Rapid Evaluation of Spermidine from 12 Bean Cultivars by Direct Real-Time Mass Spectrometry Analysis
Abstract
1. Introduction
2. Results and Discussion
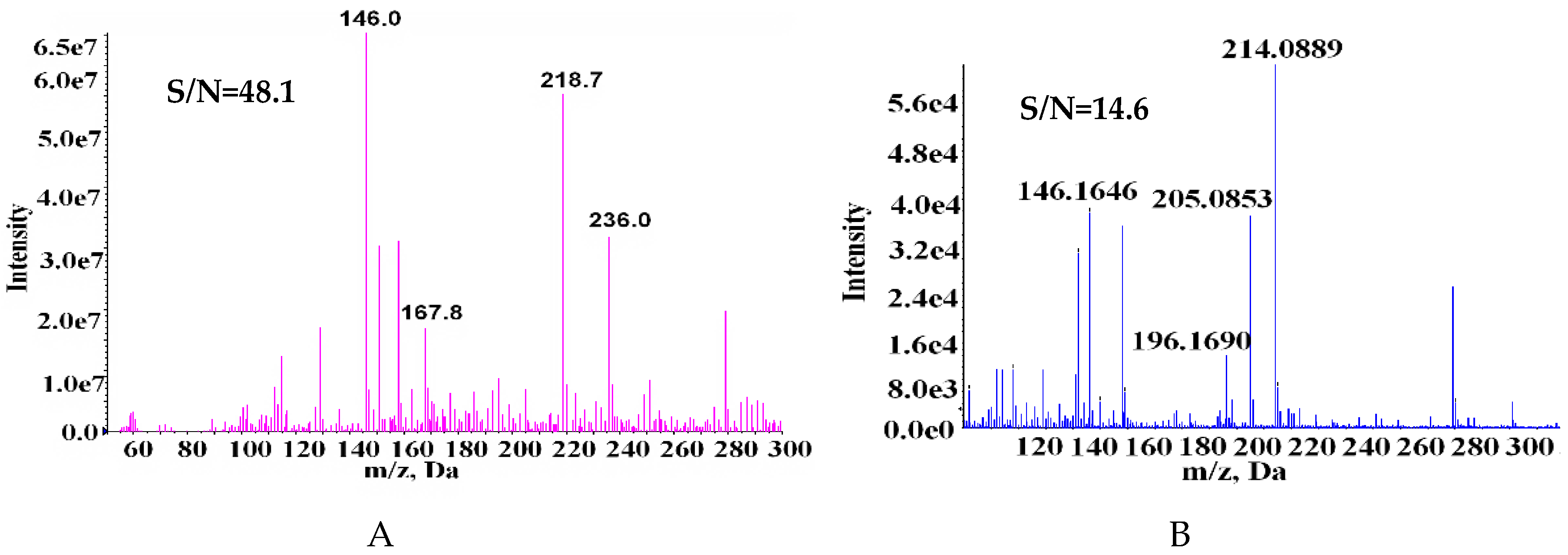
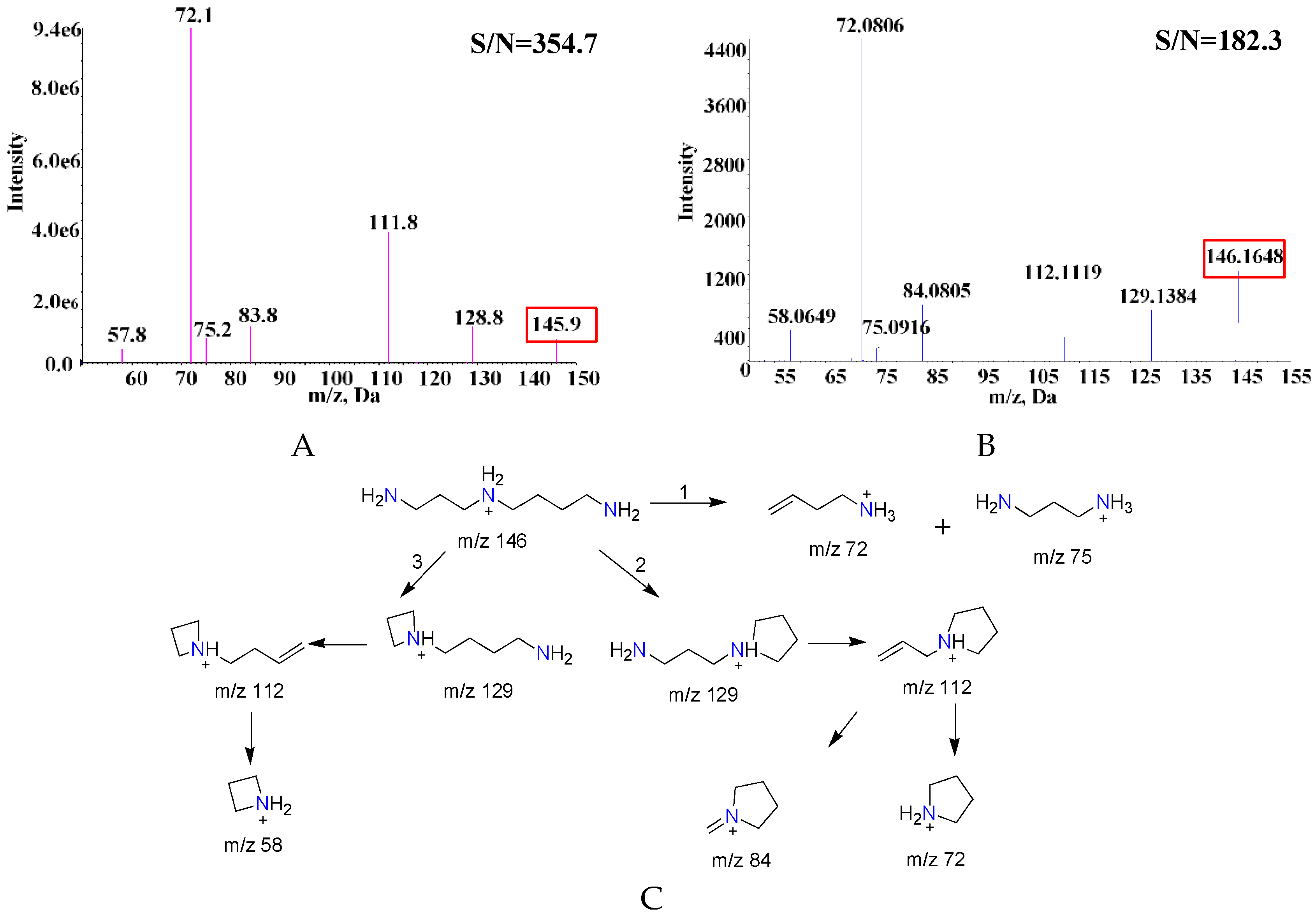
2.1. Comparison of MS and MS/MS Spectra of SPD Fragments
2.2. Optimization of the DART-MS Conditions
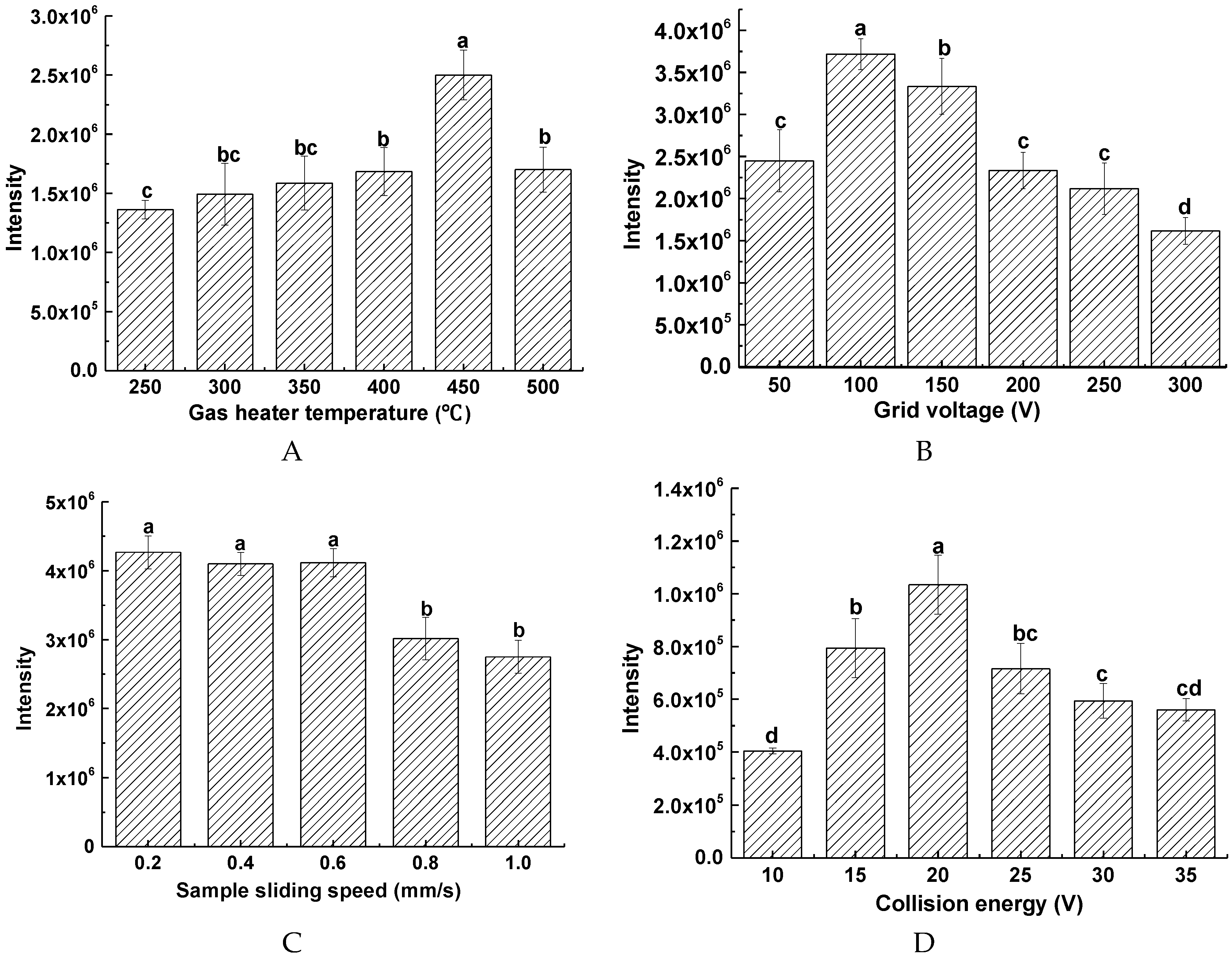
2.2.1. Effect of Gas Heating Temperature of DART Ion Source
2.2.2. Effect of Grid Voltage
2.2.3. Effect of Sample Presentation Speed
2.2.4. Effect of Collision Energy (CE)
2.2.5. Method Validation
2.3. Matrix Effect
2.4. Qualitative Analysis of SPD in Beans by DART-MS
3. Materials and Methods
3.1. Sample and Reagents
3.2. Preparation of Standard and Sample Solution
3.3. DART-MS Analysis
3.4. UHPLC-QTOF-MS Analysis
3.5. Method Validation
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalač, P. Health effects and occurrence of dietary polyamines: A review for the period 2005–mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, e2788. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Mariño, G.; Bennetzen, M.V.; Eisenberg, T.; Megalou, E.; Schroeder, S.; Cabrera, S.; Bénit, P.; Rustin, P.; Criollo, A.; et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 2011, 192, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Sagara, T.; Fiechter, G.; Pachner, M.; Mayer, H.K.; Vollmann, J. Soybean spermidine concentration: Genetic and environmental variation of a potential ‘anti-aging’ constituent. J. Food Compos. Anal. 2017, 56, 11–17. [Google Scholar] [CrossRef]
