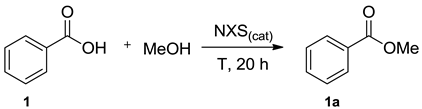
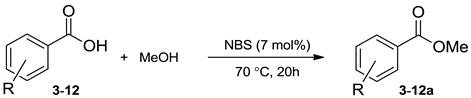Abstract
N-halosuccinimides (NXSs) are well-known to be convenient, easily manipulable and low-priced halogenation reagents in organic synthesis. In the present work, N-bromosuccinimide (NBS) has been promoted as the most efficient and selective catalyst among the NXSs in the reaction of direct esterification of aryl and alkyl carboxylic acids. Comprehensive esterification of substituted benzoic acids, mono-, di- and tri-carboxy alkyl derivatives has been performed under neat reaction conditions. The method is metal-free, air- and moisture-tolerant, allowing for a simple synthetic and isolation procedure as well as the large-scale synthesis of aromatic and alkyl esters with yields up to 100%. Protocol for the recycling of the catalyst has been proposed.
1. Introduction
Esterification reaction is one of the most important synthetic routes in organic synthesis due to the significance of its products. It is an irreplaceable reaction step during the synthesis of pharmaceuticals, cosmetics, plasticizers, perfumes, flavour chemicals, fine chemicals, electronic materials, solvents and chiral auxiliaries [1] and together with transesterification, it is a transformation of major significance in the biodiesel production [2,3,4,5]. Aside from being among the most prevalent final products and/or intermediates in the fields of science and industry, esters also play a significant role in biology, as the ester bonds are key linking groups in many primary lipid metabolites as well as secondary cyclodepsipeptide and polyketide metabolites [6]. As a result, a plethora of approaches have been reported for ester preparation, one of the most common being the reaction of direct esterification between carboxylic acids and alcohols (Fischer esterification) [7]. Conventionally, it is performed with excessive amounts of reagents/dehydrating agents or with activated carboxylic acid derivatives in the presence of a stoichiometric base, which results in significant amounts of by products and waste at the end of the process as well as in energy-, time- and solvent-consuming purification [8]. Therefore, methods of catalytic direct condensation between carboxylic acids and alcohols, which lack these disadvantages, have recently become an attractive research subject, employing a broad spectra of different catalysts, such as Brønsted acids [9], metal catalysts [10,11], Lewis acids [12,13], solid-supported catalysts [14,15] and solid acids [16,17], ionic liquids [18,19], PPh3-based catalysts [20], enzymes [21,22], zeolites [23,24], etc. From the industrial and sustainability perspective, the ideal esterification method would include the use of easily manipulable, metal-free, low-cost, water- and air-tolerant recyclable catalyst and mild solvent-free reaction conditions without the need for stoichiometric amounts of activators, large excesses of reagents and simultaneous removal of water. It should also be applicable to a broad substrate scope with high selectivity; it should be suitable for large-scale synthesis and allow a simple purification procedure, providing high product yields. The majority of known methods do not comply with at least one of the mentioned criteria; therefore, the field of sustainable design of esterification methods remains an attractive research challenge.
N-halosuccinimides (NXSs) belong to the class of N-halo reagents which are widely used in organic synthesis as halogenating, hydroxyhalogenating, oxidizing and condensing agents [25]. Their reactivity originates from the great lability of the N–X bond and various modes of its splitting [26]. Depending on the reaction conditions, different highly reactive species can be formed: N-radicals, N-cations, N-anions as well as their corresponding halogen counter particles, etc. [27]. Due to these convenient chemical properties, together with their metal-free character, low-cost, accessibility and higher stability relative to other N-halo reagents, NXSs have recently been attracting attention as mediators in substoichiometric amounts for different types of organic transformations [28,29,30,31]. The catalytic potential of N-bromosuccinimide (NBS) has recently been reviewed [32]; however, to the best of our knowledge, the use of N-bromosuccinimide as the only catalytic component in substoichiometric amounts for direct dehydrative esterification between alcohols and carboxylic acids has not been explored so far.
In our continuous pursuit of sustainable synthetic protocols [33,34,35,36,37], including those where NXSs are used as reagents [38,39,40,41,42,43,44,45], we now report and introduce the use of substoichiometric amounts of these compounds as mediators for efficient and selective comprehensive metal-free direct esterification of carboxyl functionality in organic molecules under neat reaction conditions (Scheme 1).

Scheme 1.
Direct dehydrative esterification of carboxylic acids catalysed by substoichiometric amounts of NXSs.
2. Results and Discussion
Previously, some advantages of N-bromosuccinimide (NBS) as a catalyst in reactions of transesterification have been observed, but the reaction was limited to transesterification of α-keto esters [46] or acetylation of alcohols using acetic anhydride [47]. In addition, esterification of carboxylic acids with alcohols in the presence of triphenylphosphine (PPh3) and N-bromo/iodosuccinimides has been reported as a method for ester preparation [48]. However, its significant drawback is posed by the by-product, phosphine oxide, formed in equimolar amounts, as it is difficult to remove during the purification step. Besides, the esterification was performed in halogenated solvent (dichloromethane) in the presence of equimolar amounts of base (pyridine) and NBS/NIS. Furthermore, molecular iodine has been presented as a convenient Lewis acid catalyst for direct dehydrative esterification, but attempts to prepare esters from corresponding aromatic acids have been found unsuccessful [49] or unselective [50,51]. Furthermore, bromine (Br2)-mediated esterification of carbocyclic acids with methanol has been reported [52], though this methodology carries handling safety risks due to the potentially hazardous effects of Br2/MeOH solution [53]. All the aforementioned provided us with an impetus to improve the reaction of direct esterification of aryl/alkyl acids. Since N-halosuccinimides have been widely used as more convenient and safer X2 substitutes in halogenation reactions, the catalytic activity of corresponding N-halosuccinimide derivatives in the reaction of direct condensation between various structurally different carboxylic acid and alcohols has been investigated and presented herein.
Initially, benzoic acid (1) has been chosen as an aromatic acid model molecule to verify expectations and optimize reaction protocols. In the typical experimental procedure, 1 has been refluxed with methanol (MeOH) in the presence of substoichiometric amounts of NXS: N-chlorosuccinimide (NCS), NBS, or N-iodosuccinimide (NIS). The results presented in Table 1 reveal that NBS seems to be the most promising catalyst in reactions of esterification, while without the presence of any of the NXSs, no conversion of starting material has been observed. In search of optimal reaction conditions, the effect of catalyst NBS loading has been examined by changing the amount of catalyst from 3 mol% to 15 mol% (Table 1). The amount below 7 mol% NBS furnished significantly lower conversion, while increasing the amount above 7 mol% did not significantly improve the yield of the corresponding ester. Therefore, further studies were carried out with 7 mol% of NBS. Moreover, the influence of temperature on the conversion of 1 to methyl benzoate (1a, Table 1) has been studied. The variation of temperature from 30 °C to 100 °C has been found to have a significant impact on the conversion of the acid to ester with the optimal temperature being 70 °C. Moreover, under dry reaction conditions (in the presence of Na2SO4) the efficiency of the esterification dropped considerably (entry 8). The promoting activities of NXSs, halogen mineral acids and molecular bromine were compared in the present esterification reaction. In the case of both aqueous HCl (entry 12) and molecular Br2 (entry 15), the conversions were comparable and their activity was similar to that of NBS (entry 6). On the other hand, the esterification efficiency in the case of both HBr and HI was considerably lower (entry 13 and 14) and resulted in the conversion yields of 84% and 68%, respectively. From the green-chemical point of view, NBS exhibited the highest catalytic activity among the examined catalysts.

Table 1.
Catalyst loading and temperature optimization studies 1.
The optimal reaction conditions presented above (entry 6, Table 1) have been applied to 1-octanoic acid (2) as an alkyl acid model compound. As expected, 1-octanoic acid has been quantitatively converted to the corresponding methyl octanoate (2a, Figure 1), while in the absence of NBS, no conversion of the starting material was observed.
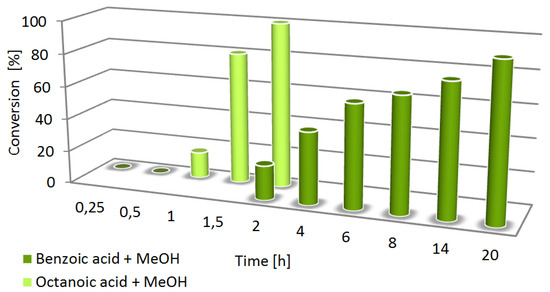
Figure 1.
The effect of reaction time on the efficiency of esterification of benzoic (1) and octanoic acid (2) with methanol in the presence of 7 mol% of NBS at 70 °C.
To compare the reactivity of the aromatic and alkyl acids, the optimization of reaction time has been performed (Figure 1). Relative to benzoic acid, significantly higher activity of alkyl acid (2) has been observed, which was in accordance with our expectations. Due to resonance stabilization of carboxyl group in aromatic acids, its lower activation resulted in longer reaction time and slightly lower conversion.
