Ruthenium on Carbonaceous Materials for the Selective Hydrogenation of HMF
Abstract
1. Introduction
2. Results and Discussion
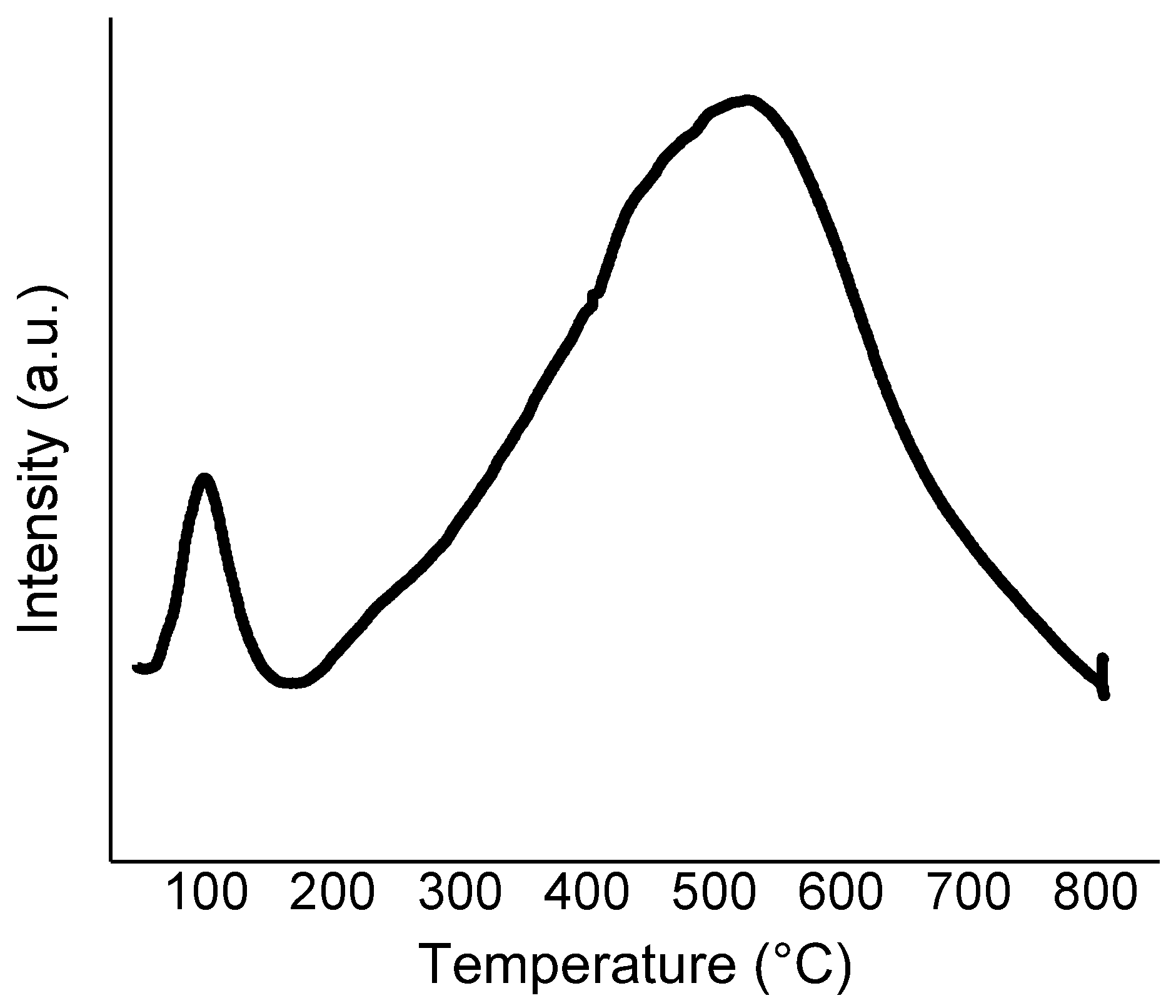
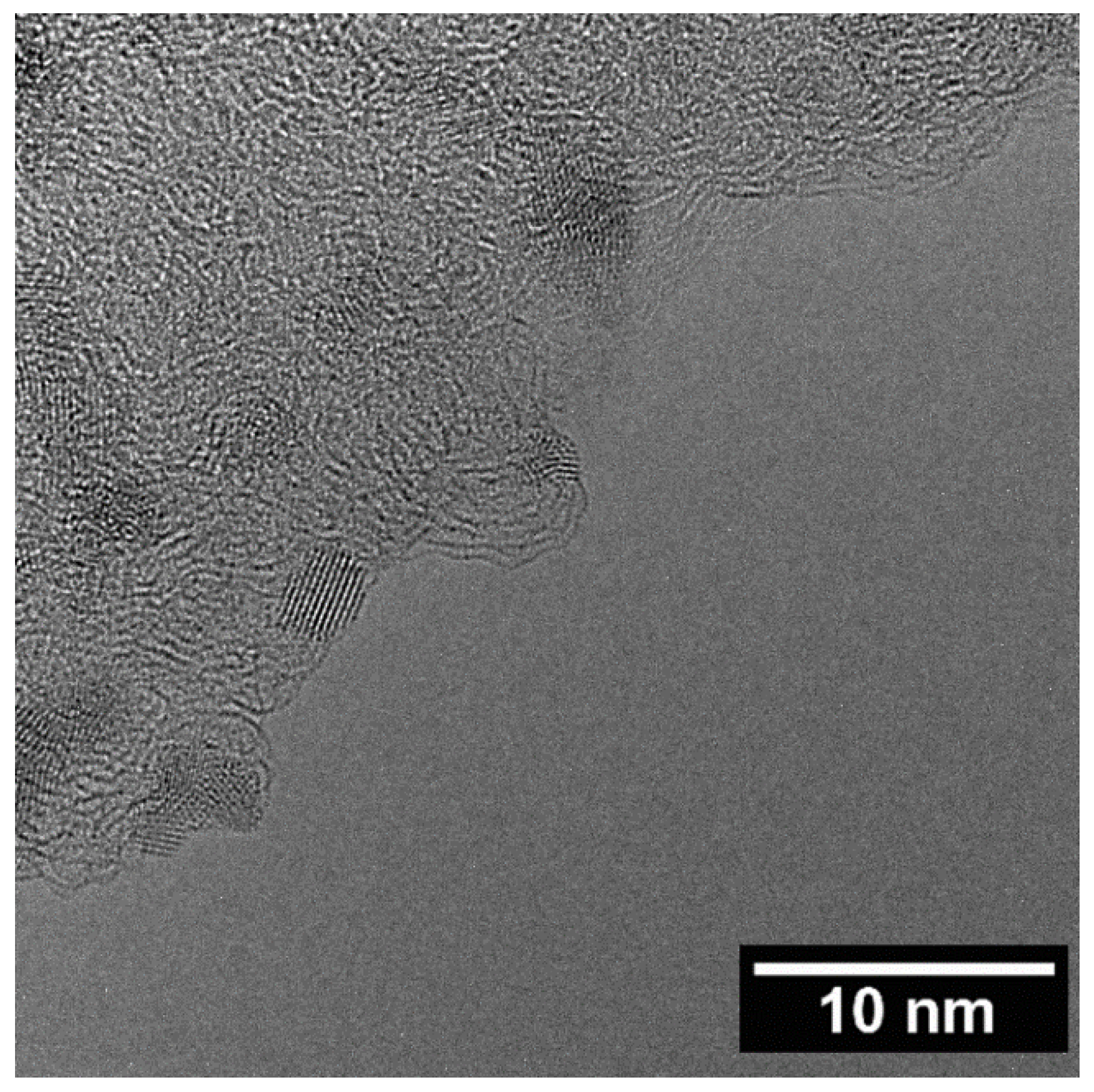
2.1. Catalysts Characterisation
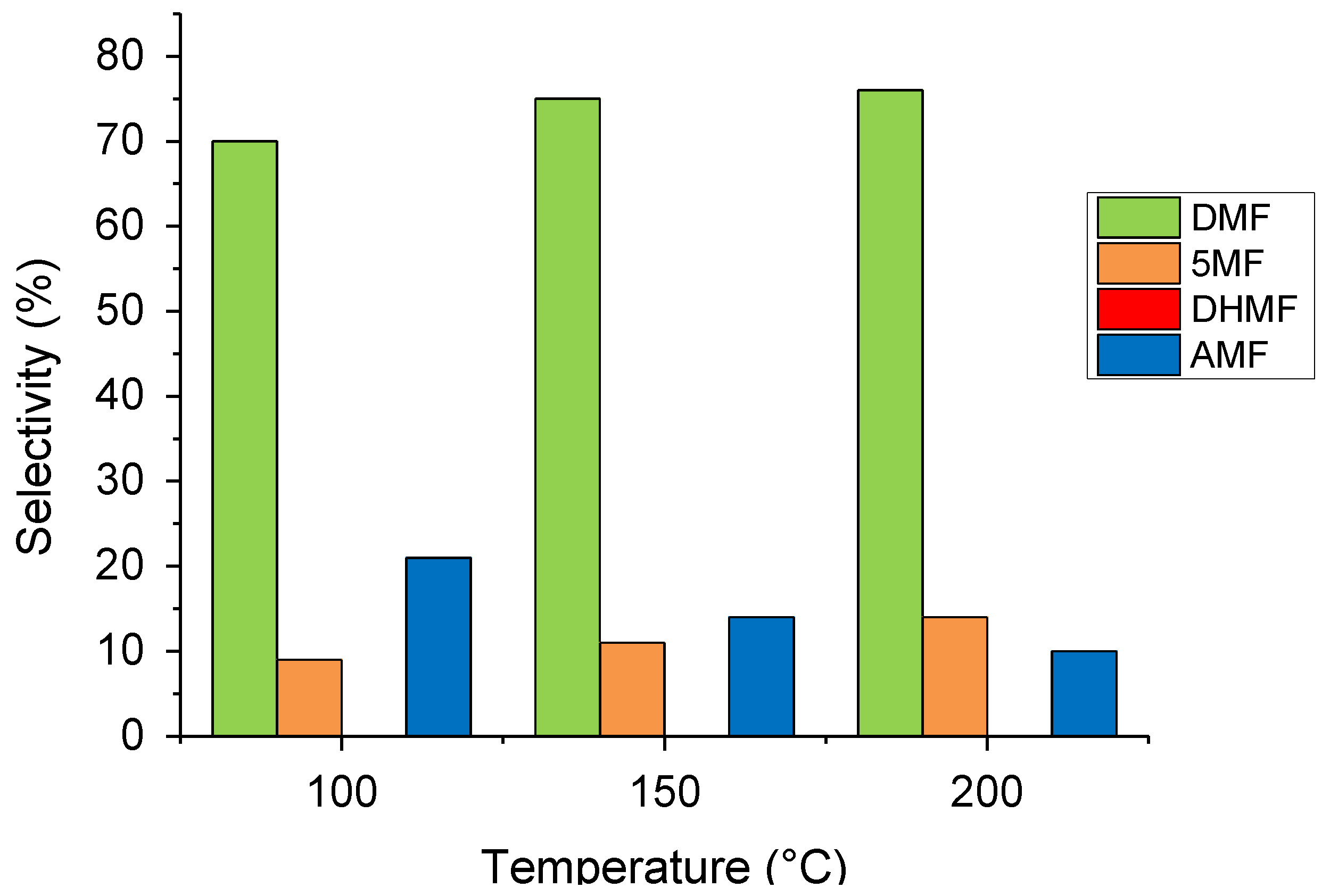
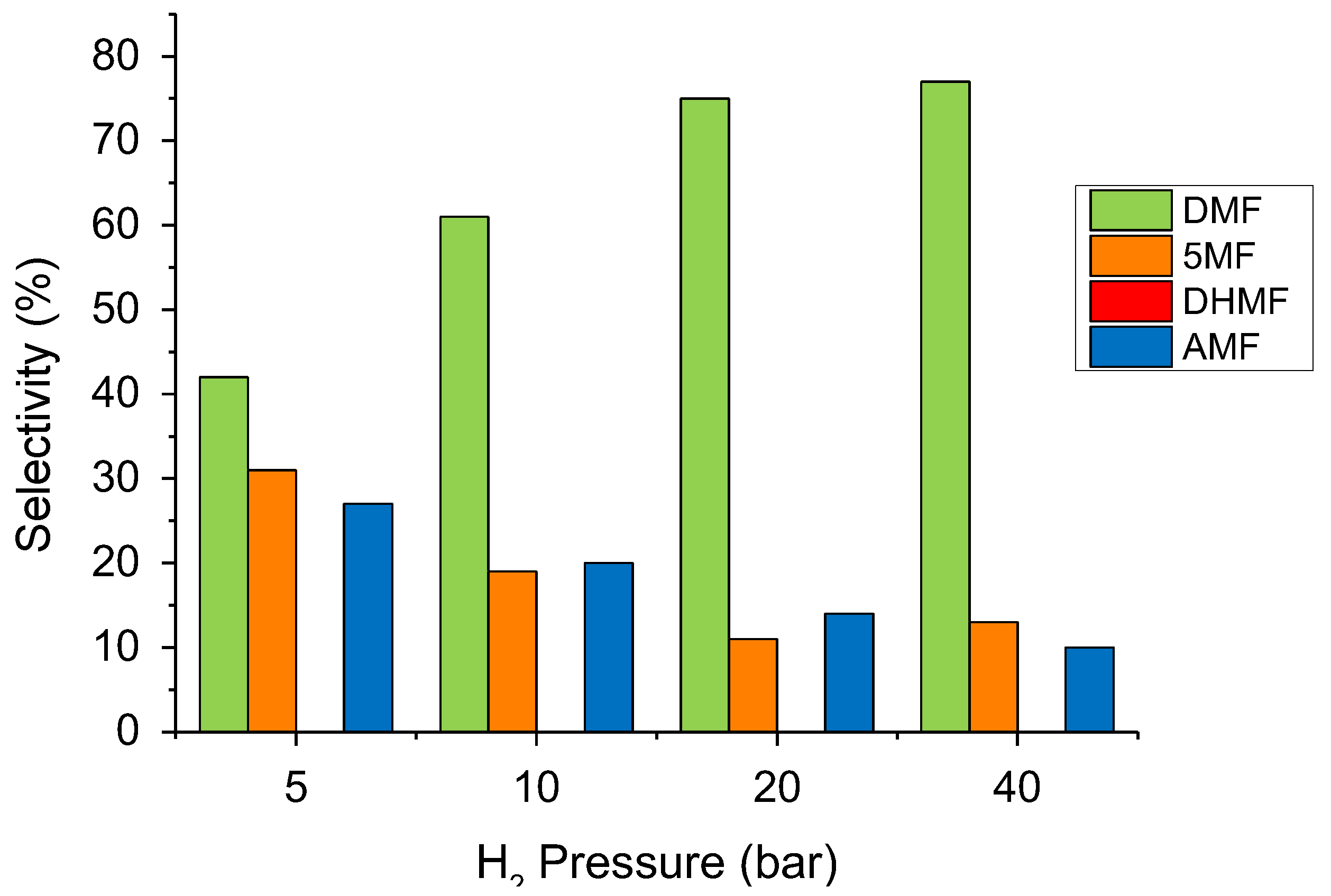
2.2. Temperature and Pressure Effect
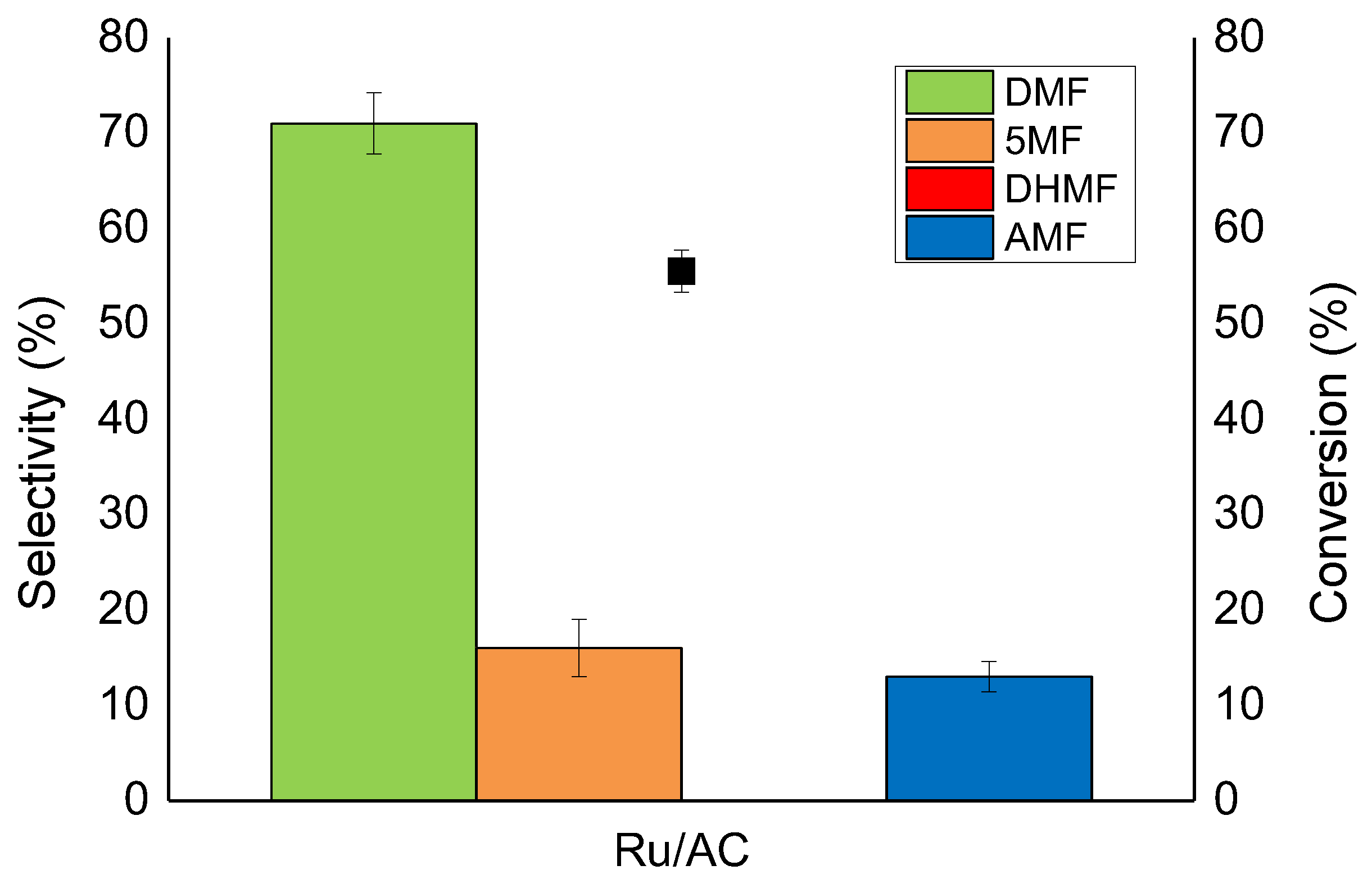
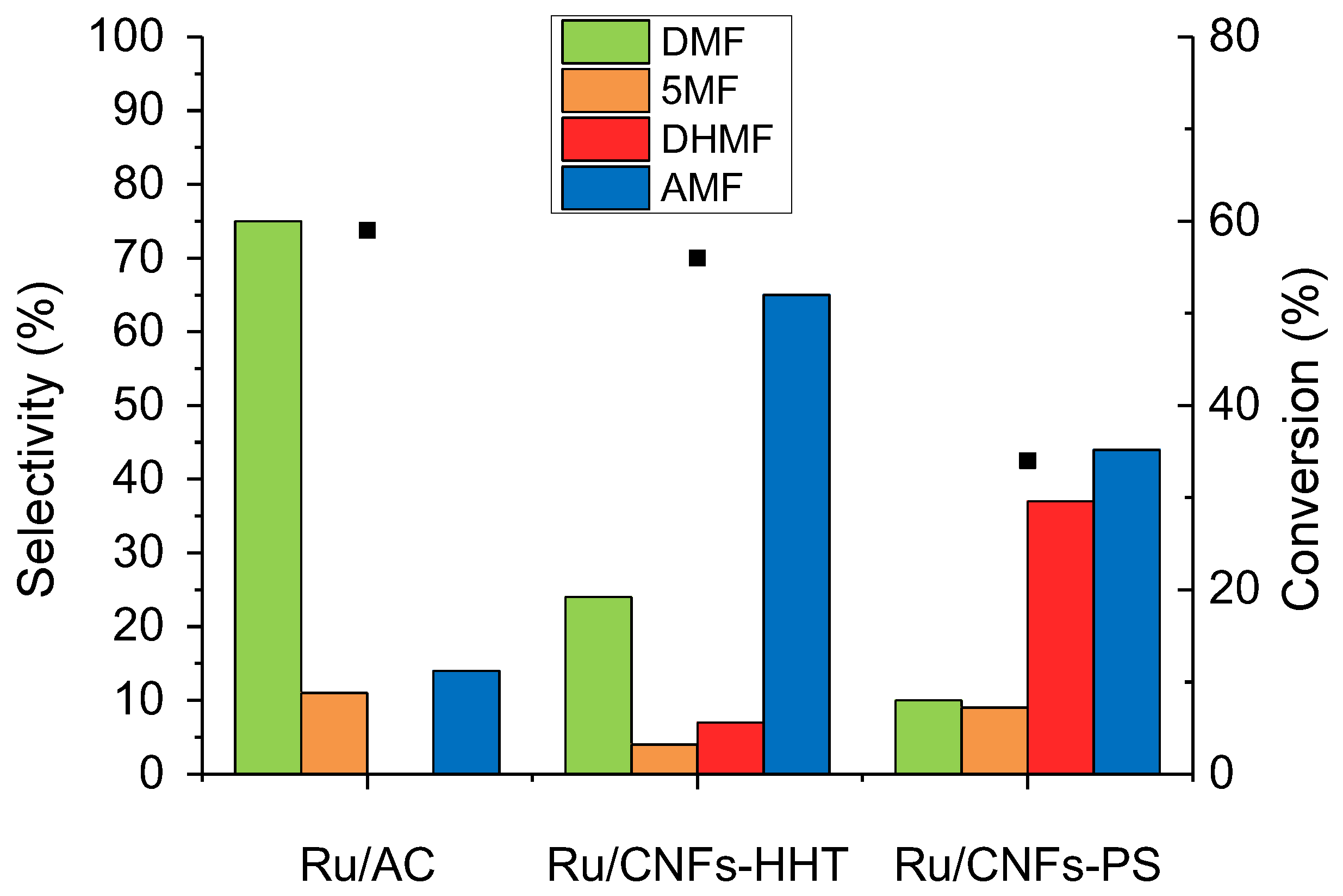
2.3. Support Effect
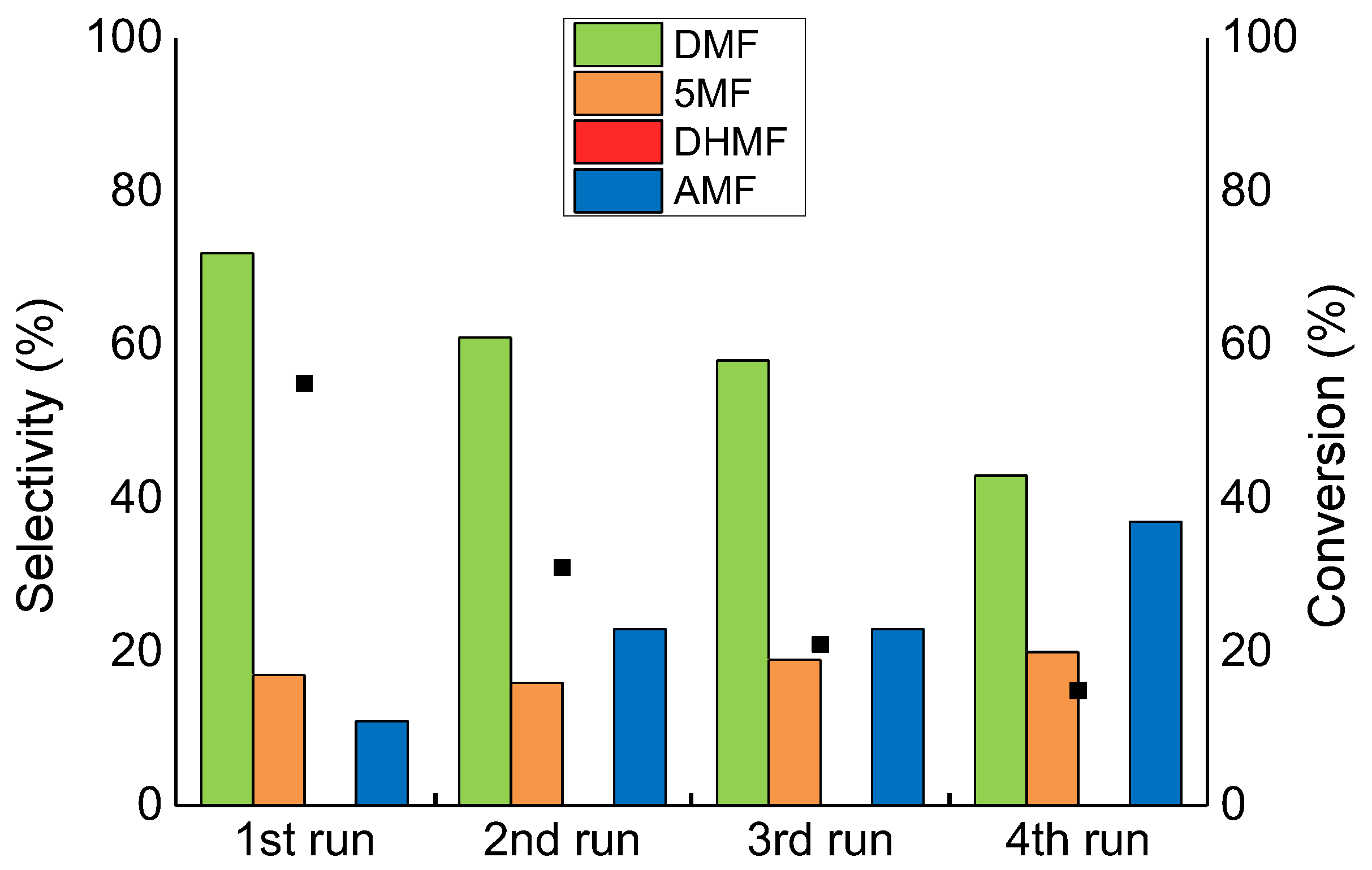
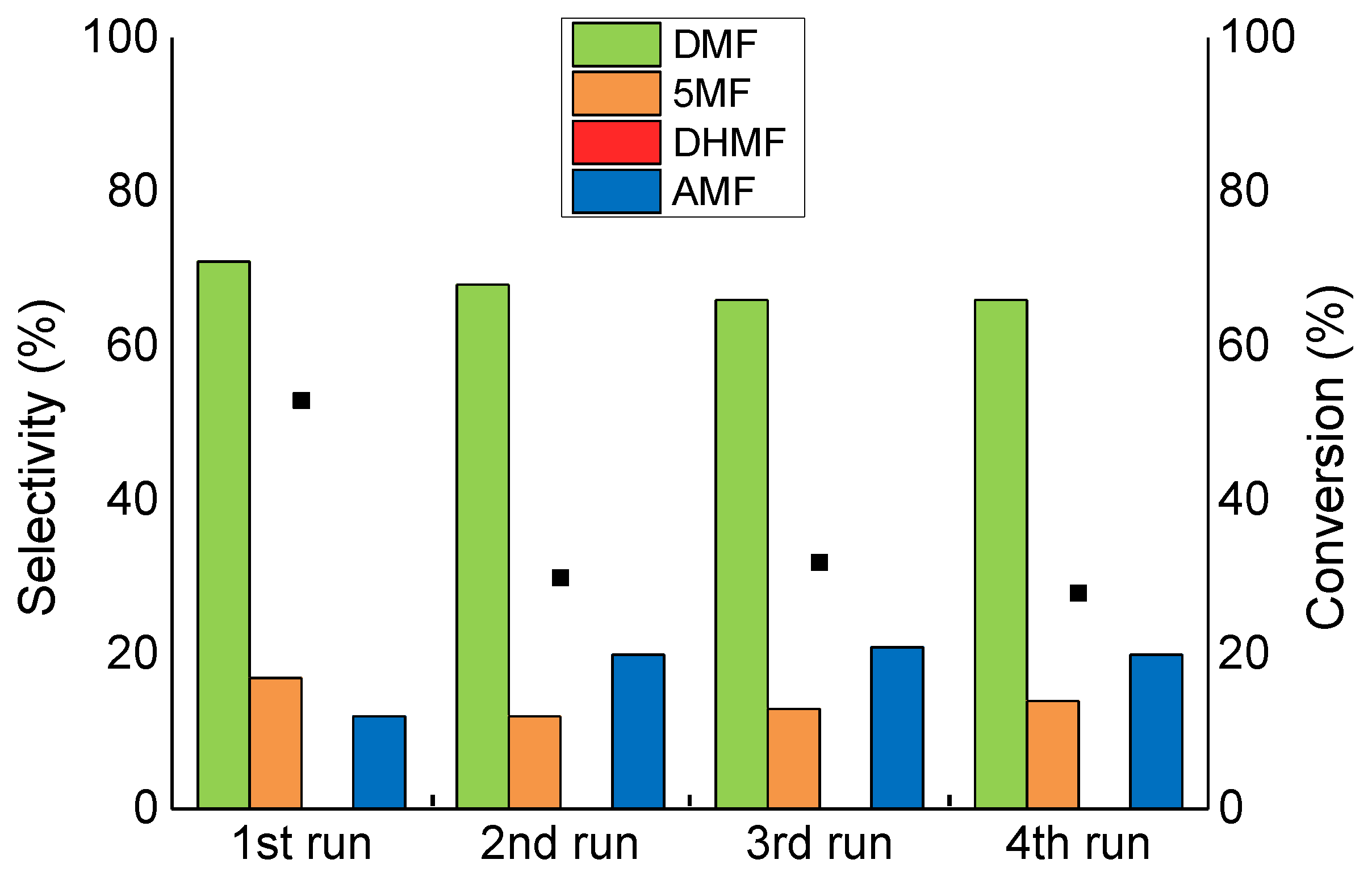
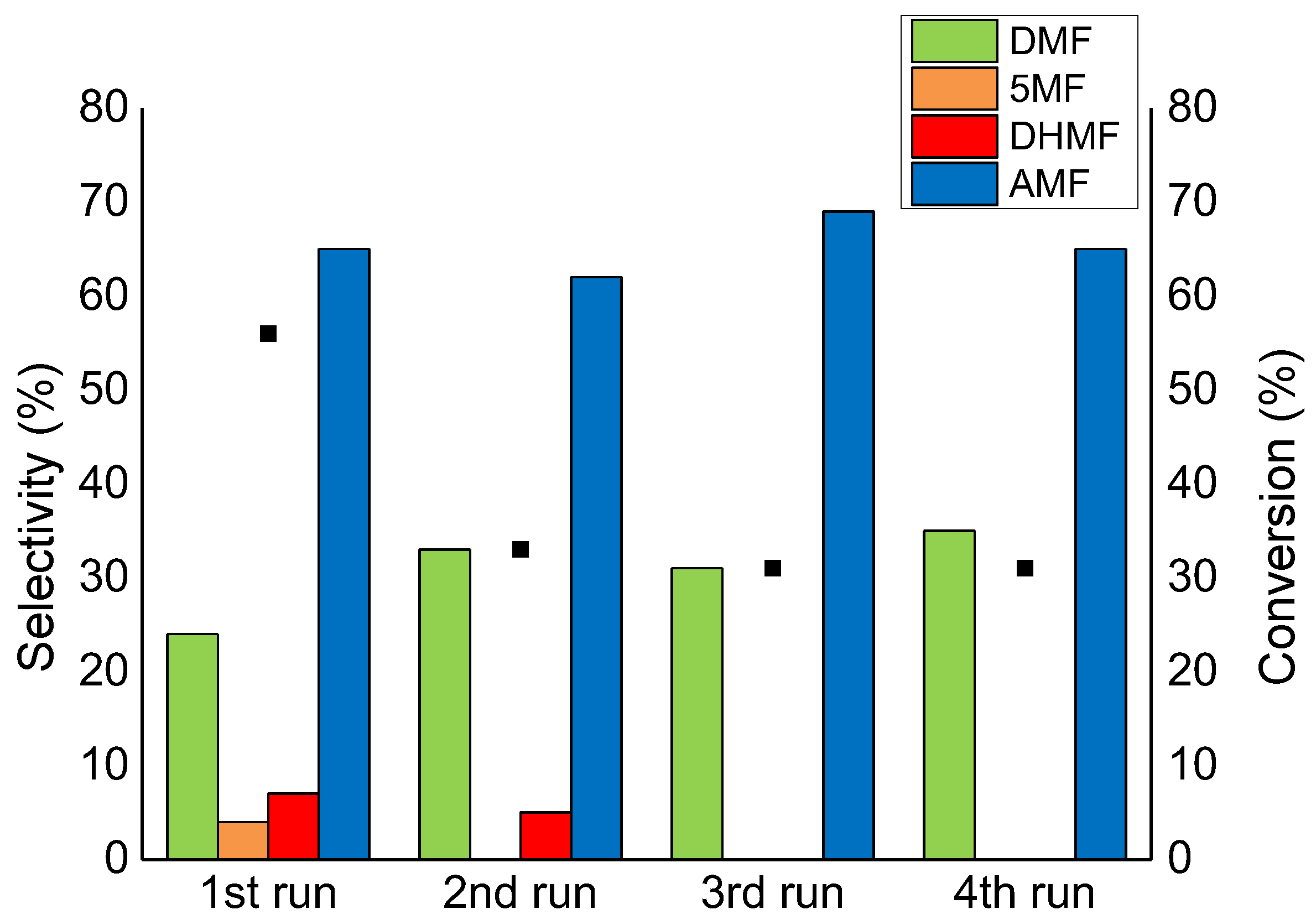
2.4. Catalyst Reusability
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Hydrogenation Reactions
3.3. Catalysts Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- BP Statistical Review of World Energy June 2014; British Petroleum Co.: London, UK, 2014.
- Binder, J.B.; Raines, R.T.; Binder, J.B.; Raines, R.T. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. J. Am. Chem. Soc. 2009, 131, 1879–1985. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Guo, X.; Guan, J.; Mu, X.; Zhang, D. A process for efficient conversion of fructose into 5-hydroxymethylfurfural in ammonium salts. Appl. Catal. A Gen. 2011, 403, 98–103. [Google Scholar] [CrossRef]
- Perego, C.; Bosetti, A. Biomass to fuels: The role of zeolite and mesoporous materials. Microporous Mesoporous Mater. 2011, 144, 28–39. [Google Scholar] [CrossRef]
- Qi, X.; Guo, H.; Li, L. Efficient conversion of fructose to 5-hydroxymethylfurfural catalyzed by sulfated zirconia in ionic liquids. Ind. Eng. Chem. Res. 2011, 50, 7985–7989. [Google Scholar] [CrossRef]
