Linear Triquinane Sesquiterpenoids: Their Isolation, Structures, Biological Activities, and Chemical Synthesis
Abstract
:1. Introduction
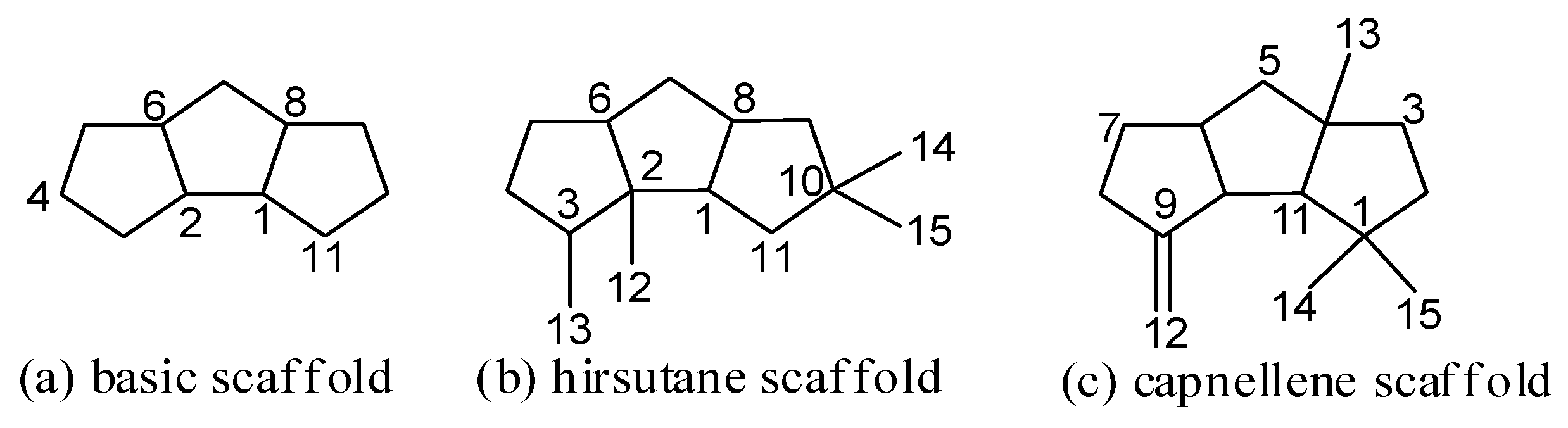
2. Isolation and Structure Elucidation
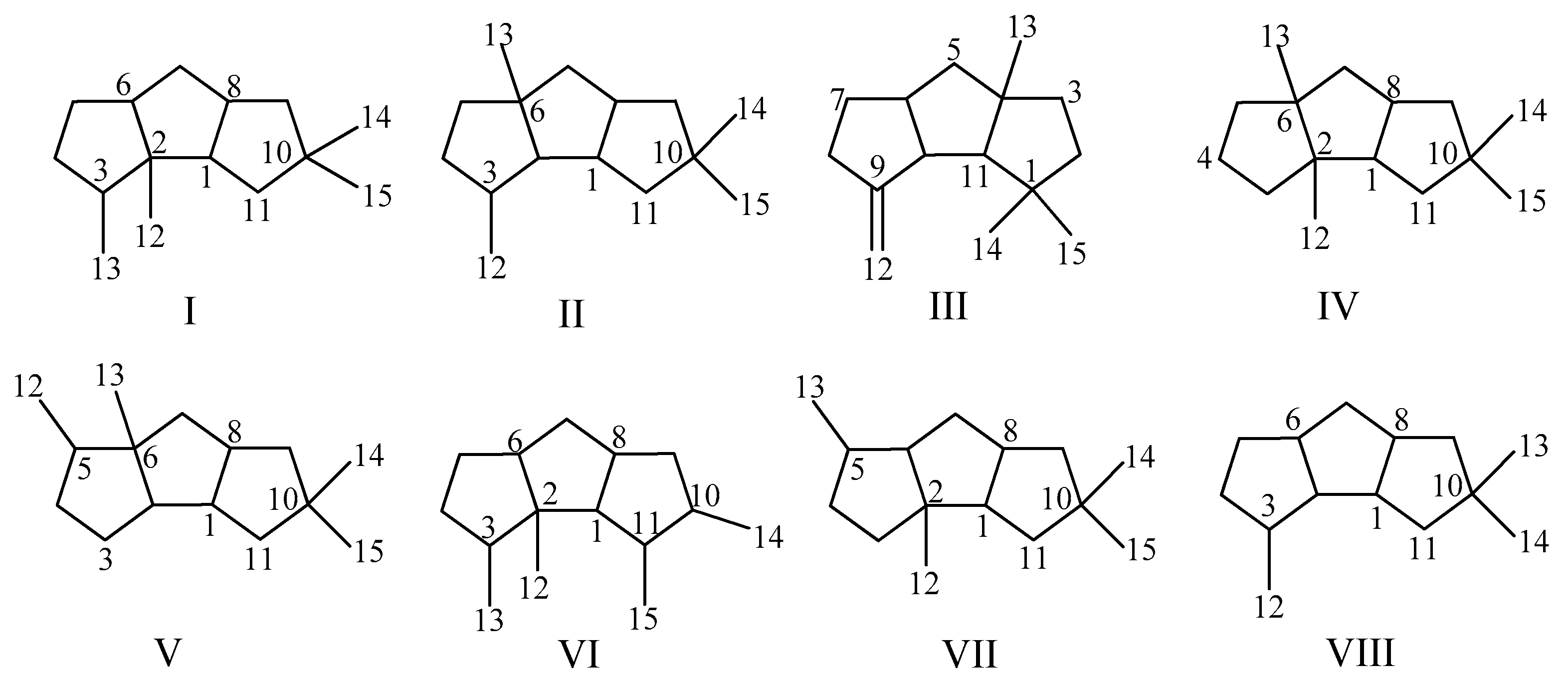
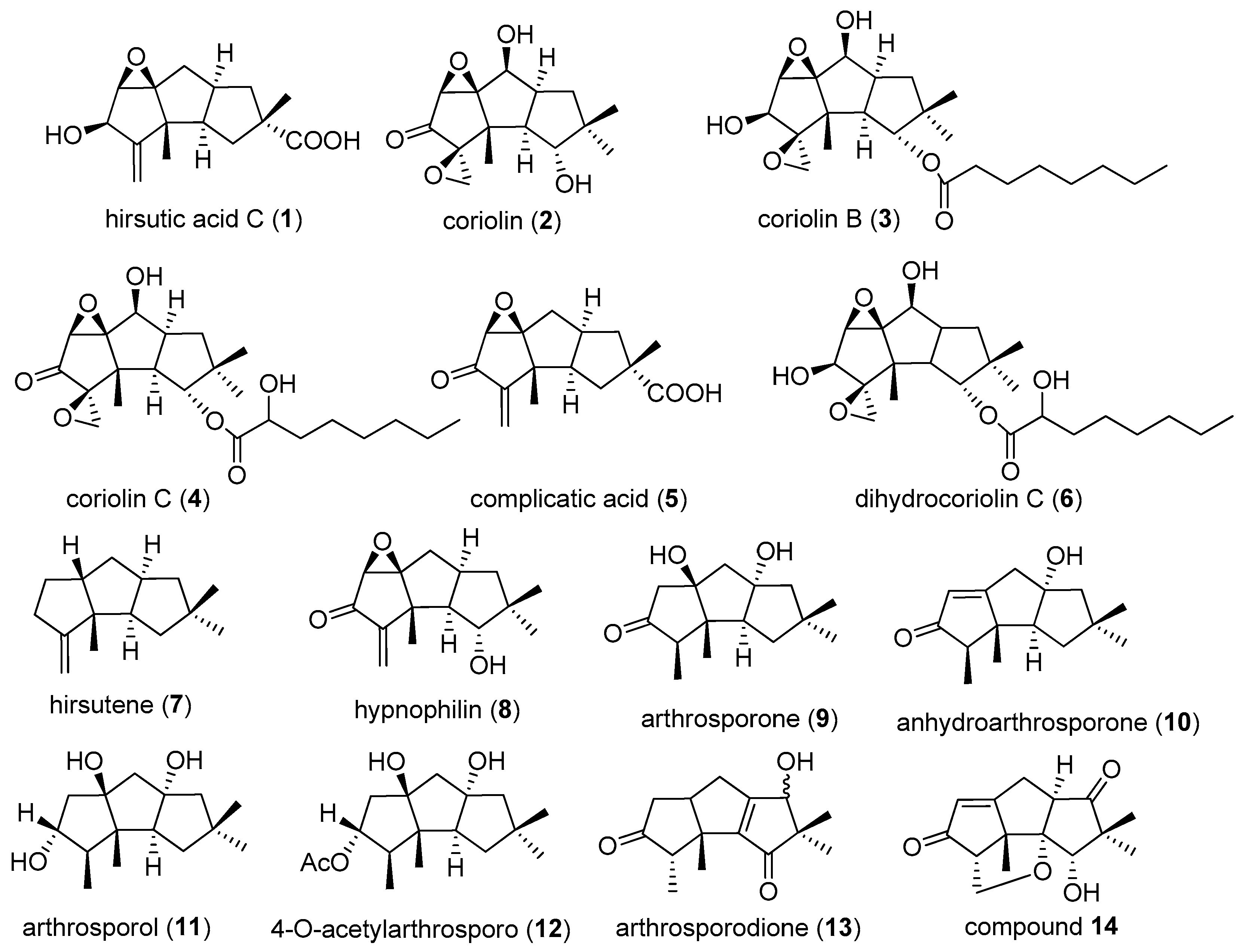
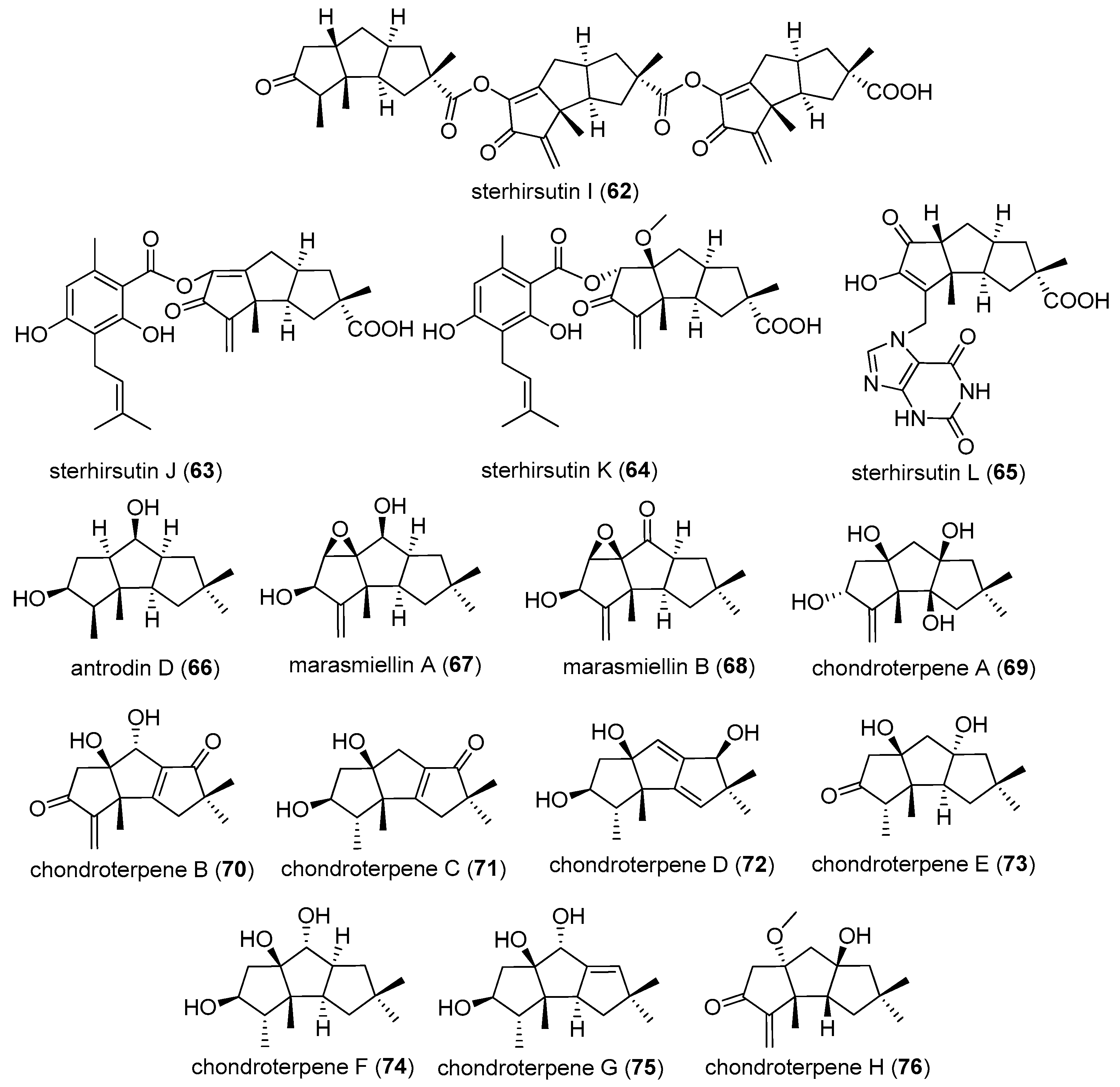
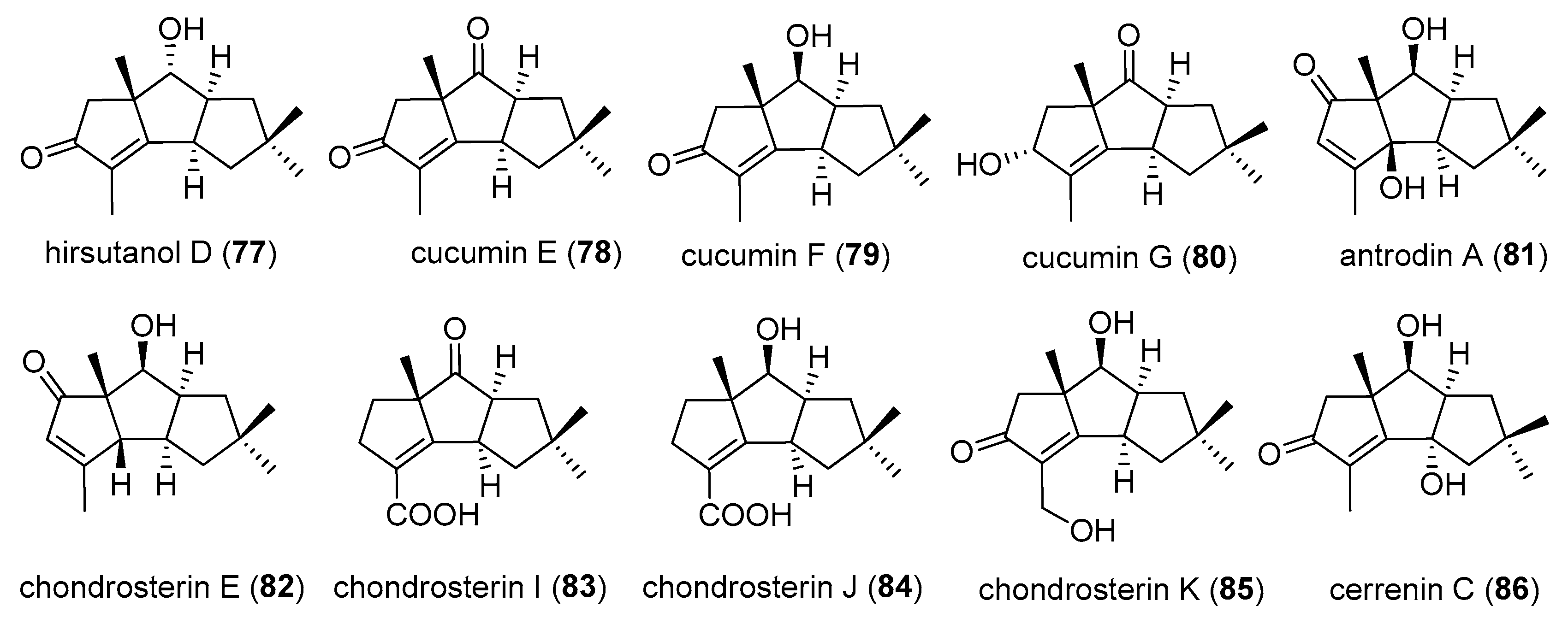
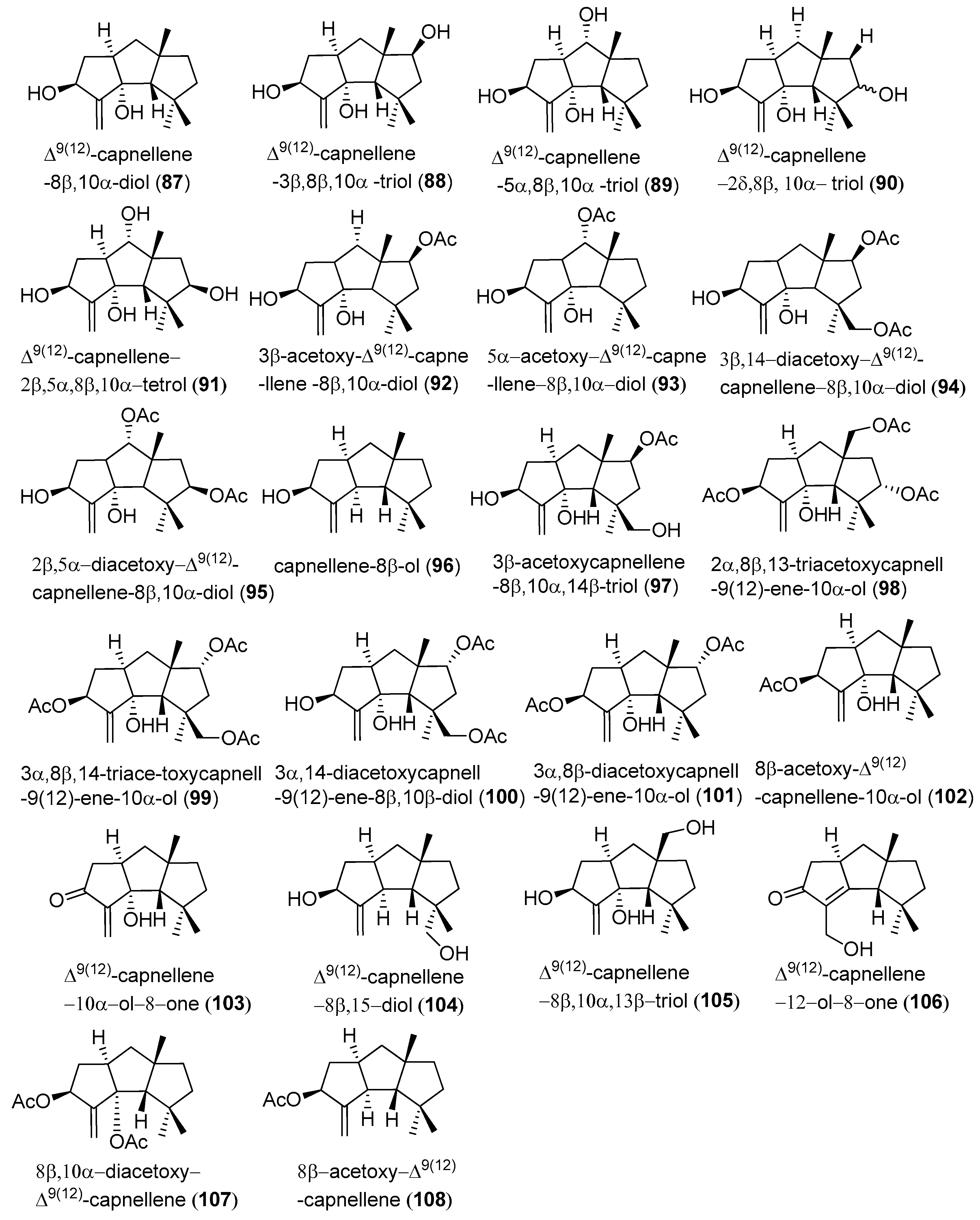
2.1. Type I
2.2. Type II
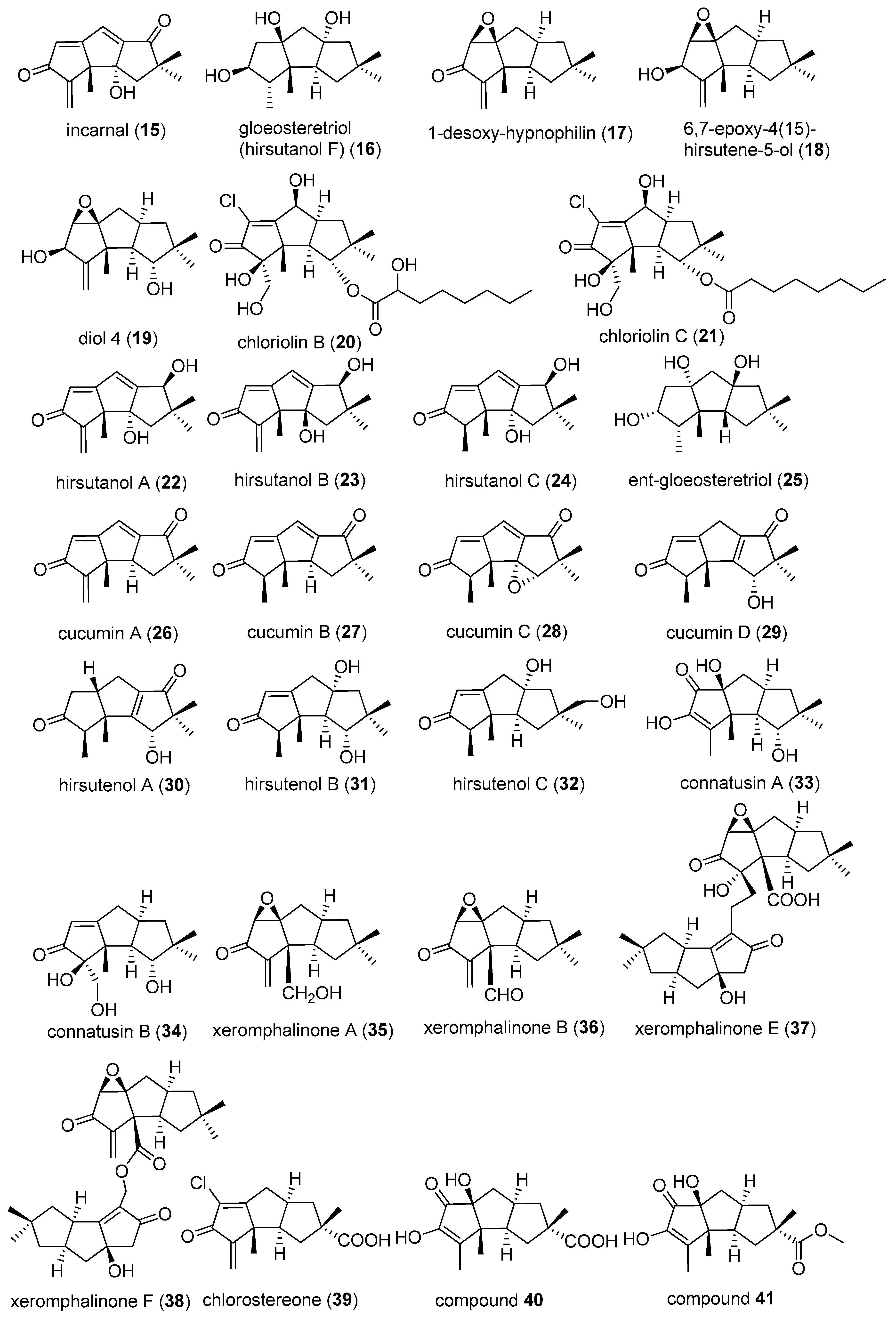
2.3. Type III
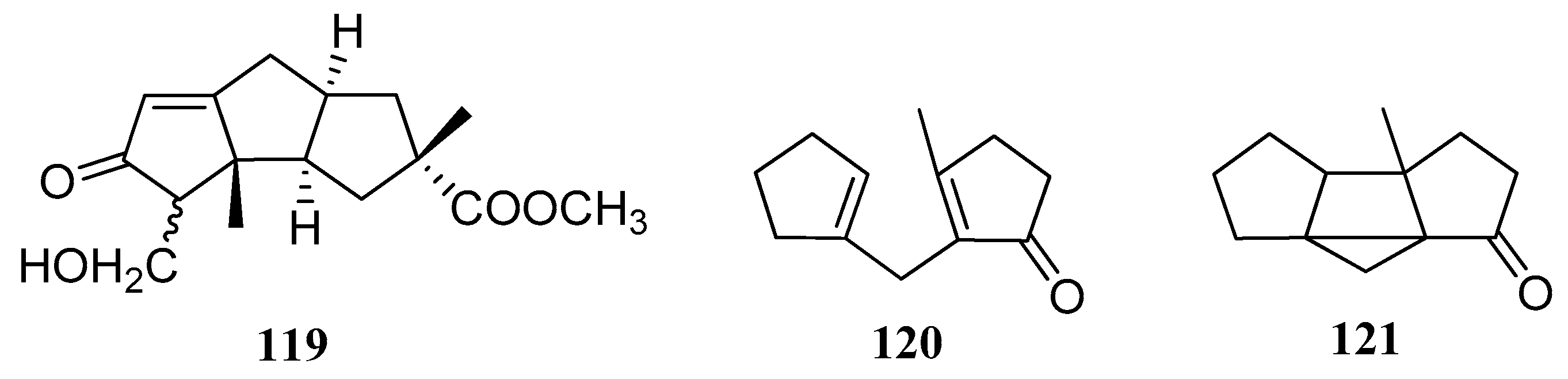
2.4. Type IV, V, VI, VII and VIII
3. Biological Activity
3.1. Cytotoxicity
3.2. Antimicrobial Activity
3.3. Other Bioactivity
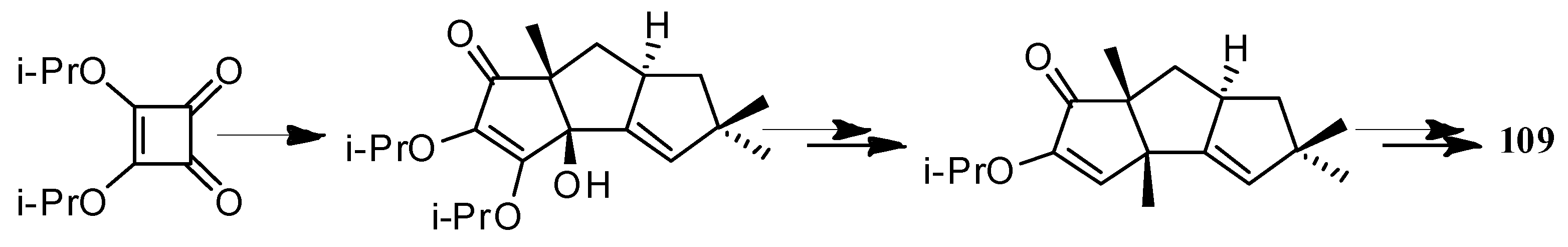
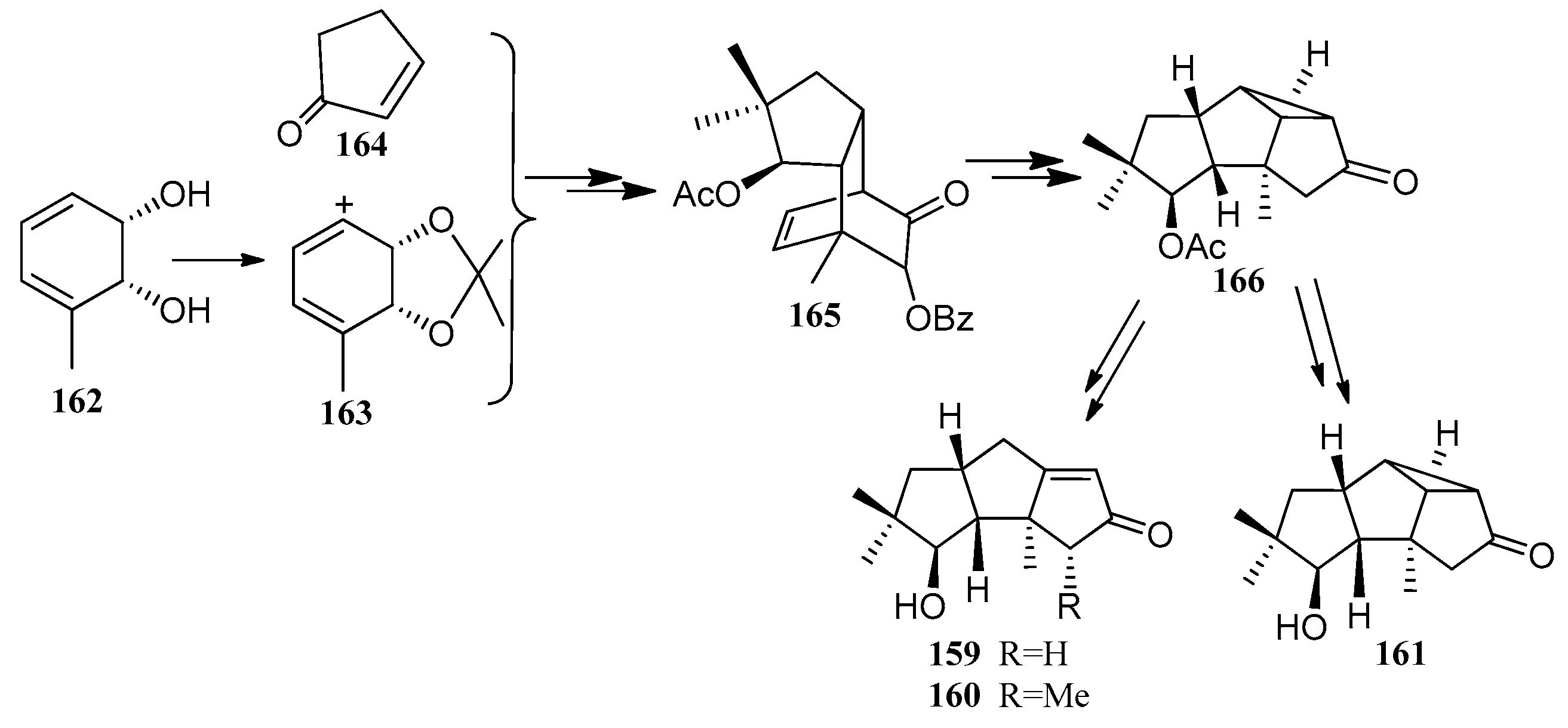
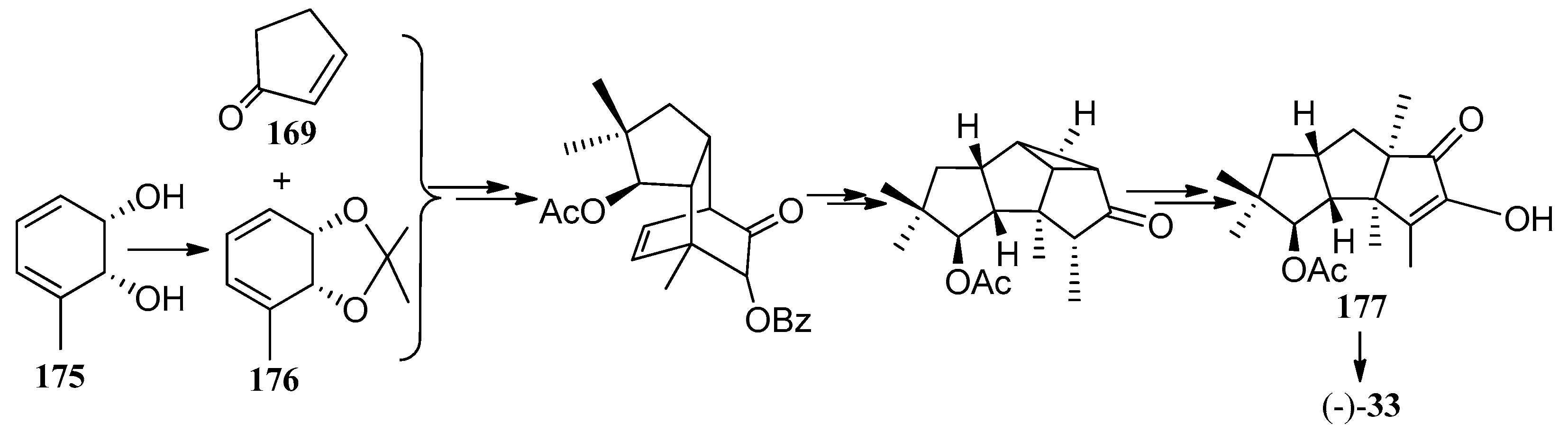
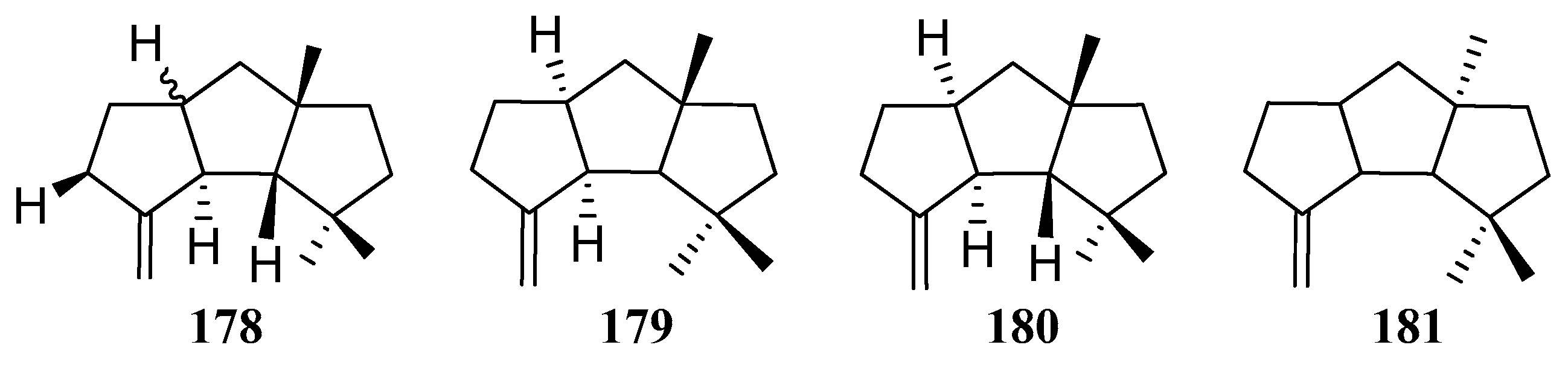
4. Chemical Synthesis
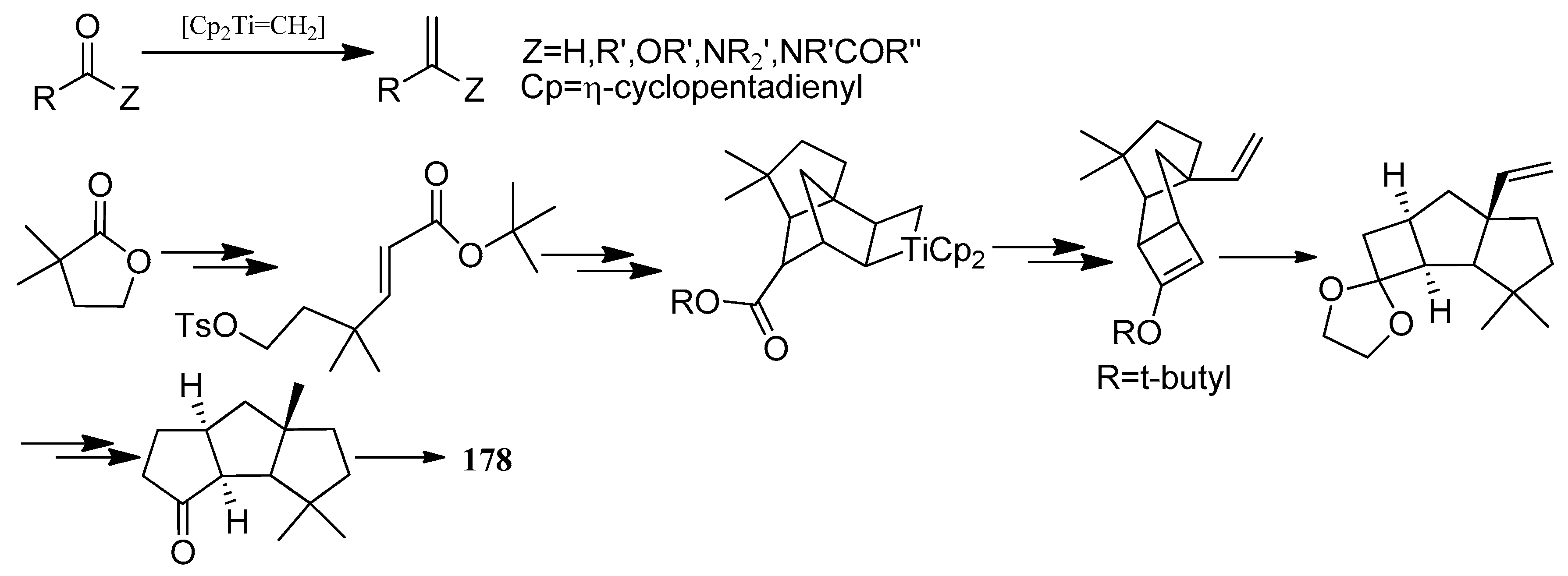
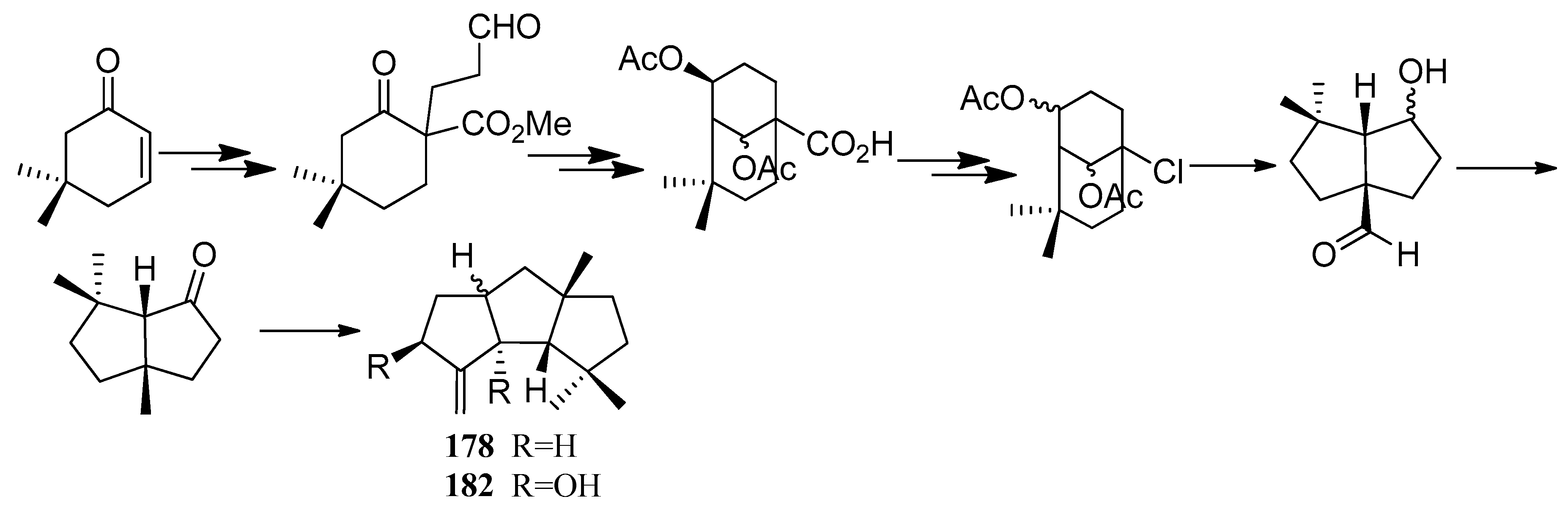
4.1. Hirsutane Type
4.2. Capnellene Type
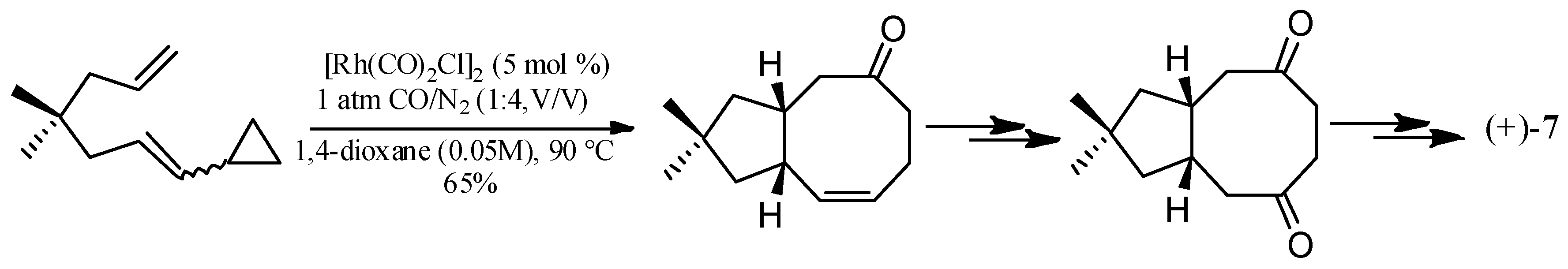
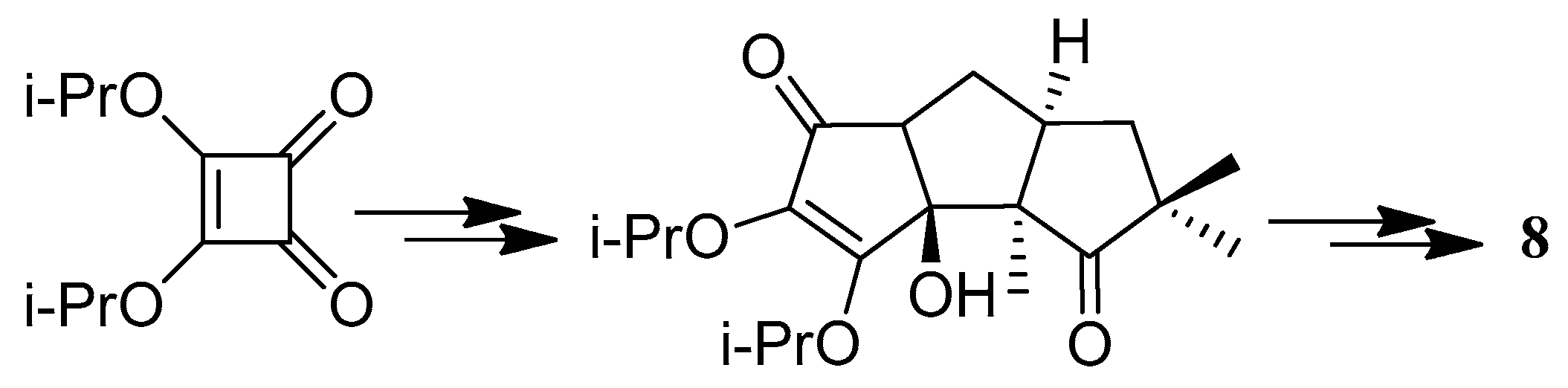
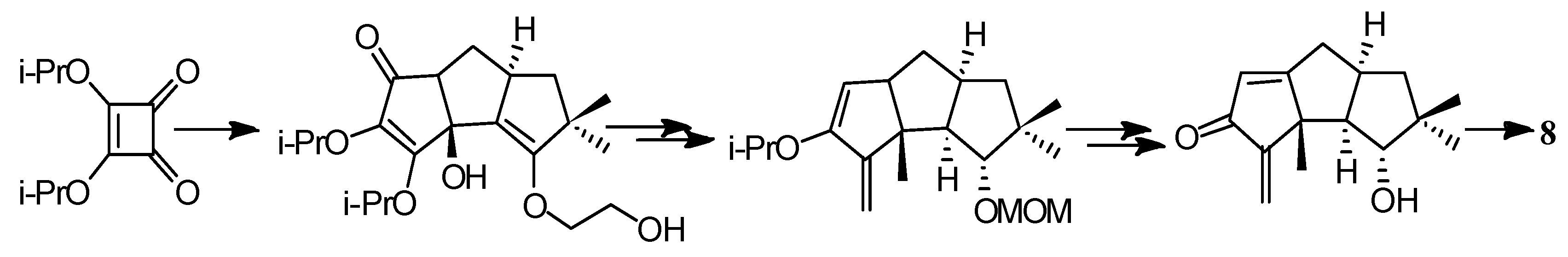
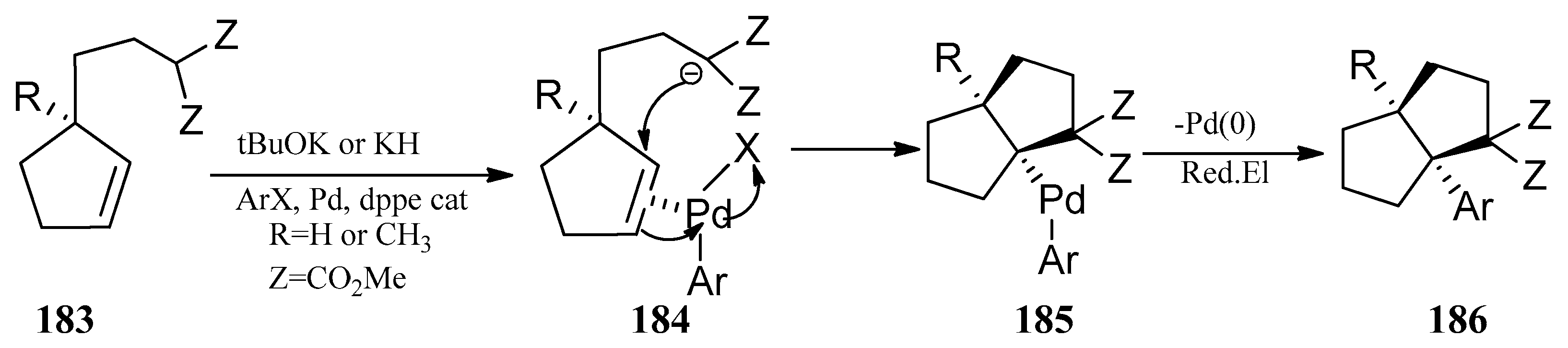
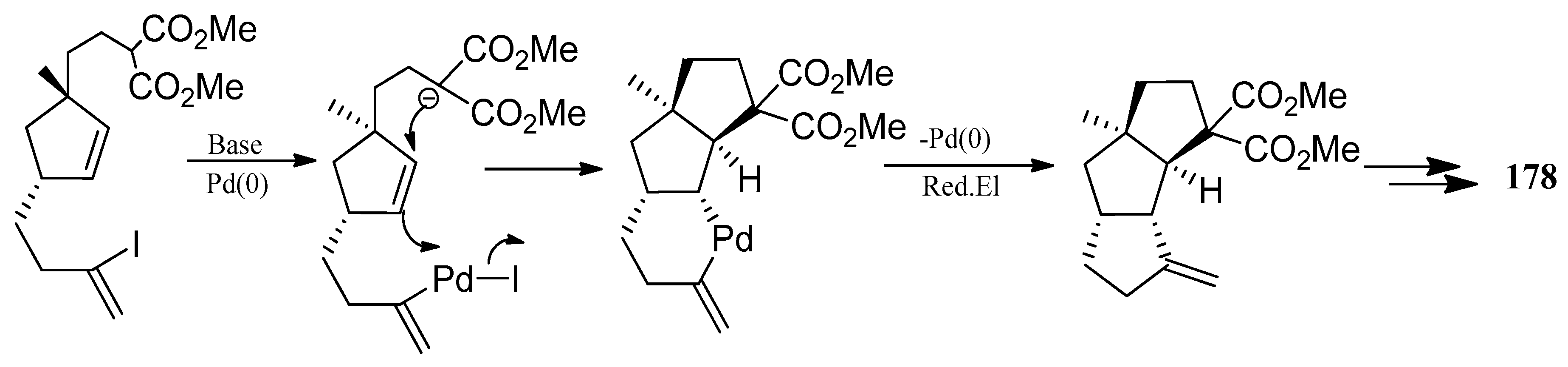
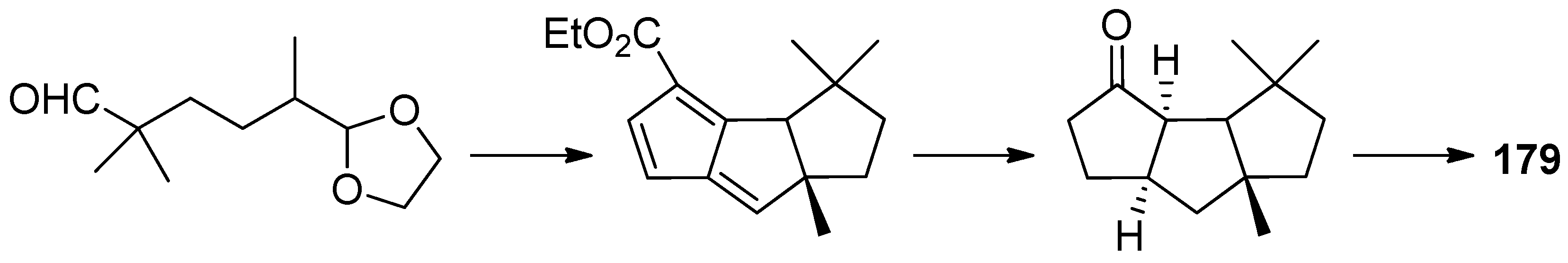
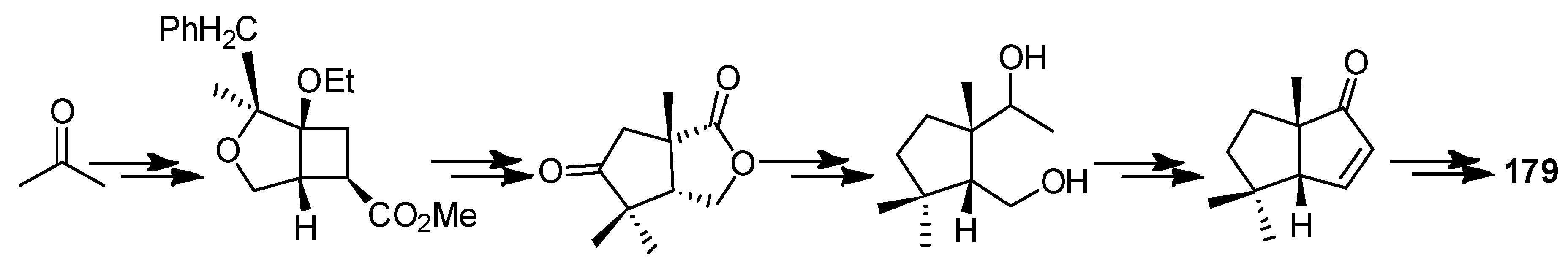
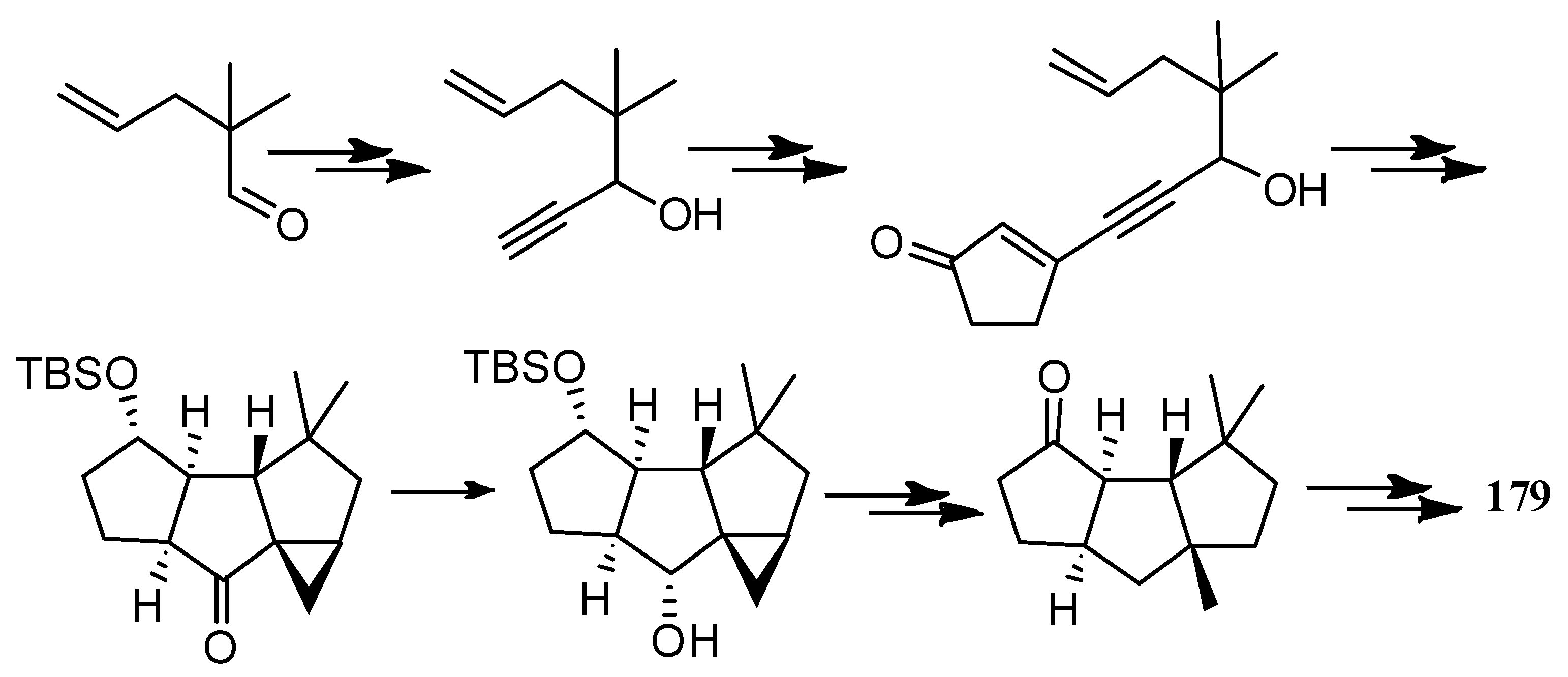
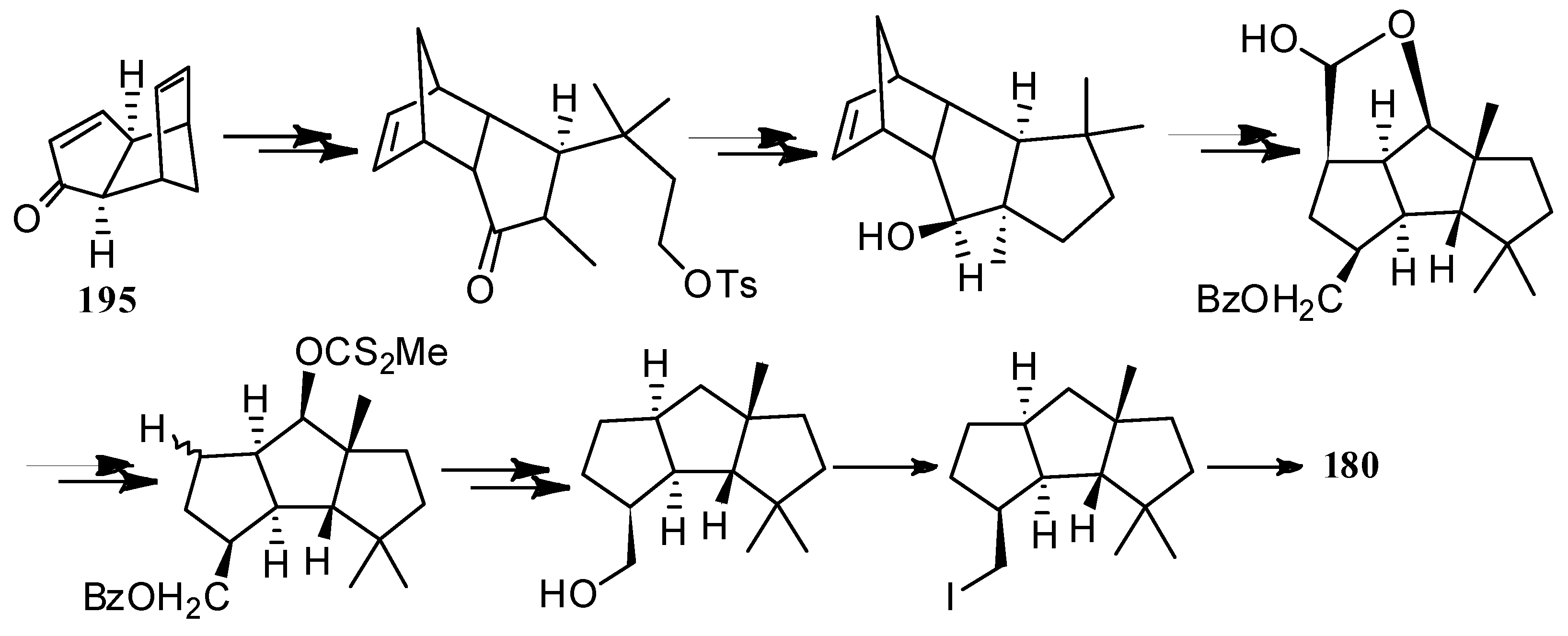
4.3. Other Type
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Le, B.F.; Kousara, M.; Chen, L.; Wei, L.; Dumas, F. Tricyclic sesquiterpenes from marine origin. Chem. Rev. 2017, 117, 6110–6159. [Google Scholar] [CrossRef]
- Comer, F.W.; Mocapra, F.; Qureshi, I.H.; Scott, A.I. The structure and chemistry of hirsutic acid. Tetrahedron 1967, 23, 4761–4768. [Google Scholar] [CrossRef]
- Heatley, N.G.; Jennings, M.A.; Florey, H.W. Antibiotics from Stereum hirsutum. Brit. J. Exp. Pathol. 1947, 28, 35–46. [Google Scholar]
- Amouzou, E.; Ayer, W.A.; Browne, L.M. Antifungal sesquiterpenoids from an arthroconidial fungus. J. Nat. Prod. 1989, 52, 1042–1054. [Google Scholar] [CrossRef]
- Wang, G.-Y.-S.; Abrell, L.M.; Avelar, A.; Borgeson, B.M.; Crews, P. New hirsutane based sesquiterpenes from salt water cultures of a marine sponge-derived fungus and the terrestrial fungus Coriolus consors. Tetrahedron 1998, 54, 7335–7342. [Google Scholar]
- Grote, D.; Hanel, F.; Dahse, H.M.; Seifert, K. Capnellenes from the soft coral Dendronephthya rubeola. Chem. Biodivers. 2007, 4, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Wen, Z.H.; Wang, S.K.; Duh, C.Y. Capnellenes from the formosan soft coral Capnella imbricate. J. Nat. Prod. 2008, 71, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Helaly, S.E.; Richter, C.; Thongbai, B.; Hyde, K.D.; Stadler, M. Lentinulactam, a hirsutane sesquiterpene with an unprecedented lactam modification. Tetrahedron Lett. 2016, 57, 5911–5913. [Google Scholar] [CrossRef]
- Takeuchi, T.; Iinuma, H.; Iwanaga, J.; Takahashi, S.; Takita, T.; Umezaw, H. Coriolin, a new basidiomycetes antibiotic. J. Antibiot. 1969, 22, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Iinuma, H.; Takita, T.; Maeda, K.; Umezawa, H. The structures of coriolin B and C. Tetrahedron Lett. 1970, 11, 1637–1639. [Google Scholar] [CrossRef]
- Nozoe, S.; Furukawa, J.; Sankawa, U.; Shibata, S. Isolation, structure and synthesis of hirsutene, a precursor hydrocarbon of coriolin biosynthesis. Tetrahedron Lett. 1976, 17, 195–198. [Google Scholar] [CrossRef]
- Takahashi, S.; Naganawa, H.; Iinuma, H.; Takita, T.; Maeda, K.; Umezawa, H. Revised structure and stereochemistry of coriolins. Tetrahedron Lett. 1971, 12, 1955–1958. [Google Scholar] [CrossRef]
