Antioxidant Activity as Biomarker of Honey Variety
Abstract
1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity of Tested Honeys
2.2. Statistical Analysis
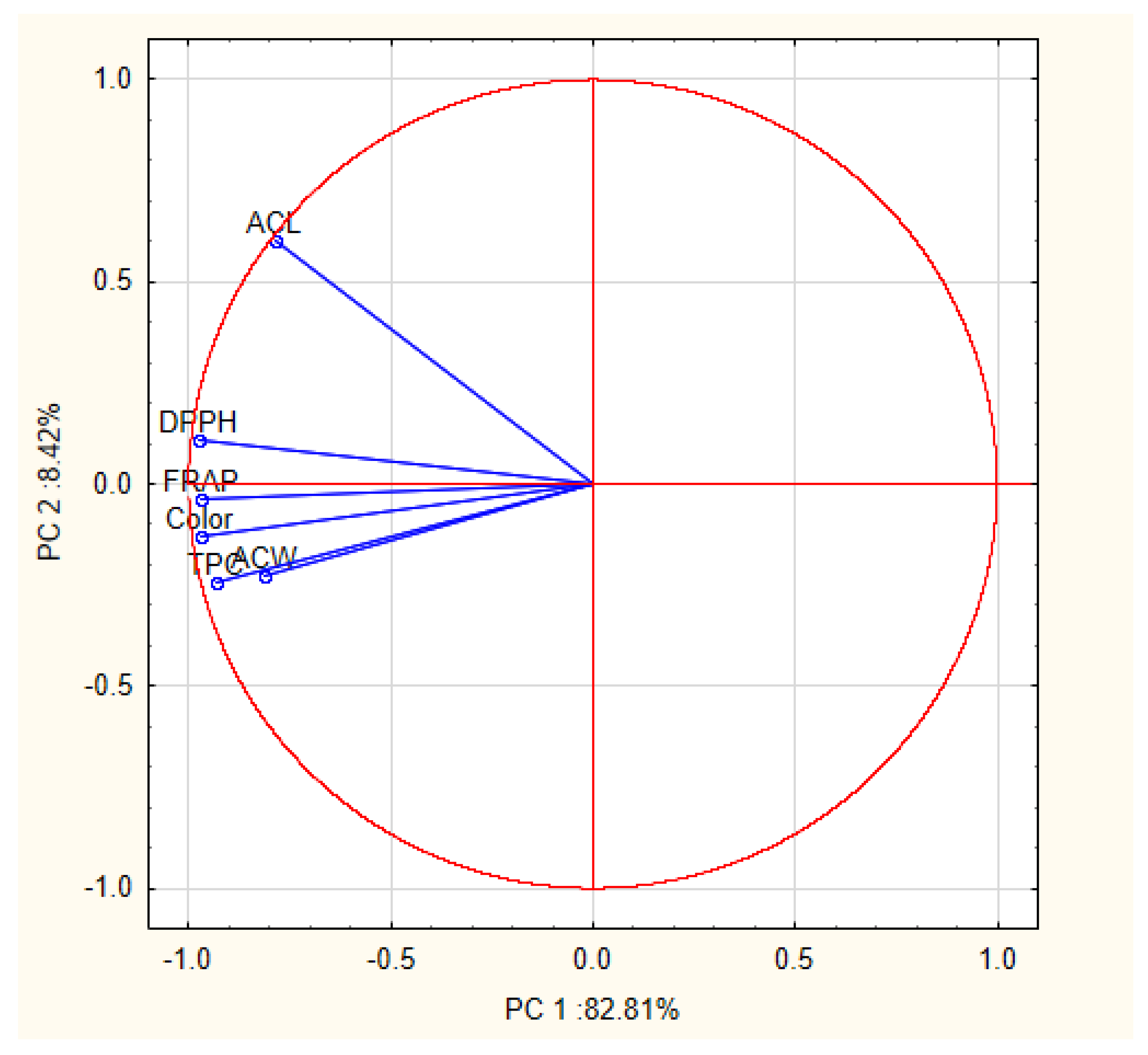
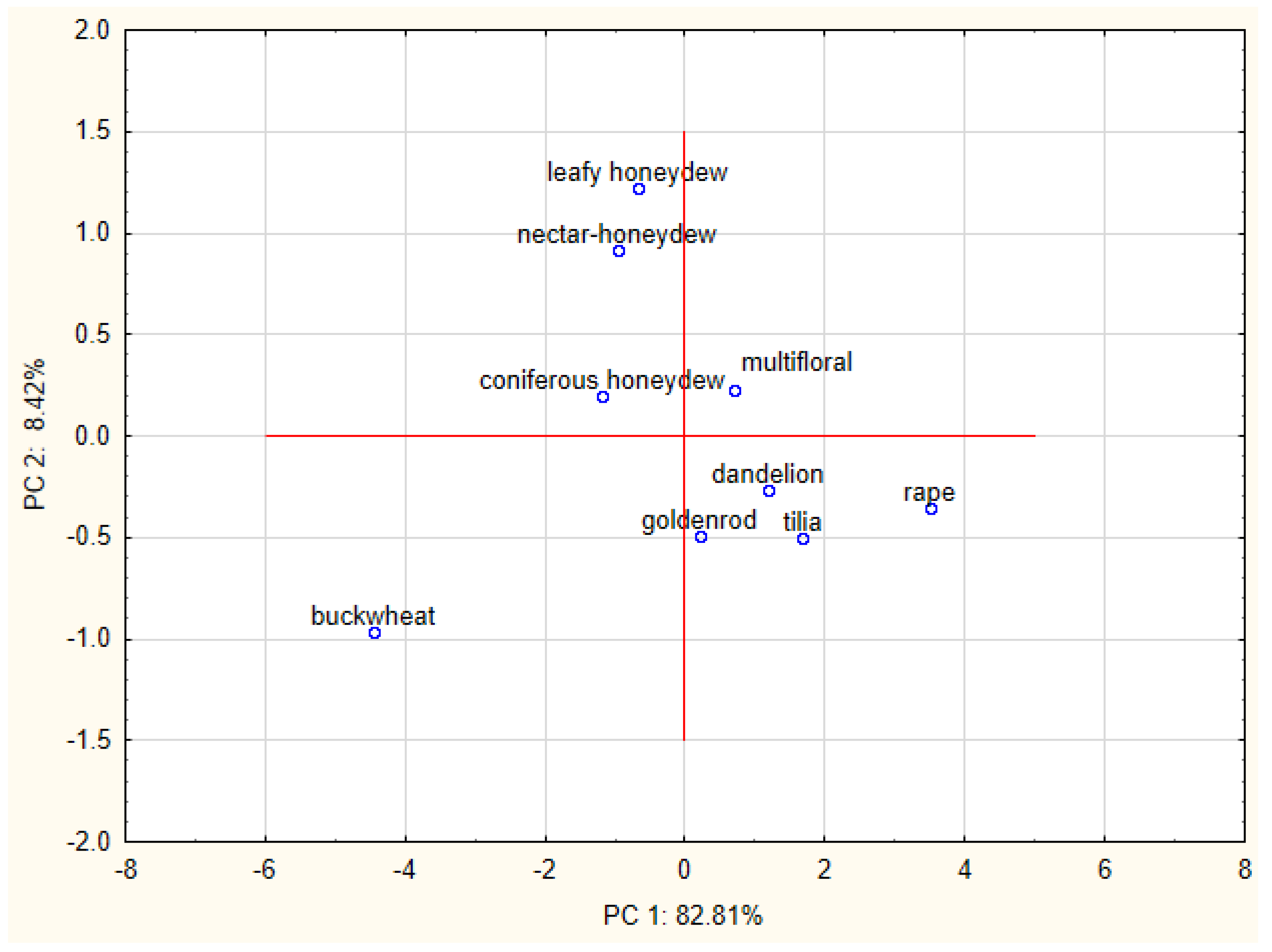
2.2.1. Principal Components Analysis (PCA)
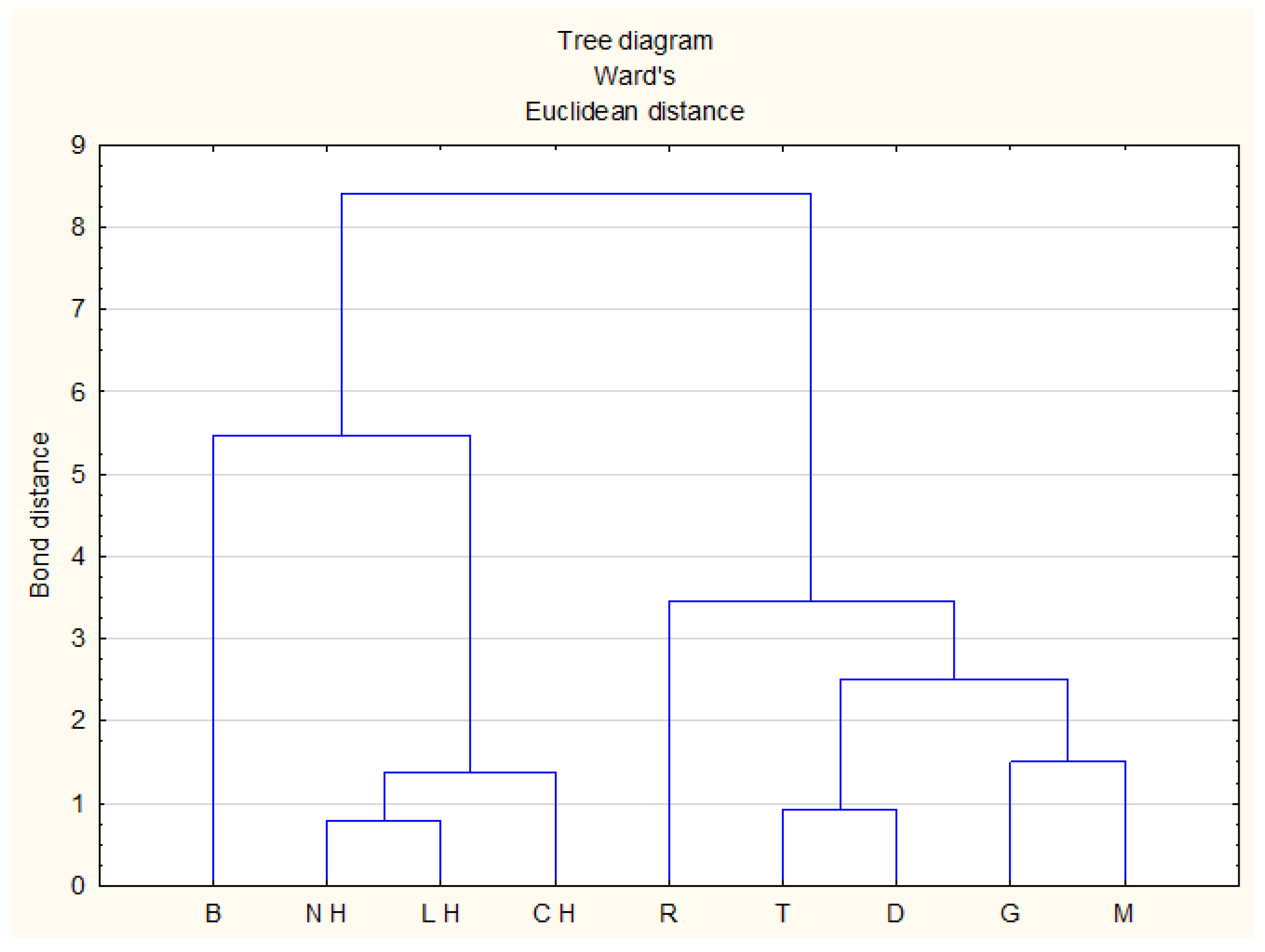
2.2.2. Cluster Analysis
3. Materials and Methods
3.1. Samples
3.2. Methods
3.2.1. Antioxidant Capacity PCL Assay
3.2.2. DPPH Assay (Radical Scavenging Activity)
3.2.3. FRAP Assay (Total Antioxidant Activity)
3.2.4. Total Phenolic Compounds (TPC)
3.2.5. Color Intensity
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramanauskiene, K.; Stelmakiene, A.; Briedis, V.; Ivanauskas, L.; Jakštas, V. The quantitative analysis of biologically active compounds in Lithuanian honey. Food Chem. 2012, 132, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, J.; Pietrzak, M.; Pomianowski, J.; Wieczorek, Z. Honey as a source of bioactive compounds. Pol. J. Food Nutr. Sci. 2014, 29, 275–285. [Google Scholar]
- Olaitan, P.B.; Oe, A.; Io, O. Honey: A reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007, 7, 159–165. [Google Scholar] [PubMed]
- Aggad, H.; Guemour, D. Honey Antibacterial Activity. Med. Arom. Plants 2014, 3, 152. [Google Scholar] [CrossRef]
