia pulcherrima. J. Ethnopharmacol. 2005, 100, 249–253. [Google Scholar] [CrossRef]
- Ribeiro, A.R.S.; Diniz, P.B.F.; Estevam, C.S.; Pinheiro, M.S.; Albuquerque-Júnior, R.L.C.; Thomazzi, S.M. Gastroprotective activity of the ethanol extract from the inner bark of Caesalpinia pyramidalis in rats. J. Ethnopharmacol. 2013, 147, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Sumalatha, S.; Padma, D.; Pai, K.S.R.; Kotian, S.R.; Kumar, N.; Bhat, K.M. Hepatoprotective activity of aqueous extract of Caesalpinia bonduc against CCl4 induced chronic hepatotoxicity. Int. J. Pharm. Pharm. Sci. 2016, 8, 207–211. [Google Scholar]
- Hikino, H.; Taguchi, T.; Fujimura, H.; Hiramatsu, Y. Antiinflammatory principles of Caesalpinia sappan wood and of Haematoxylon campechianum wood1. Planta Med. 1977, 31, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Ashour, M.L.; Sobeh, M.; El-Beshbishy, H.A.; Singab, A.N.; Wink, M. Eremophila maculata-Isolation of a rare naturally-occurring lignan glycoside and the hepatoprotective activity of the leaf extract. Phytomedicine 2016, 23, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Youssef, F.S.; Esmat, A.; Petruk, G.; El-Khatib, A.H.; Monti, D.M.; Ashour, M.L.; Wink, M. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food Chem. Toxicol. 2018, 113, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Emam, M.; El Raey, M.A.; Eisa, W.H.; El-Haddad, A.E.; Osman, S.M.; El-Ansari, M.A.; Rabie, A.-G.M. Green synthesis of silver nanoparticles from Caesalpiniagilliesii (Hook) leaves: Antimicrobial activity and in vitro cytotoxic effect against BJ-1 and MCF-7 cells. J. App. Pharm. Sci. 2017, 7, 226–233. [Google Scholar]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; El-Beshbishy, H.A.; El-Shazly, A.M.; Wink, M. Albizia harveyi: Phytochemical profiling, antioxidant, antidiabetic and hepatoprotective activities of the bark extract. Med. Chem. Res. 2017, 26, 3091–3105. [Google Scholar] [CrossRef]