- Mellows, G.; Mantle, P.G.; Feline, T.C.; William, D. Sesquiterpenoid metabolites from Stereum complicatun. J. Phytochemistry 1973, 12, 2717–2720. [Google Scholar] [CrossRef]
- Tanabe, M.; Suzuki, K.T. Biosynthetic studies with carbon-13: The FT-13C NMR spectra of the sesquiterpenoid coriolins. Tetrahedron Lett. 1974, 15, 2271–2274. [Google Scholar] [CrossRef]
- Kupka, J.; Anke, T.; Giannetti, B.M.; Steglich, W. Antibiotics from basidiomycetes XIV. * Isolation and biological characterization of hypnophilin, pleurotellol, and pleurotellic acid from Pleurotellus hypnophilus (Berk.) Sacc. Arch. Microbiol. 1981, 130, 223–227. [Google Scholar] [CrossRef]
- Giannetti, B.M.; Steffan, B.; Steglich, W. Antibiotics from basidiomycetes. Part 24.1: Antibiotics with a rearranged hirsutane skeleton from Pleurotellus Hypnophilus (agaricales). Tetrahedron 1986, 42, 3587–3593. [Google Scholar] [CrossRef]
- Takazawa, H.; Kashino, S. Incarnal, a new antibacterial sesquiterpene from basidiomycetes. Chem. Pham. Bull. 1991, 39, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yue, D.C.; Cheng, K.D.; Wang, S.C.; Yu, K.B.; Zheng, Q.T.; Yang, J.S. Gloeosteretriol, a new sesquiterpene from the fermentation products of Gloeostereum incarnatum S. Ito et Imai. Acta Pharm. Sin. 1992, 27, 33–36. [Google Scholar] [CrossRef]
- Abate, D.; Abraham, W.R. Antimicrobial metabolites from Lentinus crinitus. J. Antibiot. 1994, 47, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.C.; Varoglu, M.; Abrell, L.; Lobkovsky, E.; Clardy, J. Chloriolins A–C, chlorinated sesquiterpenes produced by fungal cultures separated from a juspis marine sponge. J. Org. Chem. 1994, 59, 6344–6348. [Google Scholar] [CrossRef]
- Hellwig, V.; Dasenbrock, J.; Schumann, S.; Steglich, W.; Leonhardt, K.; Anke, T. New triquinane-type sesquiterpenoids from Macrocystidia cucumis (Basidiomycetes). Eur. J. Org. Chem. 1998, 73–79. [Google Scholar] [CrossRef]
- Yun, B.S.; Lee, I.K.; Cho, Y.; Cho, S.M.; Yoo, I.D. New tricyclic sesquiterpenes from the fermentation broth of Stereum hirsutum. J. Nat. Prod. 2002, 65, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Tansakul, C.; Saithong, S.; Pakawatchai, C.; Isaka, M.; Suvannakad, R. Hirsutane sesquiterpenes from the fungus Lentinus connatus BCC 8996. J. Nat. Prod. 2005, 68, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Liermann, J.C.; Schuffler, A.; Wollinsky, B.; Birnbacher, J.; Kolshorn, H.; Anke, T.; Opatz, T. Hirsutane-type sesquiterpenes with uncommon modifications from three basidiomycetes. J. Org. Chem. 2010, 75, 2955–2961. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Bao, L.; Han, J.J.; Yang, X.L.; Zhao, F.; Li, S.F.; Song, F.H.; Liu, M.M.; Liu, H.W. New benzoate derivatives and hirsutane type sesquiterpenoids with antimicrobial activity and cytotoxicity from the solid-state fermented rice by the medicinal mushroom Stereum hirsutum. Food Chem. 2014, 143, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Xie, Y.L.; Xie, Z.L.; Chen, Y.; Lam, C.K.; Lan, W.J. Chondrosterins A–E, triquinane-type sesquiterpenoids from soft coral-associated fungus Chondrostereum sp. Mar. Drugs 2012, 10, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Lan, W.J.; Lam, C.K.; Yang, F.; Zhu, X.F. Hirsutane sesquiterpenoids from the marine-derived fungus Chondrostereum sp. Chem. Biodivers. 2011, 8, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lan, W.J.; Deng, R.; Feng, G.K.; Xu, Q.Y.; Hu, Z.Y.; Zhu, X.F.; Li, H.J. Additional new cytotoxic triquinane-type sesquiterpenoids chondrosterins K–M from the marine fungus Chondrostereum sp. Mar. Drugs 2016, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lan, W.J.; Li, H.J. Two new hirsutane-type sesquiterpenoids chondrosterins N and O from the marine fungus Chondrostereum sp. Nat. Prod. Res. 2018, 32, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.Y.; Bao, L.; Ren, J.W.; Han, J.J.; Zhang, Z.Y.; Li, Y.; Yao, Y.J.; Cao, R.; Liu, H.W. Sterhirsutins A and B, two new heterodimeric sesquiterpenes with a new skeleton from the culture of Stereum hirsutum collected in Tibet plateau. Org. Lett. 2014, 16, 5092–5095. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.Y.; Ren, J.W.; Sun, L.W.; He, L.W.; Bao, L.; Yue, W.; Sun, Q.M.; Yao, Y.J.; Yin, W.B.; Liu, H.W. Stucturally diverse sesquiterpenes produced by a Chinese Tibet fungus Stereum hirsutum and their cytotoxic and immunosuppressant activities. Org. Lett. 2015, 17, 3098–3101. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.M.; Chen, H.P.; Wang, F.; Li, Z.H.; Feng, T.; Liu, J.K. New triquinane and gymnomitrane sesquiterpenes from fermentation of the basidiomycete Antrodiella albocinnamomea. Fitoterapia 2015, 102, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Palasarn, S.; Sappan, M.; Supothina, S.; Boonpratuang, T. Hirsutane sesquiterpenes from cultures of the basidiomycete Marasmiellus sp. BCC 22389. Nat. Prod. Bioprospect. 2016, 6, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, G.; Chi, W.C.; Pang, K.L.; Chen, J.J.; Kuo, Y.H.; Wang, Y.K.; Cha, H.J.; Chou, S.C.; Lee, T.H. Hirsutane-type sesquiterpenes with inhibitory activity of microglial nitric oxide production from the red alga-derived fungus Chondrostereum sp. NTOU4196. J. Nat. Prod. 2017, 80, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Jiang, W.H.; Liang, W.L.; Huang, J.X.; Mo, Y.F.; Ding, Y.Q.; Lam, C.K.; Qian, X.J.; Zhu, X.F.; Lan, W.J. Induced marine fungus Chondrostereum sp. as a means of producing new sesquiterpenoids chondrosterins I and J by using glycerol as the carbon source. Mar. Drugs 2014, 12, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Tan, H.B.; Chen, K.; Chen, Y.C.; Li, S.N.; Li, H.H.; Zhang, W.M. Cerrenins A-C, cerapicane and isohirsutane sesquiterpenoids from the endophytic fungus Cerrena sp. Fitoterapia 2018, 129, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Y.M.; Singy, G.; Kaisin, M.; Eggert, H.; Djerassi, C. Terpenoids—LXXI: Chemical studies of marine invertebrates—XIV. Four representatives of a novel sesquiterpene class—The capnellane skeleton. Tetrahedron 1976, 32, 1171–1178. [Google Scholar] [CrossRef]
- Kaisin, M.; Tursch, B.; Declercq, J.P.; Germain, G.; Meerssche, M.V. Chemical studies of marine invertebrates. xl. Δ9(12)-capnellene-2β,5α,8β, 10α-tetrol, a new sesquiterpene alcohol from the soft coral Capnella imbricate. Bull. Soc. Chim. Belg. 1979, 88, 253–258. [Google Scholar] [CrossRef]
- Kaisin, M.; Braekman, J.C.; Daloze, D.; Tursch, B. Novel acetoxycapnellenes from the alcyonacean capnella imbricate. Tetrahedron 1985, 41, 1067–1072. [Google Scholar] [CrossRef]
- Morris, L.A.; Jaspars, M.; Adamson, K.; Woods, S.; Wallace, H.M. The capnellenes revisited: New structures and new biological activity. Tetrahedron 1998, 54, 12953–12958. [Google Scholar] [CrossRef]
- Hanssen, H.P.; Abranam, W.R. Sesquiterpene alcohols with novel skeletons from the fungus Ceratocystis piceae (ascomycotina). Tetrahedron 1988, 44, 2175–2180. [Google Scholar] [CrossRef]
- Huang, Z.L.; Dan, Y.; Huang, Y.C.; Lin, L.D.; Li, T.H.; Ye, W.H.; Wei, X.Y. Sesquiterpenes from the mycelial cultures of Dichomitus squalens. J. Nat. Prod. 2004, 67, 2121–2123. [Google Scholar] [CrossRef] [PubMed]