- Kwakman, P.H.; Zaat, S.A. Antibacterial components of honey. IUBMB Life 2012, 64, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, N.; Wang, X.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Maruška, A.; Kornyšova, O. Quantitative and qualitative determination of phenolic compounds in honey. Cheminė Technologija 2009, 3, 74–80. [Google Scholar]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Popek, S. A procedure to identify a honey type. Food Chem. 2002, 79, 401–406. [Google Scholar] [CrossRef]
- Terrab, A.; Diez, M.J.; Heredia, F.J. Characterization of Moroccan unifloral honeys by their physicochemical characteristics. Food Chem. 2002, 79, 373–379. [Google Scholar] [CrossRef]
- Devilliers, J.; Morlot, M.; Pham-Dele`Gue, M.H.; Dore, J.C. Classification of monofloral honeys based on their quality control data. Food Chem. 2004, 86, 305–312. [Google Scholar] [CrossRef]
- Khalafi, R.; Goli, S.A.H.; Behjatian, M. Characterization and classification of several monofloral iranian honeys based on physicochemical properties and antioxidant activity. Int. J. Food Prop. 2016, 19, 1065–1079. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Oroian, M.; Amariei, S.; Leahu, A.; Gutt, G. Multi-element composition of honey as a suitable tool for its authenticity analysis. Pol. J. Food Nutr. Sci. 2015, 65, 93–100. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Makowicz, E.; Stanek, N. Chromatographic fingerprint, antioxidant activity, and colour characteristic of polish goldenrod (Solidago virgaurea L.) honey and flower. Eur. Food Res. Technol. 2018, 244, 1169–1184. [Google Scholar] [CrossRef]