- Vol, N. Determination of amino acids in food and feed by derivatization with 6-aminoquinolyl-n-hydroxysuccinimidyl carbamate and reversed-phase liquid chromatographic separation. J. Aoac Int. 1995, 78, 736–744. [Google Scholar]
- Wongyai, S.; Oefner, P.; Bonn, G. HPLC analysis of polyamines and their acetylated derivatives in the picomole range using benzoyl chloride and 3,5-dinitrobenzoyl chloride as derivatizing agent. Biomed. Chromatogr. 1988, 2, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Håkanson, R.; Rönnberg, A.-L. Fluorometric determination of spermidine using o-phthalaldehyde: Optimum reaction conditions and tests of identity. Anal. Biochem. 1973, 54, 353–361. [Google Scholar] [CrossRef]
- Weston, D.J. Ambient ionization mass spectrometry: Current understanding of mechanistic theory; analytical performance and application areas. Analyst 2010, 135, 661–668. [Google Scholar] [CrossRef] [PubMed]
- McEwen, C.N.; McKay, R.G.; Larsen, B.S. Analysis of solids, liquids, and biological tissues using solids probe introduction at atmospheric pressure on commercial LC/MS instruments. Anal. Chem. 2005, 77, 7826–7831. [Google Scholar] [CrossRef] [PubMed]
- Silina, Y.E.; Fink-Straube, C.; Hayen, H.; Volmer, D.A. Analysis of fatty acids and triacylglycerides by Pd nanoparticle-assisted laser desorption/ionization mass spectrometry. Anal. Methods 2015, 7, 3701–3707. [Google Scholar] [CrossRef]