- Wang, P.; Yu, H.; Zhan, S.; Wang, S. Catalytic hydrolysis of lignocellulosic biomass into 5-hydroxymethylfurfural in ionic liquid. Bioresour. Technol. 2011, 102, 4179–4183. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic production of liquid hydrocarbon transportation fuels. Catal. Altern. Energy Gener. 2012, 29–56. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Synergistic conversion of glucose into 5-hydroxymethylfurfural in ionic liquid-water mixtures. Bioresour. Technol. 2012, 109, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Kroh, L.W. Caramelisation in food and beverages. Food Chem. 1994, 51, 373–379. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Wu, S.; Fan, H.; Xie, Y.; Cheng, Y.; Wang, Q.; Zhang, Z.; Han, B. Effect of CO2 on conversion of inulin to 5-hydroxymethylfurfural and propylene oxide to 1{,}2-propanediol in water. Green Chem. 2010, 12, 1215–1219. [Google Scholar] [CrossRef]
- Mitra, J.; Zhou, X.; Rauchfuss, T. Pd/C-catalyzed reactions of HMF: Decarbonylation, hydrogenation, and hydrogenolysis. Green Chem. 2015, 17, 307–313. [Google Scholar] [CrossRef]
- Luo, J.; Arroyo-Ramírez, L.; Wei, J.; Yun, H.; Murray, C.B.; Gorte, R.J. Comparison of HMF hydrodeoxygenation over different metal catalysts in a continuous flow reactor. Appl. Catal. A Gen. 2015, 508, 86–93. [Google Scholar] [CrossRef]
- Hales, R.A. Process for Preparing 2,5-bis Hydroxymethyl Tetrahydrofuran. U.S. Patent 6866760, 19 June 1962. [Google Scholar]