- Tsiapara, A.; Jaakkola, M.; Chinou, I.; Graikou, K.; Tolonen, T.; Virtanen, V.; Moutsatsou, P. Bioactivity of greek honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and endometrial cancer (Ishikawa) cells: Profile analysis of extracts. Food Chem. 2009, 116, 702–708. [Google Scholar] [CrossRef]
- Elbanna, K.; Attalla, K.; Elbadry, M.; Abdeltawab, A.; Gamal-Eldin, H.; Ramadan, M.F. Impact of floral sources and processing on the antimicrobial activities of different unifloral honeys. Asian Pacific J. Trop. Dis. 2014, 4, 194–200. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, M.; Dżugan, M. The use of Photochem device in evaluation of antioxidant activity of polish honey. Food Anal. Method. 2017, 10, 1568–1574. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Advances in the analytical methods for determining the antioxidant properties of honey: A review. Afr. J. Trad. Complement Altern. Med. 2012, 9, 36–42. [Google Scholar] [CrossRef]
- Chua, L.S.; Lee, J.Y.; Chan, G.F. Honey protein extraction and determination by mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Kishore, R.K.; Halim, A.S.; Syazana, M.S.N.; Sirajudeen, K.N.S. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr. Res. 2011, 31, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Aires, E.; Barreira, J.; Estevinho, L. Antioxidant activity of portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Jerkovic, I.; Marijanovic, Z. Oak (Quercus frainetto Ten.) honeydew honey-approach to screening of volatile organic composition and antioxidant capacity (dpph and frap assay). Molecules 2010, 15, 3744–3756. [Google Scholar] [CrossRef] [PubMed]
- Besco, E.; Braccioli, E.; Vertuani, S.; Ziosi, P.; Brazzo, F.; Bruni, R.; Sacchetti, G.; Manfredini, S. The use of photochemiluminescence for the measurement of the integral antioxidant capacity of baobab products. Food Chem. 2007, 102, 1352–1356. [Google Scholar] [CrossRef]
- Zielińska, D.; Szawara-Nowak, D.; Michalska, A. Antioxidant capacity of thermally-treated buckwheat. Pol. J. Food Nutr. Sci. 2007, 57, 465–470. [Google Scholar]
- Directive, C. 110/EC of 20 December 2001 relating to honey. Off. J. Eur. Commun. 2002, 10, 47–52. [Google Scholar]
- RMRiRW. Rozporządzenie Ministra Rolnictwa i Rozwoju Wsi z dnia 29 maja 2015 r. Zmieniające Rozporządzenie w Sprawie Szczegółowych Wymagań w Zakresie Jakości Handlowej Miodu (Regulation of the Minister of Agriculture and Rural Development from 29 May 2015 Regarding the Regulation on Specific Requirements in the Field of Commercial Quality of Honey), Dz. U. z 2015, Poz. 850. 2015. Available online: http://www.dziennikustaw.gov.pl/du/2015/850 (accessed on 18 August 2018).
