Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates
Abstract
Introduction
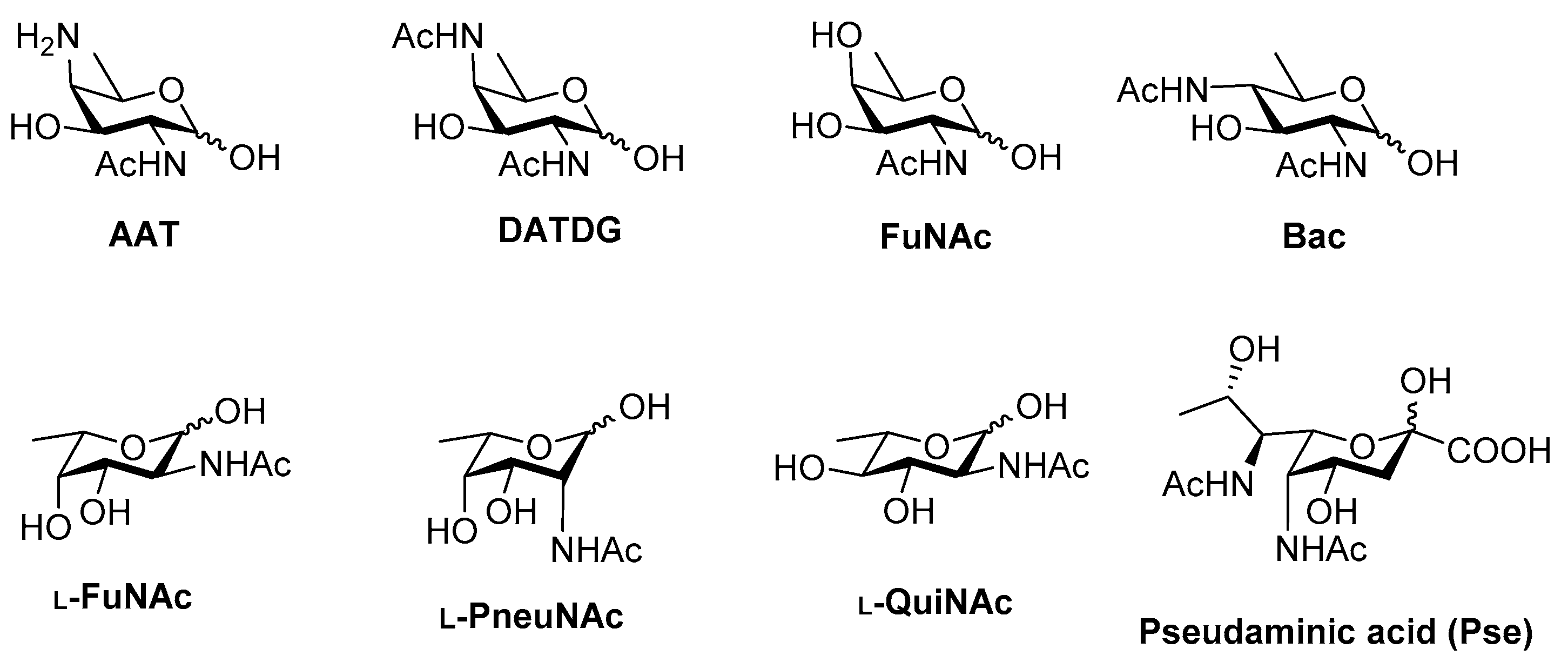
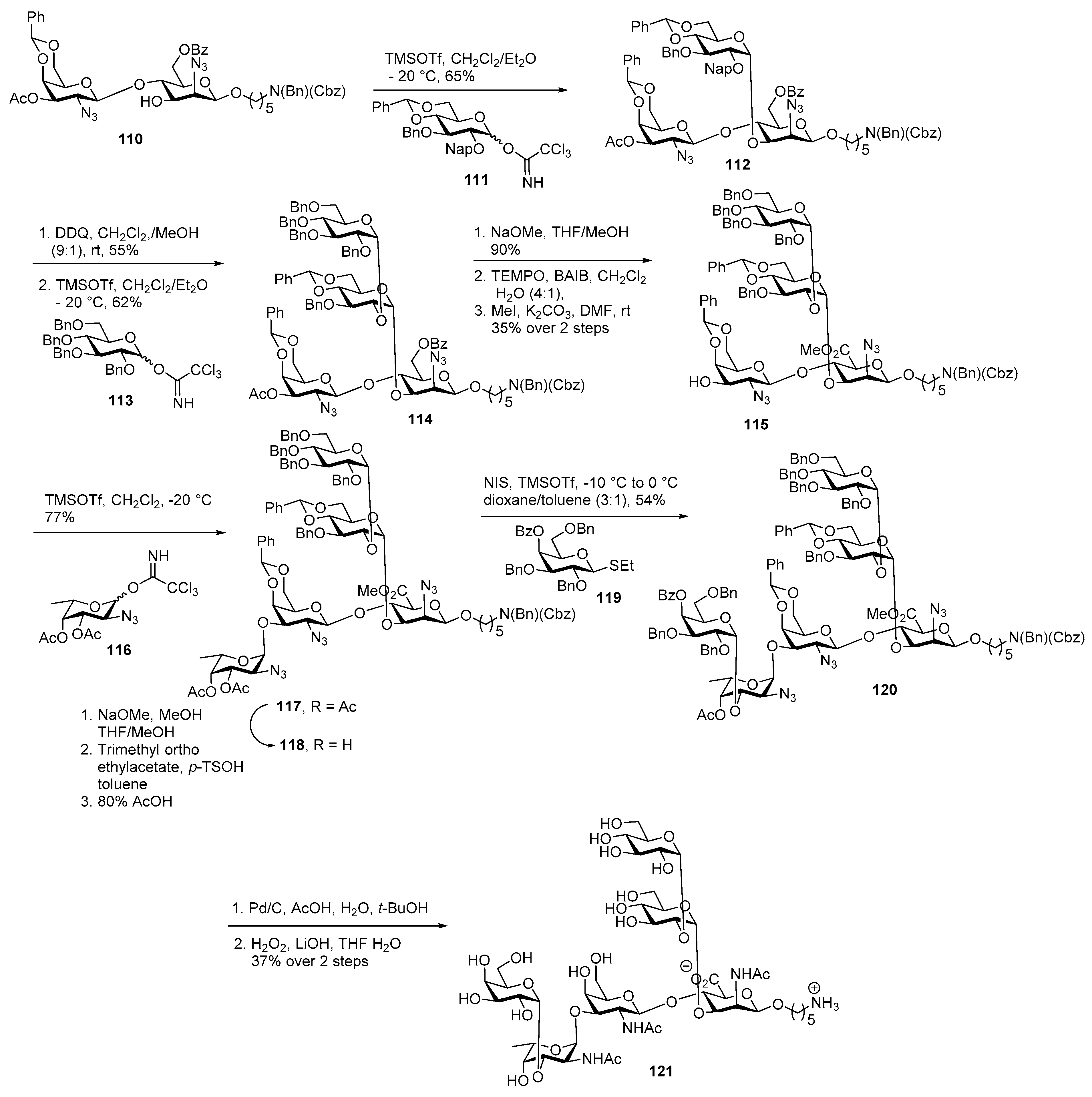
Campylobacter jejuni Heptasaccharide
Zwitterionic Polysaccharides
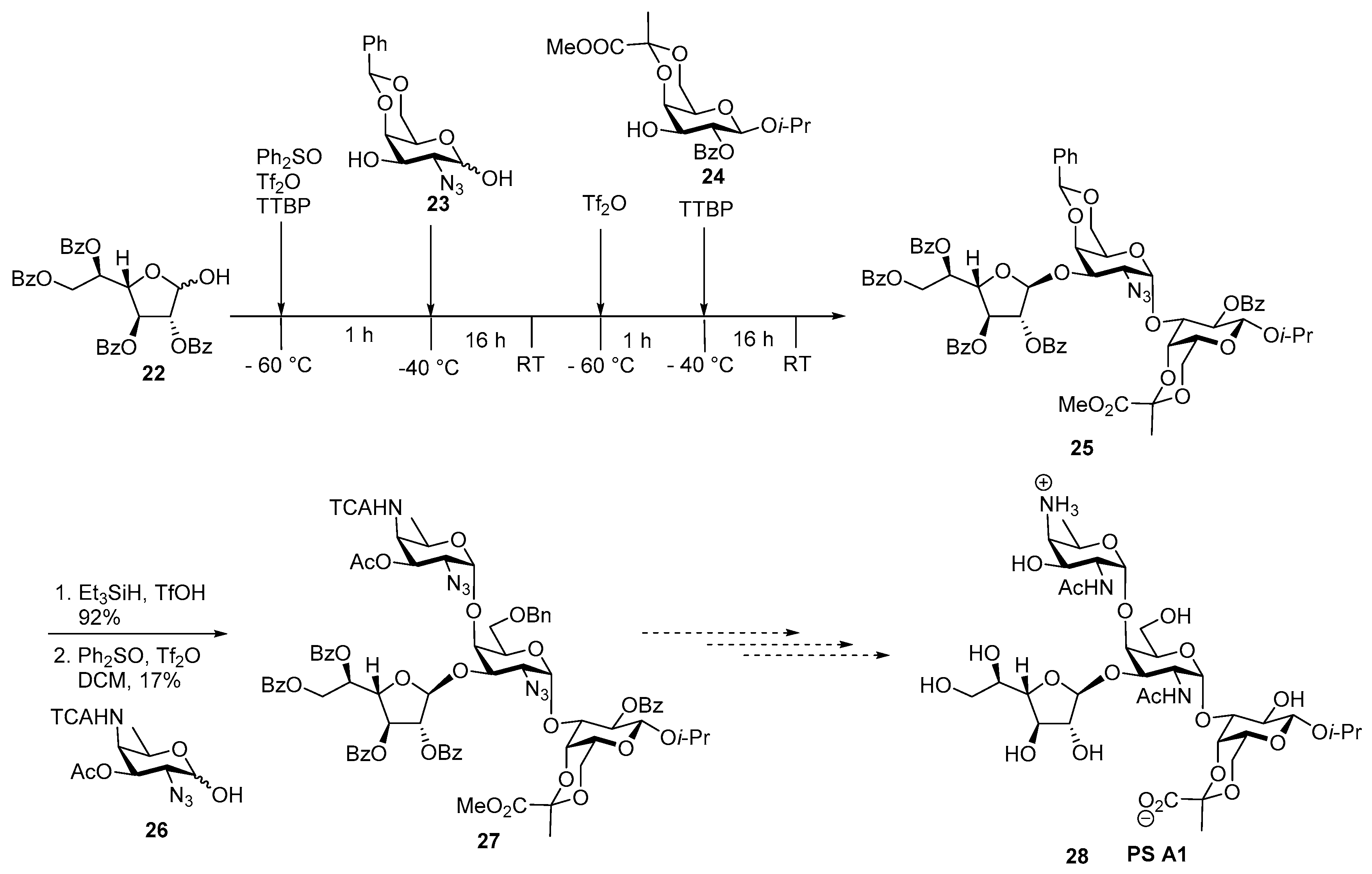
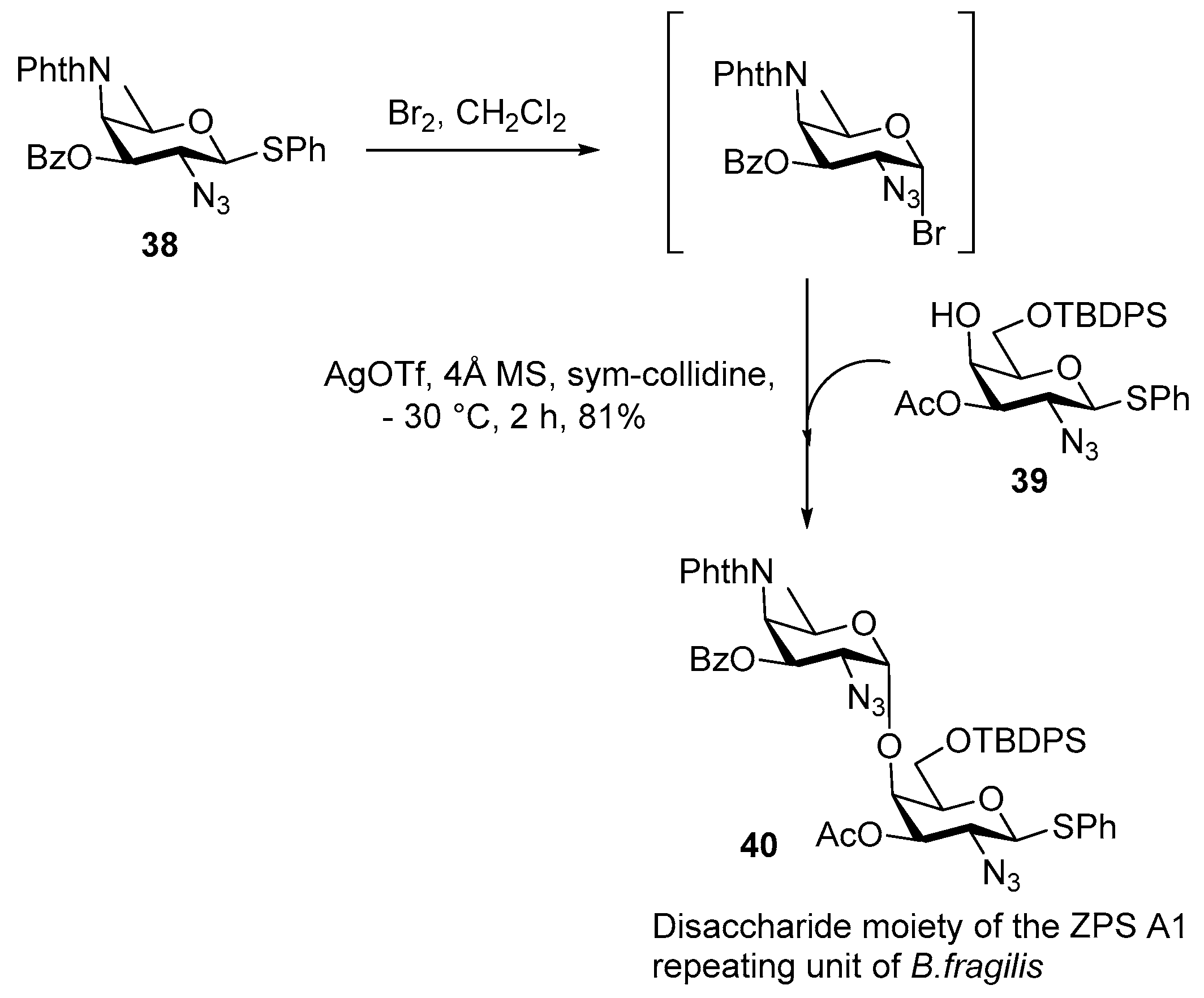
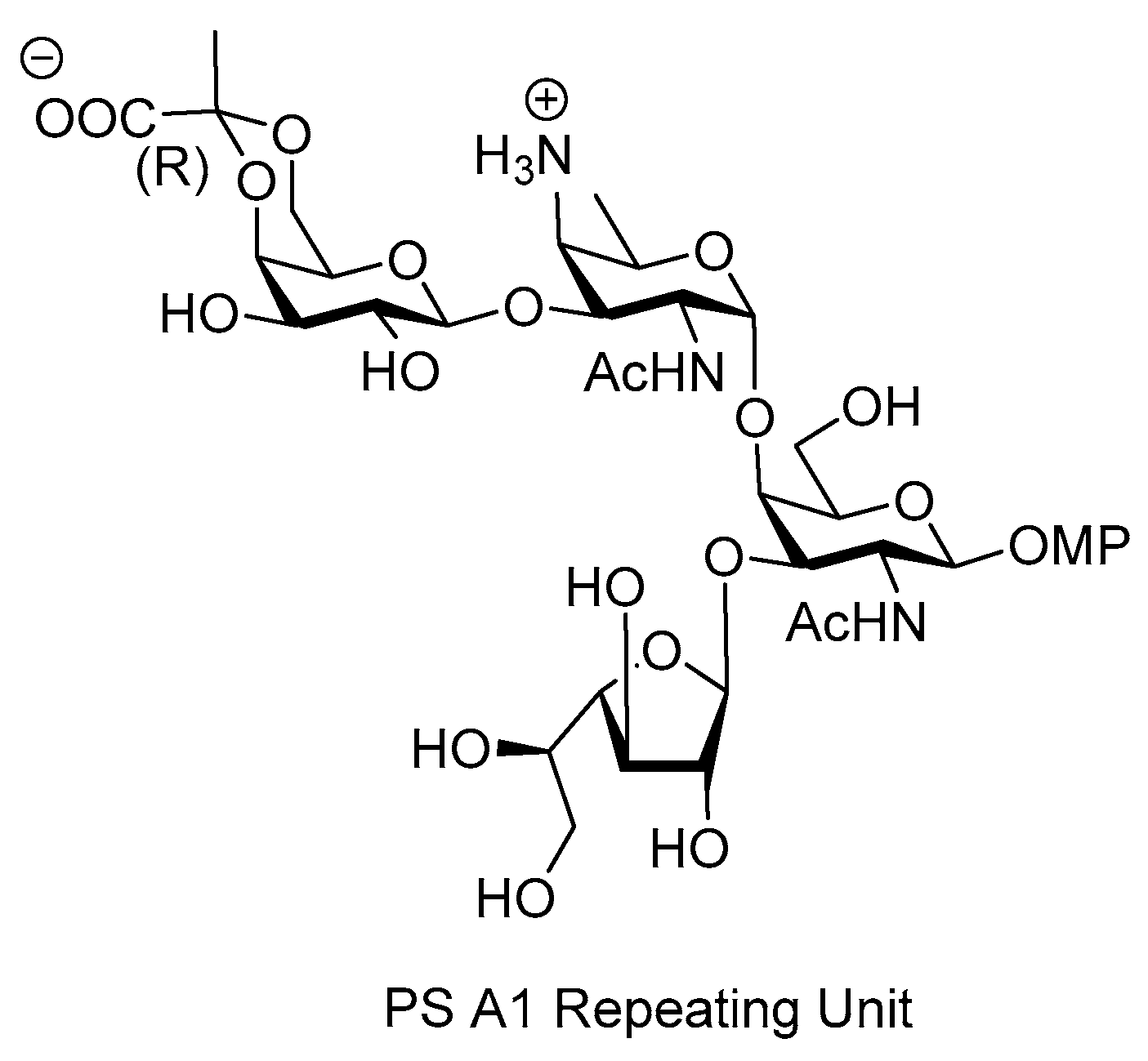
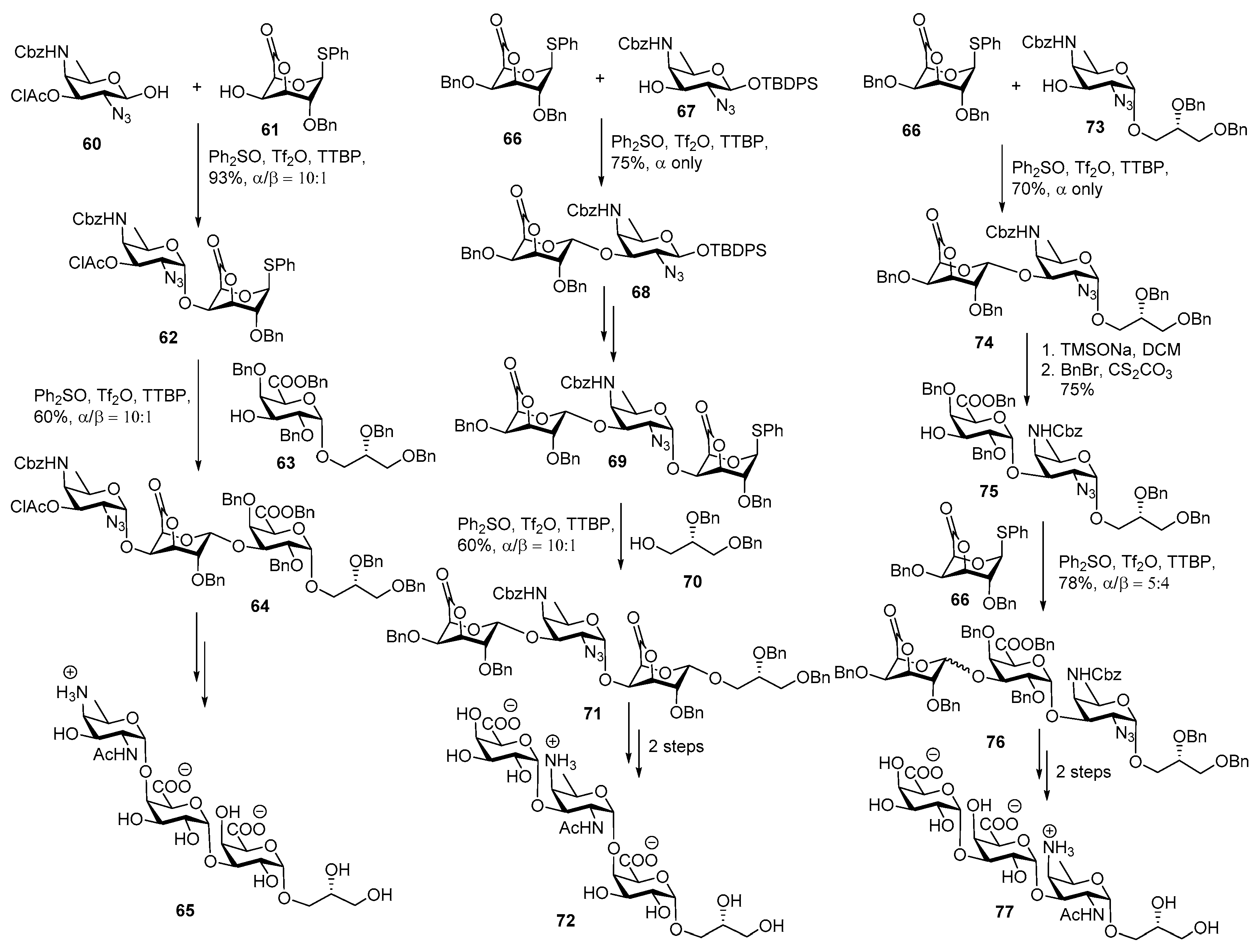
ZPS of Bacteroides fragilis
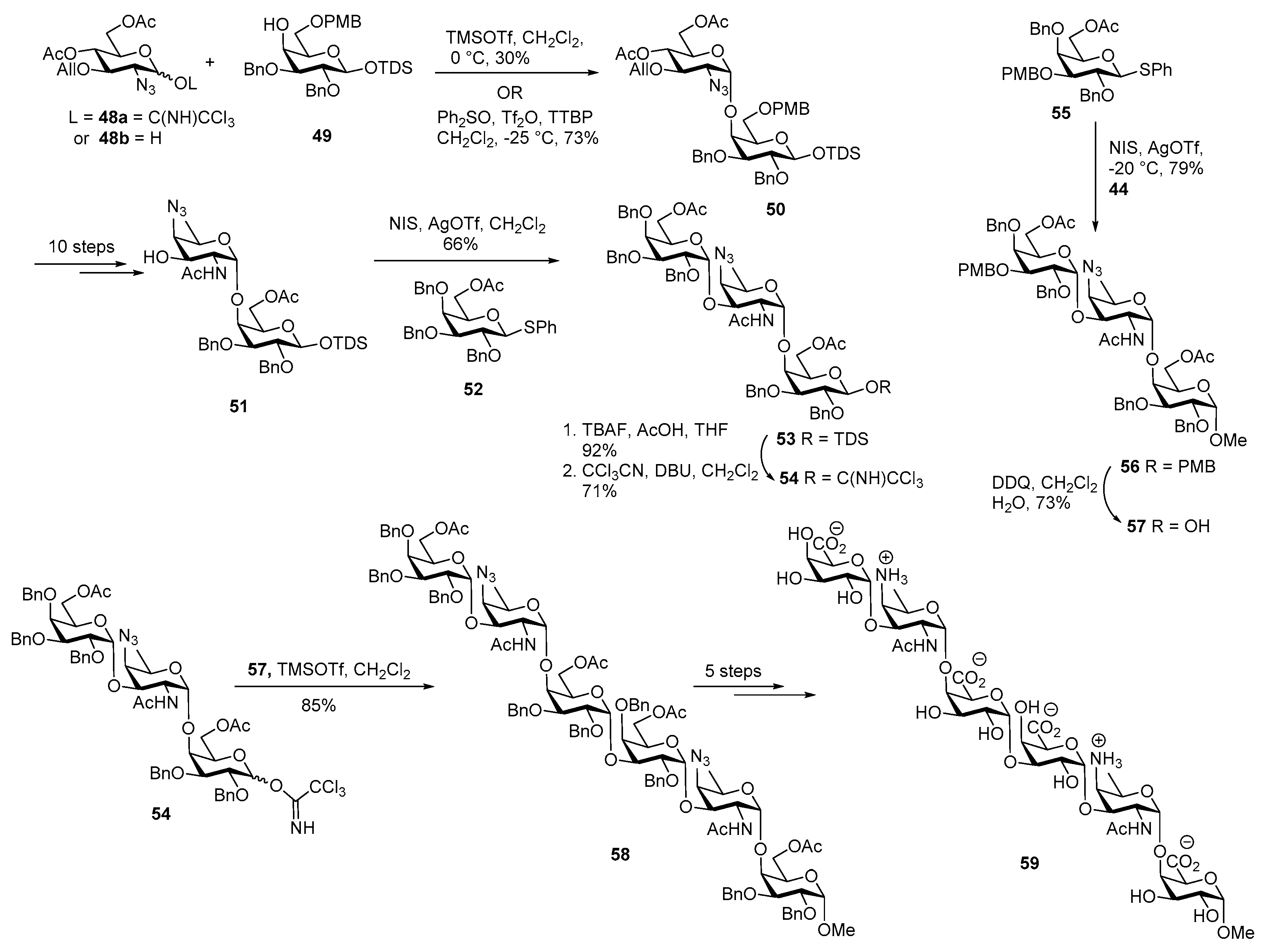
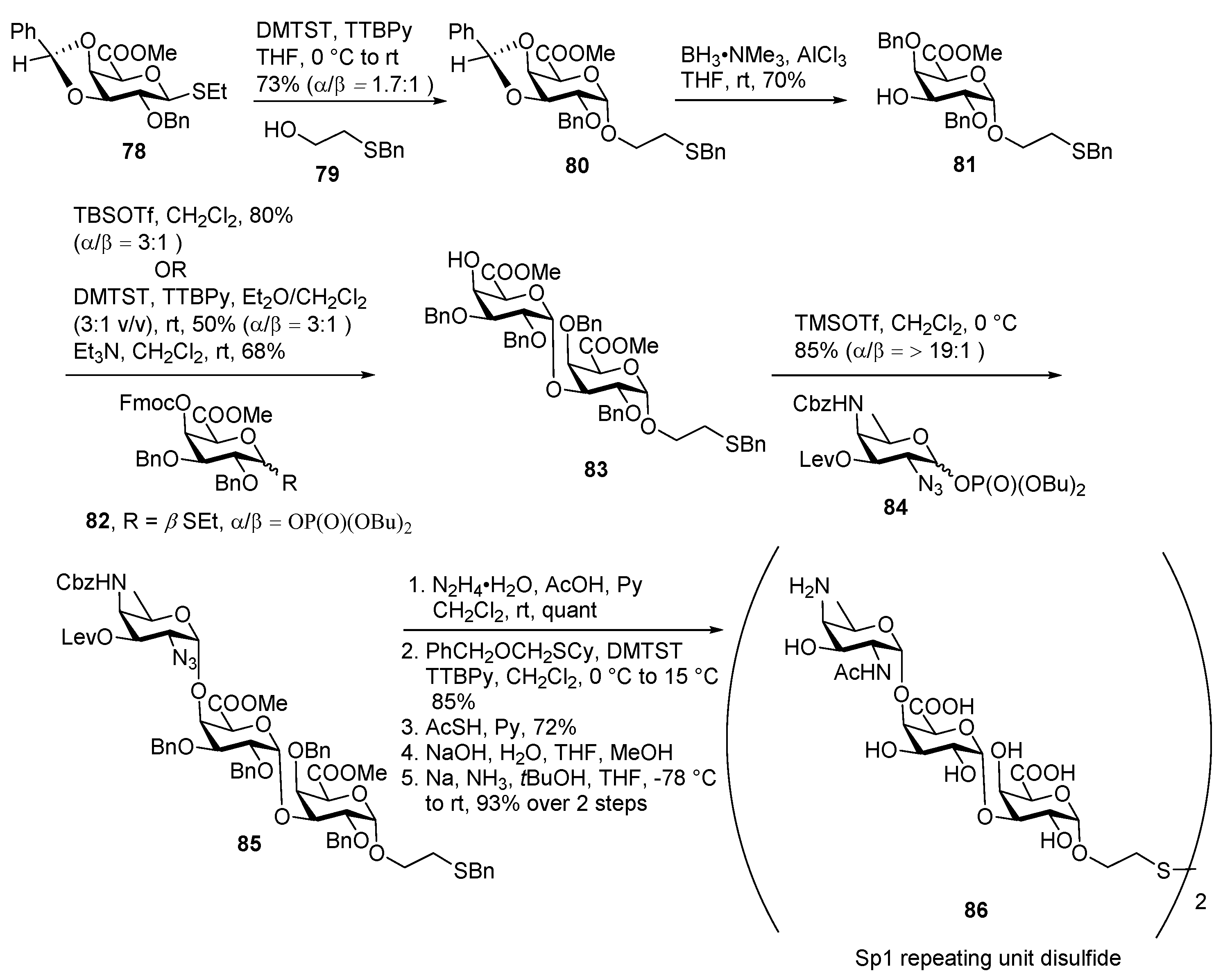
ZPS of Streptococcus pneumoniae
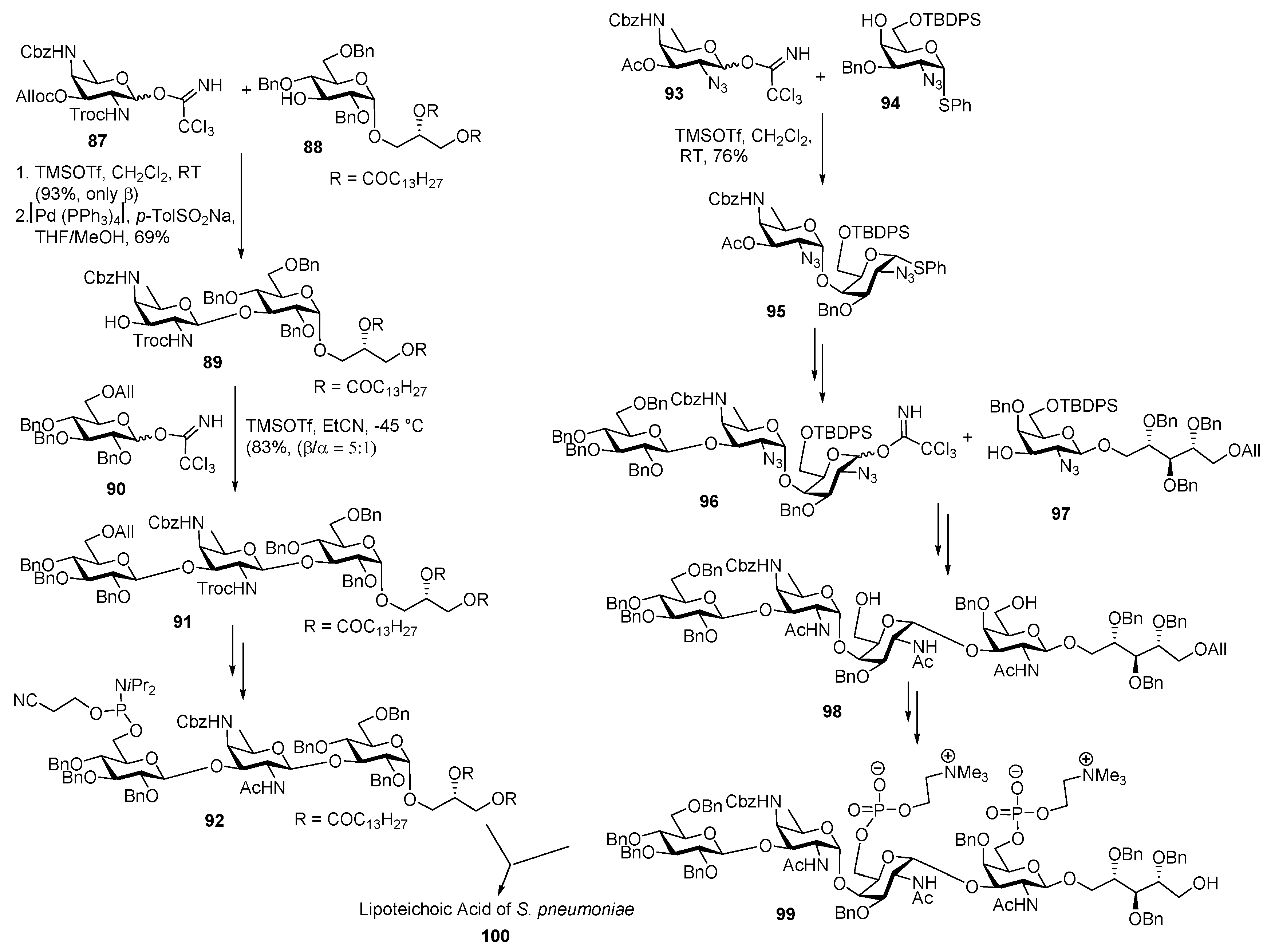
Lipoteichoic Acid of Streptococcus pneumoniae
CPS of Streptococcus pneumoniae Serotype 4
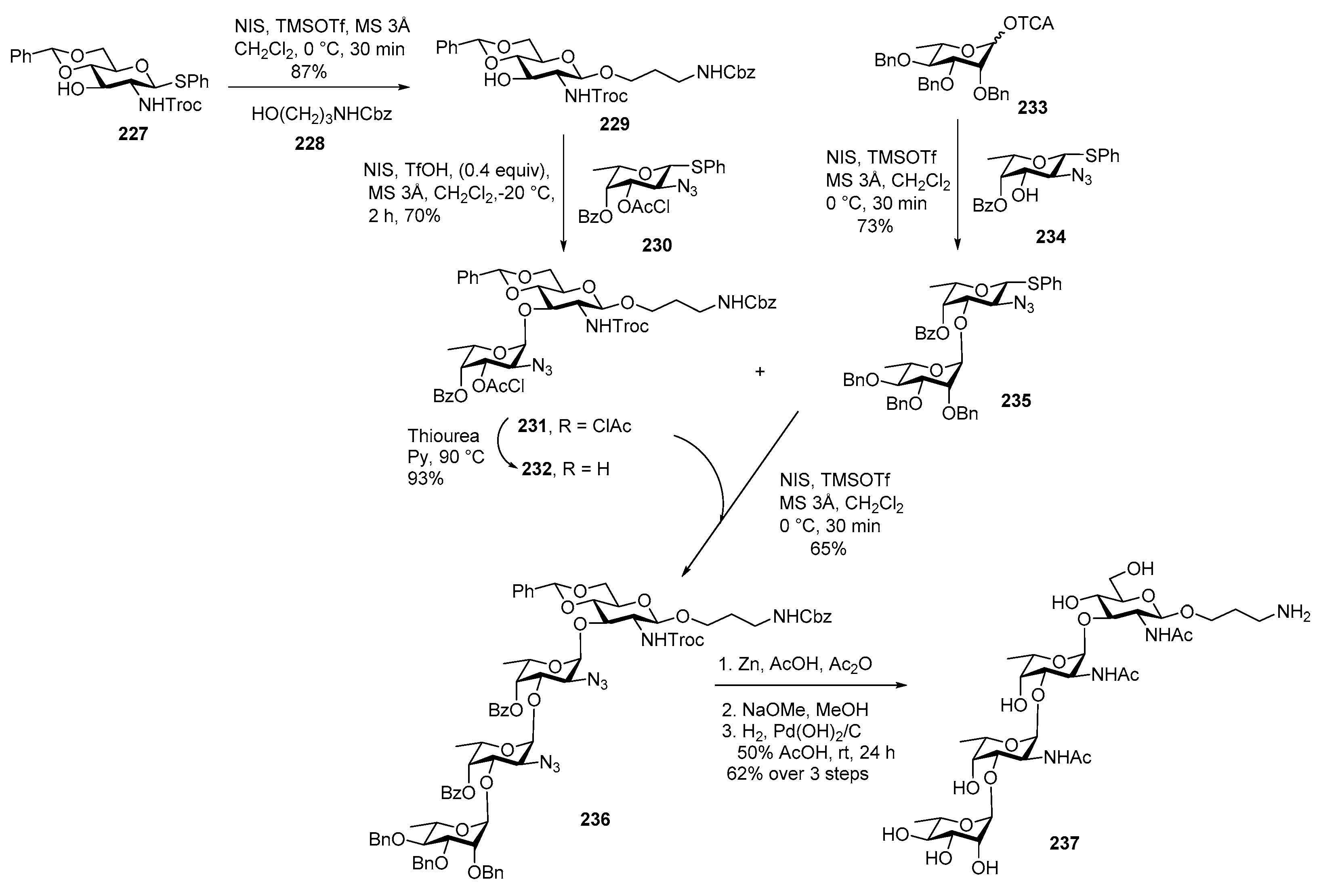
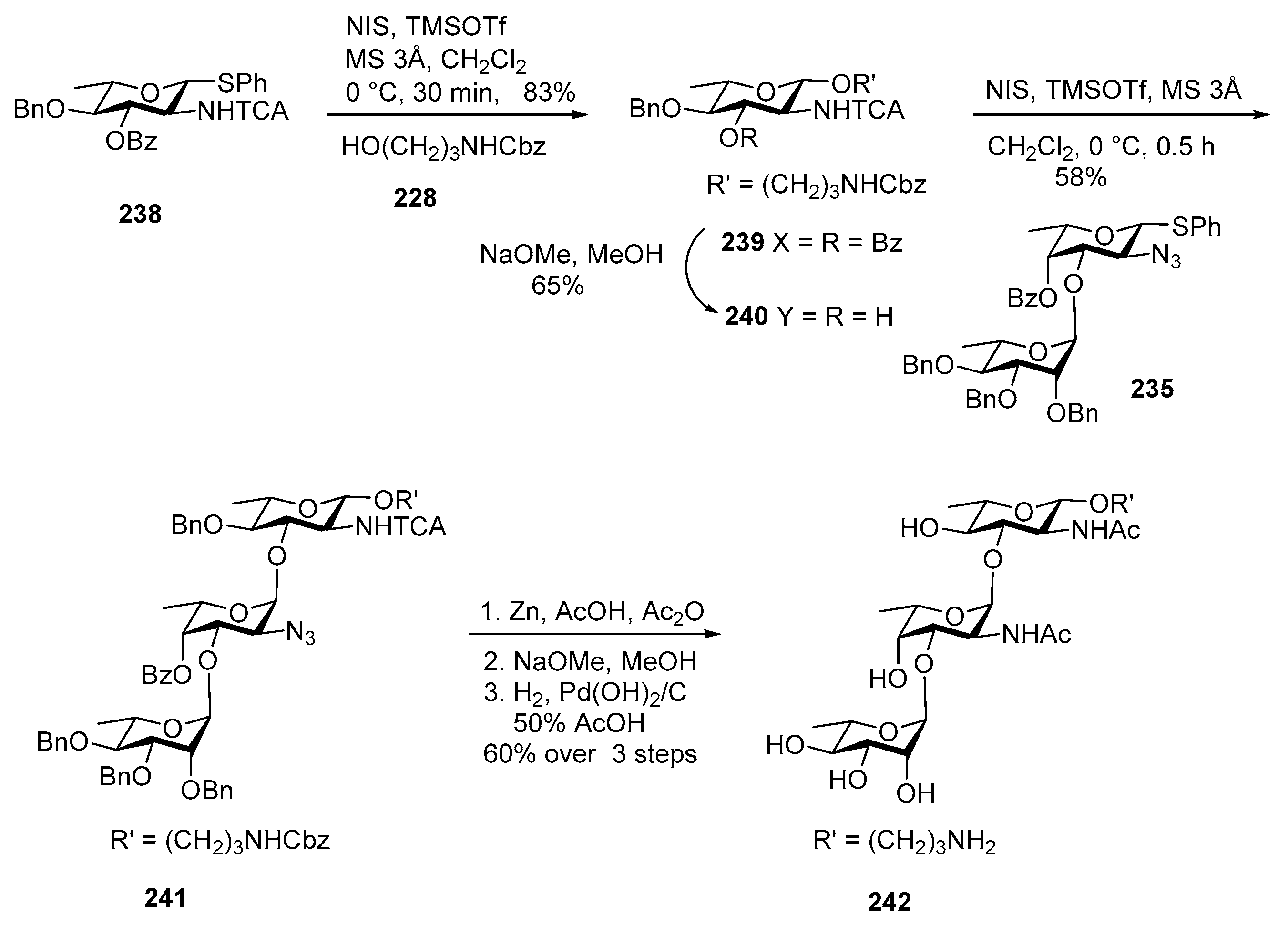
Streptococcus pneumoniae Serotype 12F CPS
CPS of Streptococcus pneumoniae Serotype 5
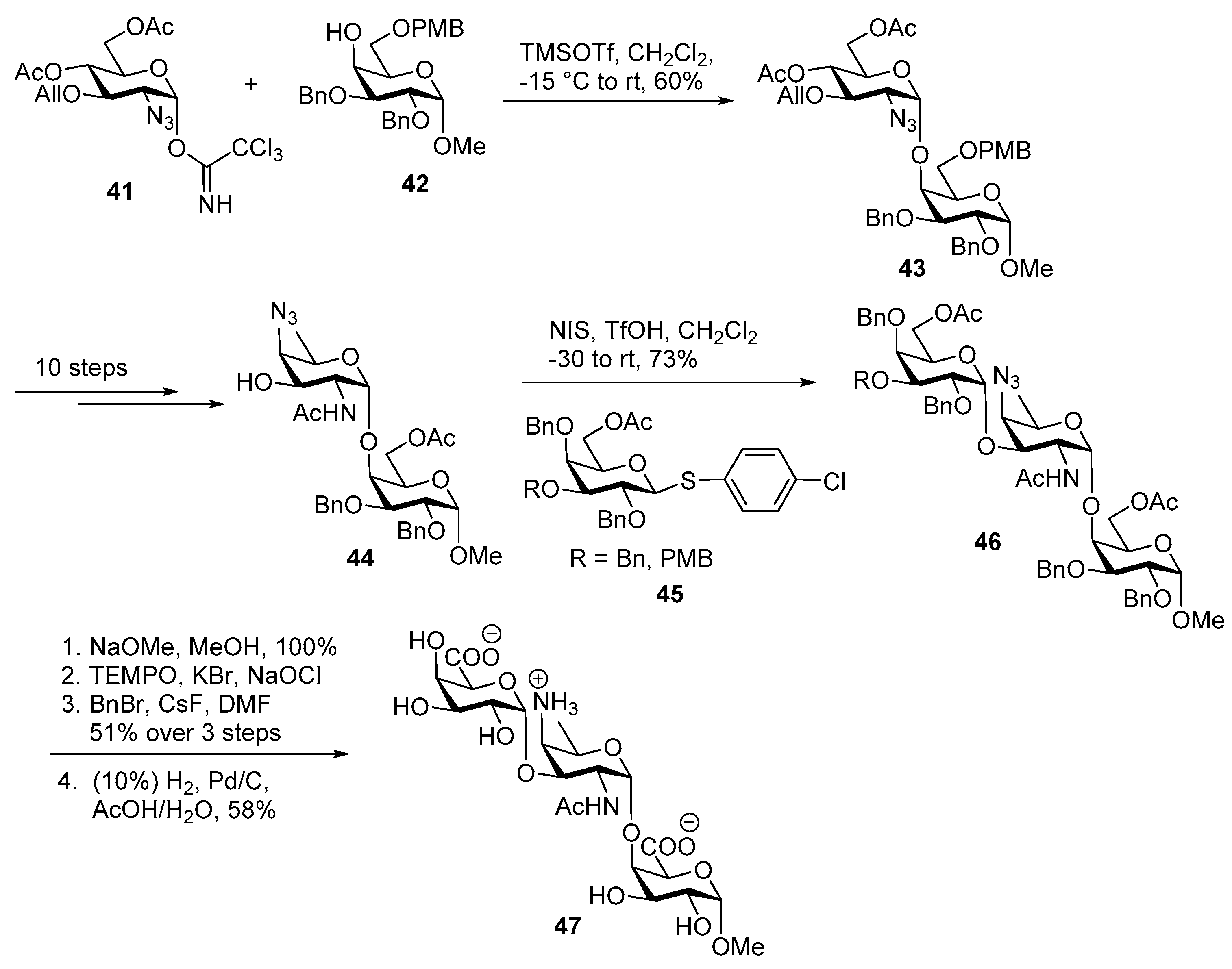
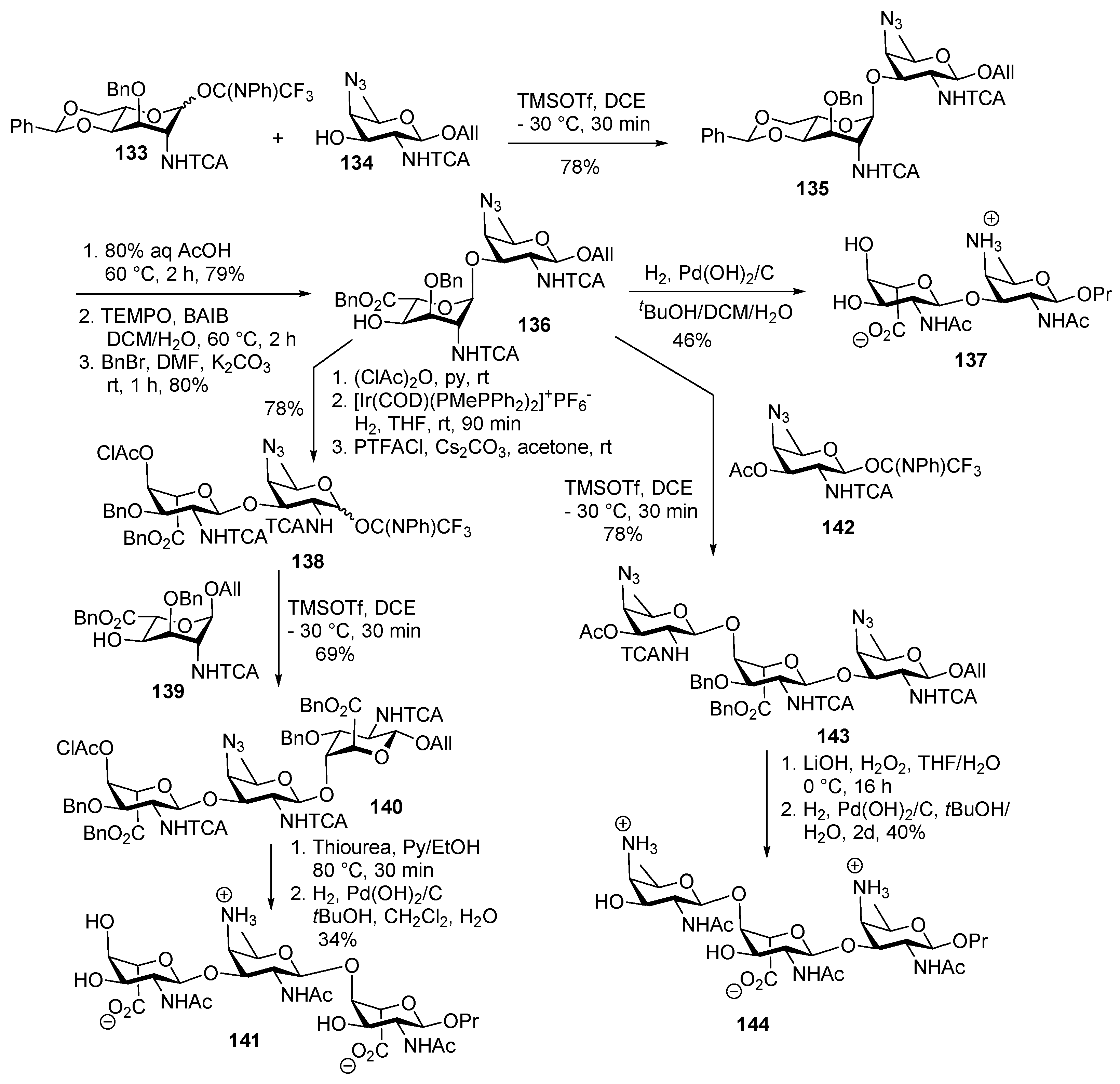
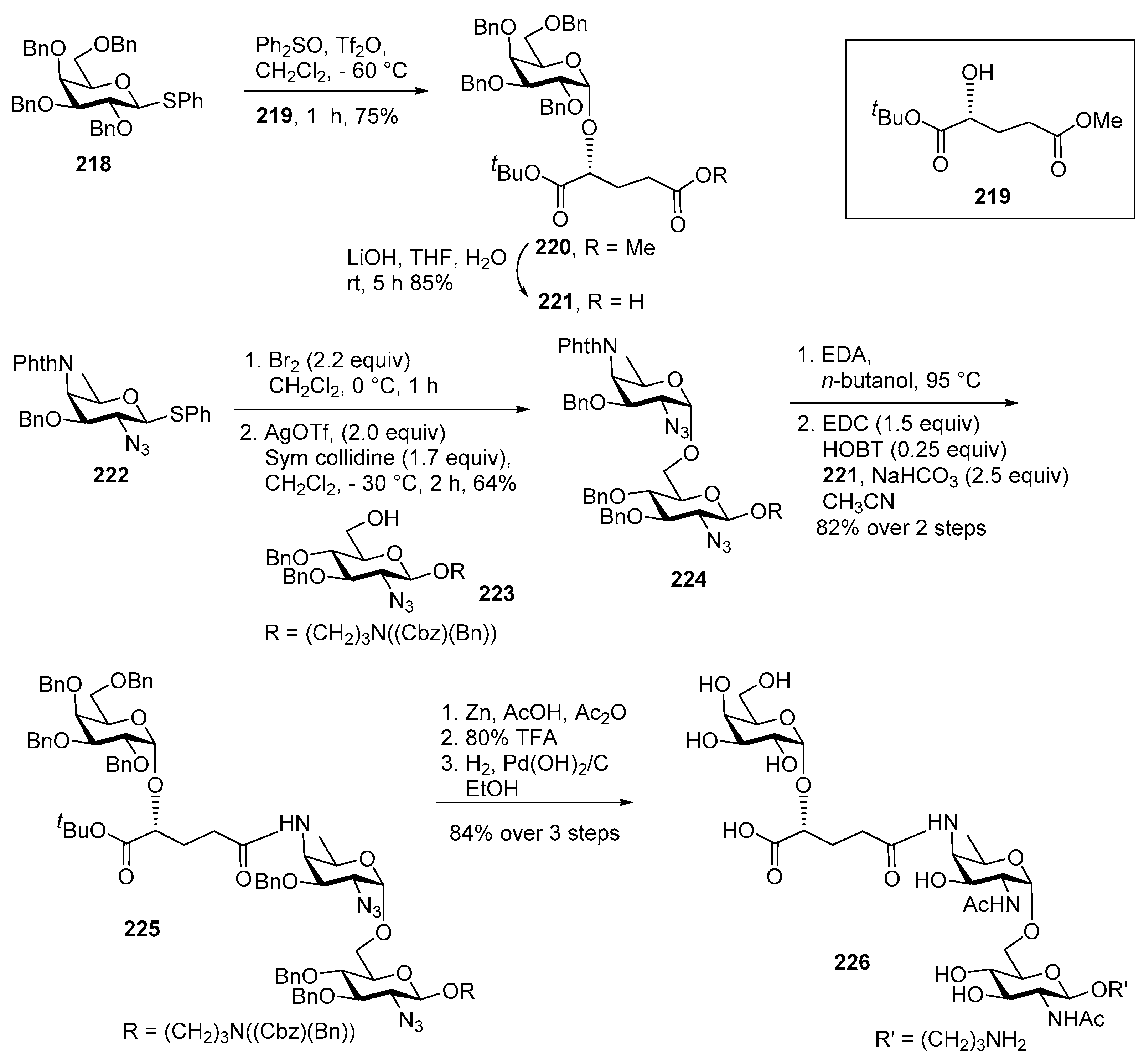
ZPS of Shigella sonnei
Synthesis of Disaccharide AB-Pr (1)
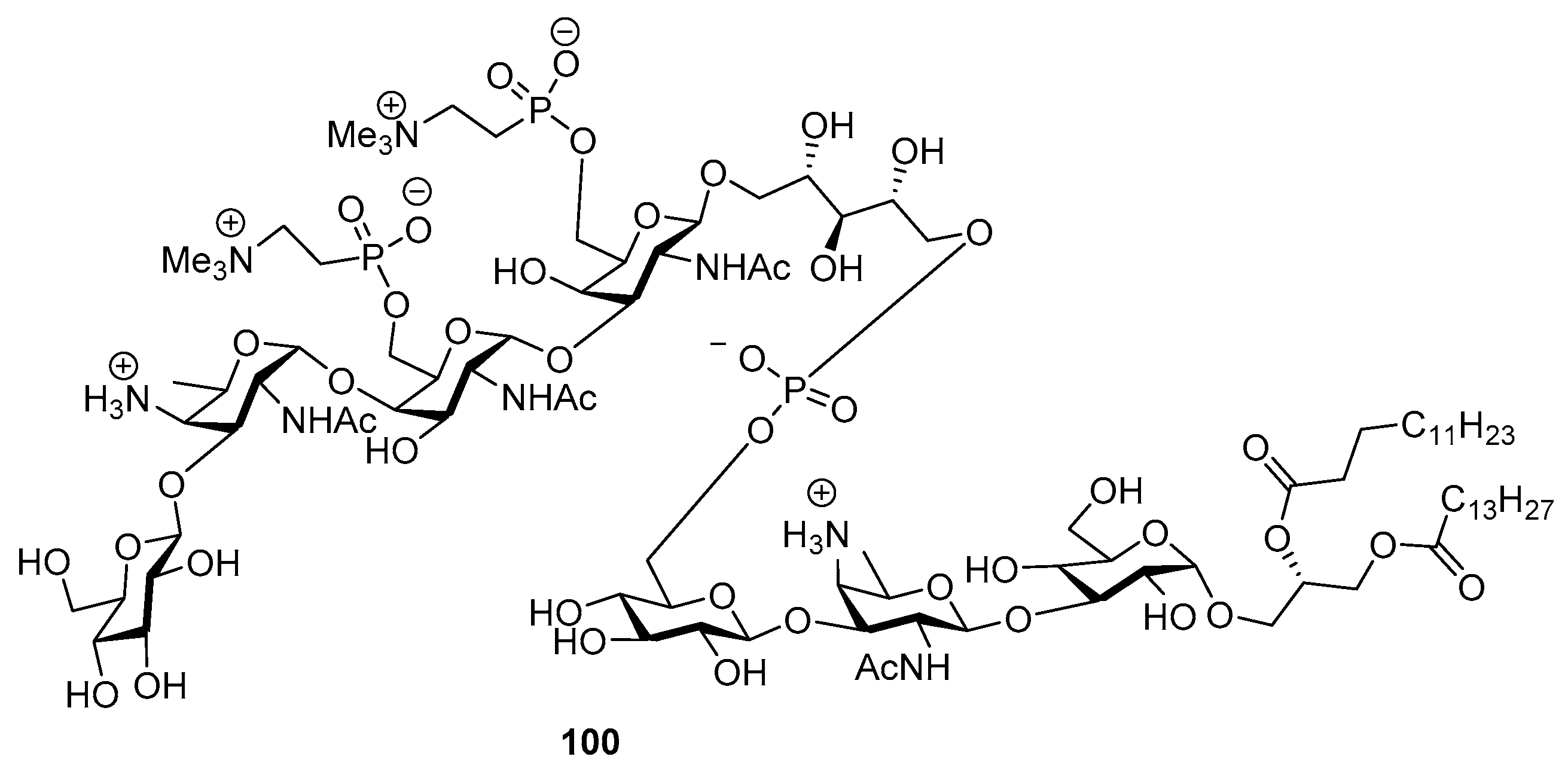
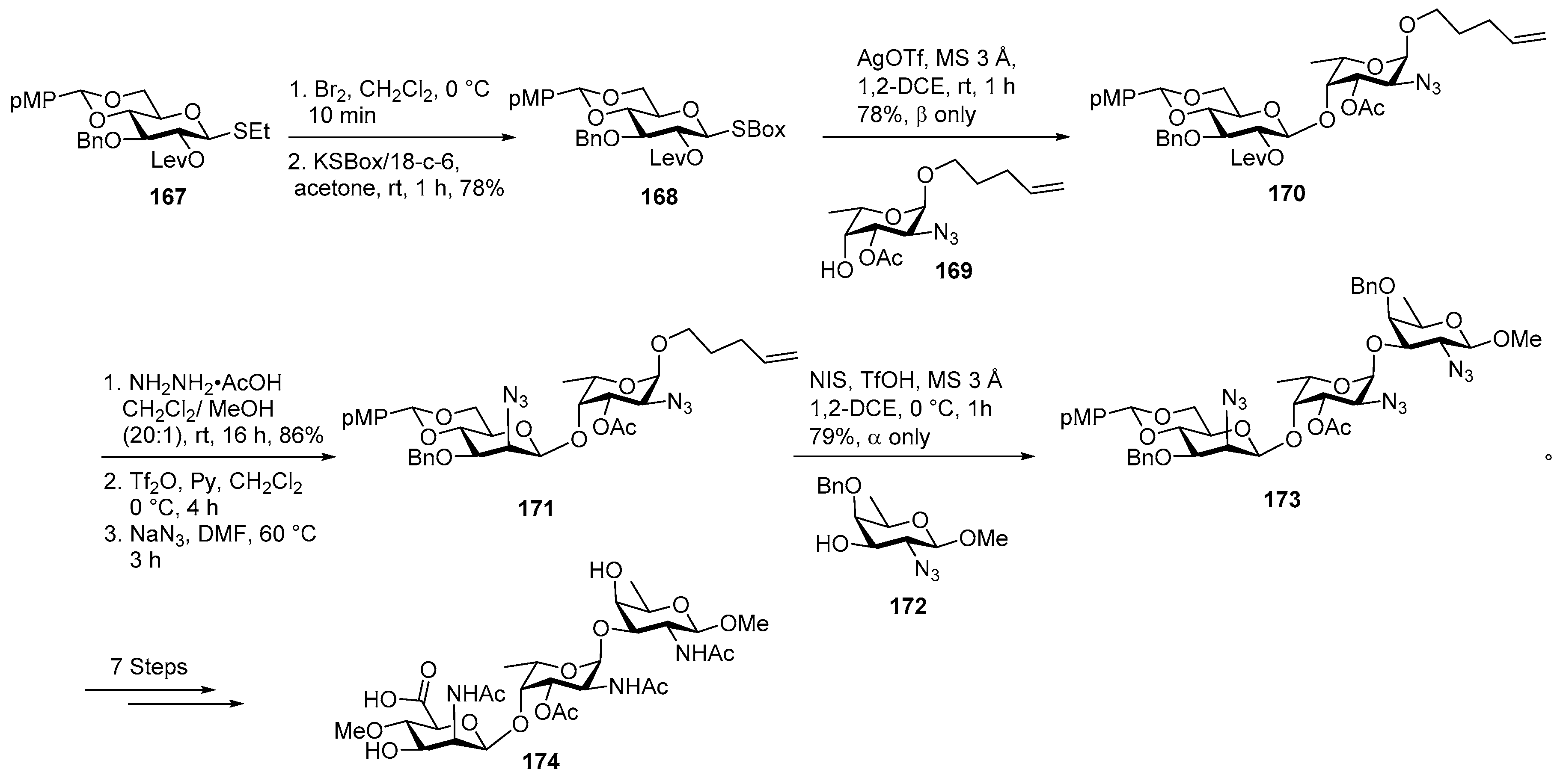
Phosphorylated ZPS of Providencia alcalifaciens O22
Staphylococcus aureus Type 5 Capsular Polysaccharide
Staphylococcus aureus Type 8 Capsular Polysaccharide
Staphylococcus aureus Strain M Capsular Polysaccharide
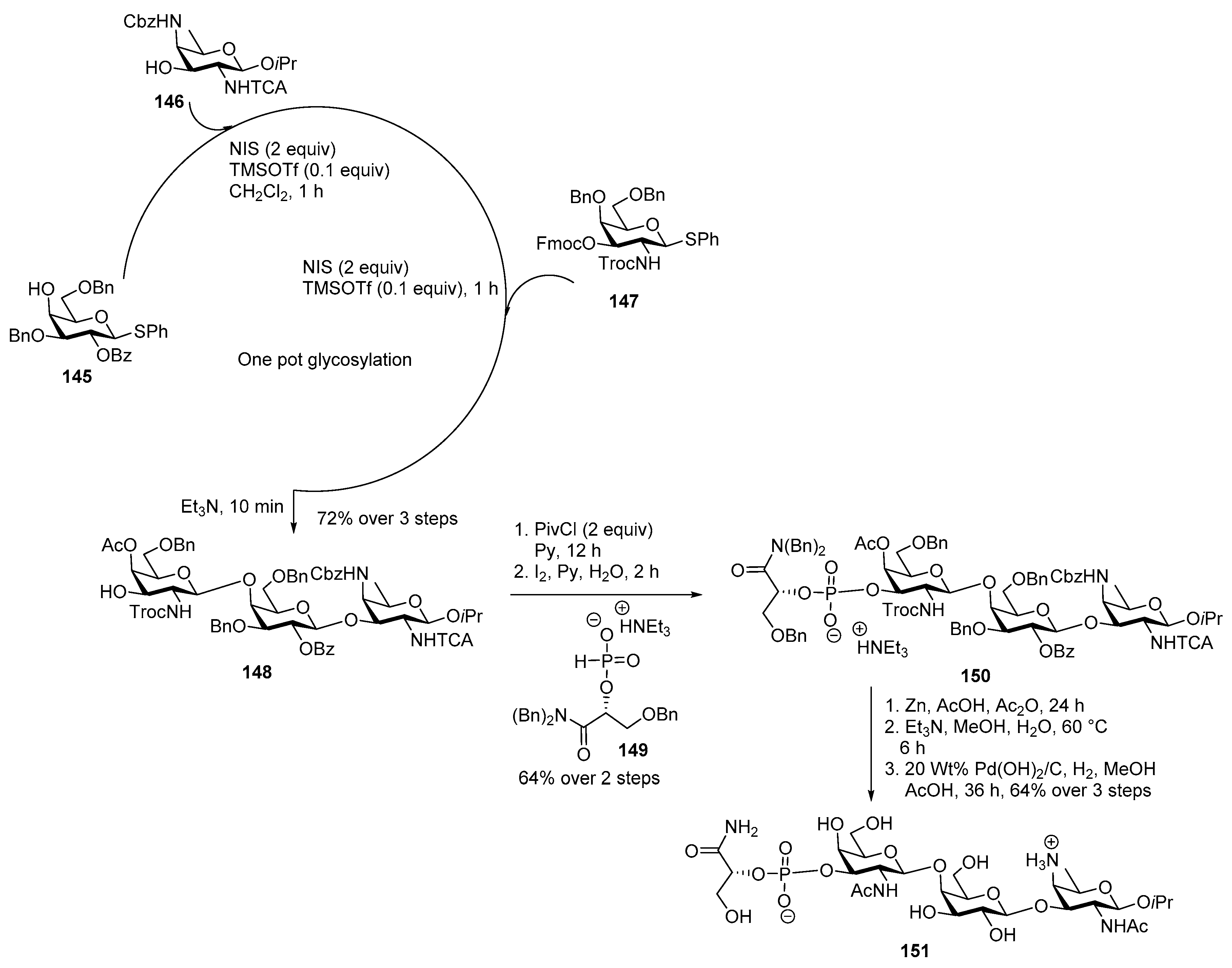
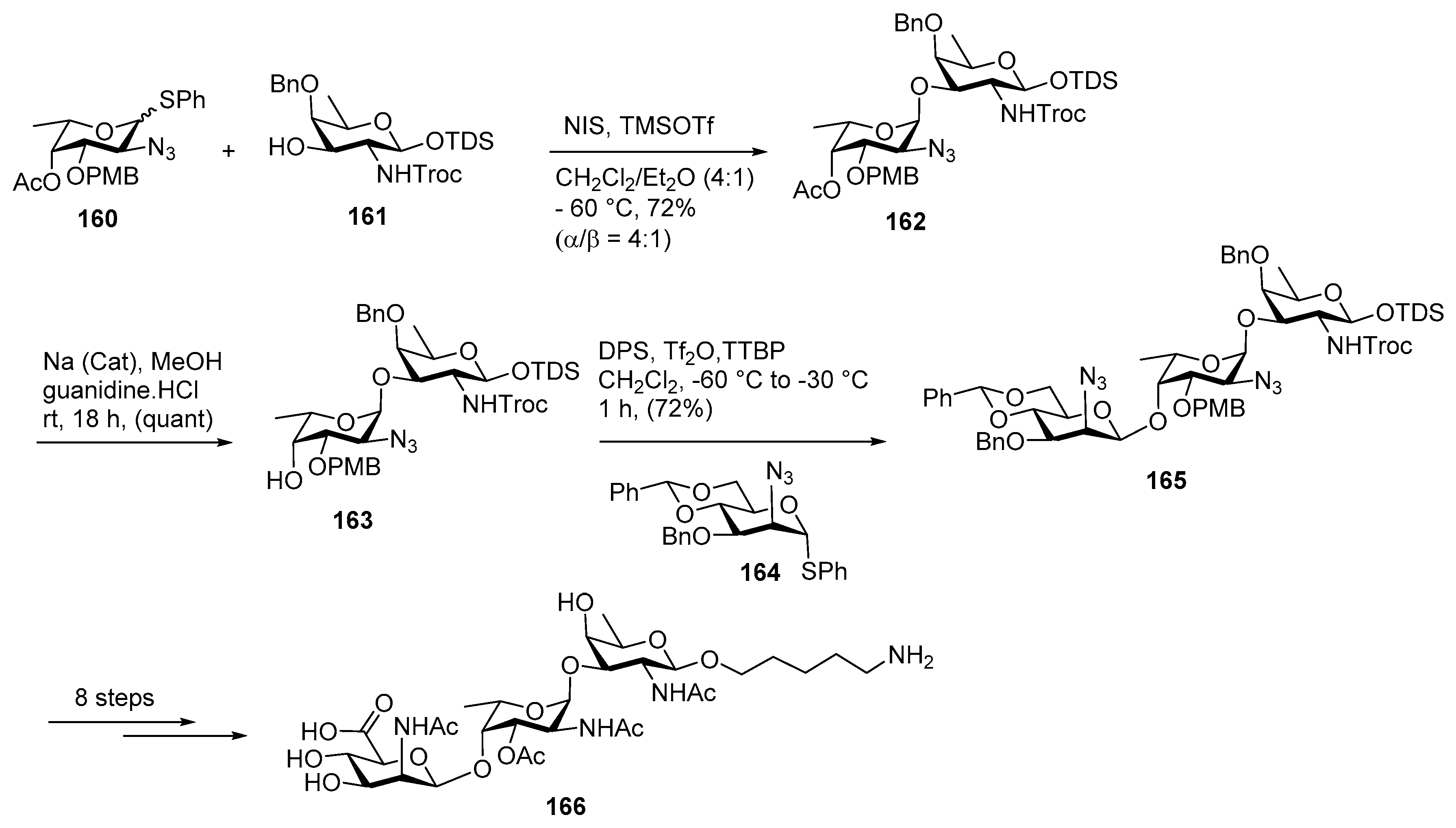
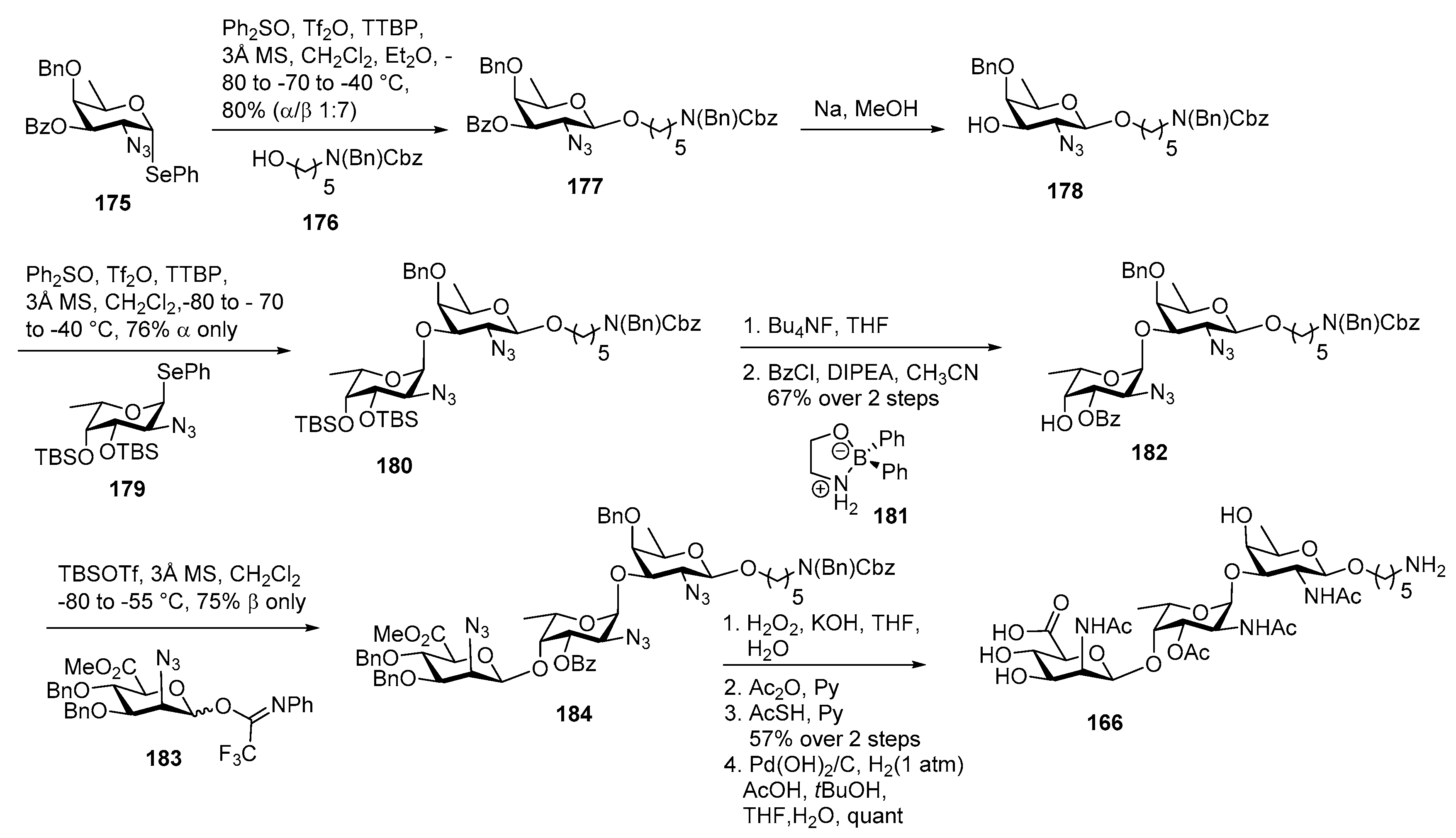
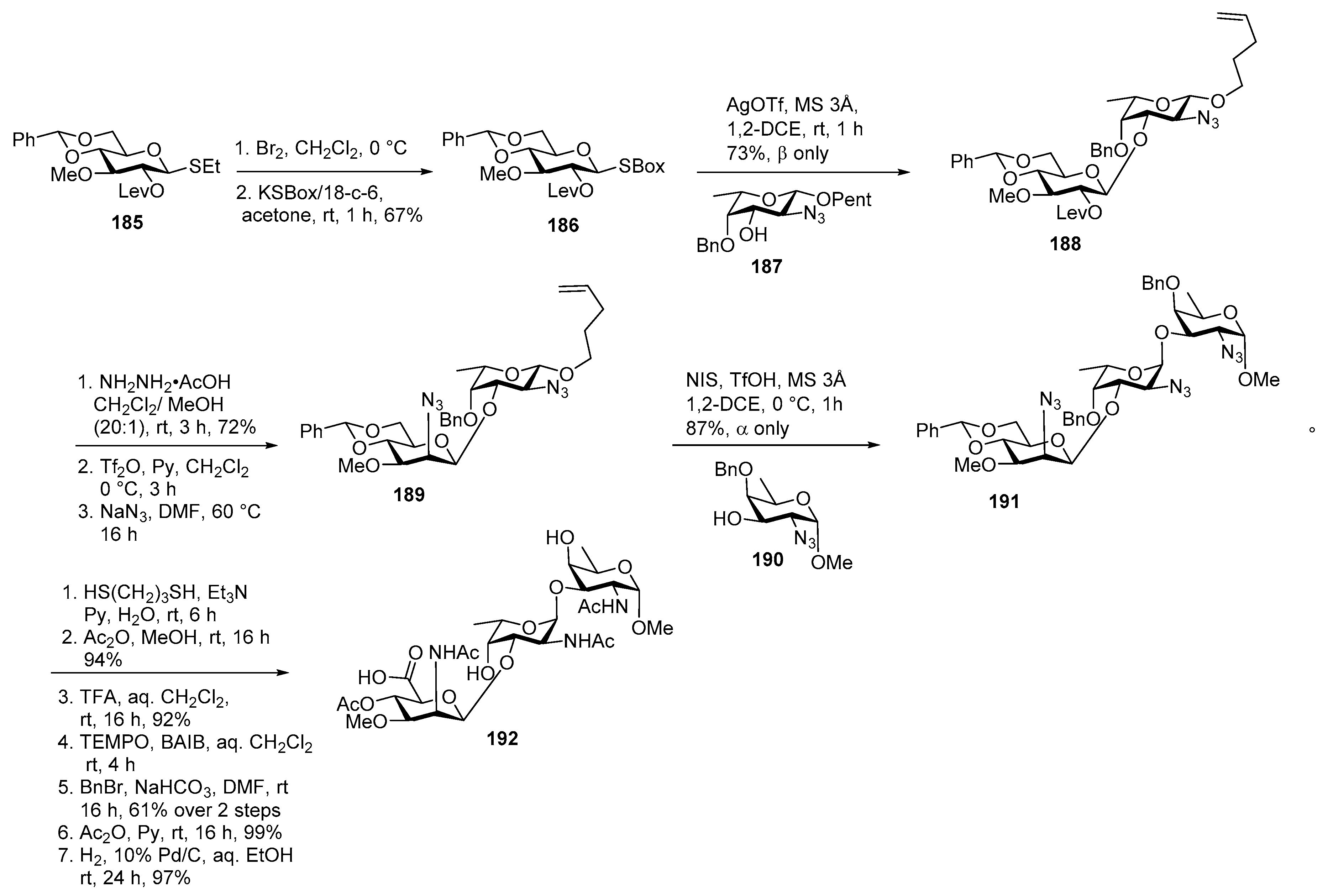
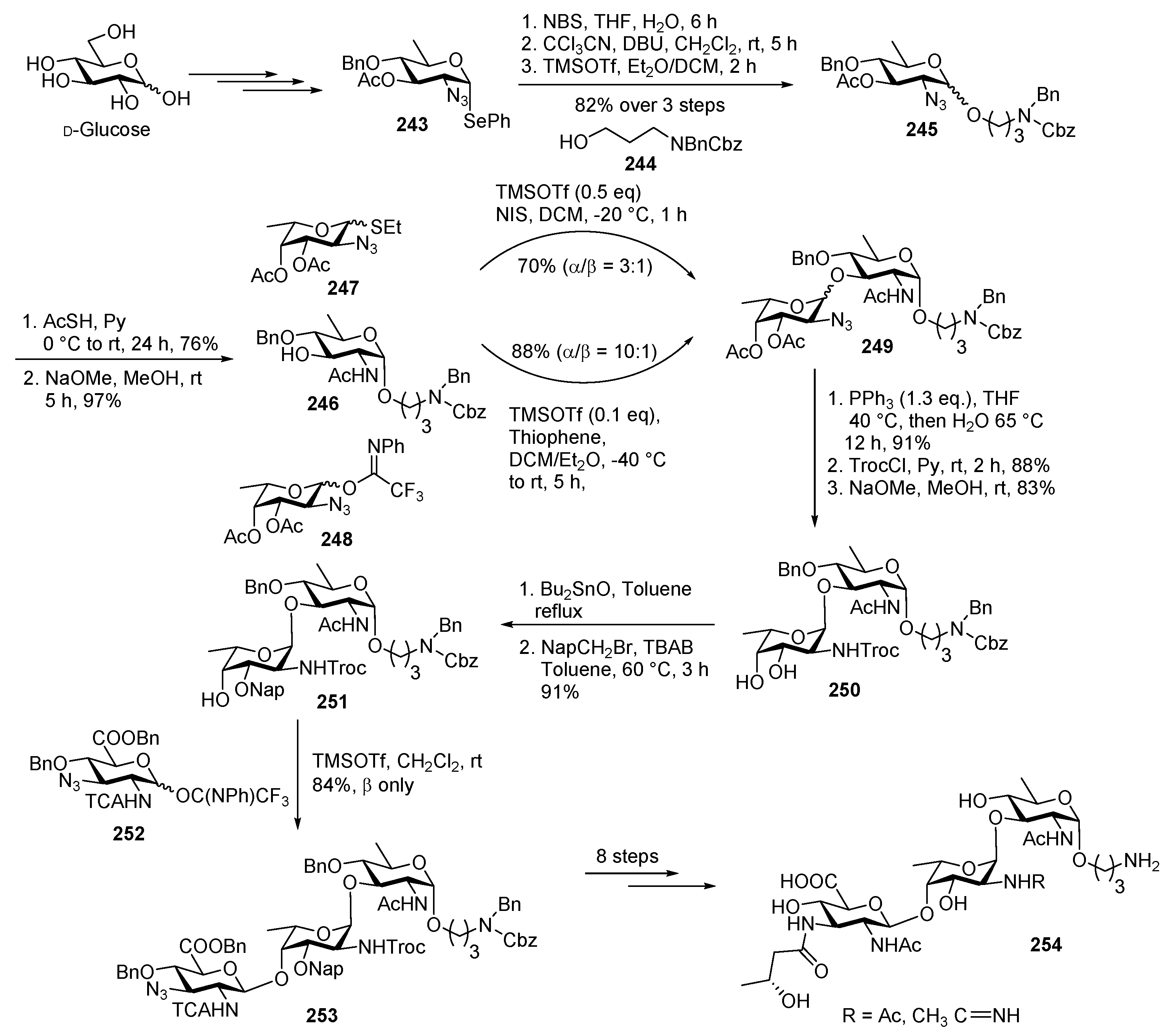
Neisseria meningitidis Pilin Glycans
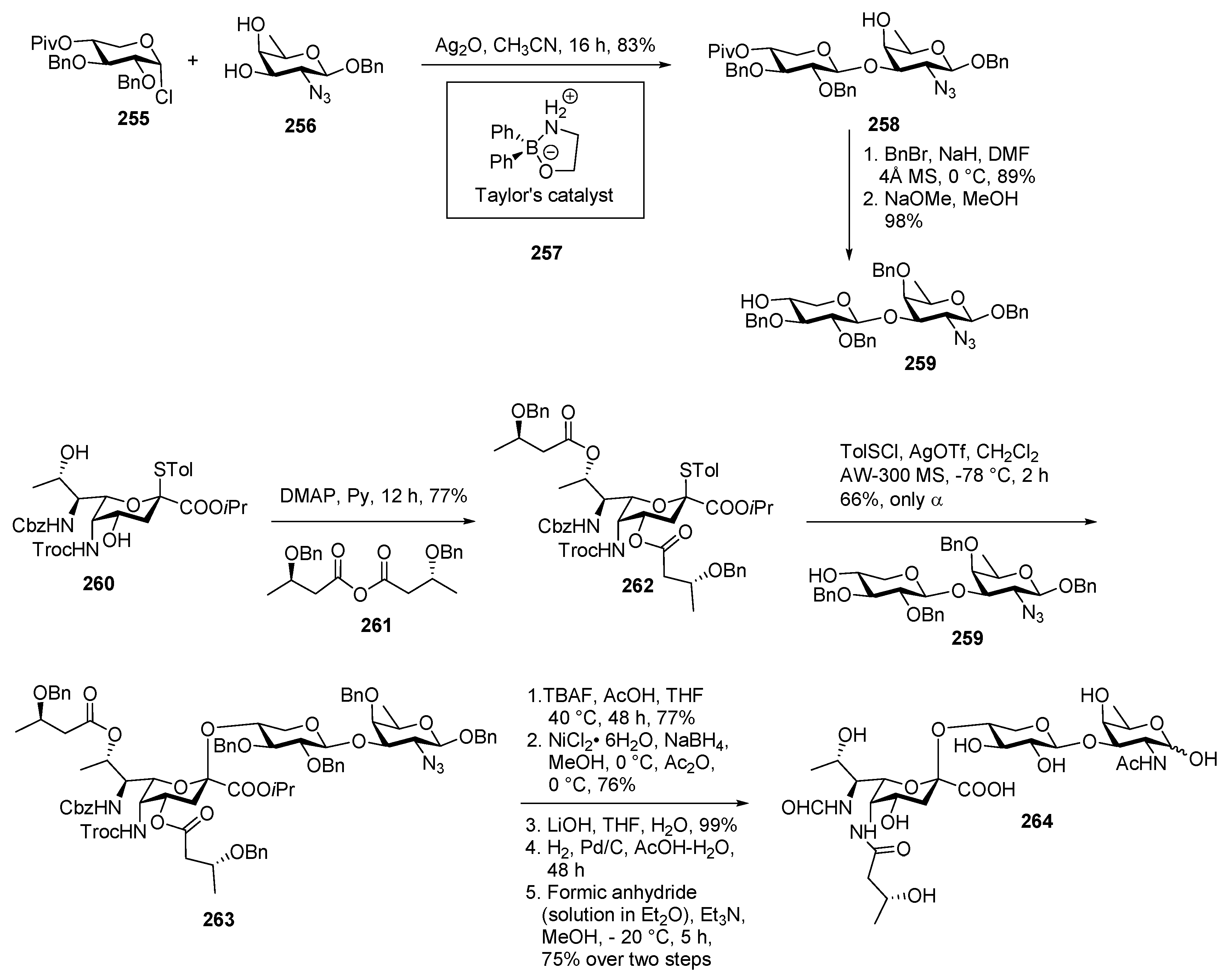
Bacillus cereus Ch HF-PS
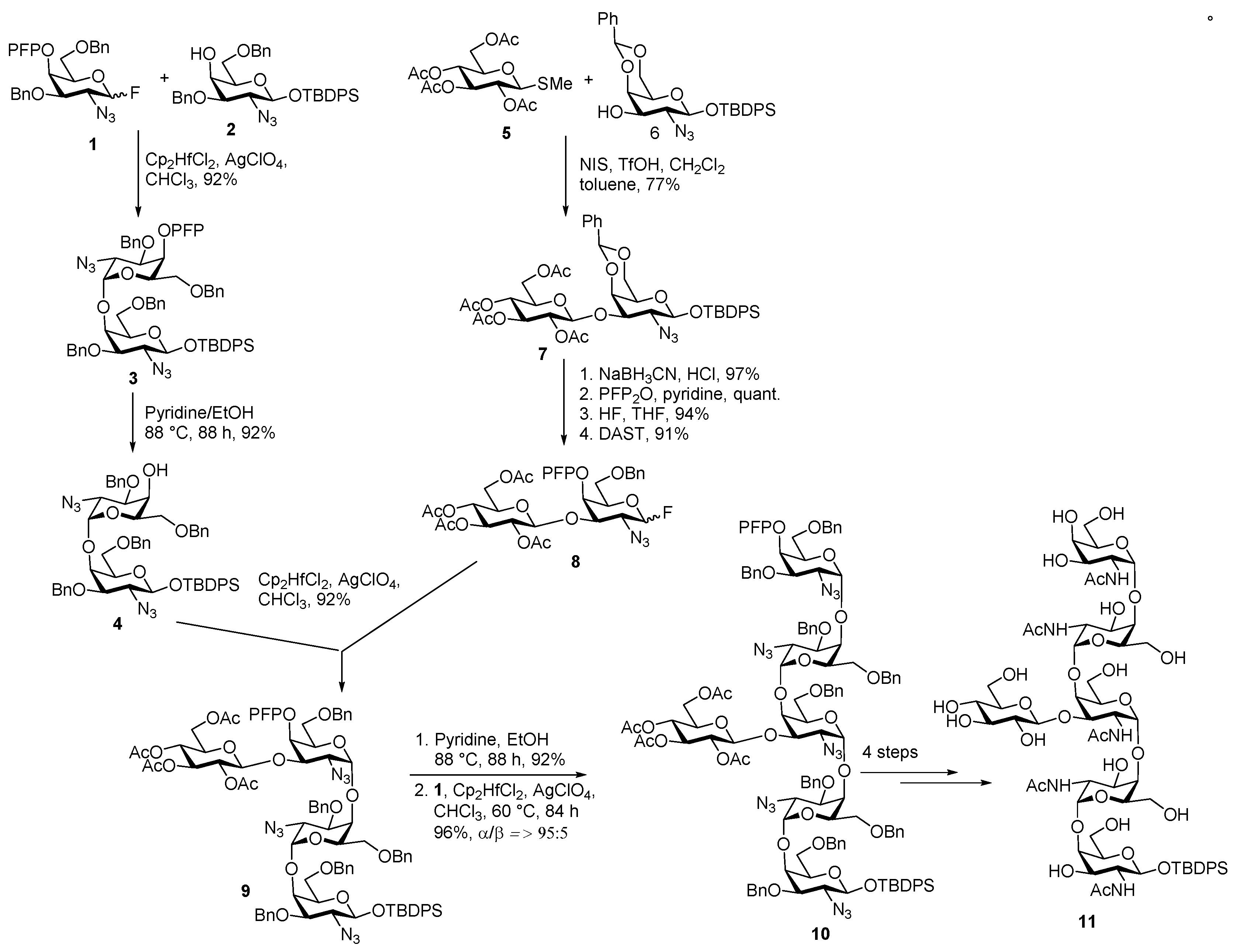
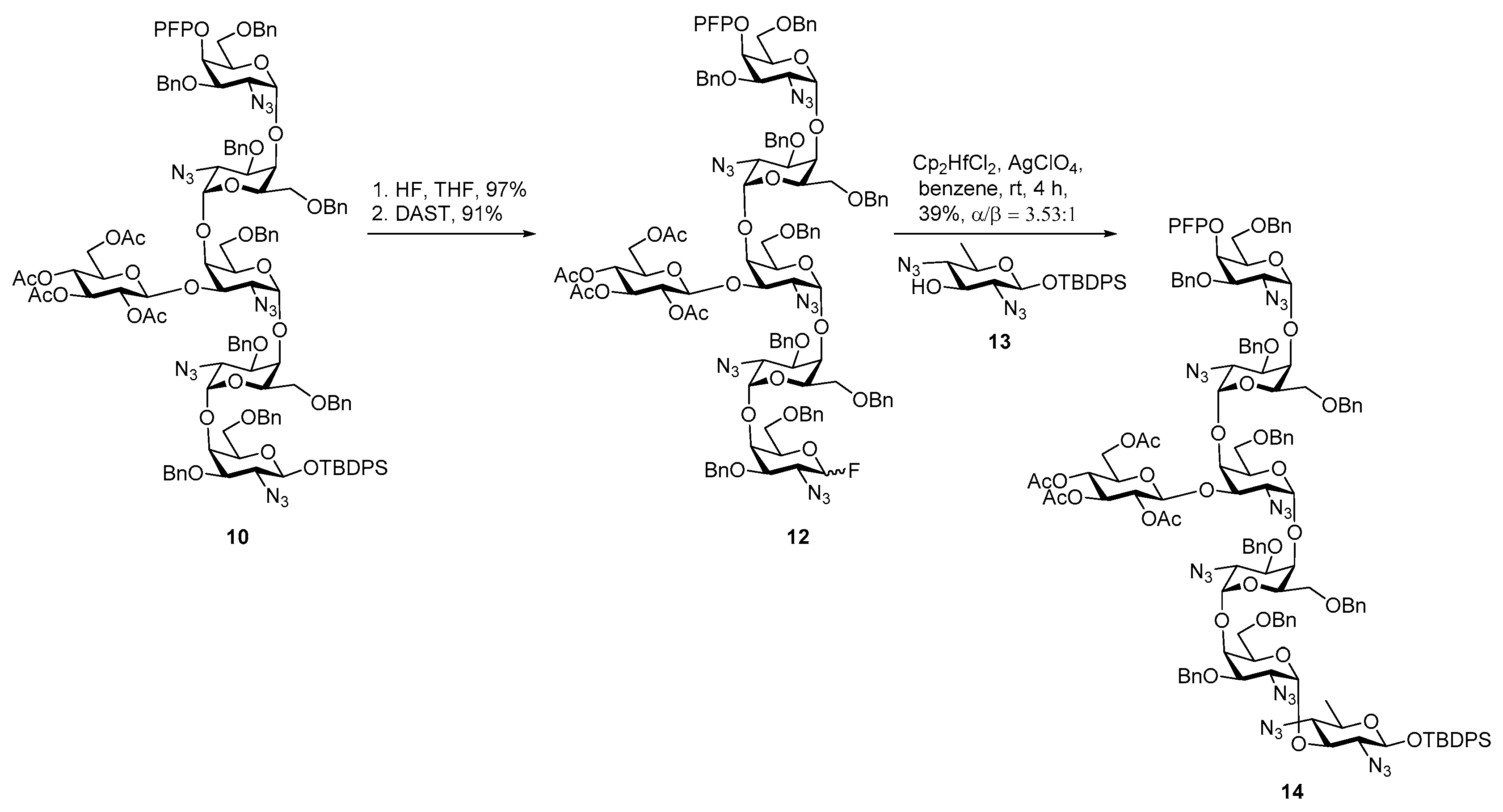
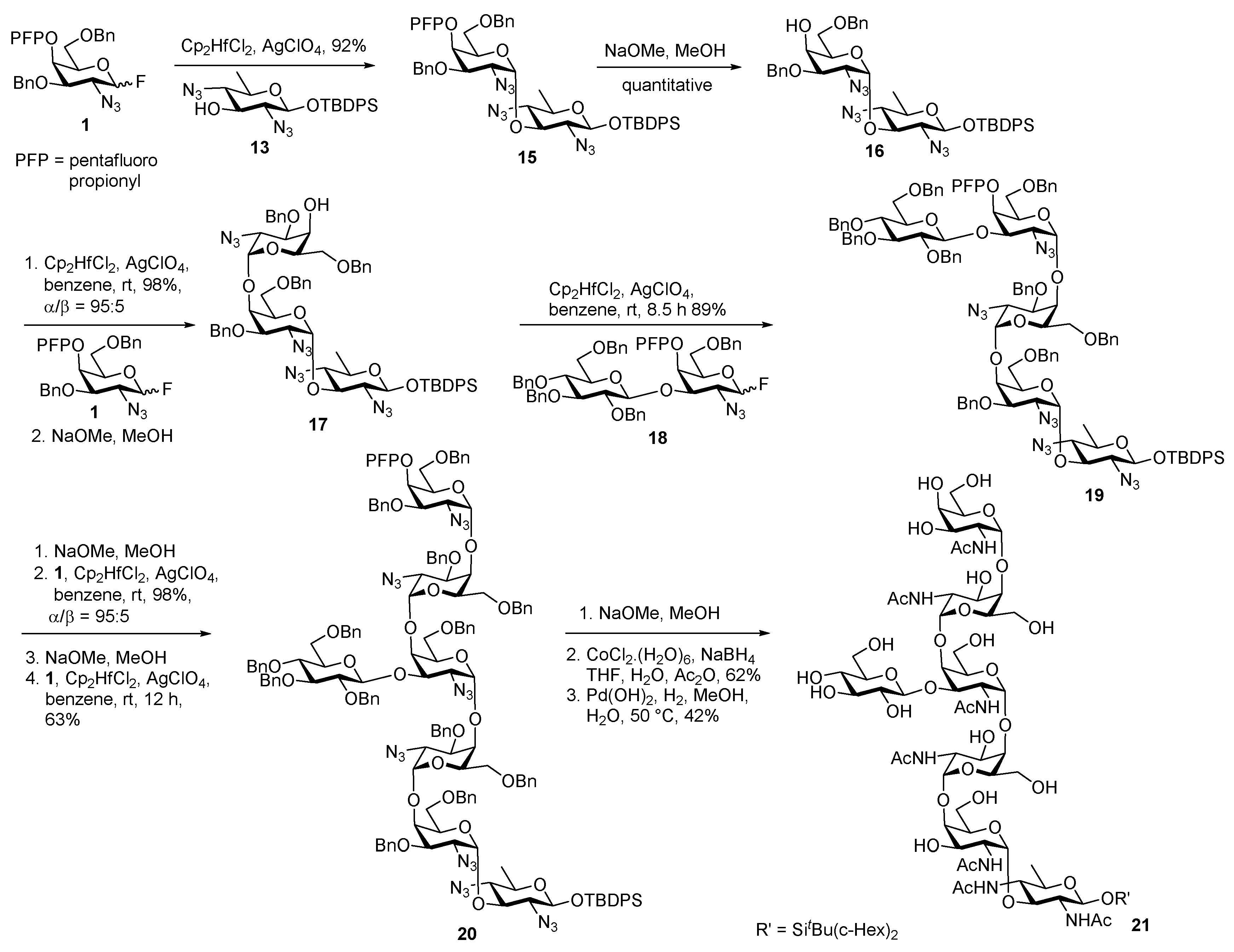
Glycan of Yersinia enterocolitica
P. chlororaphis Subsp. Aureofaciens Strain M71 Glycan
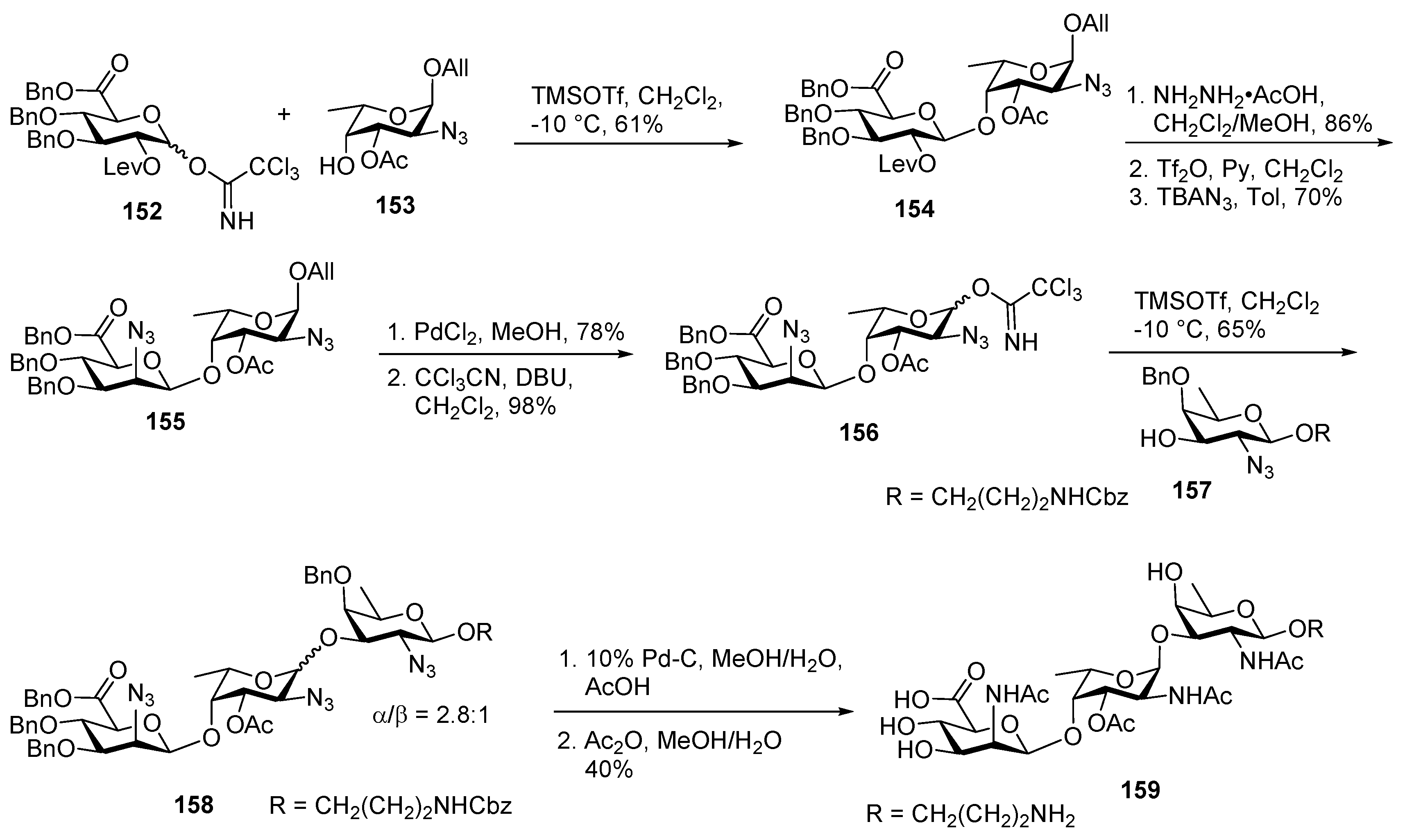
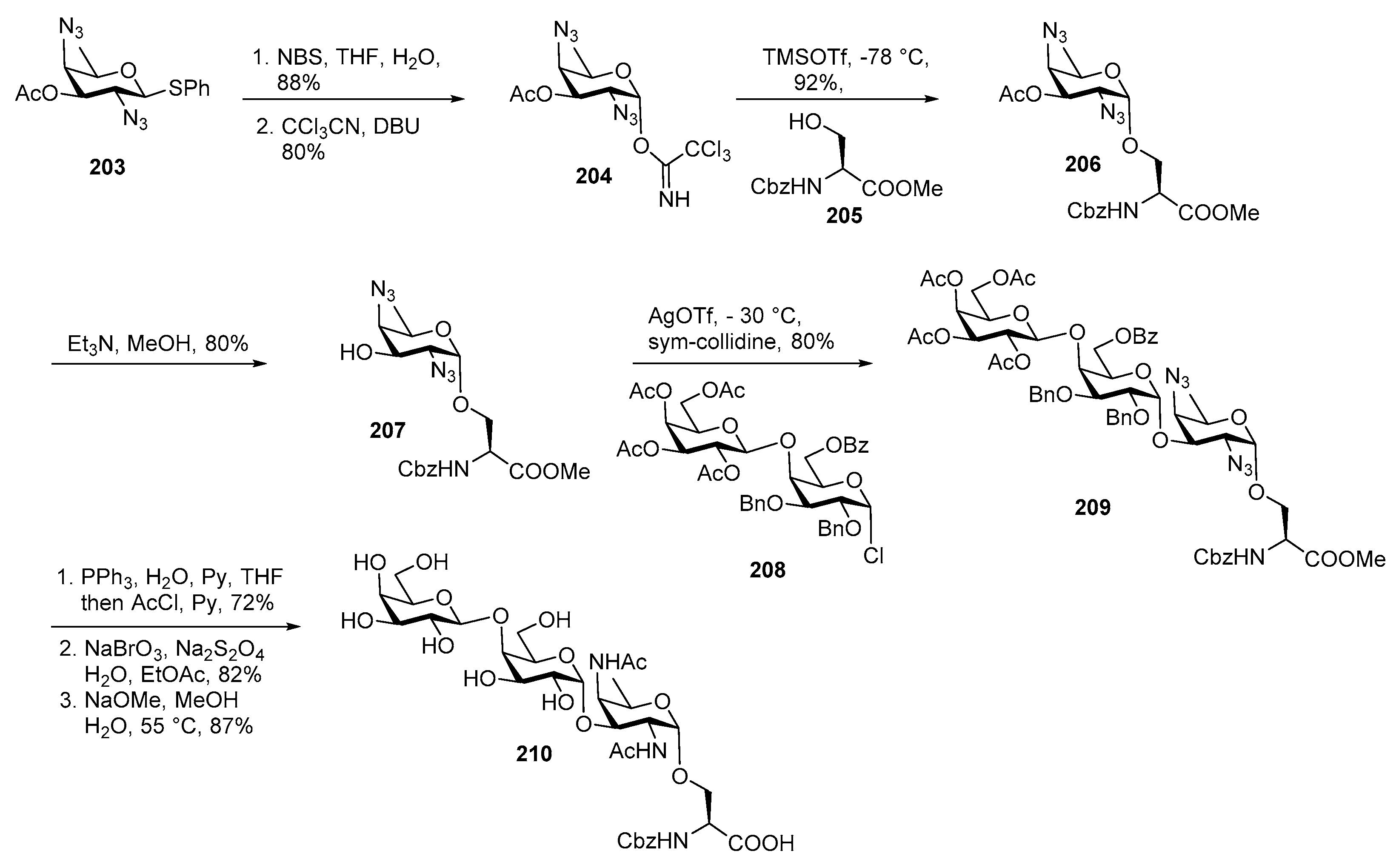
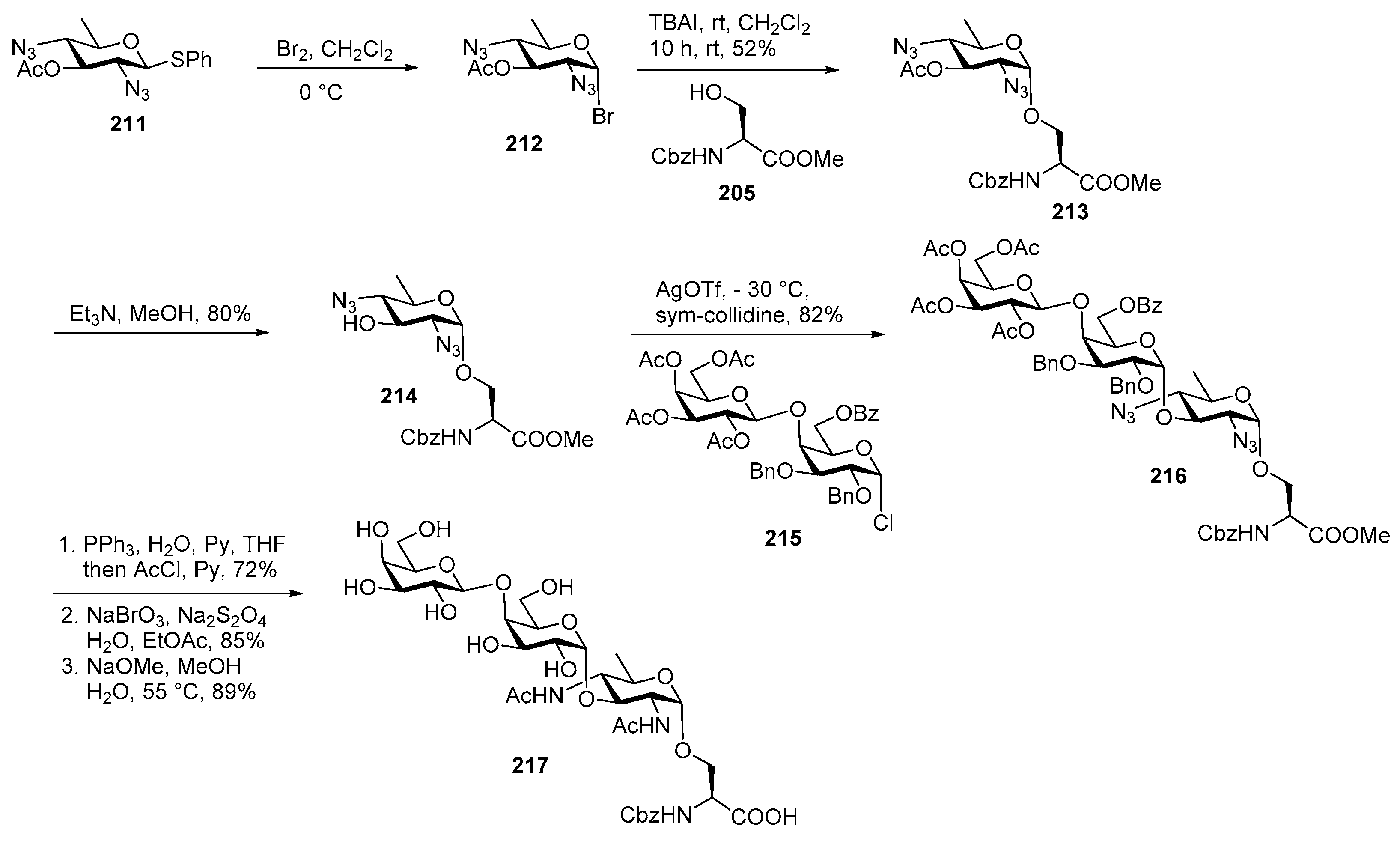
Plesiomonas shigelloides Serotype 51 Aminoglycoside Trisaccharide Antigen
Pseudomonas aeruginosa 1244 Pilin
Summary and Outlook
Acknowledgments
Conflicts of Interest
References
- Varki, A. Biological Roles of Oligosaccharides: All of the Theories Are Correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Nishat, S.; Andreana, P. Entirely Carbohydrate-Based Vaccines: An Emerging Field for Specific and Selective Immune Responses. Vaccines 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A Mechanism for Glycoconjugate Vaccine Activation of the Adaptive Immune System and Its Implications for Vaccine Design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Costantino, P.; Adamo, R. Potential Targets for next Generation Anti-Microbial Glycoconjugate Vaccines. FEMS Microbiol. Rev. 2018, 42, 388–423. [Google Scholar] [CrossRef] [PubMed]
- Dube, D.H.; Champasa, K.; Wang, B. Chemical Tools to Discover and Target Bacterial Glycoproteins. Chem. Commun. 2011, 47, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Longwell, S.A.; Dube, D.H. Deciphering the Bacterial Glycocode: Recent Advances in Bacterial Glycoproteomics. Curr. Opin. Chem. Biol. 2013, 17, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Adibekian, A.; Stallforth, P.; Hecht, M.L.; Werz, D.B.; Gagneux, P.; Seeberger, P.H. Comparative Bioinformatics Analysis of the Mammalian and Bacterial Glycomes. Chem. Sci. 2011, 2, 337–344. [Google Scholar] [CrossRef]
- Morelli, L.; Poletti, L.; Lay, L. Carbohydrates and Immunology: Synthetic Oligosaccharide Antigens for Vaccine Formulation. Eur. J. Org. Chem. 2011, 2011, 5723–5777. [Google Scholar] [CrossRef]