- Kutateladze, A.G.; Kuznetsov, D.M. Triquinanes and related sesquiterpenes revisited computationally: Structure corrections of hirsutanols B and D, hirsutenol E, cucumin B, antrodins C–E, chondroterpenes A and H, chondrosterins C and E, dichrocephone A, and pethybrene. J. Org. Chem. 2017, 82, 10795–10802. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, W.D.; Deng, R.; Zhang, H.; Tang, J.; Wu, K.W.; Li, D.D.; Feng, G.K.; Lan, W.J.; Li, H.J.; Zhu, X.F. Hirsutanol A, a novel sesquiterpene compound from fungus Chondrostereum sp., induces apoptosis and inhibits tumor growth through mitochondrial-independent ROS production: Hirsutanol A inhibits tumor growth through ROS production. J. Transl. Med. 2013, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Shindo, M.; Sato, Y.; Shishido, K. An ynolate-initiated tandem process giving cyclopentenones: Total synthesis of cucumin E. Tetrahedron Lett. 2002, 43, 5039–5041. [Google Scholar] [CrossRef]
- Mehta, G.; Murthy, A.S.K. The first total synthesis of the novel triquinane natural products pleurotellol and pleurotellic acid. Tetrahedron Lett. 2003, 44, 5243–5246. [Google Scholar] [CrossRef]
- Sakan, F.; Hashimoto, H.; Ichihara, A.; Shirahama, H.; Matsumoto, T. A synthesis of the hirsutane skeleton. Tetrahedron Lett. 1971, 12, 3703–3706. [Google Scholar] [CrossRef]
- Hashimoto, H.; Tsuzuki, K.; Sakan, F.; Shirahama, H.; Matsumoto, T. Total synthesis of dl-hirsutic acid. Tetrahedron Lett. 1974, 15, 3745–3748. [Google Scholar] [CrossRef]
- Kueh, J.S.H.; Mellor, M.; Pattenden, G. Photocyclisations of dicyclopent-1-enyl methanes to tricyclo[6.3.0.02,6]undecanes: A synthesis of the hirsutane carbon skeleton. J. Chem. Soc. Chem. Commun. 1978, 1, 5–6. [Google Scholar] [CrossRef]
- Mehta, G.; Reddy, A.V. Olefin metathesis in polycyclic frames. A total synthesis of hirsutene. J. Chem. Soc. Chem. Commun 1981, 15, 756–757. [Google Scholar] [CrossRef]
- Dawson, B.A.; Ghosh, A.K.; Jurlina, J.L.; Ragauskas, A.J.; Stothers, J.B. A synthesis of hirsutene: A simple route via β-enolization. Can. J. Chem. 1984, 62, 2521–2525. [Google Scholar] [CrossRef]
- Coverdhan, M.; Murthy, A.N.; Reddy, D.S.; Reddy, A.V. A general approach to linearly fused triquinane natural products. Total syntheses of (±)-hirsutene, (±)-coriolin, and (±)-capnellene. J. Am. Chem. Soc. 1986, 108, 3443–3452. [Google Scholar] [CrossRef]
- Iyoda, M.; Kushida, T.; Kitami, S.; Oda, M. An extremely short synthesis of hirsutene. J. Chem. Soc. Chem. Commun. 1986, 1049–1050. [Google Scholar] [CrossRef]
- Cossy, J.; Belotti, D.; Pete, J.P. Photoreductive cyclization: Application to the total synthesis of (±) Hirsutene. Tetrahedron Lett. 1987, 28, 4547–4550. [Google Scholar] [CrossRef]
- Majetich, G.; Defauw, J. Intramolecular additions of allylsilanes in triquinane synthesis. Studies directed toward the total synthesis of (±)-hirsutene. Tetrahedron 1988, 44, 3833–3849. [Google Scholar] [CrossRef]
- Franck-Neumann, M.; Miesch, M.; Lacroix, E.; Metz, B.; Kern, J.M. Access to the Linear Triquinane Skeleton via Bicyclo (2.1. 0) pentane Intermediates. Total Synthesis of the Triquinane Hirsutene. Tetrahedron 1992, 48, 1911–1926. [Google Scholar] [CrossRef]
- Franck-Neumann, M.; Miesch, M.; Lacroix, E. Total synthesis of the natural linear triquinane (±)-hirsutene from a cyclopropene compound. Tetrahedron Lett. 1989, 30, 3529–3532. [Google Scholar] [CrossRef]
- Hong, B.C.; Shr, Y.J.; Wu, J.L.; Gupta, A.K.; Lin, K.J. Novel [6 + 2] cycloaddition of fulvenes with alkenes: A facile synthesis of the anislactone and hirsutane framework. Org. Lett. 2002, 4, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Sternbach, D.D.; Ensinger, C.L. Synthesis of polyquinanes. 3. Total synthesis of (±)-hirsutene: The intramolecular Diels-Alder approach. J. Org. Chem. 1990, 55, 2725–2736. [Google Scholar] [CrossRef]