- Galighor, A.; Kozloff, E. Essentials of Practical Microtechnique, 2nd ed.; Lea and Febiger: NewYork, NY, USA, 1976. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenan-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Society Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Arunachalam, G.; Mandal, A.B.; Sur, T.K.; Mandal, S.C.; Bhattacharya, S. Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J. Ethnopharmacol. 2002, 82, 229–237. [Google Scholar] [CrossRef]
- Robert, A.; Nezamis, J.E.; Lancaster, C.; Hanchar, A.J. Cytoprotection by prostaglandins in rats: Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterol 1979, 77, 433–443. [Google Scholar]
- Mózsik, G.; Moron, F.; Jávor, T. Cellular mechanisms of the development of gastric mucosal damage and of gastrocytoprotection induced by prostacyclin in rats. A pharmacological study. Prostag. Leukotr. Med. 1982, 9, 71–84. [Google Scholar] [CrossRef]
- Ibrahim, B.M.; Salama, A.A.; Abdallah, H.M.; El Awdan, S.A.; Shaffie, N.M. Study of the protective effects of flaxseed oil on ethanol induced gastric mucosal lesions in non ovariectomized and ovariectomized rats. Int. J. Pharmacol. 2016, 12, 329–339. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds and the plant material are available from the authors. |
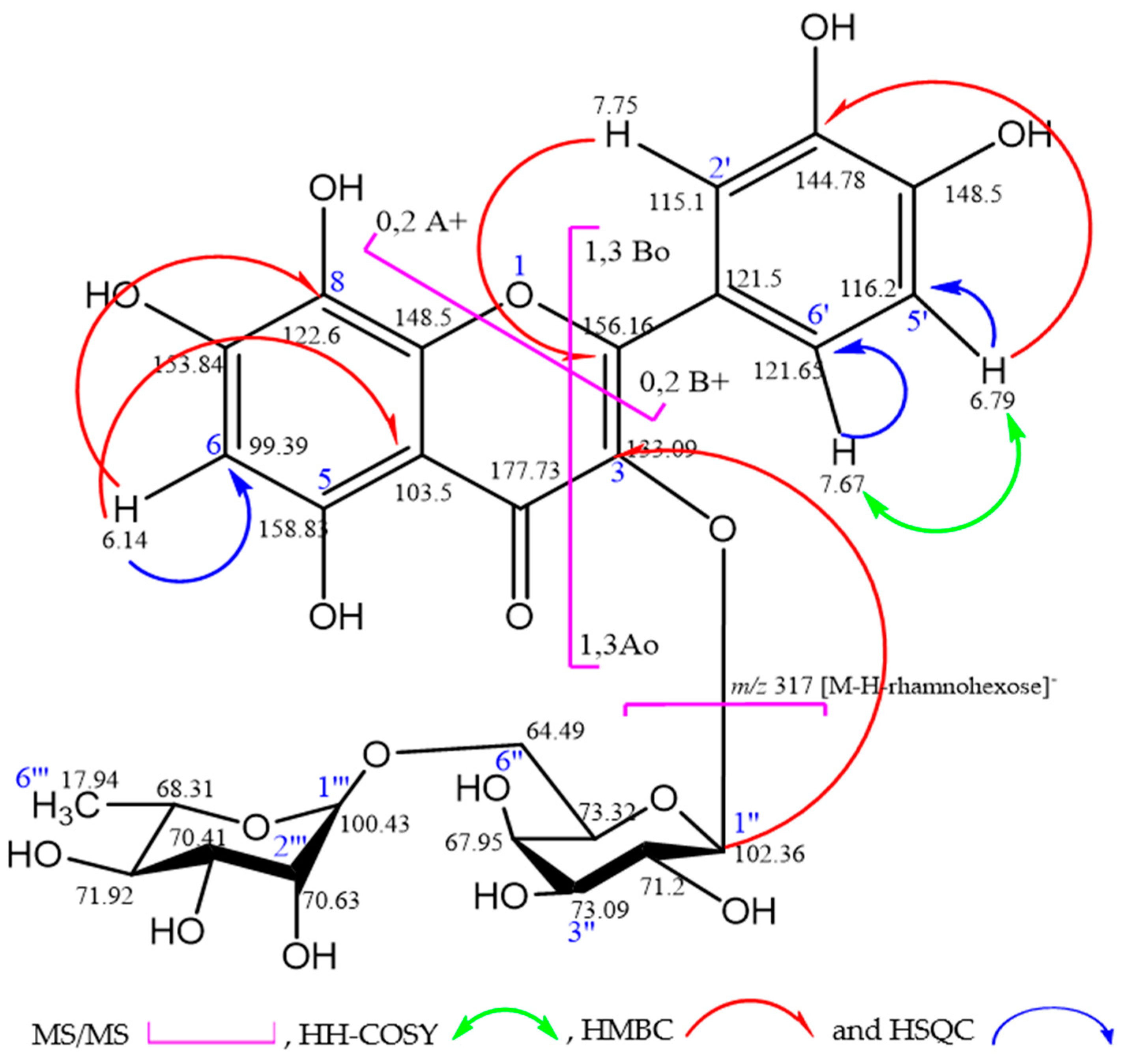

| Position | APT | δH (mult, J [Hz]) | δC | HMBC (H→C) |
|---|---|---|---|---|
| 2 | C | 156.16 | ||
| 3 | C | 133.09 | ||
| 4 | C | 177.73 | ||
| 5 | C | 158.83 | ||
| 6 | CH | 6.14 (1H, s, H-6) | 98.39 | C (8), C (10) |
| 7 | C | 153.84 | ||
| 8 | C | 122.6 | ||
| 9 | C | 148.5 | ||
| 10 | C | 103.50 | ||
| 1′ | C | 121.50 | ||
| 2′ | CH | 7.75(1H, d, J = 2.0Hz, H-2′) | 115.10 | C (2), C (4′), C (6′) |
| 3′ | C | 144.78 | ||
| 4′ | C | 148.50 | ||
| 5′ | CH | 6.79 (1H, d, J = 8.5Hz, H-5′) | 116.2 | C (1′), C (3′) |
| 6′ | CH | 7.67 (1H, dd, J = 2&8.5Hz, H-6′) | 121.65 | C (2), C (2′), C (4′) |
| 1″ | CH | 5.35 (1H, d, J = 7.7Hz, H-1″) | 102.36 | |
| 2″ | CH | 71.20 | ||
| 3″ | CH | 73.09 | ||