- Fernández-Tejada, A.; Cañada, F.J.; Jiménez-Barbero, J. Recent Developments in Synthetic Carbohydrate-Based Diagnostics, Vaccines, and Therapeutics. Chem. Eur. J. 2015, 21, 10616–10628. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H.; Werz, D.B. Synthesis and Medical Applications of Oligosaccharides. Nature 2007, 446, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.L.; Emmadi, M.; Krupp, K.L.; Podilapu, A.R.; Helble, J.D.; Kulkarni, S.S.; Dube, D.H. Development of Rare Bacterial Monosaccharide Analogs for Metabolic Glycan Labeling in Pathogenic Bacteria. ACS Chem. Biol. 2016, 11, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Dumont, A.; Malleron, A.; Awwad, M.; Dukan, S.; Vauzeilles, B. Click-Mediated Labeling of Bacterial Membranes through Metabolic Modification of the Lipopolysaccharide Inner Core. Angew. Chem. Int. Ed. 2012, 51, 3143–3146. [Google Scholar] [CrossRef] [PubMed]
- Emmadi, M.; Kulkarni, S.S. Recent Advances in Synthesis of Bacterial Rare Sugar Building Blocks and Their Applications. Nat. Prod. Rep. 2014, 31, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Linton, D.; Hitchen, P.G.; Nita-Lazar, M.; Haslam, S.M.; North, S.J.; Panico, M.; Morris, H.R.; Dell, A.; Wren, B.W. N-Linked Glycosylation in Campylobacter Jejuni and Its Functional Transfer into E. coli. Science 2002, 298, 1790–1793. [Google Scholar] [CrossRef] [PubMed]
- Young, N.M.; Brisson, J.R.; Kelly, J.; Watson, D.C.; Tessier, L.; Lanthier, P.H.; Jarrell, H.C.; Cadotte, N.; St. Michael, F.; Aberg, E.; et al. Structure of the N-Linked Glycan Present on Multiple Glycoproteins in the Gram-Negative Bacterium, Campylobacter Jejuni. J. Biol. Chem. 2002, 277, 42530–42539. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, C.M.; Burr, D.H.; Guerry, P. Campylobacter Protein Glycosylation Affects Host Cell Interactions. Infect. Immun. 2002, 70, 2242–2244. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, A.H.M.; Ketley, J.M. Pathogenesis of Enteric Campylobacter Infection. J. Appl. Microbiol. 2001, 90, 45S–56S. [Google Scholar] [CrossRef]
- Nachamkin, I.; Allos, B.M.; Ho, T. Campylobacter Species and Guillain-Barre Syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [Google Scholar] [PubMed]
- Glover, K.J.; Weerapana, E.; Imperiali, B. In Vitro Assembly of the Undecaprenylpyrophosphate-Linked Heptasaccharide for Prokaryotic N-Linked Glycosylation. Proc. Natl. Acad. Sci. USA 2005, 102, 14255–14259. [Google Scholar] [CrossRef] [PubMed]
- Weerapana, E.; Glover, K.J.; Chen, M.M.; Imperiali, B. Investigating Bacterial N-Linked Glycosylation: Synthesis and Glycosyl Acceptor Activity of the Undecaprenyl Pyrophosphate-Linked Bacillosamine. J. Am. Chem. Soc. 2005, 127, 13766–13767. [Google Scholar] [CrossRef] [PubMed]
- Glover, K.J.; Weerapana, E.; Numao, S.; Imperiali, B. Chemoenzymatic Synthesis of Glycopeptides with PglB, a Bacterial Oligosaccharyl Transferase from Campylobacter Jejuni. Chem. Biol. 2005, 12, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, A.; Ohta, S.; Ito, Y. A Stereoselective 1,2-Cis Glycosylation toward the Synthesis of a Novel N-Linked Glycan from the Gram-Negative Bacterium, Campylobacter Jejuni. Carbohydr. Res. 2006, 341, 1557–1573. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Ishiwata, A.; Ito, Y. Synthesis of Asparagine-Linked Bacillosamine. Carbohydr. Res. 2006, 341, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Ishiwata, A.; Ito, Y. Synthesis of N-Linked Glycan Derived from Gram-Negative Bacterium, Campylobacter Jejuni. Tetrahedron 2007, 63, 8181–8198. [Google Scholar] [CrossRef]
- Cobb, B.A.; Kasper, D.L. Zwitterionic Capsular Polysaccharides: The New MHCII-Dependent Antigens. Cell. Microbiol. 2005, 7, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Kasper, D.L. The Love-Hate Relationship between Bacterial Polysaccharides and the Host Immune System. Nat. Rev. Immunol. 2006, 6, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Kasper, D.L. How Bacterial Carbohydrates Influence the Adaptive Immune System. Annu. Rev. Immunol. 2010, 28, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Cobb, B.A.; Wang, Q.; Tzianabos, A.O.; Kasper, D.L. Polysaccharide Processing and Presentation by the MHCII Pathway. Cell 2004, 117, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Cobb, B.A.; Kasper, D.L. Characteristics of Carbohydrate Antigen Binding to the Presentation Protein HLA-DR. Glycobiology 2008, 18, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.; Tzianabos, A.O.; Brisson, J.R.; Kasper, D.L.; Jennings, H.J. Structural Elucidation of Two Capsular Polysaccharides from One Strain of Bacteroides Fragilis Using High-Resolution NMR Spectroscopy. Biochemistry 1992, 31, 4081–4089. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; McLoughlin, R.M.; Cobb, B.A.; Charrel-Dennis, M.; Zaleski, K.J.; Golenbock, D.; Tzianabos, A.O.; Kasper, D.L. A Bacterial Carbohydrate Links Innate and Adaptive Responses through Toll-Like Receptor 2. J. Exp. Med. 2006, 203, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Cui, H.L.; Tzianabos, A.O.; Kasper, D.L. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Perez, B.; Chung, D.R.; Sharpe, A.H.; Yagita, H.; Kalka-Moll, W.M.; Sayegh, M.H.; Kasper, D.L.; Tzianabos, A.O. Modulation of Surgical Fibrosis by Microbial Zwitterionic Polysaccharides. Proc. Natl. Acad. Sci. USA 2005, 102, 16753–16758. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A Microbial Symbiosis Factor Prevents Intestinal Inflammatory Disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A Polysaccharide from the Human Commensal Bacteroides Fragilis Protects against CNS Demyelinating Disease. Mucosal Immunol. 2010, 3, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Van den Bos, L.J.; Boltje, T.J.; Provoost, T.; Mazurek, J.; Overkleeft, H.S.; van der Marel, G.A. A Synthetic Study towards the PSA1 Tetrasaccharide Repeating Unit. Tetrahedron Lett. 2007, 48, 2697–2700. [Google Scholar] [CrossRef]
- Pragani, R.; Seeberger, P.H. Total Synthesis of the Bacteroides Fragilis Zwitterionic Polysaccharide A1 Repeating Unit. J. Am. Chem. Soc. 2011, 133, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Pragani, R.; Seeberger, P.H. De Novo Synthesis of a 2-Acetamido-4-Amino-2,4,6-Trideoxy-d-Galactose (AAT) Building Block for the Preparation of the Zwitterionic Polysaccharide A1 (PS A1) Repeating Subunit of Bacteroides Fragilis. Org. Lett. 2010, 12, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Emmadi, M.; Kulkarni, S.S. Orthogonally Protected d-Galactosamine Thioglycoside Building Blocks via Highly Regioselective, Double Serial and Double Parallel Inversions of β-d-Thiomannoside. Org. Biomol. Chem. 2013, 11, 4825–4830. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.A.; Wang, Q.; Chidley, T.; Appulage, D.K.; Andreana, P.R. Immunological Response from an Entirely Carbohydrate Antigen: Design of Synthetic Vaccines Based on Tn-PS A1 Conjugates. J. Am. Chem. Soc. 2009, 131, 9622–9623. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kleski, K.A.; Trabbic, K.R.; Bourgault, J.-P.; Andreana, P.R. Sialyl-Tn A1 as an Entirely Carbohydrate Immunogen: Synthesis and Immunological Evaluation. J. Am. Chem. Soc. 2016, 138, 14264–14272. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Nishat, S.; Andreana, P.R. Synthesis of an Aminooxy Derivative of the Tetrasaccharide Repeating Unit of Streptococcus dysgalactiae 2023 Polysaccharide for a PS A1 Conjugate Vaccine. J. Org. Chem. 2016, 81, 4475–4484. [Google Scholar] [CrossRef] [PubMed]
- Eradi, P.; Ghosh, S.; Andreana, P.R. Total Synthesis of Zwitterionic Tetrasaccharide Repeating Unit from Bacteroides fragilis ATCC 25285/NCTC 9343 Capsular Polysaccharide PS A1 with Alternating Charges on Adjacent Monosaccharides. Org. Lett. 2018. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Roehrl, M.H.; Kasper, D.L.; Wang, J.Y. A Unique Structural Pattern Shared by T-Cell-Activating and Abscess-Regulating Zwitterionic Polysaccharides. Biochemistry 2002, 41, 15144–15151. [Google Scholar] [CrossRef] [PubMed]
- Zangwill, K.M.; Vadheim, C.M.; Vannier, A.M.; Hemenway, L.S.; Greenberg, D.P.; Ward, J.I. Epidemiology of Invasive Pneumococcal Disease in Southern California: Implications for the Design and Conduct of a Pneumococcal Conjugate Vaccine Efficacy Trial. J. Infect. Dis. 1996, 174, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejas, M.J. Pneumococcal Virulence Factors: Structure and Function. Microbiol. Mol. Biol. Rev. 2001, 65, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus Aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cui, L.; Lipinski, T.; Bundle, D.R. Synthesis of Monomeric and Dimeric Repeating Units of the Zwitterionic Type 1 Capsular Polysaccharide from Streptococcus Pneumoniae. Chem. Eur. J. 2010, 16, 3476–3488. [Google Scholar] [CrossRef] [PubMed]