- Castro, J.; Sorensen, H.; Riera, A.; Morin, C.; Moyano, A.; Greene, A.E. Asymmetric approach to Pauson-Khand bicyclization. Enantioselective formal synthesis of hirsutene. J. Am. Chem. Soc. 1990, 112, 9388–9389. [Google Scholar] [CrossRef]
- Paquette, L.A.; Moriarty, K.J.; Chang, S.C. Sequential annulation in molecular construction. A short, stereocontrolled synthesis of (+)-hirsutene. Israel J. Chem. 1991, 31, 195–198. [Google Scholar] [CrossRef]
- Sarkar, T.K.; Ghosh, S.K.; Subba Rao, P.S.V.; Mamdapur, V.R. Cyclopentanoid allylsilanes in synthesis: A stereoselective synthesis of (+)-hirsutene. Tetrahedron Lett. 1990, 31, 3465–3466. [Google Scholar] [CrossRef]
- Sarkar, T.K.; Ghosh, S.K.; Subba Rao, P.S.V.; Satapathi, T.K. Cyclopentanoid allysilanes in synthesis: Generation via intramolecular ene reaction of activated 1,6-dienes and application to the synthesis of functionalized diquinanes. Tetrahedron Lett. 1990, 31, 3461–3464. [Google Scholar] [CrossRef]
- Sarkar, T.K.; Ghosh, S.K.; Subba Rao, P.S.V.; Satapathi, T.K.; Mamdapur, V.R. Cyclopentanoid allylsilanes in synthesis of di- and triquinanes. A stereoselective synthesis of (±)-hirsutene. Tetrahedron 1992, 48, 6897–6908. [Google Scholar] [CrossRef]
- Mehta, G.; Srikrishna, A. Synthesis of polyquinane natural products: An update. Chem. Rev. 1997, 97, 671–719. [Google Scholar] [CrossRef] [PubMed]
- Ramig, K.; Kuzemko, M.A.; McNamara, K.; Cohen, T. Use of a dilithiomethane equivalent in a novel one-flask [2+1+2] cyclopentannulation reaction: A highly efficient total synthesis of (±)-hirsutene. J. Org. Chem. 1992, 57, 1969–1970. [Google Scholar] [CrossRef]
- Toyota, M.; Nishikawa, Y.; Motoki, K.; Yoshida, N.; Fukumoto, K. Pd2+-promoted cyclization in linear triquinane synthesis total synthesis of (±)-hirsutene. Tetrahedron Lett. 1993, 34, 6099–6102. [Google Scholar] [CrossRef]
- Rawal, V.H.; Fabre, A.; Iwasa, S. Photocyclization-fragmentation route to linear triquinanes: Stereocontrolled synthesis of (±)-endo-hirsutene. Tetrahedron Lett. 1995, 36, 6851–6854. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.X. Rhodium-catalyzed [5 + 2 +1] cycloaddition of ene–vinylcyclopropanes and CO: Reaction design, development, application in natural product synthesis, and inspiration for developing new reactions for synthesis of eight-membered carbocycles. Acc. Chem. Res. 2015, 48, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Liu, J.; Paquette, L.A. Three-component coupling via the squarate ester cascade as a concise route to the bioactive triquinane sesquiterpene hypnophilin. Org. Lett. 2002, 4, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Paquette, L.A.; Geng, F. Applications of the squarate ester cascade to the expeditious synthesis of hypnophilin, coriolin, and ceratopicanol. J. Am. Chem. Soc. 2002, 124, 9199–9203. [Google Scholar] [CrossRef] [PubMed]
- Bon, D.J.Y.D.; Banwell, M.G.; Ward, J.S.; Willis, A.C. From toluene to triquinanes: Formal total syntheses of the sesquiterpenoid natural products (−)-hypnophilin and (−)-coriolin. Tetrahedron 2013, 69, 1363–1368. [Google Scholar] [CrossRef]
- Bon, D.J.Y.D.; Banwell, M.G.; Willis, A.C. A chemoenzymatic total synthesis of the hirsutene-type sesquiterpene (+)-connatusin B from toluene. Tetrahedron 2010, 66, 7807–7814. [Google Scholar] [CrossRef]
- Bon, D.J.Y.D.; Banwell, M.G.; Cade, I.A.; Willis, A.C. The total synthesis of (−)-connatusin A, a hirsutane-type sesquiterpene isolated from the fungus Lentinus connatus BCC8996. Tetrahedron 2011, 67, 8348–8352. [Google Scholar] [CrossRef]
- Singh, V.; Prathap, S.; Porinehu, M. A novel, stereospecific total synthesis of (±)-∆9(12)-capnellene from p-Cresol. Tetrahedron Lett. 1997, 38, 2911–2914. [Google Scholar] [CrossRef]