- Tuteja, J.; Choudhary, H.; Nishimura, S.; Ebitani, K. Direct synthesis of 1,6-hexanediol from HMF over a heterogeneous Pd/ZrP catalyst using formic acid as hydrogen source. ChemSusChem 2014, 7, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wang, X.; Jiang, Y.; Wu, F.; Chen, X.; Mu, X. One-step conversion of biomass-derived 5-hydroxymethylfurfural to 1,2,6-hexanetriol over ni–co–al mixed oxide catalysts under mild conditions. ACS Sustain. Chem. Eng. 2014, 2, 173–180. [Google Scholar] [CrossRef]
- Luo, J.; Yu, J.; Gorte, R.J.; Mahmoud, E.; Vlachos, D.G.; Smith, M.A. The effect on oxide acidity on HMF etherification. Catal. Sci. Technol. 2014, 4, 3074–3081. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Daniel, R.; Ghafourian, A.; Herreros, J.M.; Shuai, S.; Ma, X. Combustion characteristics and emissions of 2-methylfuran compared to 2,5-dimethylfuran, gasoline and ethanol in a DISI engine. Fuel 2013, 103, 200–211. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Nagpure, A.S.; Venugopal, A.K.; Lucas, N.; Manikandan, M.; Thirumalaiswamy, R.; Chilukuri, S. Renewable fuels from biomass-derived compounds: Ru-containing hydrotalcites as catalysts for conversion of HMF to 2,5-dimethylfuran. Catal. Sci. Technol. 2015, 5, 1463–1472. [Google Scholar] [CrossRef]
- Zu, Y.; Yang, P.; Wang, J.; Liu, X.; Ren, J.; Lu, G.; Wang, Y. Efficient production of the liquid fuel 2,5-dimethylfuran from 5-hydroxymethylfurfural over Ru/Co3O4 catalyst. Appl. Catal. B Environ. 2014, 146, 244–248. [Google Scholar] [CrossRef]
- Jae, J.; Zheng, W.; Lobo, R.F.; Vlachos, D.G. Production of dimethylfuran from hydroxymethylfurfural through catalytic transfer hydrogenation with ruthenium supported on carbon. ChemSusChem 2013, 6, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Jae, J.; Mahmoud, E.; Lobo, R.F.; Vlachos, D.G. Cascade of liquid-phase catalytic transfer hydrogenation and etherification of 5-hydroxymethylfurfural to potential biodiesel components over Lewis acid zeolites. ChemCatChem 2014, 6, 508–513. [Google Scholar] [CrossRef]