- Boebel, T.A.; Gin, D.Y. Sulfoxide Covalent Catalysis: Application to Glycosidic Bond Formation. Angew. Chem. Int. Ed. 2003, 42, 5874–5877. [Google Scholar] [CrossRef] [PubMed]
- Christina, A.E.; Van Den Bos, L.J.; Overkleeft, H.S.; Van Der Marel, G.A.; Codée, J.D.C. Galacturonic Acid Lactones in the Synthesis of All Trisaccharide Repeating Units of the Zwitterionic Polysaccharide Sp1. J. Org. Chem. 2011, 76, 1692–1706. [Google Scholar] [CrossRef] [PubMed]
- Schumann, B.; Pragani, R.; Anish, C.; Pereira, C.L.; Seeberger, P.H. Synthesis of Conjugation-Ready Zwitterionic Oligosaccharides by Chemoselective Thioglycoside Activation. Chem. Sci. 2014, 5, 1992–2002. [Google Scholar] [CrossRef]
- Schumann, B.; Reppe, K.; Kaplonek, P.; Wahlbrink, A.; Anish, C.; Witzenrath, M.; Pereira, C.L.; Seeberger, P.H. Development of an Efficacious, Semisynthetic Glycoconjugate Vaccine Candidate against Streptococcus pneumoniae Serotype 1. ACS Cent. Sci. 2018, 4, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.; Behr, T.; Hartmann, R.; Peter-Katalinic, J.; Egge, H. Teichoic Acid and Lipoteichoic Acid of Streptococcus Pneumoniae Possess Identical Chain Structures: A Reinvestigation of Teichoid Acid (C Polysaccharide). Eur. J. Biochem. 1993, 215, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.W.; Fischer, W.; Joiner, K.A. Influence of Lipoteichoic Acid Structure on Recognition by the Macrophage Scavenger Receptor. Infect. Immun. 1996, 64, 3318–3325. [Google Scholar] [PubMed]
- Fischer, W. Pneumococcal Lipoteichoic and Teichoic Acid. Microb. Drug Resist. 1997, 3, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.M.; Figueroa-Perez, I.; Lindner, B.; Ulmer, A.J.; Zähringer, U.; Schmidt, R.R. Total Synthesis of Lipoteichoic Acid of Streptococcus Pneumoniae. Angew. Chem. Int. Ed. 2010, 49, 2585–2590. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.M.; Figueroa-Perez, I.; Boruwa, J.; Lindner, B.; Ulmer, A.J.; Zähringer, U.; Schmidt, R.R. Synthesis of the Core Structure of the Lipoteichoic Acid of Streptococcus Pneumoniae. Chem. Eur. J. 2010, 16, 12627–12641. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.E.; Lindberg, B.; Lindquist, U. Structural Studies of the Capsular Polysaccharide from Streptococcus Pneumoniae Type 4. Carbohydr. Res. 1981, 95, 73–80. [Google Scholar] [CrossRef]
- Jones, C.; Currie, F. The Pneumococcal Polysaccharide S4: A Structural Re-Assessment. Carbohydr. Res. 1988, 184, 279–284. [Google Scholar] [CrossRef]
- Higginbotham, J.D.; Heidelberger, M. The Specific Capsular Polysaccharide of Pneumococcus Type IV. Carbohydr. Res. 1972, 23, 165–173. [Google Scholar] [CrossRef]
- Jow, Y.L.; Heidelberger, M. Note Linkage of Pyruvyl Groups in the Specific Capsular Poiysaccharide of Pneumococcus Type IV. Carbohydr. Res. 1976, 52, 255–258. [Google Scholar]
- Jones, C. A Novel Method for the Determination of the Stereochemistry of Pyruvate Acetal Substituents Applied to the Capsular Polysaccharide from Streptococcus Pneumoniae Type 4. Carbohydr. Res. 1990, 198, 353–357. [Google Scholar] [CrossRef]
- Jones, C.; Currie, F.; Forster, M.J. Nmr and Conformational Analysis of the Capsular Polysaccharide from Streptococcus Pneumoniae Type 4. Carbohydr. Res. 1991, 221, 95–121. [Google Scholar] [CrossRef]
- Horito, S.; Lorentzen, J.P.; Paulsen, H. Bausteine von Oligosacchariden, LXXVII. Synthese Einer Trisaccharideinheit Des Kapselpolysaccharides VonStreptococcus Pneumoniae Typ 4. Liebigs Ann. Chem. 1986, 1986, 1880–1890. [Google Scholar] [CrossRef]