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Beretta, G.; Granata, P.; Ferrero, M.; Faccino, F.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta. 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Kücük, M.; Kolayli, S.; Karaoglu, S.; Ulusoy, E.; Baltaci, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Wilczyńska, A. Phenolic content and antioxidant activity of different types of Polish honey—A short report. Pol. J. Food Nutr. Sci. 2010, 60, 309–313. [Google Scholar]
- Kačániová, M.; Vukovic, N.; Bobková, A.; Fikselová, M.; Rovná, K.; Haščík, P.; Čuboň, J.; Hleba, L.; Bobko, M. Antimicrobial and antiradical activity of Slovakian honeydew honey samples. JMBFS 2011, 1, 354–368. [Google Scholar]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. A comparative study on phenolic profile, vitamin C content and antioxidant activity of Italian honeys of different botanical origin. Int. J. Food Sci. Technol. 2013, 48, 1899–1908. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Saich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, N.; Engeseth, N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef]
- Kesic, A.; Mazalovic, M.; Crnkic, A.; Catovic, B.; Hadzidedic, S.; Dragosevic, G. The influence of l-ascorbic acid content on total antioxidant activity of bee-honey. Eur. J. Sci. Res. 2009, 32, 95–101. [Google Scholar]
- Anand, S.; Pang, E.; Livanos, G.; Mantri, N. Characterization of Physico-chemical properties and antioxidant capacities of bioactive honey produced from australian grown agastache rugosa and its correlation with colour and poly-phenol content. Molecules 2018, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Mellen, M.; Fikselová, M.; Mendelová, A.; Haščík, P. antioxidant effect of natural honeys affected by their source and origin. Pol. J. Food Nutr. Sci. 2015, 65, 81–85. [Google Scholar] [CrossRef]
