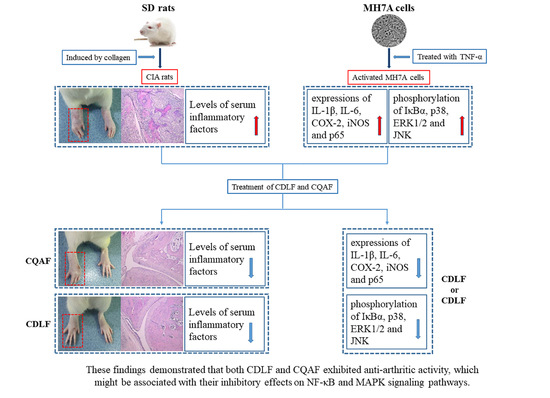
In Vivo and In Vitro Anti-Arthritic Effects of Cardenolide-Rich and Caffeoylquinic Acid-Rich Fractions of Periploca forrestii
Abstract
1. Introduction
2. Results
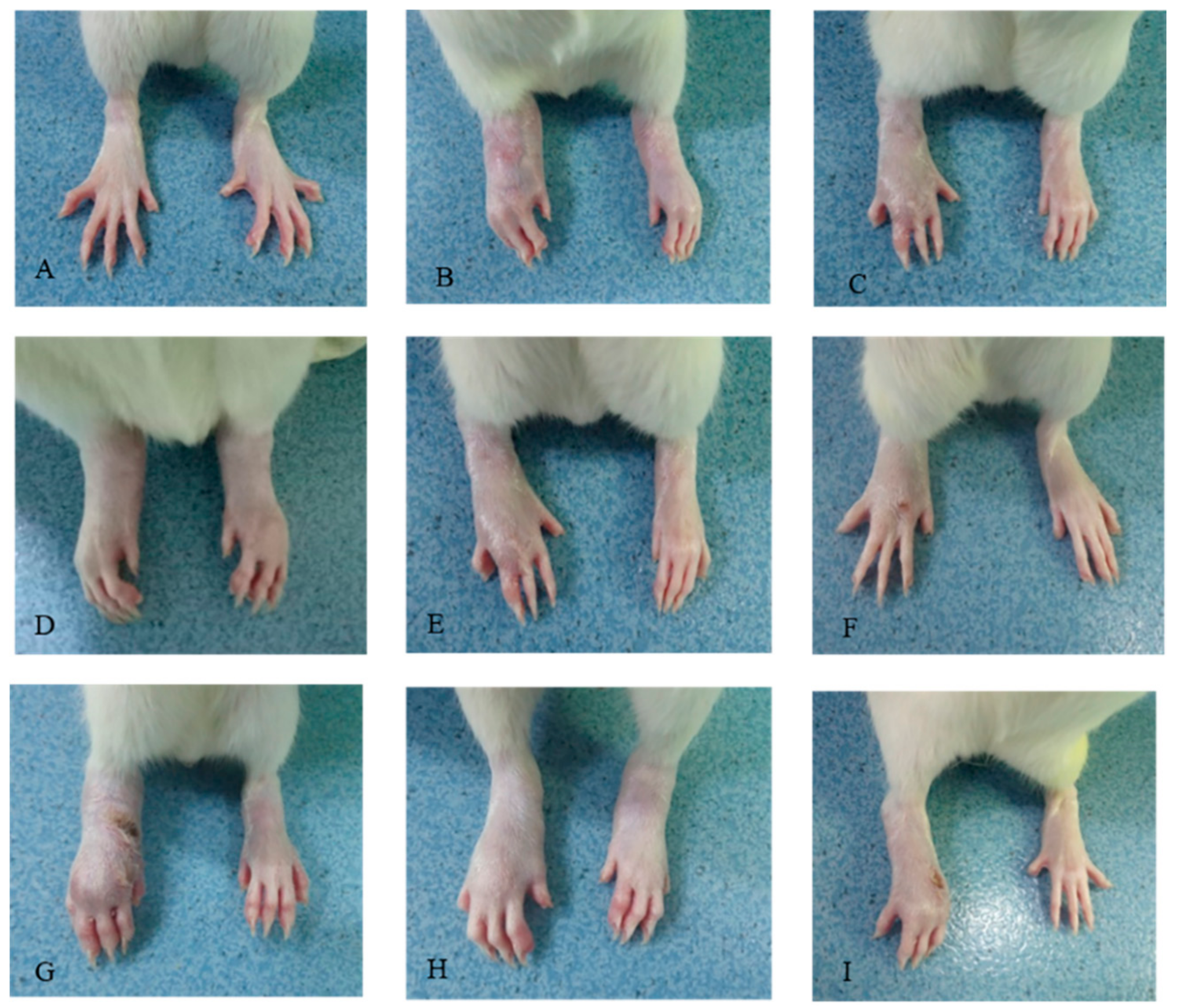
2.1. Effects of CDLF and CQAF on Weight, Paw Edema, and Histopathological Alterations
2.2. Protective Effects of CDLF and CQAF on Inflammation