Encouraged by these promising results, the scope of the alcohols, suitable for esterification with acids 1 and 2, has been studied (Table 2). It can be observed that almost in all cases, alkyl acid (2) is significantly more active than aromatic acid (1) and the conversions to the corresponding esters (2a–i) are higher. Since nucleophilic characters, as well as steric properties, have been envisioned to have a considerable impact on reaction kinetics, different alcohols have been tested (a–i, Table 2). 2-Fluoro-1-ethanol (FCH2CH2OH, b) is a significantly weaker nucleophile than MeOH, therefore the reaction time for esterification of 1 as well as of 2 had to be prolonged. The elongation of the alcohol alkyl chain (a, d, f) had a stronger impact on esterification efficiency of benzoic acid than on esterification efficiency of octanoic acid, resulting in higher yields of octanoate esters (2a, 2d, 2f) relative to benzoate esters (1a, 1d, 1f). Moreover, the effect of increased steric hindrance of the nucleophilic alcohol component was followed by varying the bulkiness of alcohol from primary to tertiary structure (a, c and g, e and i). Interestingly, the esterification of octanoic acid was considerably less affected by the structural change from primary (MeOH, 2a) to secondary alcohol (i-PrOH and cyclopentanol, 2c and 2g) than the transformation of benzoic acid, where low (i-PrOH, 1a) or no conversion (cyclopentanol, 1g) was detected. Unfortunately, the limitation of the method was observed in reactions with bulky tertiary alcohols t-BuOH (e) and adamantanol (i), where no product was detected. Similarly, when phenol (h) has been used as the nucleophile, no reaction products were noticed, which can be assigned to the low nucleophilicity originating from the relatively high acidity of phenol molecule.

Table 2.
The effect of alcohol structure on esterification of benzoic (1) and octanoic acid (2) 1,2,3.
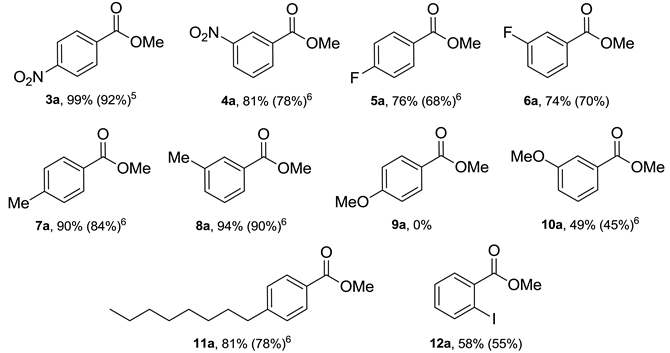
Due to the commercial importance of certain methyl esters in biodiesel production (fatty acid methyl esters, FAME) and perfumery (methyl benzoate), esterification of different types of carbocyclic acids with MeOH under optimal reaction conditions has been furtherly investigated (Table 3). As can be noticed, electronic effects of substituents, as well as their position on the phenyl ring of the investigated aromatic acids have exhibited a significant influence on the conversion of acids (3–12) to their corresponding methyl esters.

Table 3.
Esterification of substituted benzoic and different alkyl carboxylic acids with MeOH in the presence of NBS 1,2,3.
Strong electron-withdrawing (4-NO2, 3-NO2), as well as weak electron-donating groups (4-Me, 3-Me) with only positive inductive +I and no mesomeric effect (M) have expressed moderate impact on the yield, while substituents with positive mesomeric (+M) and negative inductive (–I) effects (4-F, 3-F) have resulted in a decrease in the conversion. The strong electron-donating group at para position (–OMe, compound 9) has completely inhibited the reaction due to the strong resonance interaction between the lone electron pair of the substituent and carboxyl functional group, while m–positioned methoxy group which could not resonate with carboxyl moiety, resulted in fair yield of target benzoate 10a. On the other hand, in the case of aliphatic carboxylic acids, no scope limits have been observed and methyl stearate (13a), as well as methyl oleate (14a), methyl 2-cyanoacetate (20a), (S)-methyl 2-acetamido-3-phenylpropanoate (21a), methyl 2-(1H-indol-3-yl)acetate (22a) and methyl 4-(4-chlorophenyl)-4-oxobutanoate (23a) have been obtained almost quantitatively, while dimethyl oxalate (15a), trimethyl citrate (16a), and adamantane-1-carboxylic acid methyl ester (17a) have been gained in excellent yields.
Furthermore, the applicability of the method on more complex structural backbones has been demonstrated by performing the esterification of two significant steroidal carboxylic acids: cholic acid as one of the most common bile acids, formed as an end product of cholesterol metabolism in the liver [54], and its derivative dehydrocholic acid, which is the main component in many drugs against cholestatic liver disease and for dissolution of cholesterol gallstones [55]. In both cases, an excellent conversion of the starting material was achieved already after 1 h. Although in the crude reaction mixture obtained after esterification of dehydrocholic acid, partial conversion to ketal was observed, it was quantitatively converted into the corresponding ester during the isolation step by washing the mixture with 10% HCl (aq).
Moreover, to confirm the synthetic value of the presented methodology, synthesis of methyl benzoate (1a), methyl stearate (13a) and methyl citrate (16a) has been performed on 10–40 mmol scale with high to excellent yields (85–100%).
3. Materials and Methods
3.1. General Information
All reactions were performed in Mettler-Toledo Easymax 102 Advanced Synthesis Workstation using 25 mL reactor tubes. NMR spectra were recorded on Varian Inova 300 spectrometer (300 MHz 1H, 75 MHz 13C, 285 MHz 19F) at 25 °C. 1H-NMR spectra were obtained as solutions in CDCl3 with TMS as the internal standard. 19F-NMR spectra were obtained as solutions in CDCl3 with CFCl3 as the internal standard. N-bromosuccinimide was freshly recrystallized before use. All other chemicals used for synthetic procedures were obtained from commercial sources and were of reagent grade purity or better (Merck, Sigma Aldrich, Carlo Erba, Fluka, Fisher Scientific, Apollo Scientific, etc.). Reactions were monitored by TLC with silica gel coated plates Silica gel/TLC cards, DC-Alufolien-Kieselgel with 60 Å medium pore diameter (Sigma Aldrich) and detection was conducted by UV absorption (254 nm). Purification of certain products was conducted on preparative silica gel glass plates PLC Kieselgel 60 F254 with 2 mm layer thickness. Succinimide, isolated at the end of the reaction, can easily be recycled back to N-bromosuccinimide according to the standard procedure by NaOH, as elaborated in other reports [56]. Copies of 1H-NMR, 13C-NMR and 19F-NMR spectra of isolated final products are available in Supplementary material file online.
3.2. Experimental Procedures
3.2.1. General Procedure for the Esterification between Carboxylic Acids and Alcohols
The mixture of carboxylic acid, alcohol and N-bromosuccinimide was stirred in a 25 mL reactor tube at 70 °C for 2–40 h. After the completion of the reaction, the mixture was cooled to room temperature and alcohol was evaporated under reduced pressure. The isolation procedure was as follows, except where noted differently in Section 3.2.6. The residue was dissolved in ethyl acetate and consecutively washed with 10 mL of 10% Na2S2O3 (aq), 5 mL of saturated NaHCO3 (aq) and 10 mL of distilled water. The water phase was extracted with ethyl acetate (3 × 5 mL). The organic layers were combined, dried over Na2SO4 and the solvent was evaporated under reduced pressure.
3.2.2. Scale-Up Procedure for Preparation of Methyl Benzoate (1a) and Isolation of Succinimide
The mixture of benzoic acid (40 mmol, 4.88 g), MeOH (20 mL) and N-bromosuccinimide (2.80 mmol, 0.50 g) was stirred in a 25 mL reactor tube at 70 °C for 20 h. After the completion of the reaction, the mixture was cooled to room temperature and alcohol was evaporated under reduced pressure. The residue was washed with distilled water (20 mL) and the water phase was extracted with ethyl acetate (2 × 20 mL). The organic layers were combined and washed with the mixture of 10 mL of saturated NaHCO3 (aq), 10 mL of 10% Na2S2O3 (aq) and 15 mL of distilled water. The water layer was again extracted with ethyl acetate (2 × 20 mL). The organic layers were combined, dried over Na2SO4 and the solvent was evaporated under reduced pressure to furnish methyl benzoate as colourless oil. The water layer from the first washing of the crude reaction mixture was evaporated under the reduced pressure to give succinimide as a white solid.
- Yield (methyl benzoate): 4.60 g, 85%.
- Yield (succinimide [57]): 272 mg, 98%.
- 1H NMR (300 MHz, CDCl3) δ 10.04 (s, 1H), 2.72 (s, 4H).
- 13C NMR (76 MHz, CDCl3) δ 178.8, 29.4.