- Dżugan, M.; Sowa, P.; Kwaśniewska, M.; Wesołowska, M.; Czernicka, M. Physicochemical parameters and antioxidant activity of bee honey enriched with herbs. Plant Foods Hum. Nutr. 2017, 72, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Sowa, P.; Grabek-Lejko, D.; Wesołowska, M.; Swacha, S.; Dżugan, M. Hydrogen peroxide-dependent antibacterial action of Melilotus albus honey. Lett. Appl. Microbiol. 2017, 65, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.M.; Purcarea, C.; Dżugan, M.; Teusdea, A. Comparitive antioxidant content and activity of selected Romanian and polish honey. Rev. Chim. 2016, 67, 214–218. [Google Scholar]
- Sant’Ana, L.D.; Sousa, J.P.L.M.; Salgueiro, F.B.; Lorenzon, M.C.A.; Castro, R.N. Characterization of monofloral honeys with multivariate analysis of their chemical profile and antioxidant activity. J. Food. Sci. 2012, 71, C135–C140. [Google Scholar] [CrossRef] [PubMed]
- Nayik, G.A.; Nanda, V. Physico-chemical, enzymatic, mineral and colour characterization of three different varieties of honeys from kashmir valley of india with multivariate approach. Pol. J. Food. Nutr. Sci. 2015, 65, 101–108. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Tezcan, F.; Erim, F.B.; Yildiz, O.; Sahin, H.; Can, Z.; Kolayli, S. Characterization of anatolian honeys based on minerals, bioactive components and principal component analysis. LWT-Food Sci. Technol. 2016, 68, 273–279. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Classification of entomological origin of honey based on its physicochemical and antioxidant properties. Int. J. Food Prop. 2017, 20, S2723–S2738. [Google Scholar] [CrossRef]
- Purcarea, C.; Dżugan, M.; Wesolowska, M.; Chis, A.M.; Zagula, G.; Teusdea, A.C.; Puchalski, C. A comparative study of metal content in selected polish and romanian honey samples. Rev. Chim. 2017, 68, 1163–1169. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Piljac-Žegarac, J.; Stipčević, T.; Belščak, A. Antioxidant properties and phenolic content of different floral origin honeys. J. ApiProd. ApiMed. Sci. 2009, 1, 43–50. [Google Scholar] [CrossRef]
Sample Availability: The honey samples are available from the authors for limited time. |
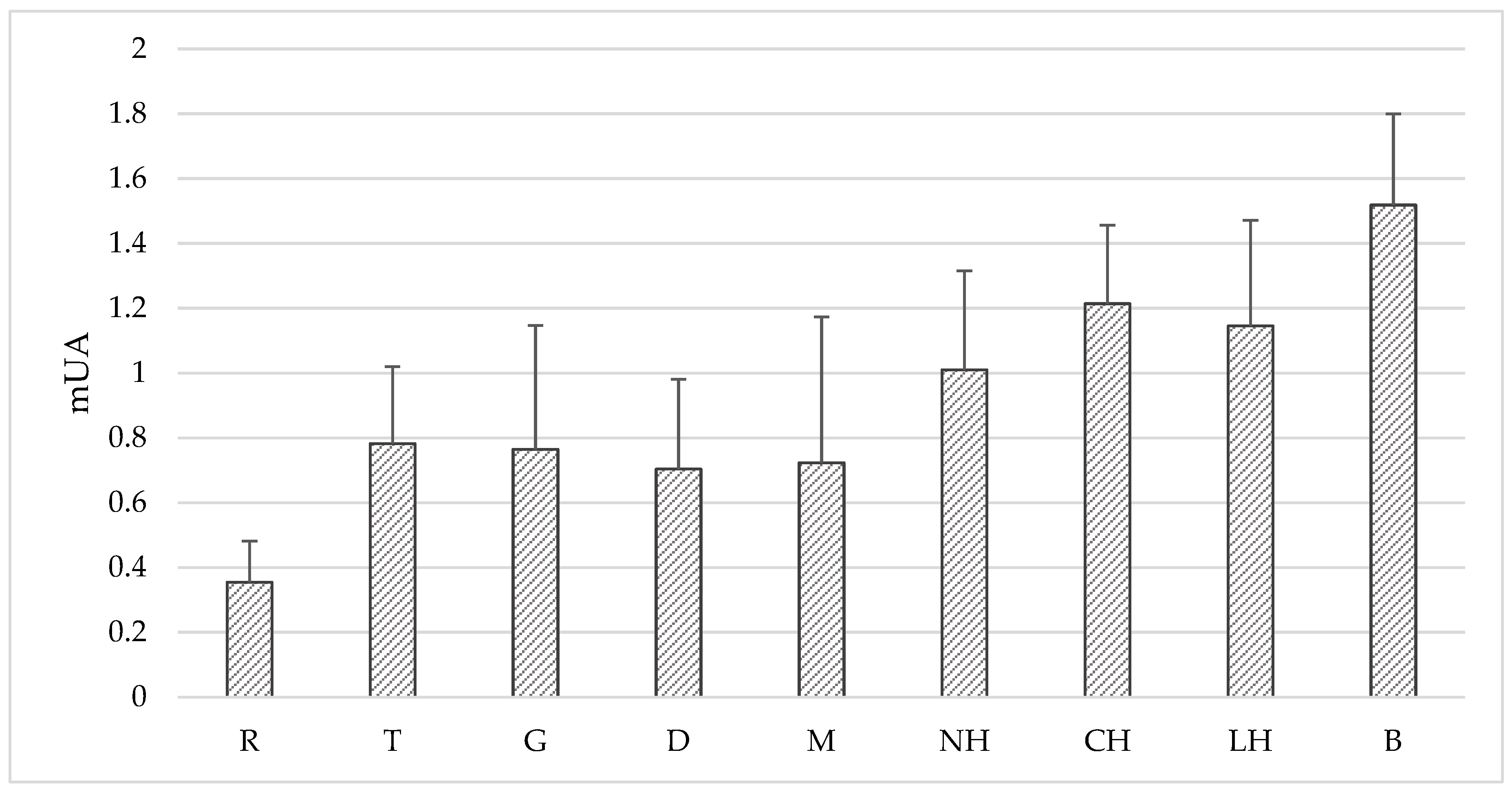
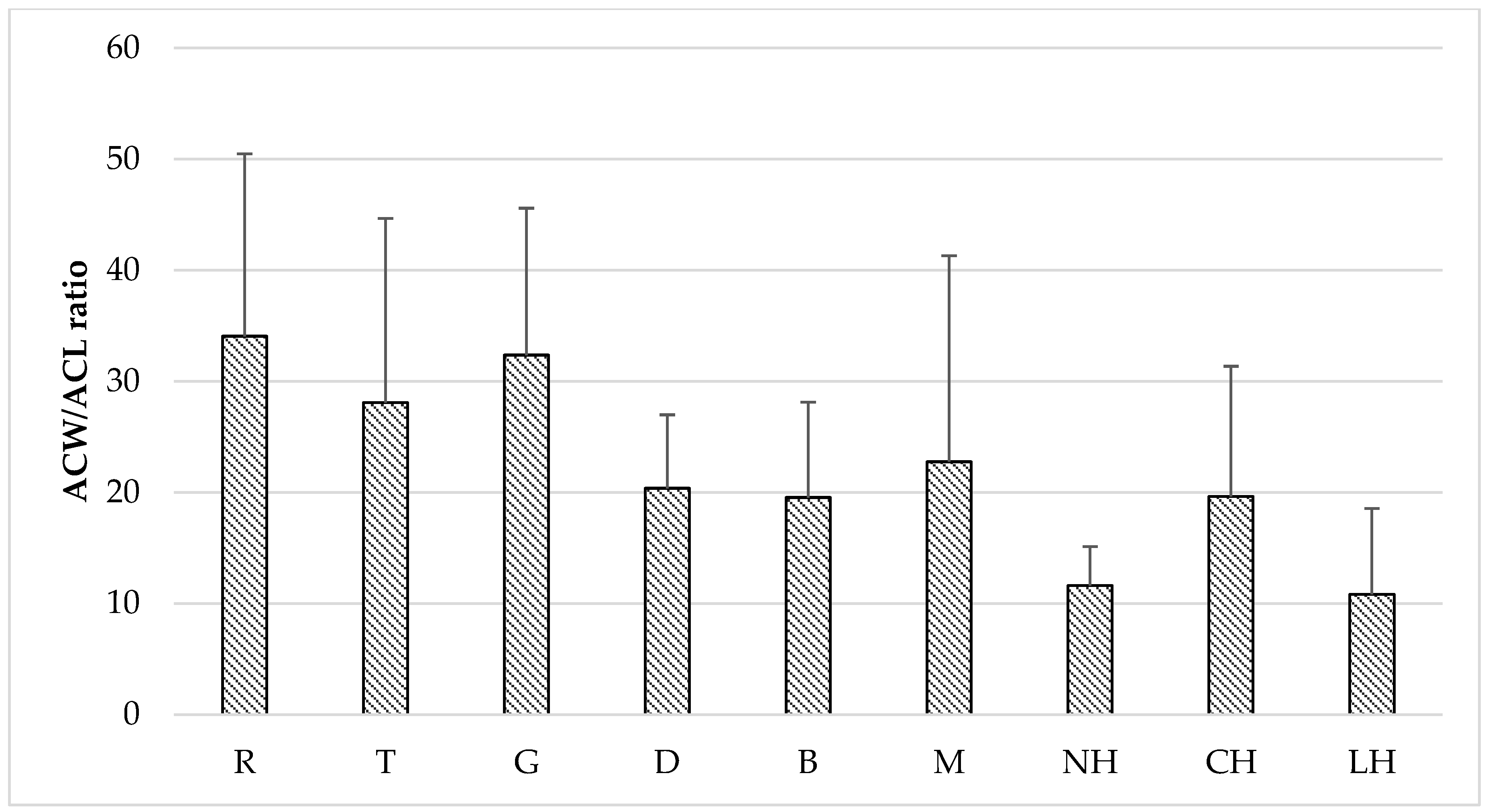