- Hu, L.; Tang, X.; Xu, J.; Wu, Z.; Lin, L.; Liu, S. Selective transformation of 5-hydroxymethylfurfural into the liquid fuel 2,5-dimethylfuran over carbon-supported ruthenium. Ind. Eng. Chem. Res. 2014, 53, 3056–3064. [Google Scholar] [CrossRef]
- Thananatthanachon, T.; Rauchfuss, T.B. Efficient production of the liquid fuel 2,5-dimethylfuran from fructose using formic acid as a reagent. Angew. Chem. 2010, 122, 6766–6768. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Yu, X.; Du, W.; Hou, Z. Production of 2,5-dimethylfuran from 5-hydroxymethylfurfural over reduced graphene oxides supported Pt catalyst under mild conditions. Fuel 2016, 163, 74–79. [Google Scholar] [CrossRef]
- Kong, X.; Zheng, R.; Zhu, Y.; Ding, G.; Zhu, Y.; Li, Y.-W. Rational design of Ni-based catalysts derived from hydrotalcite for selective hydrogenation. Green Chem. 2015, 17, 2504–2514. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, X.; Zheng, H.; Ding, G.; Zhu, Y.; Li, Y.-W. Efficient synthesis of 2,5-dihydroxymethylfuran and 2,5-dimethylfuran from 5-hydroxymethylfurfural using mineral-derived Cu catalysts as versatile catalysts. Catal. Sci. Technol. 2015, 5, 4208–4217. [Google Scholar] [CrossRef]
- Nishimura, S.; Ikeda, N.; Ebitani, K. Selective hydrogenation of biomass-derived 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF) under atmospheric hydrogen pressure over carbon supported PdAu bimetallic catalyst. Catal. Today 2014, 232, 89–98. [Google Scholar] [CrossRef]