3.2.3. Scale-Up Procedure for Preparation of Trimethyl Citrate (16a)
The mixture of citric acid (11 mmol, 2.11 g), MeOH (20 mL) and N-bromosuccinimide (0.77 mmol, 0.138 g) was stirred in a 25 mL reactor tube at 70 °C for 20 h. After the completion of the reaction, the mixture was cooled to room temperature and alcohol was evaporated under reduced pressure. The residue was dissolved in 50 mL of ethyl acetate, washed with the mixture of 10 mL of saturated NaHCO3 (aq), 10 mL of 10% Na2S2O3 (aq) and 25 mL of distilled water and the water phase was extracted with ethyl acetate (2 × 25 mL). The organic layers were combined, dried with Na2SO4 and the solvent was evaporated under reduced pressure to furnish methyl citrate as a white solid.
- Yield: 2.55 g, 99%.
3.2.4. Scale-Up Procedure for Preparation of Methyl Stearate (13a)
The mixture of stearic acid (10 mmol, 2.85 g), MeOH (20 mL) and N-bromosuccinimide (0.70 mmol, 0.125 g) was stirred in a 25 mL reactor tube at 70 °C for 20 h. After the completion of the reaction, the mixture was cooled to room temperature and alcohol was evaporated under reduced pressure. The residue was dissolved in 50 mL of ethyl acetate, washed with the mixture of 10 mL of saturated NaHCO3 (aq), 10 mL of 10% Na2S2O3 (aq) and 15 mL of distilled water and the water phase was extracted with ethyl acetate (2 × 25 mL). The organic layers were combined, washed with distilled water (2 × 20 mL), dried with Na2SO4 and the solvent was evaporated under reduced pressure to furnish methyl stearate as a white solid.
- Yield: 2.99 g, 100%.
3.2.5. Procedure for Recycling of N-bromosuccinimide (NBS) from Waste Succinimide
Succinimide (0.272 g, 2.75 mmol) was dissolved in a mixture of 1.36 g (3.29 mmol) NaOH, 0.5 g crushed ice and 1.5 mL of cold water. To this mixture, 0.156 mL (3.02 mmol, 0.483 g) of Br2 was added while stirring. It was stirred for five minutes and then the product was filtered, washed with cold water and dried in a desiccator to isolate 0.348 g (71%) of NBS.
3.2.6. Detailed Procedures for the Preparation of Synthesized Compounds
Methyl benzoate (1a) [17]. Synthesized according to the general procedure. Reaction conditions: benzoic acid (1 mmol, 122.1 mg), MeOH (0.5 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 122 mg of colourless oil (90%); 1H-NMR (300 MHz, CDCl3) δ 8.07–8.01 (m, 2H), 7.59–7.51 (m, 1H), 7.43 (ddt, J = 8.2, 6.8, 1.1 Hz, 2H), 3.91 (s, 3H); 13C-NMR (76 MHz, CDCl3) δ 167.2, 133.0, 130.3, 129.7, 128.4, 52.2; HRMS (ESI) for C8H8O2: calculated m/z = 137.0603 (MH+); found m/z = 137.0606 (MH+).
Methyl octanoate (2a) [58]. Synthesized according to the general procedure. Reaction conditions: octanoic acid (1 mmol, 158.5 μL), MeOH (0.5 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 2 h. Purification: Not necessary. Yield: 153 mg of colourless oil (97%); 1H-NMR (300 MHz, CDCl3) δ 3.67 (s, 3H), 2.30 (t, J = 7.5 Hz, 2H), 1.69–1.56 (m, 2H), 1.35–1.25 (m, 8H), 0.88 (t, J = 6.9 Hz, 3H); 13C-NMR (76 MHz, CDCl3) δ 174.5, 51.5, 34.2, 31.8, 29.2, 29.0, 25.1, 22.7, 14.2; HRMS (ESI) for C9H18O2: calculated m/z = 159.1385 (MH+); found m/z = 159.1389 (MH+).
Methyl 4-nitrobenzoate (3a) [17]. Synthesized according to the general procedure. Reaction conditions: 4-nitrobenzoic acid (1 mmol, 167.1 mg), MeOH (1.5 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 167 mg of white solid (92%); 1H-NMR (300 MHz, CDCl3) δ 8.34–8.19 (m, 4H), 3.99 (s, 3H); 13C-NMR (76 MHz, CDCl3) δ 165.2, 150.6, 135.6, 130.8, 123.6, 77.2, 52.9.
Methyl 3-nitrobenzoate (4a) [59]. Synthesized according to the general procedure. Reaction conditions: 3-nitrobenzoic acid (1 mmol, 167.1 mg), MeOH (2 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 141 mg of yellow solid (78%); 1H-NMR (300 MHz, CDCl3) δ 8.94–8.75 (m, 1H), 8.50–8.30 (m, 2H), 7.68 (t, J = 8.0 Hz, 1H), 4.00 (s, 3H); 13C-NMR (76 MHz, CDCl3) δ 165.0, 148.3, 135.3, 131.9, 129.7, 127.4, 124.6, 52.8; HRMS (ESI) for C8H7NO4: calculated m/z = 182.0453 (MH+); found m/z = 182.0457 (MH+).
Methyl 4-fluorobenzoate (5a) [60]. Synthesized according to the general procedure. Reaction conditions: 4-fluorobenzoic acid (1 mmol, 140.1 mg), MeOH (2 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 105 mg of colourless oil (68%); 1H-NMR (300 MHz, CDCl3) δ 8.02 (ddd, J = 10.1, 5.2, 2.5 Hz, 2H), 7.13–7.03 (m, 2H), 3.89 (s, 3H); 13C-NMR (76 MHz, CDCl3) δ 166.2, 165.8 (d, J = 253.5 Hz) 132.2 (d, J = 9.4 Hz), 126.5 (d, J = 3.0 Hz), 115.6 (d, J = 22.0 Hz), 52.2; 19F-NMR (285 MHz, CDCl3) δ -106.34 (tt, J = 8.5, 5.4 Hz); HRMS (ESI) for C8H7FO2: calculated m/z = 155.0508 (MH+); found m/z = 155.0505 (MH+).
Methyl 3-fluorobenzoate (6a) [61]. Synthesized according to the general procedure. Reaction conditions: 3-fluorobenzoic acid (1 mmol, 140.1 mg), MeOH (0.5 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 108 mg of colourless oil (70%); 1H-NMR (300 MHz, CDCl3) δ 7.83 (dt, J = 7.7, 1.2 Hz, 1H), 7.71 (ddd, J = 9.4, 2.6, 1.5 Hz, 1H), 7.41 (td, J = 8.0, 5.6 Hz, 1H), 7.25 (tdd, J = 8.3, 2.5, 1.0 Hz, 1H), 3.92 (s, 3H); 13C-NMR (76 MHz, CDCl3) δ 166.0, 162.6 (d, J = 246.6 Hz), 132.4 (d, J = 7.6 Hz), 130.1 (d, J = 7.6 Hz), 125.4 (d, J = 2.9 Hz), 120.1 (d, J = 21.4 Hz), 116.6 (d, J = 23.0 Hz), 52.4; 19F-NMR (285 MHz, CDCl3) δ -112.92 (ddd, J = 9.4, 8.4, 5.6 Hz); HRMS (ESI) for C8H7FO2: calculated m/z = 155.0508 (MH+); found m/z = 155.0511 (MH+).
Methyl 4-methylbenzoate (7a) [60]. Synthesized according to the general procedure. Reaction conditions: 4-methylbenzoic acid (1 mmol, 136.1 mg), MeOH (2 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 126 mg of colourless oil (84%); 1H-NMR (300 MHz, CDCl3) δ 7.97–7.86 (m, 2H), 7.22 (d, J = 8.2 Hz, 2H), 3.88 (s, 3H), 2.39 (s, 3H); 13C-NMR (76 MHz, CDCl3) δ 167.2, 143.6, 129.6, 129.1, 127.5, 52.0, 21.7; HRMS (ESI) for C9H10O2: calculated m/z = 151.0759 (MH+); found m/z = 151.0755 (MH+).
Methyl 3-methylbenzoate (8a) [60]. Synthesized according to the general procedure. Reaction conditions: 3-methylbenzoic acid (1 mmol, 136.1 mg), MeOH (2 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 136 mg of colourless oil (90%); 1H-NMR (300 MHz, CDCl3) δ 7.88–7.81 (m, 1H), 7.39–7.28 (m, 1H), 3.91 (s, 2H), 2.40 (s, 1H); 13C-NMR (76 MHz, CDCl3) δ 167.3, 138.2, 133.7, 130.2, 130.2, 128.3, 126.8, 52.1, 21.3; HRMS (ESI) for C9H10O2: calculated m/z = 151.0759 (MH+); found m/z = 151.0762 (MH+).