| Honey Variety | PCL-ACW (mM AA kg−1) | PCL-ACL (mM TE kg−1) | DPPH ** (%inhibition) | FRAP (μmol TE kg−1) | TPC (mg GAE kg−1) | |
|---|---|---|---|---|---|---|
| Rape [R] n = 12 | mean ± SD | 10.59 ± 3.96 | 0.40 ± 0.19 | 21.81 ± 3.15 | 656.73 ± 119.40 | 254.52 ± 34.71 |
| min-max | 5.37–17.95 | 0.21–0.73 | 17.34–27.65 | 486.54–859.62 | 205.41–310.81 | |
| %VC | 37.43 | 47.87 | 14.42 | 18.18 | 13.46 | |
| Tilia [T] n = 10 | mean ± SD | 12.71 ± 2.06 | 0.57 ± 0.28 | 40.53 ± 13.01 | 1060.19 ± 307.37 | 409.10 ± 69.76 |
| min-max | 8.29–15.08 | 0.19–1.22 | 24.35-65.36 | 619.23–1626.92 | 302.70–549.55 | |
| %VC | 16.19 | 49.49 | 32.11 | 28.99 | 17.05 | |
| Goldenrod [G] n = 11 | mean ± SD | 22.77 ± 12.46 | 0.97 ± 0.79 | 45.34 ± 21.44 | 1259.97 ± 721.13 | 456.84 ± 220.20 |
| min-max | 7.01–45.24 | 0.17–2.32 | 22.49–82.47 | 605.77–2350.00 | 284.68–966.67 | |
| %VC | 54.75 | 80.84 | 47.27 | 57.23 | 48.20 | |
| Dandelion [D] n = 5 | mean ± SD | 13.58 ± 4.78 | 0.76 ± 0.40 | 42.59 ± 17.65 | 1593.85 ± 567.98 | 508.11 ± 180.95 |
| min-max | 7.65–18.98 | 0.33–1.16 | 28.36–64.25 | 1038.46-2257.69 | 326.13–738.74 | |
| %VC | 35.19 | 52.45 | 41.44 | 35.64 | 35.61 | |
| Buckwheat [B] n = 6 | mean ± SD | 24.03 ± 2.68 | 1.41 ± 0.60 | 82.41 ± 4.59 | 3635.49 ± 1328.22 | 1353.66 ± 314.15 |
| min-max | 19.80–26.88 | 0.66–2.41 | 76.42–89.03 | 1973.08–5051.92 | 922.52–1876.58 | |
| %VC | 11.17 | 42.51 | 5.57 | 36.53 | 23.21 | |
| Multifloral [M] n = 17 | mean ± SD | 16.82 ± 6.07 | 1.14 ± 0.66 | 39.89 ± 15.08 | 1562.67 ± 995.11 | 490.09 ± 225.30 |
| min-max | 8.74–27.49 | 0.23–2.40 | 22.45–65.78 | 580.77–3340.38 | 236.94–1021.62 | |
| %VC | 36.09 | 58.07 | 37.84 | 63.68 | 45.97 | |
| Nectar-honeydew [NH] n = 8 | mean ± SD | 17.98 ± 7.78 | 1.62 ± 0.64 | 59.72 ± 15.19 | 2013.70 ± 721.08 | 630.29 ± 170.17 |
| min-max | 10.50–33.44 | 0.67–2.62 | 35.26–79.58 | 911.54–2767.31 | 409.01–962.16 | |
| %VC | 43.28 | 39.78 | 25.44 | 35.81 | 27.00 | |
| Coniferous honeydew [CH] n = 16 | mean ± SD | 19.98 ± 6.08 | 1.29 ± 0.55 | 66.82 ± 11.21 | 2153.37 ± 663.92 | 600.11 ± 161.52 |
| min-max | 7.83–33.87 | 0.33-2.17 | 51.39–85.29 | 1180.77–3701.92 | 372.97–1001.02 | |