- Wang, G.-H.; Hilgert, J.; Richter, F.H.; Wang, F.; Bongard, H.-J.; Spliethoff, B.; Weidenthaler, C.; Schüth, F. Platinum-cobalt bimetallic nanoparticles in hollow carbon nanospheres for hydrogenolysis of 5-hydroxymethylfurfural. Nat. Mater. 2014, 13, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yun, H.; Mironenko, A.V.; Goulas, K.; Lee, J.D.; Monai, M.; Wang, C.; Vorotnikov, V.; Murray, C.B.; Vlachos, D.G.; et al. Mechanisms for High Selectivity in the Hydrodeoxygenation of 5-Hydroxymethylfurfural over PtCo Nanocrystals. ACS Catal. 2016, 6, 4095–4104. [Google Scholar] [CrossRef]
- Luo, J.; Monai, M.; Wang, C.; Lee, J.D.; Duchoň, T.; Dvořák, F.; Matolín, V.; Murray, C.B.; Fornasiero, P.; Gorte, R.J. Unraveling the surface state and composition of highly selective nanocrystalline Ni-Cu alloy catalysts for hydrodeoxygenation of HMF. Catal. Sci. Technol. 2017, 7, 1735–1743. [Google Scholar] [CrossRef]
- Bicker, M.; Kaiser, D.; Ott, L.; Vogel, H. Dehydration of d-fructose to hydroxymethylfurfural in sub- and supercritical fluids. J. Supercrit. Fluids 2005, 36, 118–126. [Google Scholar] [CrossRef]
- Gruter, G.J.M.; Manzer, L.E.; De Sousa Dias, A.S.V.; Dautzenberg, F.; Purmova, J. Hydroxymethylfurfural Ethers and Esters Prepared in Ionic Liquids. U.S. Patent 8314260B2, 20 November 2012. [Google Scholar]
- Gruter, G.J.M.; Dautzenberg, F. Method for the Synthesis of S-Alkoxymethyl Furfural Ethers and Their Use. U.S. Patent 8133289B2, 13 March 2012. [Google Scholar]