Methyl 3-methoxybenzoate (10a) [62]. Synthesized according to the general procedure. Reaction conditions: 3-methoxybenzoic acid (1 mmol, 152.1 mg), MeOH (2 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 75 mg of yellow oil (45%); 1H-NMR (300 MHz, CDCl3) δ 7.66–7.53 (m, 2H), 7.33 (t, J = 7.9 Hz, 1H), 7.09 (ddd, J = 8.2, 2.6, 0.9 Hz, 1H), 3.91 (s, 3H), 3.84 (s, 3H); 13C-NMR (76 MHz, CDCl3) δ 167.0, 159.6, 131.5, 129.4, 122.0, 119.5, 114.0, 55.4, 52.2; HRMS (ESI) for C9H10O3: calculated m/z = 167.0708 (MH+); found m/z = 167.0717 (MH+).
Methyl 4-octylbenzoate (11a) [63]. Synthesized according to the general procedure. Reaction conditions: 4-octylbenzoic acid (1 mmol, 234.3 mg), MeOH (2 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 194 mg of yellow oil, (78%); 1H-NMR (300 MHz, CDCl3) δ 8.02–7.87 (m, 2H), 7.31–7.16 (m, 2H), 3.89 (s, 3H), 2.73–2.57 (m, 2H), 1.69–1.54 (m, 2H), 1.43–1.19 (m, 10H), 0.87 (t, J = 6.7 Hz, 3H); 13C-NMR (76 MHz, CDCl3) δ 167.3, 148.6, 129.7, 128.5, 127.7, 52.0, 36.1, 32.0, 31.2, 29.5, 29.4, 29.3, 22.8, 14.2; HRMS (ESI) for C16H24O2: calculated m/z = 249.1855 (MH+); found m/z = 249.1853 (MH+).
Methyl 2-iodobenzoate (12a) [64]. Synthesized according to the general procedure. Reaction conditions: 2-iodobenzoic acid (1 mmol, 248.0 mg), MeOH (0.5 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 144 mg of colourless oil (55%); 1H-NMR (300 MHz, CDCl3) δ 7.95 (dd, J = 7.9, 1.1 Hz, 1H), 7.76 (dd, J = 7.8, 1.7 Hz, 1H), 7.36 (td, J = 7.6, 1.1 Hz, 1H), 7.11 (td, J = 7.7, 1.7 Hz, 1H), 3.90 (s, 3H); 13C-NMR (76 MHz, CDCl3) δ 166.9, 141.3, 135.1, 132.7, 130.9, 127.9, 94.1, 52.5; HRMS (ESI) for C8H7IO2: calculated m/z = 262.9569 (MH+); found m/z = 262.9563 (MH+).
Methyl stearate (13a) [14]. Synthesized according to the general procedure. Reaction conditions: stearic acid (1 mmol, 284.5 mg), MeOH (0.5 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 285 mg of white solid (95%); 1H-NMR (300 MHz, CDCl3) δ 3.67 (s, 3H), 2.30 (t, J = 7.5 Hz, 2H), 1.69–1.55 (m, 2H), 1.37–1.20 (m, 28H), 0.88 (t, J = 7.0 Hz, 3H); 13C-NMR (76 MHz, CDCl3) δ 174.5, 51.6, 34.3, 32.1, 29.8, 29.8, 29.8, 29.8, 29.8, 29.8, 29.8, 29.7, 29.6, 29.5, 29.4, 29.3, 25.1, 22.8, 14.3; HRMS (ESI) for C19H38O2: calculated m/z = 299.2950 (MH+); found m/z = 299.2957 (MH+).
Methyl oleate (14a) [14]. Synthesized according to the general procedure. Reaction conditions: oleic acid (1 mmol, 315.6 µL), MeOH (2 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 15 h. Purification: Not necessary. Yield: 289 mg of colorless oil, (97%); 1H-NMR (300 MHz, CDCl3) δ 5.34 (ddd, J = 5.6, 3.5, 2.1 Hz, 2H), 3.66 (s, 3H), 2.30 (t, J = 7.5 Hz, 2H), 2.10–1.91 (m, 4H), 1.71–1.54 (m, 2H), 1.42–1.17 (m, 18H), 0.88 (t, J = 6.7 Hz, 3H); 13C-NMR (76 MHz, CDCl3) δ 174.4, 130.1, 129.9, 51.5, 34.2, 32.0, 29.9, 29.8, 29.7, 29.5, 29.4, 29.3, 29.3, 29.2, 27.3, 27.3, 25.1, 22.8, 14.2; HRMS (ESI) for C19H36O2: calculated m/z = 297.2794 (MH+); found m/z = 297.2798 (MH+).
Dimethyl oxalate (15a) [65]. Synthesized according to the general procedure. Reaction conditions: oxalic acid (1 mmol, 90.0 mg), MeOH (2 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 15 h. Purification: Not necessary. Yield: 110 mg of white solid (93%); 1H-NMR (300 MHz, CDCl3) δ 3.92 (s, 6H); 13C-NMR (76 MHz, CDCl3) δ 157.8, 53.5; HRMS (ESI) for C4H6O4: calculated m/z = 119.0344 (MH+); found m/z = 119.0342 (MH+).
Trimethyl citrate (16a) [66]. Synthesized according to the general procedure. Reaction conditions: citric acid (1 mmol, 210.1 mg), MeOH (2 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 220 mg of white solid (94%); 1H-NMR (300 MHz, CDCl3) δ 4.10 (s, 1H), 3.81 (s, 3H), 3.67 (s, 6H), 2.89 (d, J = 15.6 Hz, 2H), 2.79 (d, J = 15.6 Hz, 2H); 13C-NMR (76 MHz, CDCl3) δ 173.8, 170.2, 73.3, 53.2, 52.0, 43.1; HRMS (ESI) for C9H14O7: calculated m/z = 235.0818 (MH+); found m/z = 235.0815 (MH+).
Adamantane-1-carboxylic acid methyl ester (17a) [61]. Synthesized according to the general procedure. Reaction conditions: 1-adamantanecarboxylic acid (1 mmol, 180.2 mg), MeOH (0.5 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 186 mg of white solid (96%); 1H-NMR (300 MHz, CDCl3) δ 3.60 (s, 3H), 2.01–1.92 (m, 3H), 1.88–1.80 (m, 6H), 1.74–1.59 (m, 3H); 13C-NMR (76 MHz, CDCl3) δ 178.08, 51.44, 40.64, 38.81, 36.46, 27.91; HRMS (ESI) for C12H18O2: calculated m/z = 195.1385 (MH+); found m/z = 195.1382 (MH+).
Methyl 3,7,12-trioxo-5β-cholan-24-oate (18a) [67]. The mixture of dehydrocholic acid (0.25 mmol, 101.6 mg), MeOH (2 mL) and N-bromosuccinimide (0.018 mmol, 3.1 mg) was stirred in a 25 mL reactor tube at 70 °C for 1 h. After the completion of the reaction, the mixture was cooled to room temperature and MeOH was evaporated under the reduced pressure. The residue was dissolved in ethyl acetate and first washed with the mixture of 10 mL of 10% Na2S2O3 (aq), 5 mL of saturated NaHCO3 (aq) and 10 mL of distilled water and then with 10 mL of 10% HCl (aq). The water phase was extracted with ethyl acetate (3 × 5 mL). The organic layers were combined, dried with Na2SO4 and the solvent was evaporated under the reduced pressure. Purification: Not necessary. Yield: 93 mg of white solid (89%); 1H-NMR (300 MHz, CDCl3) δ 3.66 (s, 3H), 3.01–2.75 (m, 3H), 2.51–1.16 (m, 21H), 1.41 (s, 3H), 1.08 (s, 3H), 0.85 (d, J = 6.5 Hz, 3H); 13C-NMR (76 MHz, CDCl3) δ 212.0, 209.1, 208.8, 174.5, 56.9, 51.8, 51.5, 49.0, 46.9, 45.7, 45.6, 45.0, 42.8, 38.7, 36.5, 36.1, 35.6, 35.3, 31.3, 30.5, 27.7, 25.2, 22.0, 18.7, 11.9; HRMS (ESI) for C25H36O5: calculated m/z = 417.2641 (MH+); requires m/z = 417.2644 (MH+).
Methyl 3α,7α,12α-trihydroxy-5β-cholan-24-oate (19a) [68]. The mixture of cholic acid (0.25 mmol, 102.1 mg), MeOH (2 mL) and N-bromosuccinimide (0.018 mmol, 3.1 mg) was stirred in a 25 mL reactor tube at 70 °C for 1 h. After the completion of the reaction, the mixture was cooled to room temperature and MeOH was evaporated under the reduced pressure. The residue was dissolved in ethyl acetate and washed with the mixture of 10 mL of 10% Na2S2O3 (aq), 5 mL of saturated NaHCO3(aq) and 10 mL of distilled water. The water phase was extracted with ethyl acetate (3 × 5 mL). The organic layers were combined, dried with Na2SO4 and the solvent was evaporated under the reduced pressure. Purification: Not necessary. Yield: 93 mg of white solid (88%); 1H-NMR (300 MHz, CDCl3) δ 3.95 (s, 1H), 3.83 (s, 1H), 3.66 (s, 3H), 3.55 (s, 3H), 3.49–3.32 (m, 1H), 2.48–1.07 (m, 24H), 0.98 (d, J = 5.8 Hz, 3H), 0.88 (s, 3H), 0.67 (s, 3H); 13C-NMR (76 MHz, CDCl3) δ 174.7, 72.9, 71.7, 68.3, 51.3, 46.8, 46.2, 41.4, 41.4, 39.3, 39.3, 35.2, 35.2 34.6, 34.6, 31.0, 30.8, 30.2, 28.0, 27.4, 26.1, 23.1, 22.3, 17.2, 12.3; HRMS (ESI) for C25H42O5: calculated m/z = 423.3110 (MH+); found m/z = 423.3128 (MH+).