- Takats, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Cody, R.B.; Laramée, J.A.; Durst, H.D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-H.; Hsieh, H.-Y.; Hsu, C.-C. Clinical application of ambient ionization mass spectrometry. Mass Spectrom. 2017, 6, S0060. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lv, H.; Wang, F.; Wang, Y. Characterization of polyphenols from lycium ruthenicum fruit by UPLC-Q-TOF/MSE and their antioxidant activity in caco-2 cells. J. Agric. Food Chem. 2016, 64, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.L.G.; Sturgeon, R.E.; Welz, B.; Curtius, A.J.; Mester, Z. Ambient mass spectrometric detection of organometallic compounds using direct analysis in real time. Anal. Chem. 2009, 81, 9834–9839. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.A.; Fernández, F.M. Simulations and experimental investigation of atmospheric transport in an ambient metastable-induced chemical ionization source. Anal. Chem. 2009, 81, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Glória, M.B.A.; Tavares-Neto, J.; Labanca, R.A.; Carvalho, M.S. Influence of cultivar and germination on bioactive amines in soybeans (Glycine max L. Merril). J. Agric. Food Chem. 2005, 53, 7480–7485. [Google Scholar] [CrossRef] [PubMed]
- Kaczyński, P. Clean-up and matrix effect in LC-MS/MS analysis of food of plant origin for high polar herbicides. Food Chem. 2017, 230, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xiao, K. Modified quechers combined with ultra high performance liquid chromatography tandem mass spectrometry to determine seven biogenic amines in Chinese traditional condiment soy sauce. Food Chem. 2017, 229, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, D.-Y.; Liu, Z.-Y.; Zhang, Y.; Liu, L.; Li, L.; Liu, C.C.; Wu, G.