- Mascal, M.; Nikitin, E.B. Direct, high-yield conversion of cellulose into biofuel. Angew. Chem. Int. Ed. Engl. 2008, 47, 7924–7926. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Sacia, E.R.; Bell, A.T. Etherification and reductive etherification of 5-(hydroxymethyl)furfural: 5-(alkoxymethyl)furfurals and 2{,}5-bis(alkoxymethyl)furans as potential bio-diesel candidates. Green Chem. 2012, 14, 1626–1634. [Google Scholar] [CrossRef]
- Lanzafame, P.; Temi, D.M.; Perathoner, S.; Centi, G.; Macario, A.; Aloise, A.; Giordano, G. Etherification of 5-hydroxymethyl-2-furfural (HMF) with ethanol to biodiesel components using mesoporous solid acidic catalysts. Catal. Today 2011, 175, 435–441. [Google Scholar] [CrossRef]
- Cao, Q.; Liang, W.; Guan, J.; Wang, L.; Qu, Q.; Zhang, X.; Wang, X.; Mu, X. Catalytic synthesis of 2,5-bis-methoxymethylfuran: A promising cetane number improver for diesel. Appl. Catal. A Gen. 2014, 481, 49–53. [Google Scholar] [CrossRef]
- Alipour, S.; Omidvarborna, H.; Kim, D.S. A review on synthesis of alkoxymethyl furfural, a biofuel candidate. Renew. Sustain. Energy Rev. 2017, 71, 908–926. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.L.; Zhou, H.J.; Fu, Y. Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol over Nitrogen-Doped Carbon-Supported Iron Catalysts. ChemSusChem 2016, 9, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, I.; Pernicone, N.; Forni, L. Characterisation of Ru/C catalysts for ammonia synthesis by oxygen chemisorption. Appl. Catal. A Gen. 2003, 248, 97–103. [Google Scholar] [CrossRef]
- Peng, G.; Steib, M.; Ludwig, C.; Gramm, F.; Vogel, F. Synthesis factors affecting the catalytic performance and stability of Ru/C catalysts for supercritical water gasification. Catal. Sci. Technol. 2014, 4, 3329–3339. [Google Scholar] [CrossRef]