Methyl 2-cyanoacetate (20a) [69]. Synthesized according to the general procedure. Reaction conditions: Cyanoacetic acid (1 mmol, 85.1 mg), MeOH (0.5 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 96 mg of colourless oil (97%); 1H-NMR (300 MHz, CDCl3) δ 3.76 (s, 3H), 3.46 (s, 2H); 13C-NMR (76 MHz, CDCl3) δ 163.6, 113.2, 53.5, 24.4; HRMS (ESI) for C4H5NO2: calculated m/z = 100.0399 (MH+); found m/z = 100.0398 (MH+).
(S)-Methyl 2-acetamido-3-phenylpropanoate (21a) [70]. Synthesized according to the general procedure. Reaction conditions: N-Acetyl-l-phenylalanine (1 mmol, 207.2 mg), MeOH (0.5 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 208 mg of white solid (91%); 1H-NMR (300 MHz, CDCl3) δ 7.33–7.18 (m, 3H), 7.18–7.05 (m, 2H), 6.38 (d, J = 7.5 Hz, 1H), 4.95–4.77 (m, 1H), 3.69 (s, 3H), 3.21–2.93 (m, 2H), 1.95 (s, 3H); 13C-NMR (76 MHz, CDCl3) δ 172.2, 169.8, 136.0, 129.2, 128.5, 127.0, 53.2, 52.2, 37.8, 22.9; HRMS (ESI) for C12H15NO3: calculated m/z = 222.1130 (MH+); found m/z = 222.1135 (MH+).
Methyl 2-(1H-indol-3-yl)acetate (22a) [71]. Synthesized according to the general procedure. Reaction conditions: Heteroauxin (1 mmol, 175.2 mg), MeOH (0.5 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 185 mg of brown oil (98%); 1H-NMR (300 MHz, CDCl3) δ 8.16 (s, 1H), 7.56 (dd, J = 6.7, 1.6 Hz, 2H), 7.24–6.98 (m, 3H), 6.84 (d, J = 2.4 Hz, 1H), 3.72 (s, 2H), 3.63 (s, 3H); 13C-NMR (76 MHz, CDCl3) δ 173.0, 136.1, 127.1, 123.4, 122.0, 119.5, 118.6, 111.4, 107.8, 52.0, 31.1; HRMS (ESI) for C11H11NO2: calculated m/z = 190.0868 (MH+); found m/z = 190.0865 (MH+).
Methyl 4-(4-chlorophenyl)-4-oxobutanoate (23a) [72]. Synthesized according to the general procedure. Reaction conditions: 3-(4-Chlorobenzoyl)propionic acid (1 mmol, 212.6 mg), MeOH (0.5 mL), NBS (0.070 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 224 mg of white solid (99%); 1H-NMR (300 MHz, CDCl3) δ 7.90–7.81 (m, 2H), 7.41–7.33 (m, 2H), 3.64 (s, 3H), 3.22 (t, J = 6.6 Hz, 2H), 2.70 (t, J = 6.6 Hz, 2H); 13C-NMR (76 MHz, CDCl3) δ 196.8, 173.2, 139.6, 134.8, 129.4, 128.9, 51.8, 33.3, 27.9; HRMS (ESI) for C12H18O2: calculated m/z = 249.0294 (MNa+); found m/z = 249.0297 (MNa+).
2-Fluoroethyl benzoate (1b) [73]. Synthesized according to the general procedure. Reaction conditions: benzoic acid (1 mmol, 122.1 mg), 2-fluoroethanol (0.5 mL), NBS (0.07 mmol, 12.5 mg), 70 °C, 40 h. Purification: Preparative TLC (CH2Cl2/MeOH = 200:1). Yield: 160 mg of colourless oil (95%); 1H-NMR (300 MHz, CDCl3) δ 8.20–7.86 (m, 2H), 7.60–7.52 (m, 1H), 7.49–7.37 (m, 2H), 4.85–4.44 (m, 4H); 13C-NMR (76 MHz, CDCl3) δ 166.4, 133.3, 129.8, 128.5, 81.5 (d, J = 170.6 Hz), 63.9 (d, J = 20.2 Hz); 19F NMR (285 MHz, CDCl3) δ 5.03 (tt, J = 47.4, 28.6 Hz); HRMS (ESI) for C9H9FO2: calculated m/z = 169.0665 (MH+); found m/z = 169.0668 (MH+).
Isopropyl benzoate (1c) [74]. Synthesized according to the general procedure. Reaction conditions: benzoic acid (1 mmol, 122.1 mg), isopropanol (0.5 mL), NBS (0.07 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 12 mg of colourless oil (7%); 1H-NMR (300 MHz, CDCl3) δ 8.09–7.99 (m, 2H), 7.59–7.50 (m, 1H), 7.47–7.38 (m, 2H), 5.26 (hept, J = 6.3 Hz, 1H), 1.37 (d, J = 6.3 Hz, 6H); 13C-NMR (76 MHz, CDCl3) δ 166.3, 132.8, 131.1, 129.6, 128.4, 68.5, 22.1.
2-Fluoroethyl octanoate (2b) [75]. Synthesized according to the general procedure. Reaction conditions: octanoic acid (1 mmol, 158.5 μL), 2-fluoroethanol (0.5 mL), NBS (0.07 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 175 mg of yellow oil (92%); 1H-NMR (300 MHz, CDCl3) δ 4.73–4.20 (m, 4H), 2.36 (t, J = 7.5 Hz, 2H), 1.71–1.57 (m, 2H), 1.42–1.19 (m, 8H), 0.88 (t, J = 7.1 Hz, 3H); 13C-NMR (76 MHz, CDCl3) δ 173.7, 81.5 (d, J = 170.3 Hz), 63.2 (d, J = 20.1 Hz), 34.2, 31.7, 29.1, 29.0, 25.0, 22.7, 14.1; 19F-NMR (285 MHz, CDCl3) δ 4.86 (tt, J = 47.4, 28.7 Hz); HRMS (ESI) for C10H19FO2: calculated m/z = 191.1447 (MH+); found m/z = 191.1446 (MH+).
Isopropyl octanoate (2c) [76]. Synthesized according to the general procedure. Reaction conditions octanoic acid (1 mmol, 158.5 μL), isopropanol (0.5 mL), NBS (0.07 mmol, 12.5 mg), 70 °C, 20 h. Purification: Not necessary. Yield: 149 mg of colourless oil (80%); 1H-NMR (300 MHz, CDCl3) δ 5.00 (hept, J = 6.3 Hz, 1H), 2.25 (t, J = 7.5 Hz, 2H), 1.67–1.55 (m, 2H), 1.35–1.25 (m, 8H), 1.23 (d, J = 6.3 Hz, 6H), 0.88 (t, J = 7.0 Hz, 3H); 13C-NMR (76 MHz, CDCl3) δ 173.4, 67.3, 34.8, 31.7, 29.2, 29.0, 25.1, 22.7, 21.9, 14.1; HRMS (ESI) for C11H22O2: calculated m/z = 187.1698 (MH+); found m/z = 187.1703 (MH+).
n-Butyl benzoate (1d) [77]. Synthesized according to the general procedure. Reaction conditions: benzoic acid (1 mmol, 122.1 mg), n-butanol (0.5 mL), NBS (0.07 mmol, 12.5 mg), 70 °C, 20 h. Purification: Preparative TLC (CH2Cl2/MeOH = 200:1). Yield: 134 mg of colourless oil (75%); 1H-NMR (300 MHz, CDCl3) δ 8.10–7.99 (m, 2H), 7.58–7.49 (m, 1H), 7.42 (t, J = 7.5 Hz, 2H), 4.32 (t, J = 6.6 Hz, 2H), 1.84–1.66 (m, 2H), 1.56–1.39 (m, 2H), 0.98 (t, J = 7.4 Hz, 3H); 13C-NMR (76 MHz, CDCl3) δ 166.7, 132.8, 130.6, 129.6, 128.4, 64.9, 30.9, 19.4, 13.8; HRMS (ESI) for C11H14O2: calculated m/z = 179.1072 (MH+); found m/z = 179.1070 (MH+).