- Pereira, C.L.; Geissner, A.; Anish, C.; Seeberger, P.H. Chemical Synthesis Elucidates the Immunological Importance of a Pyruvate Modification in the Capsular Polysaccharide of Streptococcus Pneumoniae Serotype 4. Angew. Chem. Int. Ed. 2015, 54, 10016–10019. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.; Henrichsen, J. Laboratory Diagnosis, Serology and Epidemiology of Streptococcus Pneumoniae. Methods Microbiol. 1978, 12, 241–262. [Google Scholar]
- Robbins, J.B.; Austrian, R.; Lee, C.-J.; Rastogi, S.C.; Schiffman, G.; Henrichsen, J.; Makela, P.H.; Broome, C.V.; Facklam, R.R.; Tiesjema, R.H.; et al. Considerations for Formulating the Second-Generation Pneumococcal Capsular Polysaccharide Vaccine with Emphasis on the Cross-Reactive Types within Groups. J. Infect. Dis. 1983, 148, 1136–1159. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, M.; Avery, O.T. The Soluble Specific Subtance of Pneumococcus. J. Exp. Med. 1923, 38, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H.; Pereira, C.L.; Govindan, S. Total Synthesis of a Streptococcus Pneumoniae Serotype 12F CPS Repeating Unit Hexasaccharide. Beilstein J. Org. Chem. 2017, 13, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, M.P.; Khan, N.; Martin, C.; Xu, F.-F.; Reppe, K.; Geissner, A.; Govindan, S.; Witzenrath, M.; Pereira, C.L.; Seeberger, P.H. Semisynthetic Glycoconjugate Vaccine Candidate against Streptococcus Pneumoniae Serotype 5. Proc. Natl. Acad. Sci. USA 2017, 114, 11063–11068. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.L.; Deloria-Knoll, M.; Levine, O.S.; Stoszek, S.K.; Hance, L.F.; Reithinger, R.; Muenz, L.R.; O’Brien, K.L. Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children under Five: The Pneumococcal Global Serotype Project. PLoS Med. 2010, 7, e1000348. [Google Scholar] [CrossRef] [PubMed]
- How, M.J.; Brimacombe, J.S.; Stacey, M. The Pneumococcal Polysaccharides. Adv. Carbohydr. Chem. 1964, 19, 303–358. [Google Scholar] [PubMed]
- Niyogi, S.K. Shigellosis. J. Microbiol. 2005, 43, 133–143. [Google Scholar] [PubMed]
- Barry, E.M.; Pasetti, M.F.; Sztein, M.B.; Fasano, A.; Kotloff, K.L.; Levine, M.M. Progress and Pitfalls in Shigella Vaccine Research. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Kenne, L.; Lindberg, B.; Petersson, K.; Katzenellenbogen, E.; Romanowska, E. Structural Studies of the O-Specific Side-Chains of the Shigella Sonnei Phase I Lipopolysaccharide. Carbohydr. Res. 1980, 78, 119–126. [Google Scholar] [CrossRef]
- Pfister, H.B.; Mulard, L.A. Synthesis of the Zwitterionic Repeating Unit of the O-Antigen from Shigella Sonnei and Chain Elongation at Both Ends. Org. Lett. 2014, 16, 4892–4895. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, O.G.; Kocharova, N.A.; Bialczak-Kokot, M.; Shashkov, A.S.; Rozalski, A.; Knirel, Y.A. Structure of the O-Polysaccharide of Providencia Alcalifaciens O22 Containing d-Glyceramide 2-Phosphate. Eur. J. Org. Chem. 2012, 2012, 3500–3506. [Google Scholar] [CrossRef]
- Yoh, M.; Matsuyama, J.; Ohnishi, M.; Takagi, K.; Miyagi, H.; Mori, K.; Park, K.S.; Ono, T.; Honda, T. Importance of Providencia Species as a Major Cause of Travellers’ Diarrhoea. J. Med. Microbiol. 2005, 54, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Odoyo, E.; Larson, P.S.; Apondi, E.; Kathiiko, C.; Miringu, G.; Nakashima, M.; Ichinose, Y. First Report of a Foodborne Providencia Alcalifaciens Outbreak in Kenya. Am. J. Trop. Med. Hyg. 2015, 93, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Dieli, M.; Brucato, A.; Pedrotti, P.; Brambilla, P.; Curri, S.F.; Senni, M.; Pericotti, S.; Suter, F.; Ferrazzi, P. Images in Cardiovascular Medicine. Bacterial Pericarditis Due to Providencia Stuartii: An Atypical Case of Relapsing Pericarditis. Circulation 2010, 122, e401–e403. [Google Scholar] [CrossRef] [PubMed]
- Krake, P.R.; Tandon, N. Infective Endocarditis Due to Providenca Stuartii. South. Med. J. 2004, 97, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, O.R.; Bardak-Ozcem, S.; Ozgiray, E.; Aydemir, S.; Yurtseven, T.; Yamazhan, T.; Tasbakan, M.; Ulusoy, S. Meningitis Due to Providencia Stuartii. J. Clin. Microbiol. 2010, 48, 4667–4668. [Google Scholar] [CrossRef] [PubMed]