- Guerrero-Ruiz, A.; Badenes, P.; Rodrı́guez-Ramos, I. Study of some factors affecting the Ru and Pt dispersions over high surface area graphite-supported catalysts. Appl. Catal. A Gen. 1998, 173, 313–321. [Google Scholar] [CrossRef]
- Villa, A.; Schiavoni, M.; Chan-Thaw, C.E.; Fulvio, P.F.; Mayes, R.T.; Dai, S.; More, K.L.; Veith, G.M.; Prati, L. Acid-Functionalized Mesoporous Carbon: An Efficient Support for Ruthenium-Catalyzed γ-Valerolactone Production. ChemSusChem 2015, 8, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Prati, L.; Bergna, D.; Villa, A.; Spontoni, P.; Bianchi, C.L.; Hu, T.; Romar, H.; Lassi, U. Carbons from second generation biomass as sustainable supports for catalytic systems. Catal. Today 2018, 301, 239–243. [Google Scholar] [CrossRef]
- Yu, L.; He, L.; Chen, J.; Zheng, J.; Ye, L.; Lin, H.; Yuan, Y. Robust and recyclable nonprecious bimetallic nanoparticles on carbon nanotubes for the hydrogenation and hydrogenolysis of 5-hydroxymethylfurfural. ChemCatChem 2015, 7, 1701–1707. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Catalyst | Particle Size (nm) |
|---|---|
| Ru/AC | 1.7 ± 0.3 |
| Ru/CNFs-HHT | 1.0 ± 0.2 |
| Ru/CNFs-PS | 1.9 ± 1.8 |
| Catalyst | C1s | Ru 3p3/2 | Surface Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C-C | C-O | C=O | O-C=O | Aromatic Ring | Ru/C | O/C | |||
| Ru/AC | BE (eV) | 284.5 | 285.8 | 287.2 | 288.7 | 290.3 | 464.8 461.2 | 0.009 | 0.15 |
| Rel. am. % | 57 | 20 (53) | 12 (32) | 6 (16) | 5 | 77 23 | |||
| Ru/CNFs-PS | BE (eV) | 284.5 | 285.9 | 287.4 | 288.8 | 291.1 | 463.8 | 0.003 | 0.26 |