n-Butyl octanoate (2d) [4]. Synthesized according to the general procedure. Reaction conditions octanoic acid (1 mmol, 158.5 μL), n-butanol (1 mmol, 92 μL), NBS (0.07 mmol, 12.5 mg), 70 °C, 15 h. Purification: Not necessary. Yield: 190 mg of colourless oil (95%); 1H-NMR (300 MHz, CDCl3) δ 4.07 (t, J = 6.6 Hz, 2H), 2.29 (t, J = 7.5 Hz, 2H), 1.70–1.52 (m, 4H), 1.47–1.21 (m, 10H), 0.94 (t, J = 7.3 Hz, 3H), 0.87 (t, J = 6.9 Hz, 3H); 13C-NMR (76 MHz, CDCl3) δ 174.0, 64.1, 34.4, 31.8, 30.8, 29.2, 29.0, 25.1, 22.7, 19.2, 14.1, 13.7; HRMS (ESI) for C12H24O2: calculated m/z = 201.1855 (MH+); found m/z = 201.1857 (MH+).
n-Octyl benzoate (1f) [58]. Synthesized according to the general procedure. Reaction conditions: benzoic acid (1 mmol, 122.1 mg), n-octanol (1 mmol, 158 μL), NBS (0.07 mmol, 12.5 mg), 70 °C, 20 h. Purification: Preparative TLC (CH2Cl2/Hexane = 1:1). Yield: 134 mg of white solid (57%); 1H-NMR (300 MHz, CDCl3) δ 8.10–8.00 (m, 2H), 7.59–7.51 (m, 1H), 7.47–7.39 (m, 2H), 4.31 (t, J = 6.7 Hz, 2H), 1.83–1.70 (m, 2H), 1.51–1.21 (m, 10H), 0.88 (t, J = 6.6 Hz, 3H); 13C-NMR (76 MHz, CDCl3) δ 166.8, 132.9, 130.7, 129.7, 128.4, 65.3, 31.9, 29.4, 29.3, 28.9, 26.2, 22.8, 14.2; HRMS (ESI) for C15H22O2: calculated m/z = 235.1698 (MH+); found m/z = 235.1697 (MH+).
n-Octyl octanoate (2f) [78]. Synthesized according to the general procedure. Reaction conditions: octanoic acid (1 mmol, 158.5 μL), n-octanol (1 mmol, 158 μL), NBS (0.07 mmol, 12.5 mg), 70 °C, 20 h. Purification: Preparative TLC (CH2Cl2/MeOH = 200:1). Yield: 223 mg of colourless oil (87%); 1H-NMR (300 MHz, CDCl3) δ 4.06 (t, J = 6.7 Hz, 2H), 2.28 (t, J = 7.5 Hz, 2H), 1.68–1.54 (m, 4H), 1.39–1.19 (m, 18H), 0.88 (t, J = 6.3 Hz, 6H); 13C-NMR (76 MHz, CDCl3) δ 173.9, 64.4, 34.4, 31.9, 31.8, 29.3, 29.3, 29.2, 29.0, 28.8, 26.0, 25.1, 22.7, 22.7, 14.1, 14.1; HRMS (ESI) for C16H32O2: calculated m/z = 257.2481 (MH+); found m/z = 257.2486 (MH+).
Cyclopentyl octanoate (2g) [79]. Synthesized according to the general procedure. Reaction conditions: octanoic acid (1 mmol, 158.5 μL), cyclopentanol (1 mmol, 91 μL), NBS (0.07 mmol, 12.5 mg), 70 °C, 15 h. Purification: Not necessary. Yield: 200 mg of colourless oil (94%); 1H-NMR (300 MHz, CDCl3) δ 5.16 (tt, J = 5.9, 2.6 Hz, 1H), 2.25 (t, J = 7.5 Hz, 2H), 1.95–1.47 (m, 10H), 1.41–1.20 (m, 8H), 0.88 (d, J = 7.0 Hz, 3H); 13C-NMR (76 MHz, CDCl3) δ 173.8, 76.8, 34.8, 32.8, 31.8, 29.2, 29.0, 25.2, 23.8, 22.7, 14.1; HRMS (ESI) for C13H24O2: calculated m/z = 213.1855 (MH+); found m/z = 213.1860 (MH+).
4. Conclusions
In conclusion, a convenient and selective metal-free method for direct dehydrative esterification of free aromatic and aliphatic acids with different alcohols has been developed, using inexpensive and easy-to-handle N-bromosuccinimide as a moisture- and air-tolerant recyclable catalyst. The synthesis has been performed under neat reaction conditions without the need for simultaneous removal of water and excessive reagents. In spite of some scope limitations, the method provides good to excellent product yields and in the majority of cases, enables simple isolation procedure only by extraction. Even in the case of di- and tri-carboxy aliphatic acids, esterification has been successfully accomplished with the same amount of NBS catalyst as in the case of mono-carboxy alkyl acids. The applicability of the method has been successfully demonstrated also on steroidal carboxylic acids. The large-scale synthesis of methyl benzoate, methyl stearate and trimethyl citrate as examples of commercially significant esters has been performed with high to excellent yields (85–100%).
Supplementary Materials
The following are available online, 1H-NMR, 13C-NMR and 19F-NMR spectra of isolated final products.
Author Contributions
Conceptualization, S.S; Formal analysis, K.Č, B.B and S.S; Investigation, K.Č, B.B and S.S; Methodology, K.Č, B.B and S.S; Supervision, S.S; Writing—original draft, K.Č, B.B and S.S; Writing–review & editing, K.Č and S.S.
Funding
Financial support of the Slovenian Research Agency (Programme P1-0134 and Young Researcher Programme–ARRS-SP-2990/17) is greatly appreciated. B. Đ. B. is grateful to the Serbian Ministry of Education, Science and Technological Development (project no. 173052).
Acknowledgments
The authors are grateful to the Slovenian NMR Centre at the National Institute of Chemistry.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Otera, J. Esterification. Esterification; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 3–144. ISBN 9783527601844. [Google Scholar]
- Baskar, G.; Aiswarya, R. Trends in catalytic production of biodiesel from various feedstocks. Renew. Sustain. Energy Rev. 2016, 57, 496–504. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Sodagar, E. Esterification of free fatty acids (Biodiesel) using nano sulfated-titania as catalyst in solvent-free conditions. C R. Chim. 2013, 16, 229–238. [Google Scholar] [CrossRef]
- Mallesham, B.; Govinda Rao, B.; Reddy, B.M. Production of biofuel additives by esterification and acetalization of bioglycerol. C R. Chim. 2016, 19, 1194–1202. [Google Scholar] [CrossRef]
- Tsakos, M.; Schaffert, E.S.; Clement, L.L.; Villadsen, N.L.; Poulsen, T.B. Ester coupling reactions-An enduring challenge in the chemical synthesis of bioactive natural products. Nat. Prod. Rep. 2015, 32, 605–632. [Google Scholar] [CrossRef] [PubMed]
- Larock, R.C.; Dubrovskiy, A.V.; Markina, N.A.; Pletnev, A.A.; Kesharwani, T.; Raminelli, C.; Yao, T.; Zeni, G.; Zhang, L.; Rozhkov, R. Comprehensive Organic Transformations, 4 Volume Set: A Guide to Functional Group Preparations, 3rd ed.; Larock, R.C., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; Volume 1, pp. 3753–3764. ISBN 9780470927953. [Google Scholar]
- Ishihara, K. Dehydrative condensation catalyses. Tetrahedron 2009, 65, 1085–1109. [Google Scholar] [CrossRef]
- Sakakura, A.; Koshikari, Y.; Ishihara, K. Open-air and solvent-free ester condensation catalyzed by sulfonic acids. Tetrahedron Lett. 2008, 49, 5017–5020. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Yang, T.; Cai, B.; Chang, X.; Pan, H. Highly efficient metal salt catalyst for the esterification of biomass derived levulinic acid under microwave irradiation. RSC Adv. 2016, 6, 2106–2111. [Google Scholar] [CrossRef]
- Jeschke, J.; Korb, M.; Rüffer, T.; Gäbler, C.; Lang, H. Atom economic ruthenium-catalyzed synthesis of bulky β-Oxo esters. Adv. Synth. Catal. 2015, 357, 4069–4081. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Liberto, N.A.; De Andrade Leles, L.C.; Pereira, U.A. Fe4(SiW12O40)3-catalyzed glycerol acetylation: Synthesis of bioadditives by using highly active Lewis acid catalyst. J. Mol. Catal. A Chem. 2016, 422, 69–83. [Google Scholar] [CrossRef]
- Kato, C.N.; Ogasawara, T.; Kondo, A.; Kato, D. Heterogeneous esterification of fatty acids with methanol catalyzed by Lewis acidic organozirconium complexes with Keggin-type mono-aluminum-substituted polyoxotungstates. Catal. Commun. 2017, 96, 41–45. [Google Scholar] [CrossRef]