- Koreishi, A.F.; Schechter, B.A.; Karp, C.L. Ocular Infections Caused by Providencia Rettgeri. Ophthalmology 2006, 113, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, C.M.; Brenner, F.W.; Miller, J.M. Classification, Identification, and Clinical Significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 2000, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Podilapu, A.R.; Kulkarni, S.S. Total Synthesis of Repeating Unit of O-Polysaccharide of Providencia Alcalifaciens O22 via One-Pot Glycosylation. Org. Lett. 2017, 19, 5466–5469. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.; Lee, J.C. Staphylococcus Aureus Capsular Polysaccharides. Clin. Microbiol. Rev. 2004, 17, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Daum, R.S.; Spellberg, B. Progress toward a Staphylococcus Aureus Vaccine. Clin. Infect. Dis. 2012, 54, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, A.; Cervera, C.; Moreno, A.; Moreillon, P.; Miró, J.M. Patients at Risk of Complications of Staphylococcus Aureus Bloodstream Infection. Clin. Infect. Dis. 2009, 48, S246–S253. [Google Scholar] [CrossRef] [PubMed]
- Jones, C. Revised Structures for the Capsular Polysaccharides from Staphylococcus Aureus Types 5 and 8, Components of Novel Glycoconjugate Vaccines. Carbohydr. Res. 2005, 340, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Fattom, A.I.; Schneerson, R.; Szu, S.C.; Vann, W.F.; Shiloach, J.; Karakawa, W.W.; Robbins, J.B. Synthesis and Immunologic Properties in Mice of Vaccines Composed of Staphylococcus aureus Type 5 and Type 8 Capsular Polysaccharides Conjugated to Pseudomonas aeruginosa Exotoxin A. Infect. Immun. 1990, 58, 2367–2374. [Google Scholar] [PubMed]
- Fattom, A.I.; Sarwar, J.; Ortiz, A.; Naso, R. A Staphylococcus Aureus Capsular Polysaccharide (CP) Vaccine and CP-Specific Antibodies Protect Mice against Bacterial Challenge. Infect. Immun. 1996, 64, 1659–1665. [Google Scholar] [PubMed]
- Danieli, E.; Proietti, D.; Brogioni, G.; Romano, M.R.; Cappelletti, E.; Tontini, M.; Berti, F.; Lay, L.; Costantino, P.; Adamo, R. Synthesis of Staphylococcus Aureus Type 5 Capsular Polysaccharide Repeating Unit Using Novel L-FucNAc and D-FucNAc Synthons and Immunochemical Evaluation. Bioorg. Med. Chem. 2012, 20, 6403–6415. [Google Scholar] [CrossRef] [PubMed]
- Gagarinov, I.A.; Fang, T.; Liu, L.; Srivastava, A.D.; Boons, G.J. Synthesis of Staphylococcus Aureus Type 5 Trisaccharide Repeating Unit: Solving the Problem of Lactamization. Org. Lett. 2015, 17, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Yasomanee, J.P.; Visansirikul, S.; Pornsuriyasak, P.; Thompson, M.; Kolodziej, S.A.; Demchenko, A.V. Synthesis of the Repeating Unit of Capsular Polysaccharide Staphylococcus Aureus Type 5 to Study Chemical Activation and Conjugation of Native CP5. J. Org. Chem. 2016, 81, 5981–5987. [Google Scholar] [CrossRef] [PubMed]
- Hagen, B.; Ali, S.; Overkleeft, H.S.; Van der Marel, G.A.; Codée, J.D.C. Mapping the Reactivity and Selectivity of 2-Azidofucosyl Donors for the Assembly of N-Acetylfucosamine-Containing Bacterial Oligosaccharides. J. Org. Chem. 2017, 82, 848–868. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Taylor, M.S. Borinic Acid-Catalyzed Regioselective Acylation of Carbohydrate Derivatives. J. Am. Chem. Soc. 2011, 133, 3724–3727. [Google Scholar] [CrossRef] [PubMed]
- Visansirikul, S.; Yasomanee, J.P.; Pornsuriyasak, P.; Kamat, M.N.; Podvalnyy, N.M.; Gobble, C.P.; Thompson, M.; Kolodziej, S.A.; Demchenko, A.V. A Concise Synthesis of the Repeating Unit of Capsular Polysaccharide Staphylococcus Aureus Type 8. Org. Lett. 2015, 17, 2382–2384. [Google Scholar] [CrossRef] [PubMed]
- Ala’Aldeen, D.A.A.; Hiramatsu, K. Staphylococcus Aureus: Molecular and Clinical Aspects; Elsevier: New York, NY, USA, 2004. [Google Scholar]
- Hagen, B.; Van Dijk, J.H.M.; Zhang, Q.; Overkleeft, H.S.; Van Der Marel, G.A.; Codée, J.D.C. Synthesis of the Staphylococcus Aureus Strain M Capsular Polysaccharide Repeating Unit. Org. Lett. 2017, 19, 2514–2517. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Melly, M.A.; Harris, T.M.; Hellerqvist, C.G.; Hash, J.H. The Repeating Sequence of the Capsular Polysaccharide of Staphylococcus Aureus M. Carbohydr. Res. 1983, 117, 113–123. [Google Scholar] [CrossRef]