| 4″ | CH | 67.95 | ||
| 5″ | CH | 73.32 | ||
| 6″ | CH2 | 64.79 | ||
| 1‴ | CH | 4.45 (1H, br.s, H-1‴) | 100.43 | |
| 2‴ | CH | 70.63 | ||
| 3‴ | CH | 70.41 | ||
| 4‴ | CH | 71.92 | ||
| 5‴ | CH | 68.31 | ||
| 6‴ | CH3 | 1.08 (3H, d, J = 6.2Hz, H-6‴) | 17.94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emam, M.; El Raey, M.A.; El-Haddad, A.E.; El Awdan, S.A.; Rabie, A.-G.M.; El-Ansari, M.A.; Sobeh, M.; Osman, S.M.; Wink, M. A New Polyoxygenated Flavonol Gossypetin-3-O-β-d-Robinobioside from Caesalpinia gilliesii (Hook.) D. Dietr. and In Vivo Hepatoprotective, Anti-Inflammatory, and Anti-Ulcer Activities of the Leaf Methanol Extract. Molecules 2019, 24, 138. https://doi.org/10.3390/molecules24010138
Emam M, El Raey MA, El-Haddad AE, El Awdan SA, Rabie A-GM, El-Ansari MA, Sobeh M, Osman SM, Wink M. A New Polyoxygenated Flavonol Gossypetin-3-O-β-d-Robinobioside from Caesalpinia gilliesii (Hook.) D. Dietr. and In Vivo Hepatoprotective, Anti-Inflammatory, and Anti-Ulcer Activities of the Leaf Methanol Extract. Molecules. 2019; 24(1):138. https://doi.org/10.3390/molecules24010138
Chicago/Turabian StyleEmam, Mahmoud, Mohamed A. El Raey, Alaadin E. El-Haddad, Sally A. El Awdan, Abdel-Gawad M. Rabie, Mohamed A. El-Ansari, Mansour Sobeh, Samir M. Osman, and Michael Wink. 2019. "A New Polyoxygenated Flavonol Gossypetin-3-O-β-d-Robinobioside from Caesalpinia gilliesii (Hook.) D. Dietr. and In Vivo Hepatoprotective, Anti-Inflammatory, and Anti-Ulcer Activities of the Leaf Methanol Extract" Molecules 24, no. 1: 138. https://doi.org/10.3390/molecules24010138
APA StyleEmam, M., El Raey, M. A., El-Haddad, A. E., El Awdan, S. A., Rabie, A.-G. M., El-Ansari, M. A., Sobeh, M., Osman, S. M., & Wink, M. (2019). A New Polyoxygenated Flavonol Gossypetin-3-O-β-d-Robinobioside from Caesalpinia gilliesii (Hook.) D. Dietr. and In Vivo Hepatoprotective, Anti-Inflammatory, and Anti-Ulcer Activities of the Leaf Methanol Extract. Molecules, 24(1), 138. https://doi.org/10.3390/molecules24010138