- Stevens, K.E.; Paquette, L.A. Stereocontrolled total synthesis of (±)-∆9(12)-capnellene. Tetrahedron Lett. 1981, 22, 4393–4396. [Google Scholar] [CrossRef]
- Stille, J.R.; Grubbs, R.H. Synthesis of (±)-∆9(12)-capnellene using titanium reagents. J. Am. Chem. Soc. 1986, 108, 855–856. [Google Scholar] [CrossRef]
- Stille, J.R.; Santarsiero, B.D.; Grubbs, R.H. Rearrangement of bicyclo[2.2.1]heptane ring systems by Titanocene alkylidene complexes to bicyclo[3.2.0]heptane enol ethers. Total synthesis of (±)-∆9(12)-capnellene. J. Org. Chem. 1990, 55, 843–862. [Google Scholar] [CrossRef]
- Augusto, G.; Giovanni, F.; Paolo, B. Bicyclo[3.3.1]nonane approach to triquinanes. Formal synthesis of (±)-∆9(12)-capnellen and (±)-∆9(12)-capnellen-8β-10α-diol. Tetrahedron 1992, 48, 4459–4464. [Google Scholar]
- Balme, G.; Bouyssi, D. Total synthesis of the triquinane marine sesquiterpene (±)-∆9(12)-capnellene using a palladium-catalyzed bis-cyclization step. Tetrahedron 1994, 50, 403–414. [Google Scholar] [CrossRef]
- Singh, V.; Prathap, S.; Porinchu, M. Aromatics to triquinanes: P-cresol to (±)-∆9(12)-capnellene. J. Org. Chem. 1998, 63, 4011–4017. [Google Scholar] [CrossRef]
- Nguyen, N.N.M.; Leclere, M.; Stogaitis, N.; Fallis, A.G. Triquinanes: A “One-Pot” IMDA-Tandem metathesis cascade strategy: Ring-Closing Metathesis (RCM) dominates norbornene ROM! Org. Lett. 2010, 12, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.S.; Chou, Y.Y.; Tung, Y.S.; Liao, C.C. Photochemistry of tricyclo[5.2.2.02,6]undeca-4,10-dien-8-ones: An efficient general route to substituted linear triquinanes from 2-methoxyphenols. Total synthesis of (±)-∆9(12)-capnellene. Chem.-Eur. J. 2010, 16, 3121–3131. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.J.; Li, W.D.Z. Formal homoiodo allylsilane annulations: Dual total syntheses of (±)-hirsutene and (±)-capnellene. J. Org. Chem. 2013, 78, 7112–7120. [Google Scholar] [CrossRef] [PubMed]
- Little, R.D.; Carroll, G.L.; Petersen, J.L. Total synthesis of the marine natural product ∆9(12)-capnellene. Reversal of regiochemistry in the intramolecular 1,3-diyl trapping reaction. J. Am. Chem. Soc. 1983, 105, 928–932. [Google Scholar] [CrossRef]
- Paquette, L.A.; Stevens, K. Stereocontrolled total synthesis of the triquinane marine sesquiterpene ∆9(12)-capnellene. Can J. Chem. 1984, 62, 2415–2419. [Google Scholar] [CrossRef]
- Crisp, G.T.; Scott, W.J.; Stille, J.K. Palladium-catalyzed carbonylative coupling of vinyl triflates with organostannanes. A total synthesis of (±)-∆9(12)-capnellene. J. Am. Chem. Soc. 1984, 106, 7500–7506. [Google Scholar] [CrossRef]
- Wang, Y.; Mukherjee, D.; Birney, D.; Houk, K.N. Synthesis and reactions of ester-substituted fulvenes. A new route to ∆9(12)-capnellene. J. Org. Chem. 1990, 55, 4504–4506. [Google Scholar] [CrossRef]
- Samajdar, S.; Patra, D.; Ghosh, S. Stereocontrolled approach to highly substituted cyclopentanones. Application in a formal synthesis of ∆9(12)-capnellene. Tetrahedron 1998, 54, 1789–1800. [Google Scholar] [CrossRef]
- Lemiere, G.; Gandon, V.; Cariou, K.; Hours, A.; Fukuyama, T.; Dhimane, A.L.; Fensterbank, L.; Malacria, M. Generation and trapping of cyclopentenylidene gold species: Four pathways to polycyclic compounds. J. Am. Chem. Soc. 2009, 131, 2993–3006. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, H.R.; Nanjundiah, B.S.; Shah, V.G.; Kulkarni, D.G.; Ahuja, J.R. Synthesis of naturally occurring (+)-∆9(12)-capnellene and its antipode: An application of the photo-induced vinylcyclopropane-cyclopentene rearrangement. Tetrahedron Lett. 1991, 1991. 32, 1107–1108. [Google Scholar] [CrossRef]