- Minakawa, M.; Baek, H.; Yamada, Y.M.A.; Han, J.W.; Uozumi, Y. Direct dehydrative esterification of alcohols and carboxylic acids with a macroporous polymeric acid catalyst. Org. Lett. 2013, 15, 5798–5801. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, M.M.; Capodiferro, V.F.; Mali, M.; Mastrorilli, P. Esterification, transesterification and hydrogenation reactions of polyunsaturated compounds catalyzed by a recyclable polymer supported palladium catalyst. J. Organomet. Chem. 2016, 818, 106–114. [Google Scholar] [CrossRef]
- Furuta, A.; Fukuyama, T.; Ryu, I. Efficient flow fischer esterification of carboxylic acids with alcohols using sulfonic acid-functionalized silica as supported catalyst. Bull. Chem. Soc. Jpn. 2017, 90, 607–612. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, Y.; Fu, Y.; Chen, H.; Ye, M.; Luo, G. Graphene oxide: An efficient acid catalyst for the construction of esters from acids and alcohols. Synlett 2017, 28, 981–985. [Google Scholar] [CrossRef]
- Han, X.-X.; Du, H.; Hung, C.-T.; Liu, L.-L.; Wu, P.-H.; Ren, D.-H.; Huang, S.-J.; Liu, S.-B. Syntheses of novel halogen-free Bronsted-Lewis acidic ionic liquid catalysts and their applications for synthesis of methyl caprylate. Green Chem. 2015, 17, 499–508. [Google Scholar] [CrossRef]
- Dong, B.; Song, H.; Zhang, W.; He, A.; Yao, S. Ionic liquids as heterogeneous and homogeneous catalysts for condensation and esterification reactions. Curr. Org. Chem. 2016, 20, 2894–2910. [Google Scholar] [CrossRef]
- Phakhodee, W.; Duangkamol, C.; Pattarawarapan, M. Ph3P-I2 mediated aryl esterification with a mechanistic insight. Tetrahedron Lett. 2016, 57, 2087–2089. [Google Scholar] [CrossRef]
- Yeh, W.K.; Yang, H.C.; McCarthy, J.R. Enzyme Technologies: Metagenomics, Evolution, Biocatalysis and Biosynthesis; Wiley: Hoboken, NJ, USA, 2011; pp. 125–250. ISBN 9781118125038. [Google Scholar]
- Bezbradica, D.; Crovic, M.; Tanaskovic, S.J.; Lukovic, N.; Carevic, M.; Milivojevic, A.; Knezevic-Jugovic, Z. Enzymatic Syntheses of Esters-Green Chemistry for Valuable Food, Fuel and Fine Chemicals. Curr. Org. Chem. 2017, 21, 104–138. [Google Scholar] [CrossRef]
- Gokulakrishnan, N.; Pandurangan, A.; Sinha, P.K. Esterification of acetic acid with propanol isomers under autogeneous pressure: A catalytic activity study of Al-MCM-41 molecular sieves. J. Mol. Catal. A Chem. 2007, 263, 55–61. [Google Scholar] [CrossRef]
- Chung, K.-H.; Park, B.-G. Esterification of oleic acid in soybean oil on zeolite catalysts with different acidity. J. Ind. Eng. Chem. 2009, 15, 388–392. [Google Scholar] [CrossRef]
- Kolvari, E.; Ghorbani-Choghamarani, A.; Salehi, P.; Shirini, F.; Zolfigol, M.A. Application of N-halo reagents in organic synthesis. J. Iran. Chem. Soc. 2007, 4, 126–174. [Google Scholar] [CrossRef]
- Koval, I.V. N-Halo Reagents. N-Halosuccinimides in organic synthesis and in chemistry of natural compounds. Russ. J. Org. Chem. 2002, 38, 301–337. [Google Scholar] [CrossRef]
- Barton, D.; Ollis, W.D. Comprehensive Organic Chemistry: The Synthesis and Reactions of Organic Compounds, 1st ed.; Pergamon Press: Oxford, UK; New York, NY, USA, 1979; Volume 2. [Google Scholar]
- Kadam, S.T.; Kim, S.S. N-Iodosuccinimide (NIS) a novel and effective catalyst for the cyanosilylation of aldehydes under mild reaction conditions. Catal. Commun. 2008, 9, 1342–1345. [Google Scholar] [CrossRef]
- Nagarajappa Giridhar, B.; Pandey Krishna, K.; Shinde Aniket, S.; Vagdevi Hosadu, M. N-Bromosuccinimide (NBS)–An efficient catalyst for acetylation of wood. Holzforschung 2016, 70, 421–427. [Google Scholar] [CrossRef]
- Maleki, B.; Sedigh Ashrafi, S. N-Bromosuccinimide catalyzed three component one-pot efficient synthesis of 2,4,5-Triaryl-1H-imidazoles from aldehyde, ammonium acetate, and 1,2-Diketone or ±-Hydroxyketone. J. Mex. Chem. Soc. 2014, 58, 76–81. [Google Scholar] [CrossRef]
- Karimi, B.; Zamani, A.; Zareyee, D. N-Iodosuccinimide (NIS) as a mild and highly chemoselective catalyst for deprotection of tert-butyldimethylsilyl ethers. Tetrahedron Lett. 2004, 45, 9139–9141. [Google Scholar] [CrossRef]
- Saikia, I.; Borah, A.J.; Phukan, P. Use of bromine and bromo-organic compounds in organic synthesis. Chem. Rev. 2016, 116, 6837–7042. [Google Scholar] [CrossRef] [PubMed]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic oxidative iodination of organic compounds with iodide catalyzed by sodium nitrite. Adv. Synth. Catal. 2008, 350, 2921–2929. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic oxidative iodination of ketones catalysed by sodium nitrite "on water" or in a micelle-based aqueous system. Green Chem. 2009, 11, 1262–1267. [Google Scholar] [CrossRef]
- Stavber, G.; Stavber, S. Towards Greener Fluorine Organic Chemistry: Direct Electrophilic Fluorination of Carbonyl Compounds in Water and Under Solvent-Free Reaction Conditions. Adv. Synth. Catal. 2010, 352, 2838–2846. [Google Scholar] [CrossRef]
- Prebil, R.; Stavber, G.; Stavber, S. Aerobic oxidation of alcohols by using a completely metal-free catalytic system. Eur. J. Org. Chem. 2014, 2014, 395–402. [Google Scholar] [CrossRef]
- Ajvazi, N.; Stavber, S. Direct halogenation of alcohols with halosilanes under catalyst- and organic solvent-free reaction conditions. Tetrahedron Lett. 2016, 57, 2430–2433. [Google Scholar] [CrossRef]
- Ajvazi, N.; Stavber, S. Transformation of tertiary benzyl alcohols into the vicinal halo-substituted derivatives using N-Halosuccinimides. Molecules 2016, 21, 1325. [Google Scholar] [CrossRef] [PubMed]
- Vražič, D.; Jereb, M.; Laali, K.; Stavber, S. Brønsted acidic ionic liquid accelerated halogenation of organic compounds with N-Halosuccinimides (NXS). Molecules 2013, 18, 74–96. [Google Scholar] [CrossRef] [PubMed]
- Podgoršek, A.; Stavber, S.; Zupan, M.; Iskra, J. Environmentally benign electrophilic and radical bromination ‘on water’: H2O2–HBr system versus N-bromosuccinimide. Tetrahedron 2009, 65, 4429–4439. [Google Scholar] [CrossRef]
- Jereb, M.; Zupan, M.; Stavber, S. Visible-light-promoted wohl–ziegler functionalization of organic molecules with N-Bromosuccinimide under solvent-free reaction conditions. Helv. Chim. Acta 2009, 92, 555–566. [Google Scholar] [CrossRef]
- Pravst, I.; Zupan, M.; Stavber, S. Halogenation of ketones with N-halosuccinimides under solvent-free reaction conditions. Tetrahedron 2008, 64, 5191–5199. [Google Scholar] [CrossRef]
- Pravst, I.; Zupan, M.; Stavber, S. Directed regioselectivity of bromination of ketones with NBS: Solvent-free conditions versus water. Tetrahedron Lett. 2006, 47, 4707–4710. [Google Scholar] [CrossRef]
- Podgoršek, A.; Stavber, S.; Zupan, M.; Iskra, J. Visible light induced ‘on water’ benzylic bromination with N-bromosuccinimide. Tetrahedron Lett. 2006, 47, 1097–1099. [Google Scholar] [CrossRef]
- Pravst, I.; Zupan, M.; Stavber, S. Solvent-free bromination of 1,3-diketones and β-keto esters with NBS. Green Chem. 2006, 8, 1001–1005. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Uppalla, L.S.; Sadavarte, V.S. Chemoselective transesterification of β-Keto esters under neutral conditions using NBS as a catalyst. Synlett 2001, 2001, 1715–1718. [Google Scholar] [CrossRef]
- Karimi, B.; Seradj, H. N-Bromosuccinimide (NBS), a novel and highly effective catalyst for acetylation of alcohols under mild reaction conditions. Synlett 2001, 2001, 519–520. [Google Scholar] [CrossRef]
- Sucheta, K.; Reddy, G.S.R.; Ravi, D.; Rama Rao, N. A novel general route to the synthesis of carboxylic acid esters and thiolesters. Tetrahedron Lett. 1994, 35, 4415–4416. [Google Scholar] [CrossRef]