-H. Rapid determination of 1-deoxynojirimycin in Morus alba L. Leaves by direct analysis in real time (DART) mass spectrometry. J. Pharmaceut. Biomed. Anal. 2015, 114, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Wu, X.; Xu, J.; Dong, F.; Liu, X.; Wei, D.; Zheng, Y. Determination and dissipation of mesotrione and its metabolites in rice using uplc and triple-quadrupole tandem mass spectrometry. Food Chem. 2017, 229, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dong, F.; Liu, X.; Xu, J.; Meng, W.; Liu, N.; Chen, Z.; Tao, Y.; Zheng, Y. Simultaneous determination of fipronil and its major metabolites in corn and soil by ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Methods 2014, 6, 1788–1795. [Google Scholar] [CrossRef]
Sample Availability: Samples of the 12 bean cultivars are available from the authors. |

| Method | Linear | R2 | LOD (mg/kg) | LOQ (mg/kg) | Precision | |
|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | |||||
| DART-MS | y = 292923x − 45007 | 0.990 | 0.025 | 0.078 | 3.6% | 7.50% |
| UHPLC-ESI-QTOF | y = 89517x − 8931 | 0.999 | 0.036 | 0.131 | 2.15% | 5.30% |
| Variety | Sample Measurement (mg/L) | Spiked (mg/L) | DART-MS Recovery (%) | UHPLC-ESI-MS Recovery (%) |
|---|---|---|---|---|
| NO.12 | 1.20 ± 0.03 | 0.625 | 102.79 ± 3.13 | 95.64 ± 1.44 |
| 1.25 | 115.70 ± 4.01 | 93.31 ± 1.91 | ||
| 2.5 | 148.44 ± 3.83 | 103.60 ± 1.75 |
| Matrix | Method | Equation of Calibration Curve | Matrix Effect (%) |
|---|---|---|---|
| Blank | DART-MS | y = 292923x − 45007 | - |
| UHPLC-ESI-QTOF | y = 89517x − 8931 | - | |