- WHO Fact Sheets: Meningococcal Meningitis. Available online: http://www.who.int/mediacentre/factsheets/fs141/en/index.html (accessed on 9 August 2018).
- Stimson, E.; Virji, M.; Makepeace, K.; Dell, A.; Morris, H.R.; Payne, G.; Saunders, J.R.; Jennings, M.P.; Barker, S.; Panico, M.; et al. Meningococcal pilin: A glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol. Microbiol. 1995, 17, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Emmadi, M.; Kulkarni, S.S. Expeditious Synthesis of Bacterial, Rare Sugar Building Blocks to Access the Prokaryotic Glycome. Org. Biomol. Chem. 2013, 11, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Emmadi, M.; Kulkarni, S.S. Synthesis of Orthogonally Protected Bacterial, Rare-Sugar and d-Glycosamine Building Blocks. Nat. Protoc. 2013, 8, 1870–1889. [Google Scholar] [CrossRef] [PubMed]
- Emmadi, M.; Kulkarni, S.S. Total Synthesis of the Bacillosamine Containing α-l-Serine Linked Trisaccharide of Neisseria Meningitidis. Carbohydr. Res. 2014, 399, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Candela, T.; Maes, E.; Garénaux, E.; Rombouts, Y.; Krzewinski, F.; Gohar, M.; Guérardel, Y. Environmental and Biofilm-Dependent Changes in a Bacillus Cereus Secondary Cell Wall Polysaccharide. J. Biol. Chem. 2011, 286, 31250–31262. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Available online: http://www.phac-aspc.gc.ca/labbio/res/psds-ftss/bacillus-cereus-eng.php (accessed on 9 August 2018).
- Logan, N.A.; Rodrigez-Diaz, M. Bacillus Spp. and Related Genera. In Principles and Practice of Clinical Bacteriology, 2nd ed.; Gillespie, S.H., Hawkey, P.M., Eds.; John Wiley and Sons Ltd.: West Sussex, UK, 2006; pp. 139–158. [Google Scholar]
- Rosovitz, M.J.; Voskuil, M.I.; Chambliss, G.H. Bacillus. In Topley & Wilson’s Microbiology and Microbial Infection: Systematic Bacteriology, 9th ed.; Collier, L., Balows, A., Sussman, M., Balows, A., Duerden, B.I., Eds.; Hodder Education Publishers: London, UK, 1998; pp. 709–729. [Google Scholar]
- Drobniewski, F.A. Bacillus Cereus and Related Species. Am. Soc. Microbiol. 1993, 6, 324–338. [Google Scholar] [CrossRef]
- Pinna, A.; Sechi, L.A.; Zanetti, S.; Usai, D.; Delogu, G.; Cappuccinelli, P.; Carta, F. Bacillus Cereus Keratitis Associated with Contact Lens Wear. Ophthalmology 2001, 108, 1830–1834. [Google Scholar] [CrossRef]
- Cowan, C.L.; Madden, W.M.; Hatem, G.F.; Merritt, J.C. Endogenous Bacillus Cereus Panophthalmitis. Ann. Ophthalmol. 1987, 19, 65–68. [Google Scholar] [PubMed]
- O’Day, D.M.; Smith, R.S.; Gregg, C.R.; Turnbull, P.C.B.; Head, W.S.; Ives, J.A.; Ho, P.C. The Problem of Bacillus Species Infection with Special Emphasis on the Virulence of Bacillus Cereus. Ophthalmology 1981, 88, 833–838. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Salazar, L.G.; True, L.D.; Disis, M.L. Fatal Bacillus Cereus Sepsis Following Resolving Neutropenic Enterocolitis during the Treatment of Acute Leukemia. Am. J. Hematol. 2003, 72, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Podilapu, A.R.; Kulkarni, S.S. First Synthesis of Bacillus Cereus Ch HF-PS Cell Wall Trisaccharide Repeating Unit. Org. Lett. 2014, 16, 4336–4339. [Google Scholar] [CrossRef] [PubMed]