| %VC | 30.57 | 43.05 | 16.77 | 30.83 | 26.91 | |
| Leafy honeydew [LH] n = 5 | mean ± SD | 14.41 ± 4.07 | 1.62 ± 0.68 | 61.07 ± 7.87 | 2019.62 ± 574.85 | 585.95 ± 166.35 |
| min-max | 8.89–18.60 | 0.73–2.41 | 50.47–69.27 | 1080.77–2448.08 | 345.95–754.95 | |
| %VC | 28.24 | 41.85 | 12.89 | 28.46 | 28.39 | |
| Significant differences * | R-B, R-G, R-CH, T-B, T-G | R-M, R-B, R-NH, R-CH, R-LH, T-LH, T-NH | R-all, B-M, B-D, B-T, B-G, CH-M, CH-D, CH-T, CH-G | B-all, R-M, R-CH, R-LH, R-NH, T-CH | B-all, R-M, R-CH, R-LH, R-NH | |
| Variable | PCL-ACW | PCL-ACL | FRAP | TPC | DPPH | Color Intensity |
|---|---|---|---|---|---|---|
| PCL-ACW | 1.000 | |||||
| PCL-ACL | 0.422 | 1.000 | ||||
| FRAP | 0.622 | 0.673 | 1.000 | |||
| TCP | 0.647 | 0.674 | 0.943 | 1.000 | ||
| DPPH | 0.621 | 0.648 | 0.876 | 0.912 | 1.000 | |
| Color Intensity | 0.597 | 0.566 | 0.793 | 0.831 | 0.928 | 1.000 |
| Variable | Principal Components (Correlations) | |
|---|---|---|
| PC 1 | PC 2 | |
| ACW | −0.81 | −0.23 |
| ACL | −0.79 | 0.60 |
| FRAP | −0.97 | −0.04 |
| TPC | −0.93 | −0.24 |
| DPPH | −0.97 | 0.11 |
| Color | −0.97 | −0.13 |
| Honey Variety | Number of Samples |
|---|---|
| Rape (Brassica napus) | 12 |
| Tilia (Tilia) | 10 |
| Goldenrod (Solidago virgaurea) | 11 |
| Dandelion (Taraxacum officinale) | 5 |
| Buckwheat (Fagopyrum esculentum) | 6 |
| Multifloral | 17 |
| Nectar-honeydew | 8 |
| Coniferous honeydew | 16 |
| Leafy honeydew | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. https://doi.org/10.3390/molecules23082069
Dżugan M, Tomczyk M, Sowa P, Grabek-Lejko D. Antioxidant Activity as Biomarker of Honey Variety. Molecules. 2018; 23(8):2069. https://doi.org/10.3390/molecules23082069
Chicago/Turabian StyleDżugan, Małgorzata, Monika Tomczyk, Patrycja Sowa, and Dorota Grabek-Lejko. 2018. "Antioxidant Activity as Biomarker of Honey Variety" Molecules 23, no. 8: 2069. https://doi.org/10.3390/molecules23082069
APA StyleDżugan, M., Tomczyk, M., Sowa, P., & Grabek-Lejko, D. (2018). Antioxidant Activity as Biomarker of Honey Variety. Molecules, 23(8), 2069. https://doi.org/10.3390/molecules23082069