- Sonawane, H.R.; Naik, V.G.; Bellur, N.S.; Shah, V.G.; Purohit, P.C.; Kumar, M.U.; Kulltarni, D.G.; Ahuja, J.R. Photoinduced vinylcyclopropane-cyclopentene rearrangement: A methodology for chiral bicyclo[3.2.0]heptenes. Formal syntheses of (±)-grandisol and naturally occurring (+)-∆9(12)-capnellene antipode. Tetrahedron 1991, 47, 8259–8276. [Google Scholar] [CrossRef]
- Asaoka, M.; Obuchi, K.; Takei, H. An Enantioselective route to (−)-∆9(12)-capnellene employing silyl group directed stereo control. Tetrahedron 1994, 50, 655–660. [Google Scholar] [CrossRef]
- Tanaka, K.; Ogasawara, K. Stereocontrolled synthesis of natural (−)-∆9(12)-capnellene from a (−)-oxodicyclopentadiene. Chem. Commun. 1996, 15, 1839–1840. [Google Scholar] [CrossRef]
- Ohshima, T.; Kagechika, K.; Adachi, M.; Sodeoka, M.; Shibasaki, M. Asymmetric heck reaction-carbanion capture process. Catalytic asymmetric total synthesis of (−)-∆9(12)-capnellene. J. Am. Chem. Soc. 1996, 118, 7108–7116. [Google Scholar] [CrossRef]
- Meyers, A.I.; Bienz, S. Asymmetric total synthesis of (+)-∆9(12)-capnellene. J. Org. Chem. 1990, 55, 791–798. [Google Scholar] [CrossRef]
- Shibasaki, M.; Mase, T.; Ikegami, S. The first total syntheses of ∆9(12)-capnellene-8β,10α-diol and ∆9(12)-capnellene-3β,8β,10α-triol. J. Am. Chem. Soc. 1986, 108, 2090–2091. [Google Scholar] [CrossRef]
- Kagechika, K.; Shibasaki, M. Asymmetric heck reaction: A catalytic asymmetric synthesis of the key intermediate for ∆9(12)-capnellene-3β,8β,10α-triol and ∆9(12)-capnellene-3β,8β,10α,14-tetrol. J. Org. Chem. 1991, 56, 4093–4094. [Google Scholar] [CrossRef]
- Baralotto, C.; Chanon, M.; Julliard, M. Total synthesis of the tricyclic sesquiterpene (±)-ceratopicanol. An illustration of the holosynthon concept. J. Org. Chem. 1996, 61, 3576–3577. [Google Scholar] [CrossRef] [PubMed]
- Clive, D.L.J.; Magnuson, S.R.; Manning, H.W.; Mayhew, D.L. Cyclopentannulation by an iterative process of sequential claisen rearrangement and enyne radical closure: Routes to triquinane and propellane systems and use in the synthesis of (±)-ceratopicanol. J. Org. Chem. 1996, 61, 2095–2108. [Google Scholar] [CrossRef]
- Srikrishna, A.; Dethe, D.H. Enantiospecific first total synthesis and assignment of absolute configuration of the sesquiterpene (−)-cucumin H. Org. Lett. 2003, 5, 2295–2298. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef] [PubMed]


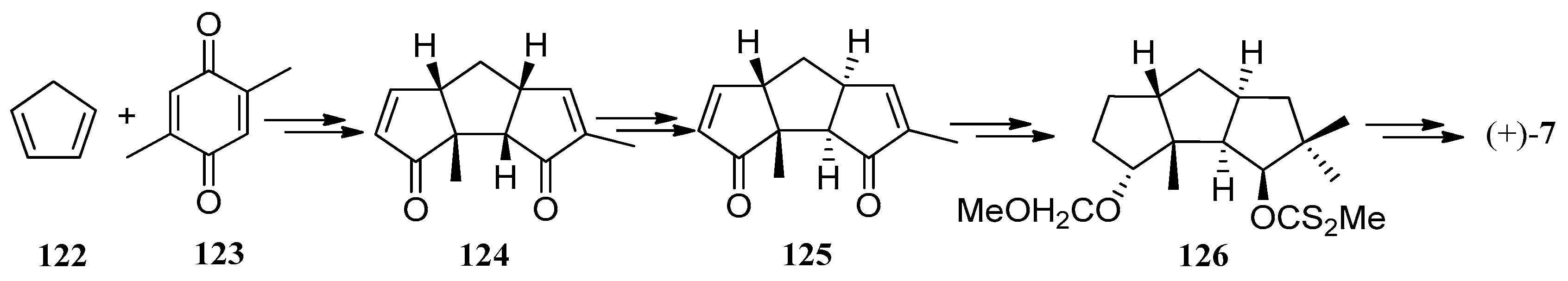
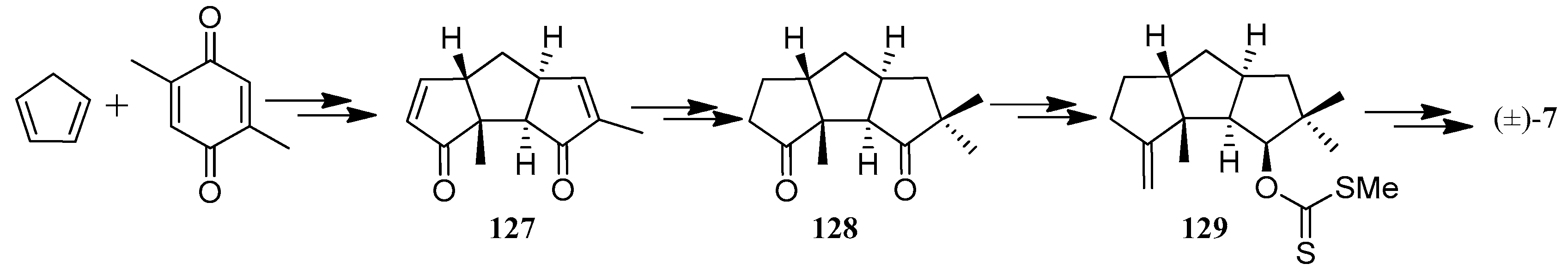
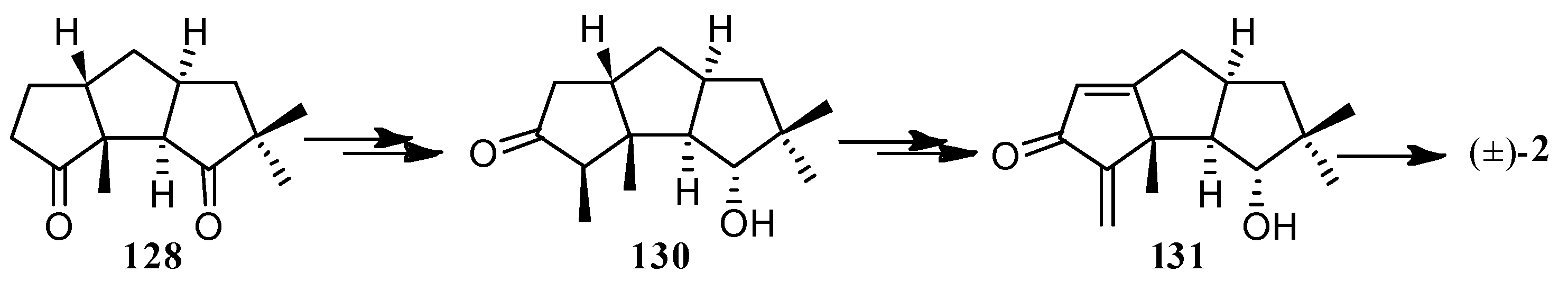
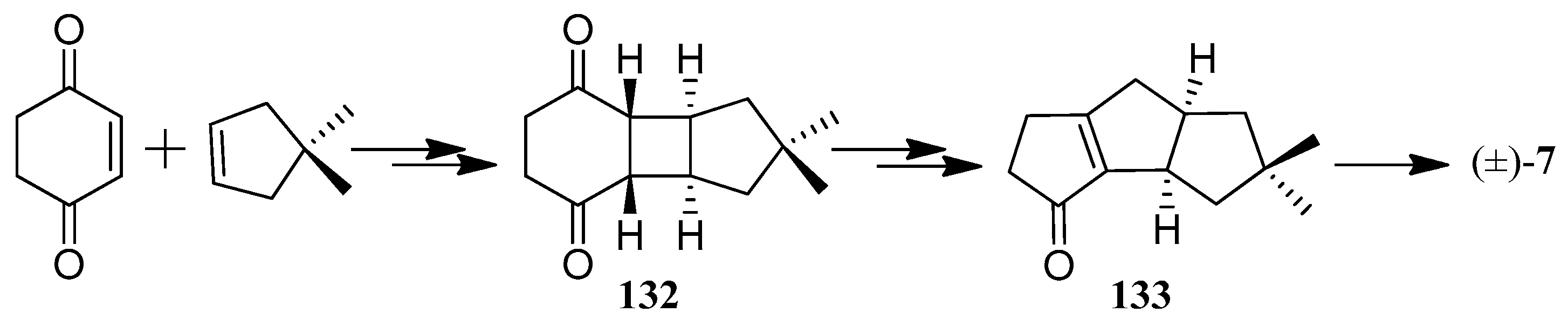
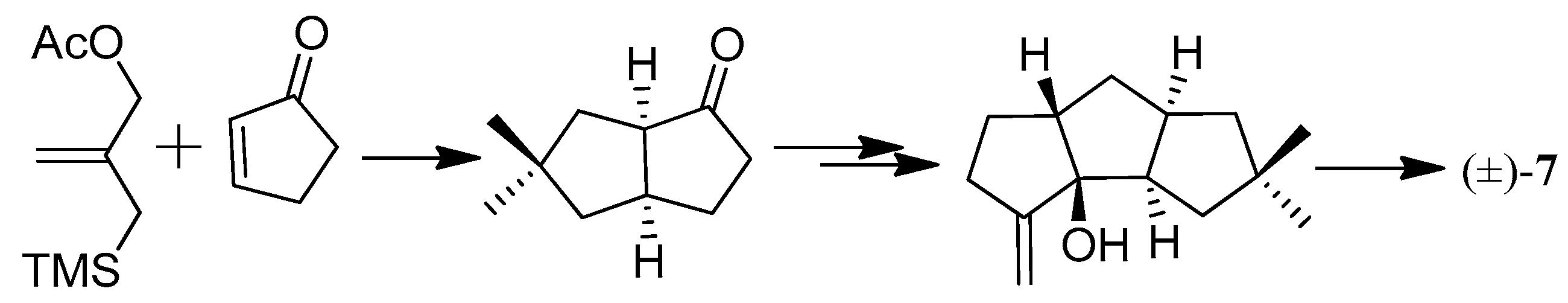
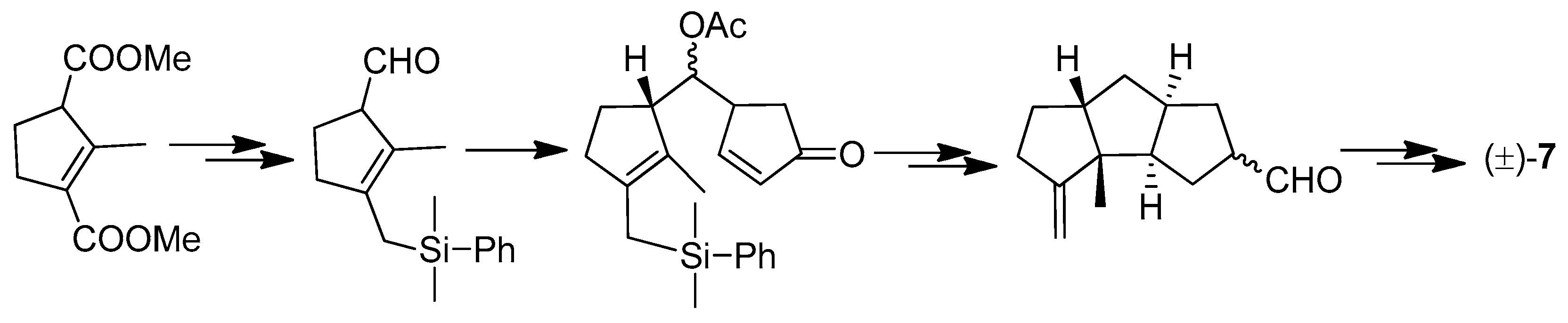
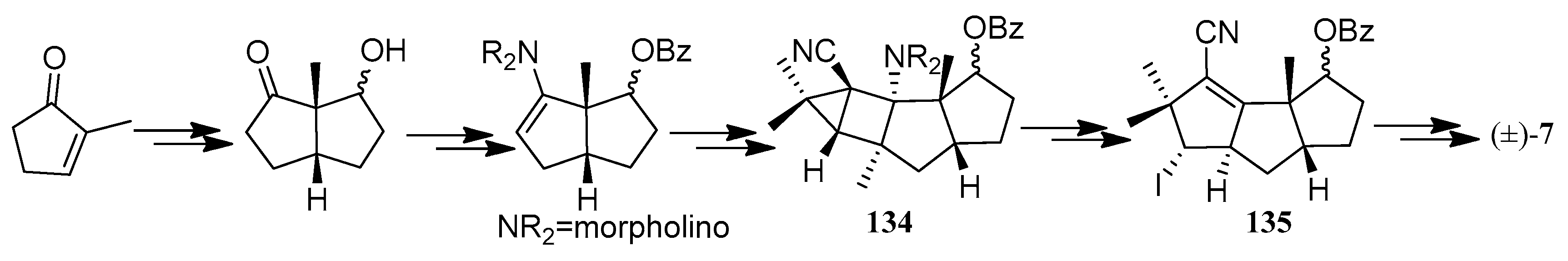

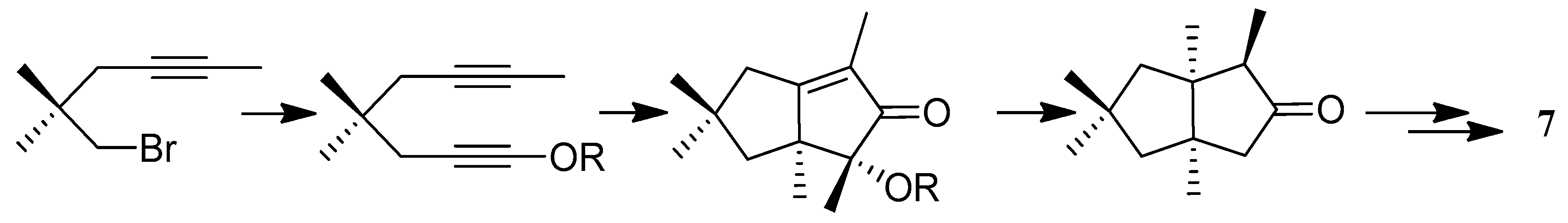

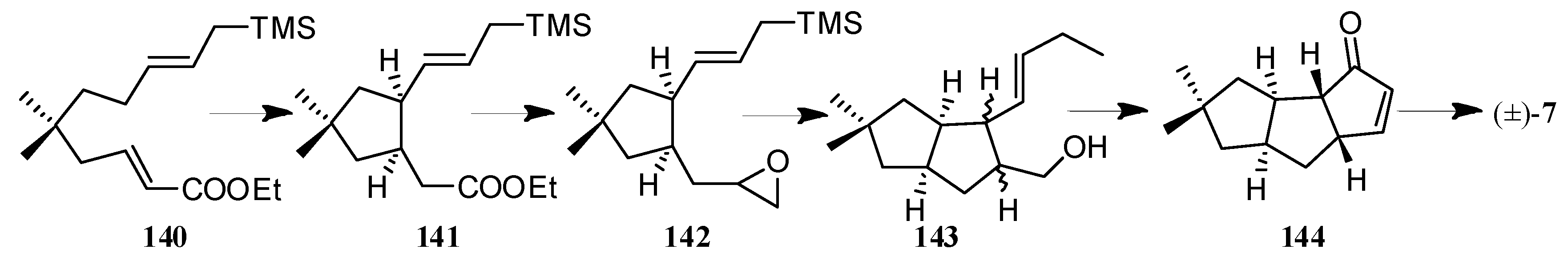
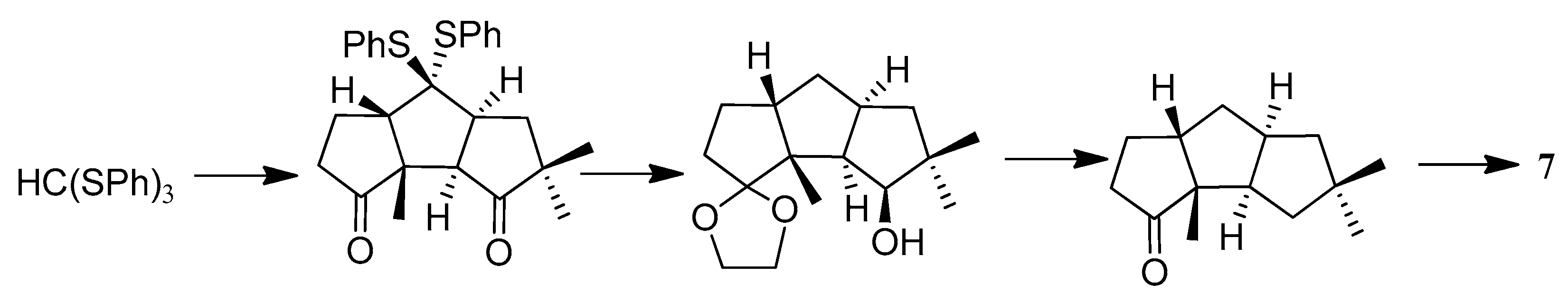
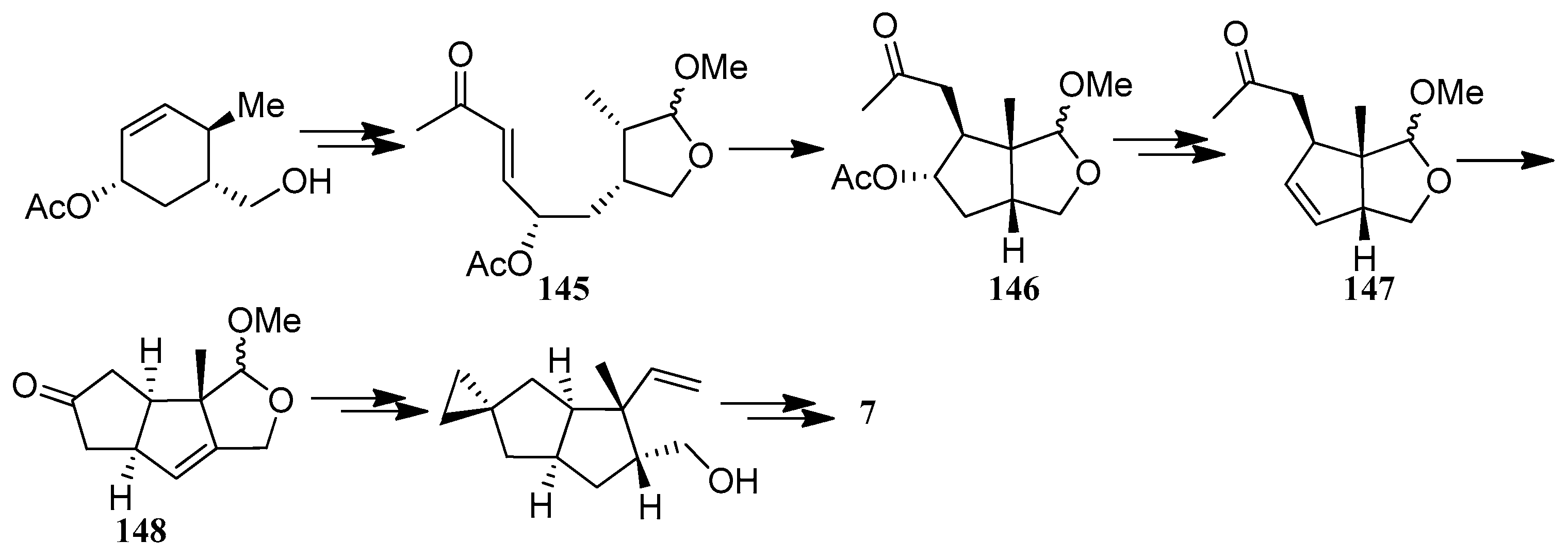

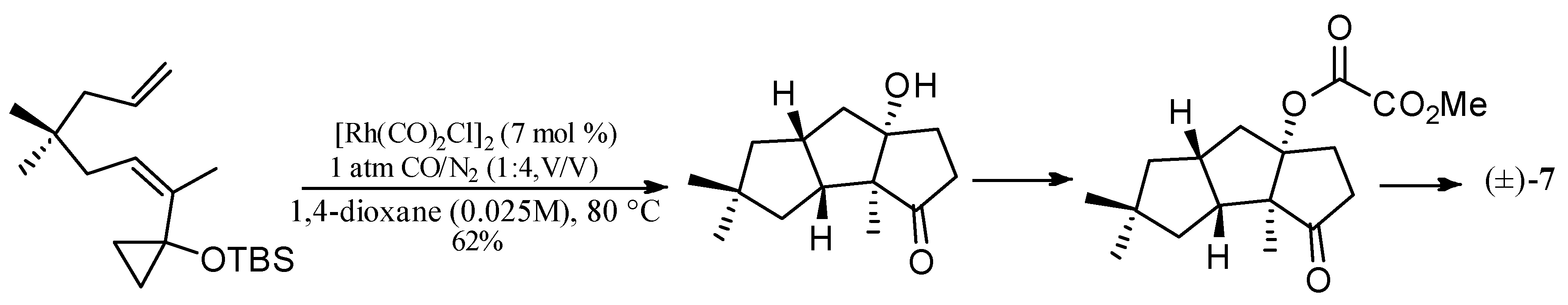
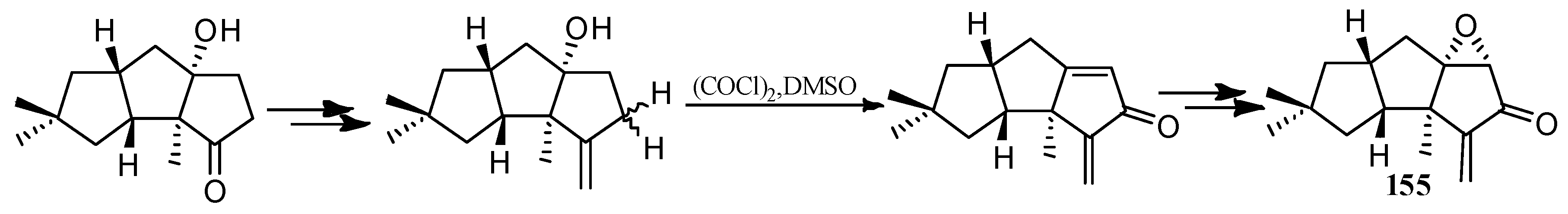





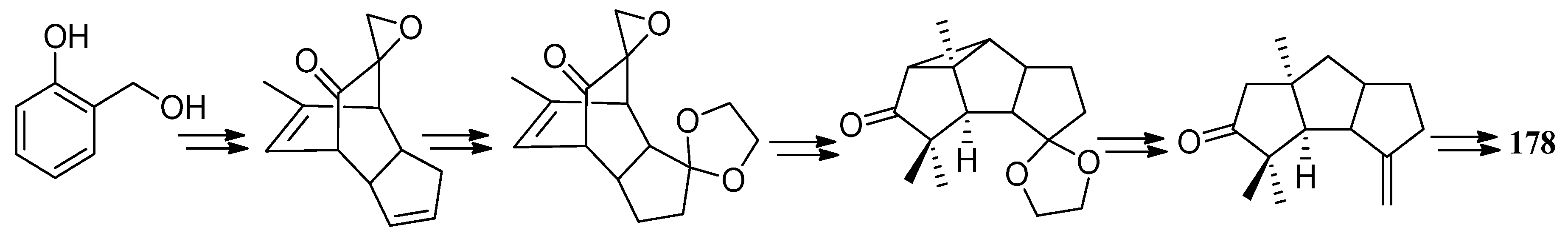



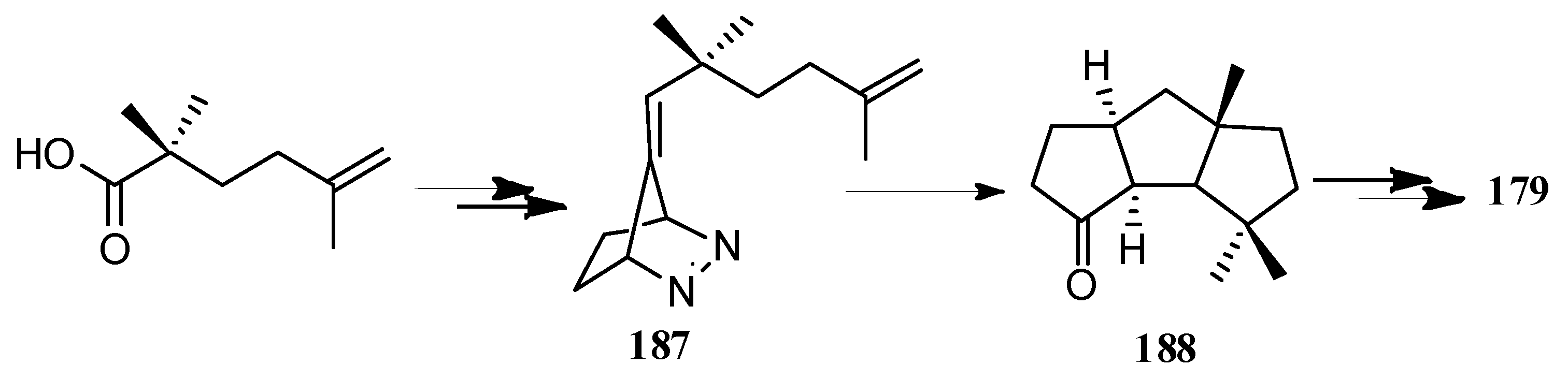
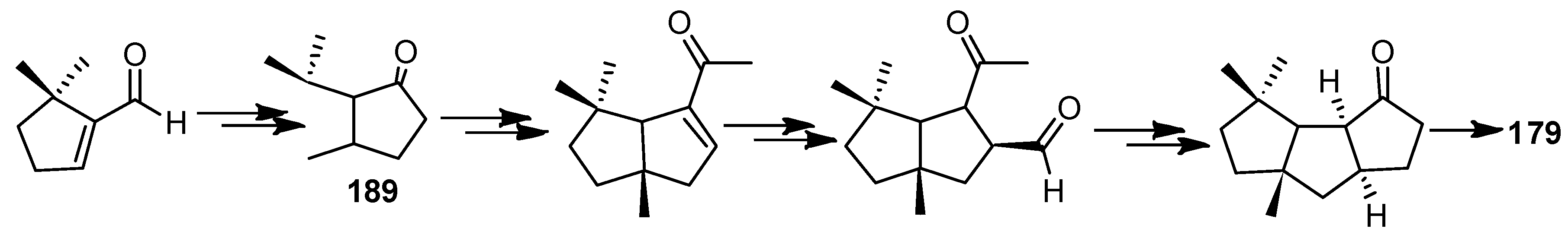
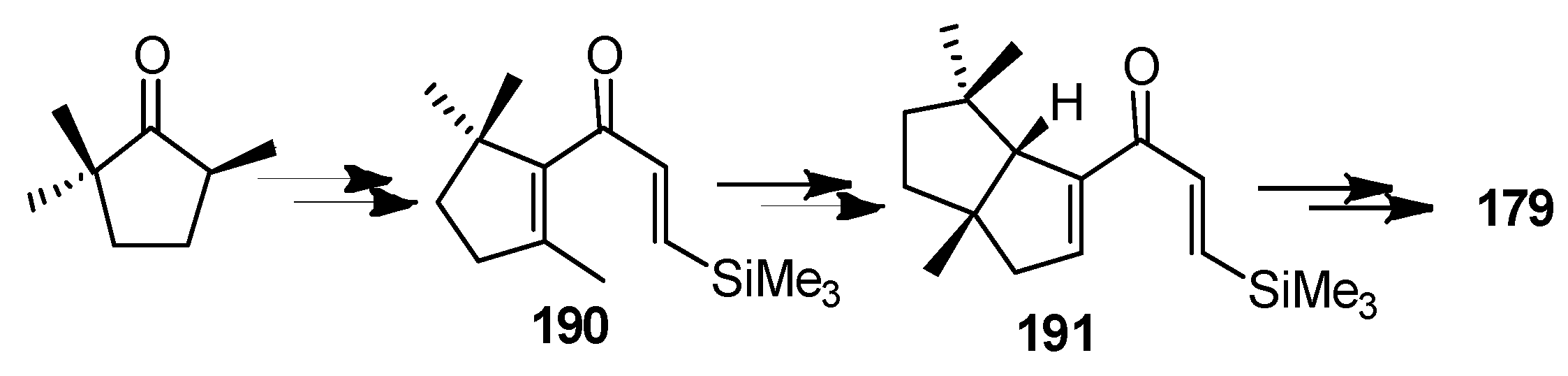


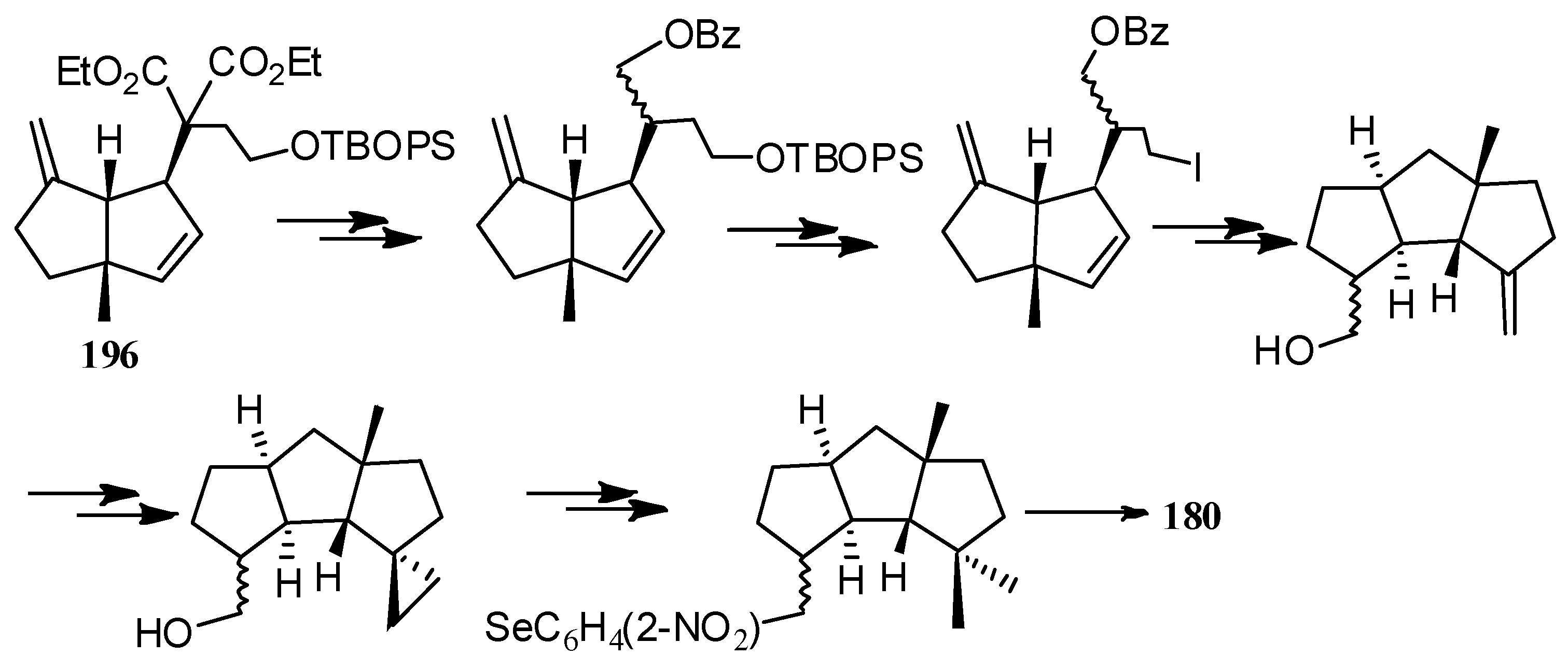
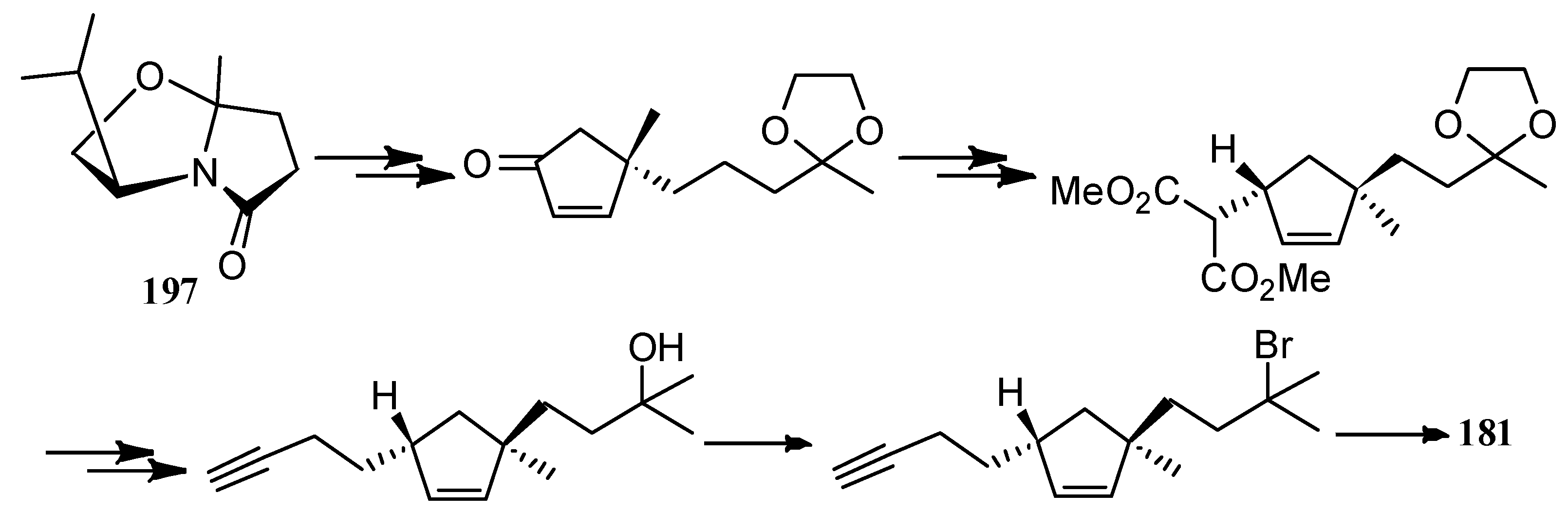

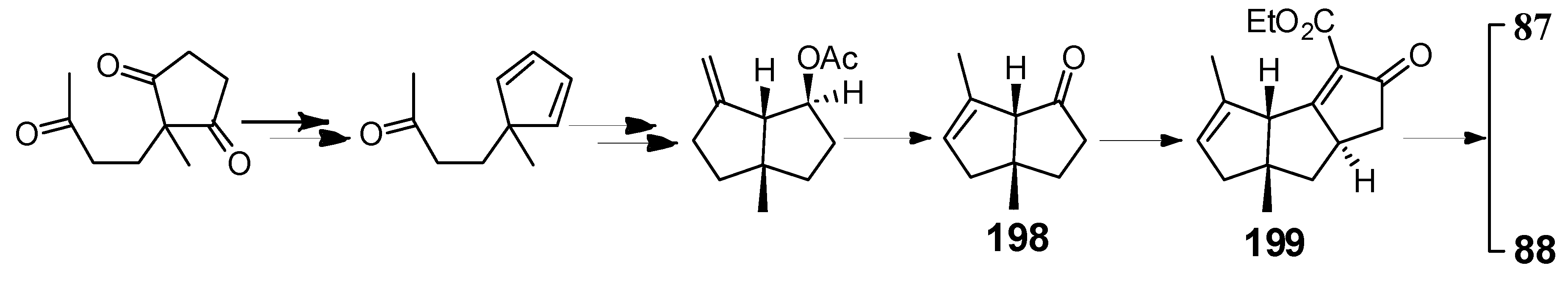
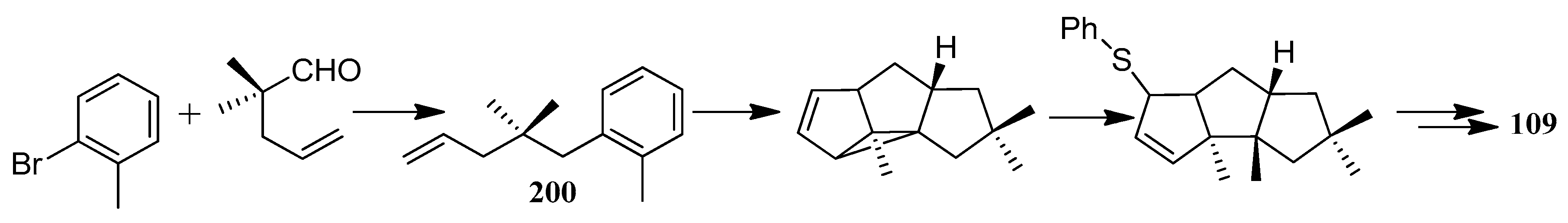
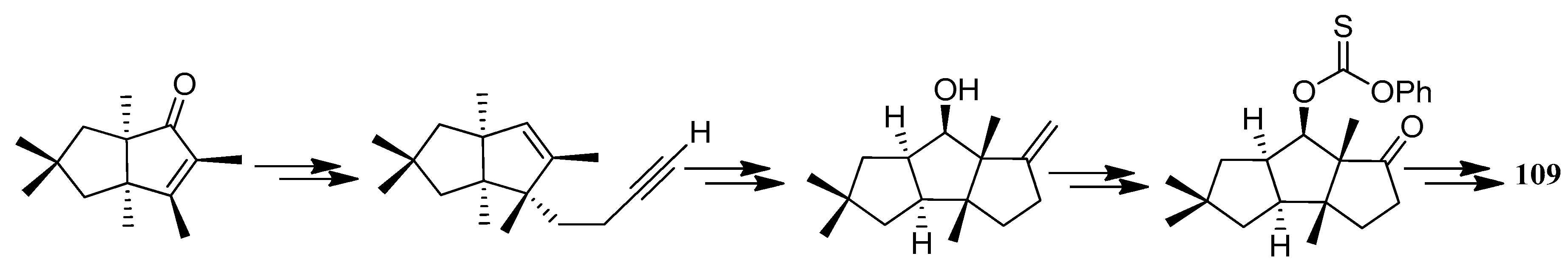
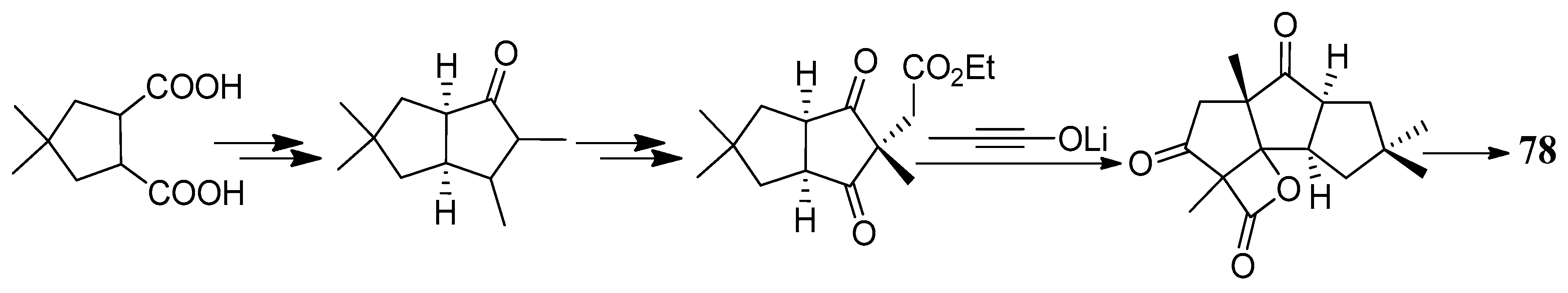
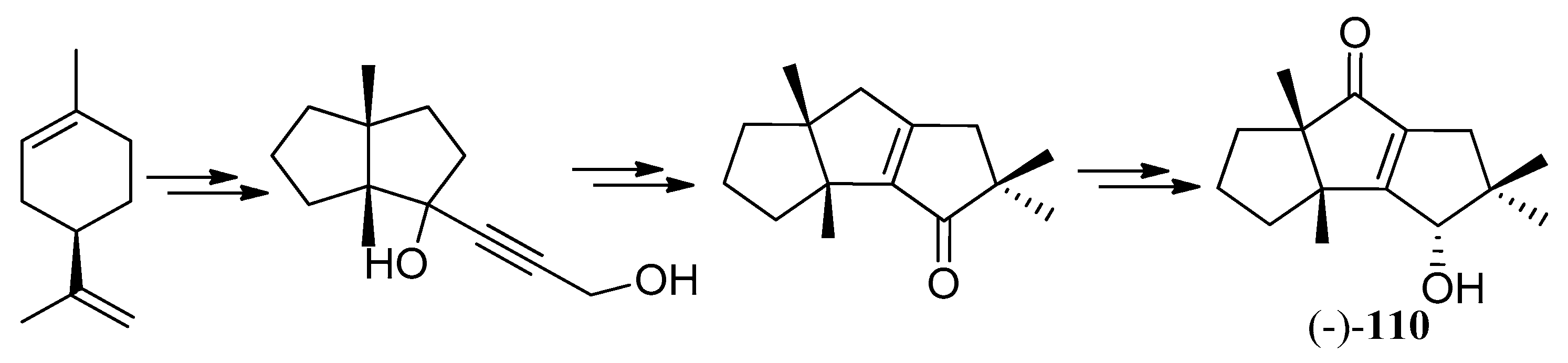
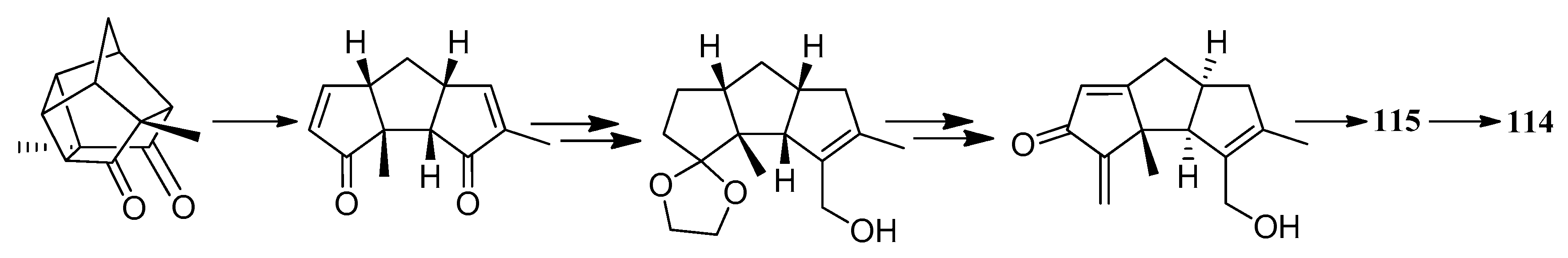
| Cell Lines | Cytotocicity |
|---|---|
| CNE1 | 15 IC50 = 34.13 μM, 22 IC50 = 10.08 μM, 48 IC50 = 33.55 μM, 49 IC50 = 42.00 μM, 84 IC50 = 1.32 μM, 85 IC50 = 17.66 μM |
| CNE2 | 15 IC50 = 24.87 μM, 22 IC50 = 12.72 μM, 43 IC50 = 4.95μM, 48 IC50 = 22.50 μM, 49 IC50 = 44.08 μM, 84 IC50 = 0.56 μM, 85 IC50 = 12.03 μM |