| Rel. am. % | 22 | 32 (44) | 28 (39) | 12 (17) | 6 | - | |||
| Ru/CNFs-HHT | BE (eV) | 284.5 | 286.1 | 287.4 | 288.8 | 290.8 | 463.6 | 0.004 | 0.18 |
| Rel. am. % | 37 | 34 (58) | 18 (31) | 7 (12) | 5 | - | |||
| Ru/AC spent | BE (eV) | 284.5 | 286.0 | 287.6 | 289.2 | 292.2 | 462.4 | 0.006 | 0.27 |
| Rel. am. % | 49 | 29 (58) | 14 (28) | 7 (14) | 1 | - | |||
| Ru/AC reactivated | BE (eV) | 284.6 | 285.8 | 287.5 | 289.1 | 291.3 | 462.8 | 0.006 | 0.21 |
| Rel. am. % | 31 | 35 (54) | 21 (32) | 9 (14) | 3 | - | |||
| Support | Pore Volume (mL/g) |
|---|---|
| AC | 0.26 |
| CNFs-PS | 1.45 |
| CNFs-HHT | 1.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattaneo, S.; Naslhajian, H.; Somodi, F.; Evangelisti, C.; Villa, A.; Prati, L. Ruthenium on Carbonaceous Materials for the Selective Hydrogenation of HMF. Molecules 2018, 23, 2007. https://doi.org/10.3390/molecules23082007
Cattaneo S, Naslhajian H, Somodi F, Evangelisti C, Villa A, Prati L. Ruthenium on Carbonaceous Materials for the Selective Hydrogenation of HMF. Molecules. 2018; 23(8):2007. https://doi.org/10.3390/molecules23082007
Chicago/Turabian StyleCattaneo, Stefano, Hadi Naslhajian, Ferenc Somodi, Claudio Evangelisti, Alberto Villa, and Laura Prati. 2018. "Ruthenium on Carbonaceous Materials for the Selective Hydrogenation of HMF" Molecules 23, no. 8: 2007. https://doi.org/10.3390/molecules23082007
APA StyleCattaneo, S., Naslhajian, H., Somodi, F., Evangelisti, C., Villa, A., & Prati, L. (2018). Ruthenium on Carbonaceous Materials for the Selective Hydrogenation of HMF. Molecules, 23(8), 2007. https://doi.org/10.3390/molecules23082007