- Skurnik, M.; Toivonen, S. Identification of Distinct Lipopolysaccharide Patterns among Yersinia Enterocolitica and Y. Enterocolitica-Like Bacteria. Biochem. 2011, 76, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Fàbrega, A.; Vila, J. Yersinia Enterocolitica: Pathogenesis, Virulence and Antimicrobial Resistance. Enferm. Infecc. Microbiol. Clin. 2012, 30, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Beczała, A.; Duda, K.A.; Skurnik, M.; Holst, O. The Structure of the O-Specific Polysaccharide of the Lipopolysaccharide from Yersinia Enterocolitica Serotype O:50 Strain 3229. Carbohydr. Res. 2012, 359, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Sanapala, S.R.; Kulkarni, S.S. Expedient Route to Access Rare Deoxy Amino L-Sugar Building Blocks for the Assembly of Bacterial Glycoconjugates. J. Am. Chem. Soc. 2016, 138, 4938–4947. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, G.; Puopolo, G.; Carillo, S.; Zoina, A.; Lanzetta, R.; Parrilli, M.; Evidente, A.; Corsaro, M.M. Structural Characterization of the O-Chain Polysaccharide from an Environmentally Beneficial Bacterium Pseudomonas Chlororaphis Subsp. Aureofaciens Strain M71. Carbohydr. Res. 2011, 346, 2705–2709. [Google Scholar] [CrossRef] [PubMed]
- Michael Janda, J.; Abbott, S.L.; McIver, C.J. Plesiomonas Shigelloides Revisited. Clin. Microbiol. Rev. 2016, 29, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Stock, I. Plesiomonas Shigelloides: An Emerging Pathogen with Unusual Properties. Rev. Med. Microbiol. 2004, 15, 129–139. [Google Scholar] [CrossRef]
- Maciejewska, A.; Lukasiewicz, J.; Niedziela, T.; Szewczuk, Z.; Lugowski, C. Structural Analysis of the O-Specific Polysaccharide Isolated from Plesiomonas Shigelloides O51 Lipopolysaccharide. Carbohydr. Res. 2009, 344, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Schumann, B.; Zou, X.; Pereira, C.L.; Tian, G.; Hu, J.; Seeberger, P.H.; Yin, J. Total Synthesis of a Densely Functionalized Plesiomonas Shigelloides Serotype 51 Aminoglycoside Trisaccharide Antigen. J. Am. Chem. Soc. 2018, 140, 3120–3127. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.J.; Imperiali, B. The Renaissance of Bacillosamine and Its Derivatives: Pathway Characterization and Implications in Pathogenicity. Biochemistry 2014, 53, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Desa, N.; Hansen, E.E.; Knirel, Y.A.; Gordon, J.I.; Gagneux, P.; Nizet, V.; Varki, A. Innovations in Host and Microbial Sialic Acid Biosynthesis Revealed by Phylogenomic Prediction of Nonulosonic Acid Structure. Proc. Natl. Acad. Sci. USA 2009, 106, 13552–13557. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ramphal, R.; Sadoff, J.C.; Pyle, M.; Silipigni, J.D. Role of Pili in the Adherence of Pseudomonas Aeruginosa to Injured Tracheal Epithelium. Infect. Immun. 1984, 44, 38–40. [Google Scholar] [PubMed]
- Castric, P.; Cassels, F.J.; Carlson, R.W. Structural Characterization of the Pseudomonas Aeruginosa 1244 Pilin Glycan. J. Biol. Chem. 2001, 276, 26479–26485. [Google Scholar] [CrossRef] [PubMed]
- Comer, J.E.; Marshall, M.A.; Blanch, V.J.; Deal, C.D.; Castric, P. Identification of the Pseudomonas Aeruginosa 1244 Pilin Glycosylation Site. Infect. Immun. 2002, 70, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Wei, R.; Andolina, G.; Li, X. Total Synthesis of Pseudomonas Aeruginosa 1244 Pilin Glycan via de Novo Synthesis of Pseudaminic Acid. J. Am. Chem. Soc. 2017, 139, 13420–13428. [Google Scholar] [CrossRef] [PubMed]
- Gouliaras, C.; Lee, D.; Chan, L.; Taylor, M.S. Regioselective Activation of Glycosyl Acceptors by a Diarylborinic Acid-Derived Catalyst. J. Am. Chem. Soc. 2011, 133, 13926–13929. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Wang, C.-C.; Sabbavarapu, N.M.; Podilapu, A.R.; Liao, P.-H.; Hung, S.-C. “One-Pot” Protection, Glycosylation, and Protection−Glycosylation Strategies of Carbohydrates. Chem. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behera, A.; Kulkarni, S.S. Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates. Molecules 2018, 23, 1997. https://doi.org/10.3390/molecules23081997
Behera A, Kulkarni SS. Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates. Molecules. 2018; 23(8):1997. https://doi.org/10.3390/molecules23081997
Chicago/Turabian StyleBehera, Archanamayee, and Suvarn S. Kulkarni. 2018. "Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates" Molecules 23, no. 8: 1997. https://doi.org/10.3390/molecules23081997
APA StyleBehera, A., & Kulkarni, S. S. (2018). Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates. Molecules, 23(8), 1997. https://doi.org/10.3390/molecules23081997