| NO. 12 | DART-MS | y = 331591x + 26566 | 13.20% |
| UHPLC-ESI-QTOF | y = 107561x + 528890 | 20.16% |
| Number | DART-MS (mg/kg) | UHPLC-ESI-QTOF (mg/kg) |
|---|---|---|
| 1 | 3.42 ± 0.05 | 3.38 ± 0.06 |
| 2 | 3.87 ± 0.17 * | 3.19 ± 0.08 |
| 3 | 9.62 ± 0.15 * | 9.16 ± 0.10 |
| 4 | 10.44 ± 0.26 * | 9.55 ± 0.14 |
| 5 | 5.28 ± 0.28 | 4.90 ± 0.10 |
| 6 | 3.58 ± 0.23 * | 5.22 ± 0.08 |
| 7 | 2.96 ± 0.10 * | 2.15 ± 0.07 |
| 8 | 3.63 ± 0.14 * | 1.95 ± 0.03 |
| 9 | 2.01 ± 0.06 * | 1.84 ± 0.01 |
| 10 | 5.11 ± 0.31 * | 3.34 ± 0.20 |
| 11 | 4.55 ± 0.22 * | 3.64 ± 0.10 |
| 12 | 12.08 ± 0.35 * | 13.45 ± 0.19 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Wu, X.; Yuan, X.; Wang, Y.; Zhou, W.; Li, W. Rapid Evaluation of Spermidine from 12 Bean Cultivars by Direct Real-Time Mass Spectrometry Analysis. Molecules 2018, 23, 2138. https://doi.org/10.3390/molecules23092138
Wu T, Wu X, Yuan X, Wang Y, Zhou W, Li W. Rapid Evaluation of Spermidine from 12 Bean Cultivars by Direct Real-Time Mass Spectrometry Analysis. Molecules. 2018; 23(9):2138. https://doi.org/10.3390/molecules23092138
Chicago/Turabian StyleWu, Tao, Xiaoyu Wu, Xv Yuan, Yi Wang, Wenhua Zhou, and Weili Li. 2018. "Rapid Evaluation of Spermidine from 12 Bean Cultivars by Direct Real-Time Mass Spectrometry Analysis" Molecules 23, no. 9: 2138. https://doi.org/10.3390/molecules23092138
APA StyleWu, T., Wu, X., Yuan, X., Wang, Y., Zhou, W., & Li, W. (2018). Rapid Evaluation of Spermidine from 12 Bean Cultivars by Direct Real-Time Mass Spectrometry Analysis. Molecules, 23(9), 2138. https://doi.org/10.3390/molecules23092138