| HONE1 | 22 IC50 = 17.40 μM, 48 IC50 = 34.60 μM, 49 IC50 = 46.11 μM, 85 IC50 = 22.06 μM |
| SUNE1 | 22 IC50 = 3.50 μM, 48 IC50 = 30.40 μM, 49 IC50 = 58.83 μM, 85 IC50 = 16.44 μM |
| A549 | 22 IC50 = 11.96 μM, 43 IC50 = 2.45μM, 48 IC50 = 29.67 μM, 49 IC50 = 49.58 μM, 85 IC50 = 23.51 μM |
| GLC82 | 22 IC50 = 10.11 μM, 48 IC50 = 37.47 μM, 49 IC50 = 55.90 μM, 85 IC50 = 18.08 μM |
| HL7702 | 22 IC50 = 9.76 μM, 48 IC50 = 34.26 μM, 49 IC50 = 56.40 μM, 85 IC50 = 22.14 μM |
| K562 | 52 IC50 = 12.97 μg/mL, 53 IC50 = 16.29 μg/mL, 54 IC50 = 6.93 μg/mL, 55 IC50 = 30.52 μg/mL, 87 IC50 = 0.7 μM, 96 IC50 = 4.6 μM, 97 IC50 = 24 μM, 98 GI50 = 126.9 ± 3.0 μm/L, 99 GI50 = 126.9 ± 2.0 μm/L, 100 GI50 = 142.0 ± 4.7 μm/L, 101 GI50 = 142.0 ± 4.7 μm/L, Doxorubicin GI50 = 1.0 ± 0.6 μm/L |
| HCT116 | 52 IC50 = 10.74 μg/mL, 53 IC50 = 16.35 μg/mL, 54 IC50 = 25.43 μg/mL, 55 IC50 = 24.17 μg/mL |
| HL-60 | 87 IC50 = 51 μM, 96 IC50 = 68 μM, 97 IC50 = 713 μM |
| G402 | 87 IC50 = 42–51 μM, 96 IC50 > 4500 μM, 97 IC50 = 52 μM |
| MCF-7 | 87 IC50 = 93 μM, 96 IC50 > 4500 μM, 97 IC50 = 1029 μM |
| HT115 | 87 IC50 = 63 μM, 96 IC50 > 4500 μM |
| A2780 | 87 IC50 = 9.7 μM, 96 IC50 = 6.6 μM, 97 IC50 = 32 μM, |
| HeLa | 98 CC50 = 126.9 ± 1.8 μm/L, 99 CC50 = 126.9 ± 2.5μm/L, 100 CC50 = 142.0 ± 2.7 μm/L, 101 CC50 = 125.0 ± 2.0 μm/L, Doxorubicin CC50 = 2.0 ± 0.8 μm/L |
| L-929 | 17 IC50 = 2.4 μg/mL, 18 IC50 = 0.9 μg/mL, 98 GI50 = 126.9 ± 2.7 μm/L, 99 GI50 = 126.9 ± 2.9 μm/L, 100 GI50 = 126.4 ± 3.0 μm/L, 101 GI50 = 99.1 ± 1.8 μm/L, Doxorubicin GI50 = 1.2 ± 0.6 μm/L |
| T-47D | 3 IC50 = 0.7 μM |
| SNB-75 | 3 IC50 = 0.5 μM |
| HepG2 | 40 IC50 = 24.41 ± 1.86 μM |
| LoVo | 43 IC50 = 5.47 μM |
| Test Organisms | 17 | 18 |
|---|---|---|
| Bacillus cereus DSM 318 | 2–5 | >100 |
| Staphylococcus aureus ATCC 13709 | 10–25 | >100 |
| Escherichia coli ATCC 9637 | >100 | >100 |
| Salmonella gallinarum ATCC 9184 | 50–100 | 50–100 |
| Mycobacterium smegmatis ATCC 607 | 25–50 | 50–100 |
| Candida albicans ATCC 10231 | 50–100 | >100 |
| Candida tropicalis DSM 1346 | >100 | >100 |
| Rhodotorula glutinis DSM 70398 | >100 | >100 |
| Aspergillus niger (spores) DSM 737 | 1–2 | 25–50 |
| Aspergillus flavus (spores) BD 27 | 2–5 | 25–50 |
| Mucor rouxii (spores) DSM 1691 | 2–5 | 25–50 |
| Compounds (20 μM) | NO (μM) |
|---|---|
| 22 | 8 ± 2 |
| 43 | 4 ± 1 |
| 44 | 4 ± 1 |
| 69 | 8 ± 2 |
| 70 | 10 ± 2 |
| 76 | 4 ± 1 |
| Compounds | Viability (%) | |||
|---|---|---|---|---|
| 2 μM | 5 μM | 10 μM | 20 μM | |
| 22 | 94 ± 1 | 87 ± 3 | 77 ± 8 | 39 ± 5 |
| 43 | nt * | nt | 22 ± 3 | 12 ± 1 |
| 44 | nt | nt | 23 ± 7 | 11 ± 1 |
| 69 | 93 ± 3 | 82 ± 5 | 73 ± 4 | 67 ± 3 |
| 70 | 95 ± 5 | 95 ± 4 | 89 ± 9 | 69 ± 5 |
| 76 | 47 ± 6 | 30 ± 5 | 11 ± 2 | 11 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Lan, W.-J.; Li, H.-J.; Chen, L.-P. Linear Triquinane Sesquiterpenoids: Their Isolation, Structures, Biological Activities, and Chemical Synthesis. Molecules 2018, 23, 2095. https://doi.org/10.3390/molecules23092095
Qiu Y, Lan W-J, Li H-J, Chen L-P. Linear Triquinane Sesquiterpenoids: Their Isolation, Structures, Biological Activities, and Chemical Synthesis. Molecules. 2018; 23(9):2095. https://doi.org/10.3390/molecules23092095
Chicago/Turabian StyleQiu, Yi, Wen-Jian Lan, Hou-Jin Li, and Liu-Ping Chen. 2018. "Linear Triquinane Sesquiterpenoids: Their Isolation, Structures, Biological Activities, and Chemical Synthesis" Molecules 23, no. 9: 2095. https://doi.org/10.3390/molecules23092095
APA StyleQiu, Y., Lan, W.-J., Li, H.-J., & Chen, L.-P. (2018). Linear Triquinane Sesquiterpenoids: Their Isolation, Structures, Biological Activities, and Chemical Synthesis. Molecules, 23(9), 2095. https://doi.org/10.3390/molecules23092095