- Ramalinga, K.; Vijayalakshmi, P.; Kaimal, T.N.B. A mild and efficient method for esterification and transesterification catalyzed by iodine. Tetrahedron Lett. 2002, 43, 879–882. [Google Scholar] [CrossRef]
- Jereb, M.; Vražič, D.; Zupan, M. Dual behavior of alcohols in iodine-catalyzed esterification under solvent-free reaction conditions. Tetrahedron Lett. 2009, 50, 2347–2352. [Google Scholar] [CrossRef]
- Jereb, M.; Vražič, D.; Zupan, M. Iodine-catalyzed transformation of molecules containing oxygen functional groups. Tetrahedron 2011, 67, 1355–1387. [Google Scholar] [CrossRef]
- Vairamani, M.; Rao, G.K.V. Use of bromine in methanol - preparation of methyl-esters. Indian J. Chem. Sect B 1985, 24, 691. [Google Scholar]
- Bowman, P.T.; Ko, E.I.; Sides, P.J. A potential hazard in preparing bromine-methanol solutions. J. Electrochem. Soc. 1990, 137, 1309–1311. [Google Scholar] [CrossRef]
- Virtanen, E.; Kolehmainen, E. Use of bile acids in pharmacological and supramolecular applications. Eur. J. Org. Chem. 2004, 2004, 3385–3399. [Google Scholar] [CrossRef]
- Cravotto, G.; Binello, A.; Boffa, L.; Rosati, O.; Boccalini, M.; Chimichi, S. Regio- and stereoselective reductions of dehydrocholic acid. Steroids 2006, 71, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Mal, P. Electrophilic aryl-halogenation using N-halosuccinimides under ball-milling. Tetrahedron Lett. 2014, 55, 2154–2156. [Google Scholar] [CrossRef]
- Annese, C.; D'Accolti, L.; Fusco, C.; Licini, G.; Zonta, C. Heterolytic (2 e) vs Homolytic (1 e) Oxidation reactivity: N−H versus C−H switch in the oxidation of lactams by Dioxirans. Chem. Eur. J. 2017, 23, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Lyons, D.J.M. A novel aromatic carbocation-based coupling reagent for esterification and amidation reactions. Chem. Commun. 2015, 51, 3131–3134. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhao, L.; Liu, W.; Huang, X.; Song, H.; Zhang, T. Convenient, metal-free ipso-nitration of arylboronic acids using nitric acid and trifluoroacetic acid. Synth. Commun. 2017, 47, 10–14. [Google Scholar] [CrossRef]
- Jia, J.; Jiang, Q.; Zhao, A.; Xu, B.; Liu, Q.; Luo, W.-P.; Guo, C.-C. Copper-catalyzed O-methylation of carboxylic acids using DMSO as a methyl source. Synthesis 2016, 48, 421–428. [Google Scholar] [CrossRef]
- Powell, A.B.; Stahl, S.S. Aerobic Oxidation of diverse primary alcohols to methyl esters with a readily accessible heterogeneous Pd/Bi/Te catalyst. Org. Lett. 2013, 15, 5072–5075. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.W.; Buchwald, S.L. Mild and General palladium-catalyzed synthesis of methyl aryl ethers enabled by the use of a palladacycle precatalyst. Org. Lett. 2013, 15, 3998–4001. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.D.; Yodsanit, N.; Melander, C. Potentiation of the fosmidomycin analogue FR 900098 with substituted 2-oxazolines against Francisella novicida. MedChemComm 2016, 7, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Offermann, D.A.; McKendrick, J.E.; Sejberg, J.J.P.; Mo, B.; Holdom, M.D.; Helm, B.A.; Leatherbarrow, R.J.; Beavil, A.J.; Sutton, B.J.; Spivey, A.C. Synthesis and incorporation into cyclic peptides of tolan amino acids and their hydrogenated congeners: construction of an array of A–B-loop mimetics of the Cε3 domain of human IgE. J. Org. Chem. 2012, 77, 3197–3214. [Google Scholar] [CrossRef] [PubMed]
- Strazzolini, P.; Gambi, A.G.; Giumanini, A.; Vancik, H. The reaction between ethanedioyl (oxalyl) dihalides and Ag2C2O4: A route to Staudinger's elusive ethanedioic (oxalic) acid anhydride. J. Chem. Soc. Perkin Trans. 1998, 1, 2553–2558. [Google Scholar] [CrossRef]
- Sun, H.-B.; Hua, R.; Yin, Y. ZrOCl2·8H2O: An efficient, cheap and reusable catalyst for the esterification of acrylic acid and other carboxylic acids with equimolar amounts of alcohols. Molecules 2006, 11, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Maslov, M.A.; Morozova, N.G.; Solomatina, T.V.; Shaforostova, N.G.; Serebrennikova, G.A. Synthesis of amino analogues of cholic acid. Russ. J. Bioorg. Chem. 2011, 37, 507–515. [Google Scholar] [CrossRef]
- Rohacova, J.; Marin, M.L.; Martinez-Romero, A.; O'Connor, J.-E.; Gomez-Lechon, M.J.; Donato, M.T.; Castell, J.V.; Miranda, M.A. Synthesis of new, UV-photoactive dansyl derivatives for flow cytometric studies on bile acid uptake. Org. Biomol. Chem. 2009, 7, 4973–4980. [Google Scholar] [CrossRef] [PubMed]
- Hutchby, M.; Houlden, C.E.; Haddow, M.F.; Tyler, S.N.G.; Lloyd-Jones, G.C.; Booker-Milburn, K.I. Switching pathways: room-temperature neutral solvolysis and substitution of amides. Angew. Chem. Int. Ed. 2012, 51, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zatolochnaya, O.V.; Wang, X.-J.; Rodríguez, S.; Qu, B.; Desrosiers, J.-N.; Mangunuru, H.P.R.; Biswas, S.; Rivalti, D.; Karyakarte, S.D.; Sieber, J.D.; Grinberg, N.; Wu, L.; Lee, H.; Haddad, N.; Fandrick, D.R.; Yee, N.K.; Song, J.J.; Senanayake, C.H. BABIPhos family of biaryl dihydrobenzooxaphosphole ligands for asymmetric hydrogenation. Org. Lett. 2018, 20, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Dethe, D.H.; Erande, R.D.; Ranjan, A. Biomimetic total syntheses of borreverine and flinderole alkaloids. J. Org. Chem. 2013, 78, 10106–10120. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Li, A.N.; Zhou, J.; Guo, Y.; Lin, L.; Chen, W.; Wang, R. Mg(OMe)2 promoted allylic isomerization of γ-hydroxy-α,β-alkenoic esters to synthesize γ-ketone esters. Org. Biomol. Chem. 2017, 15, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Suwada, M.; Fukuhara, T.; Hara, S. Selective mono-fluorination of diols via a cyclic acetal of N,N-diethyl-4-methoxybenzamide. J. Fluorine Chem. 2007, 128, 1121–1125. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, A.; Xu, B.; Jia, J.; Liu, X.; Guo, C. PIFA-Mediated Esterification Reaction of Alkynes with Alcohols via Oxidative Cleavage of Carbon Triple Bonds. J. Org. Chem. 2014, 79, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Fujita, T.; Sakamoto, M.; Kuramochi, T.; Kitazume, T. Reactions of monoesters of ethylene glycol with N,N-diethyl-1,1,2,3,3,3-hexafluoropropylamine. J. Fluorine Chem. 1987, 36, 361–372. [Google Scholar] [CrossRef]
- Umeda, R.; Nishimura, T.; Kaiba, K.; Tanaka, T.; Takahashi, Y.; Nishiyama, Y. Rhenium complex-catalyzed acylative cleavage of ethers with acyl chlorides. Tetrahedron 2011, 67, 7217–7221. [Google Scholar] [CrossRef]
- Whittaker, A.M.; Dong, V.M. Nickel-catalyzed dehydrogenative cross-coupling: direct transformation of aldehydes into esters and amides. Angew. Chem. Int. Ed. 2015, 54, 1312–1315. [Google Scholar] [CrossRef] [PubMed]
- Gianetti, T.L.; Annen, S.P.; Santiso-Quinones, G.; Reiher, M.; Driess, M.; Grützmacher, H. Nitrous Oxide as a Hydrogen Acceptor for the Dehydrogenative Coupling of Alcohols. Angew. Chem. Int. Ed. 2016, 55, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Kumar, A.; Sain, B.; Gupta, A.K. A simple and convenient one-pot synthesis of fatty acid esters from hindered alcohols using N,N-dimethylchloro-sulfitemethaniminium chloride as dehydrating agent. Synth. Commun. 2002, 32, 2885–2891. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).