2.3. Effects of CDLF and CQAF on Serum NO, SOD, and MDA
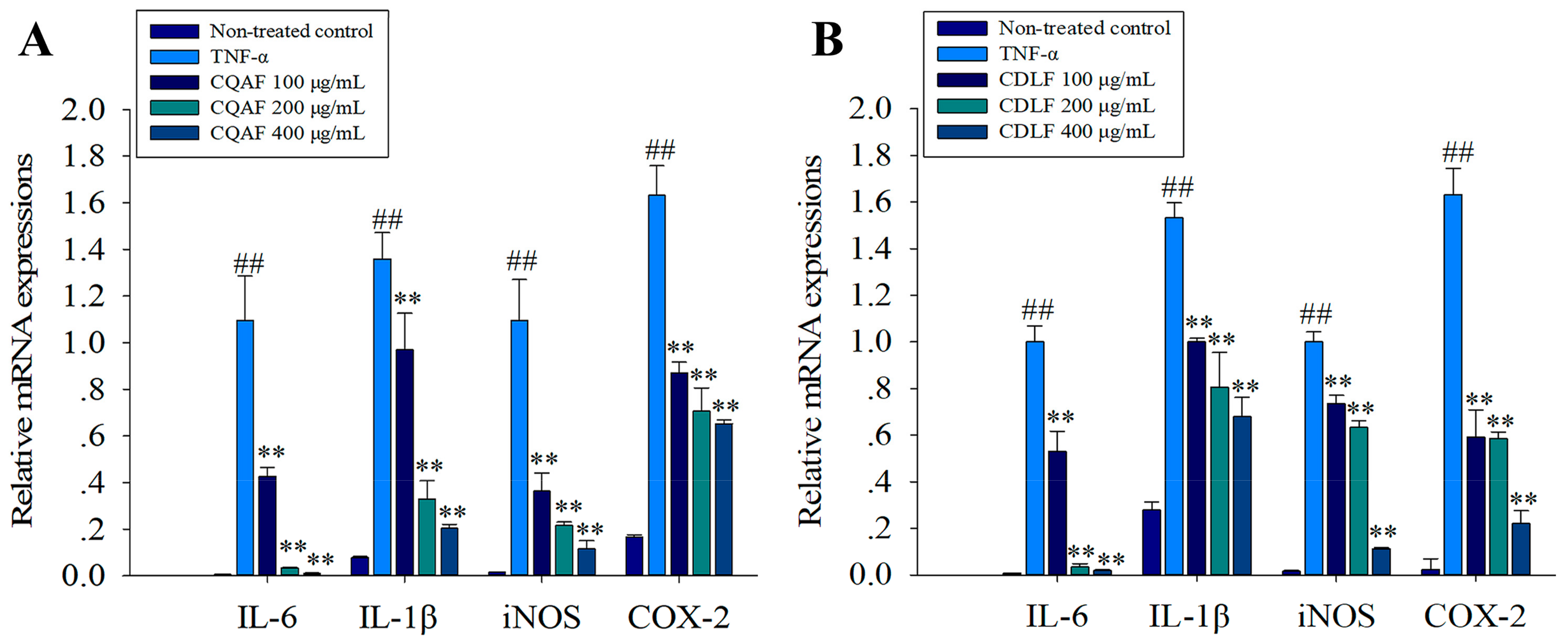
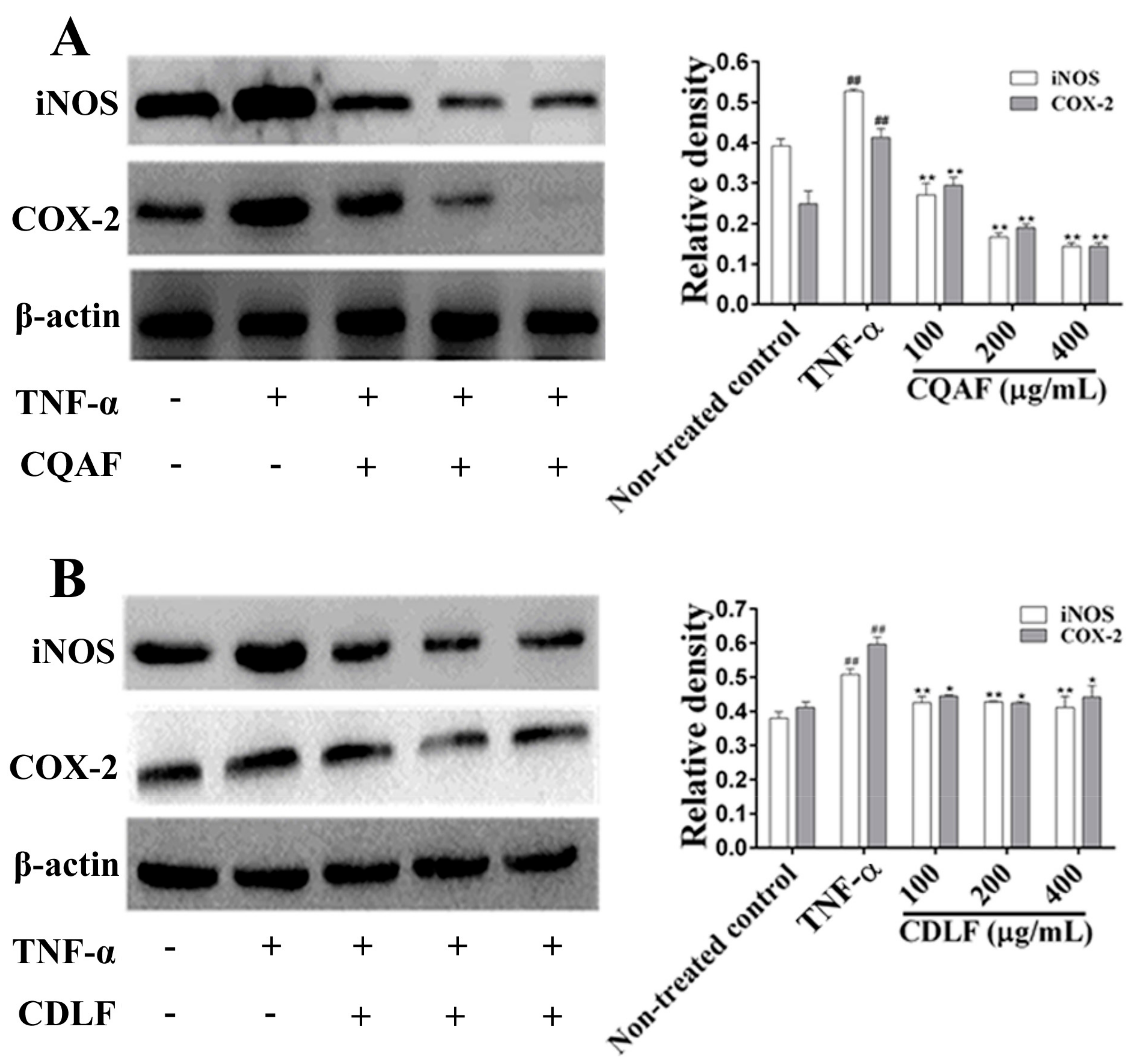
2.4. Effects of CDLF and CQAF on the Expressions of IL-1β, IL-6, iNOS, and COX-2 in MH7A Cells
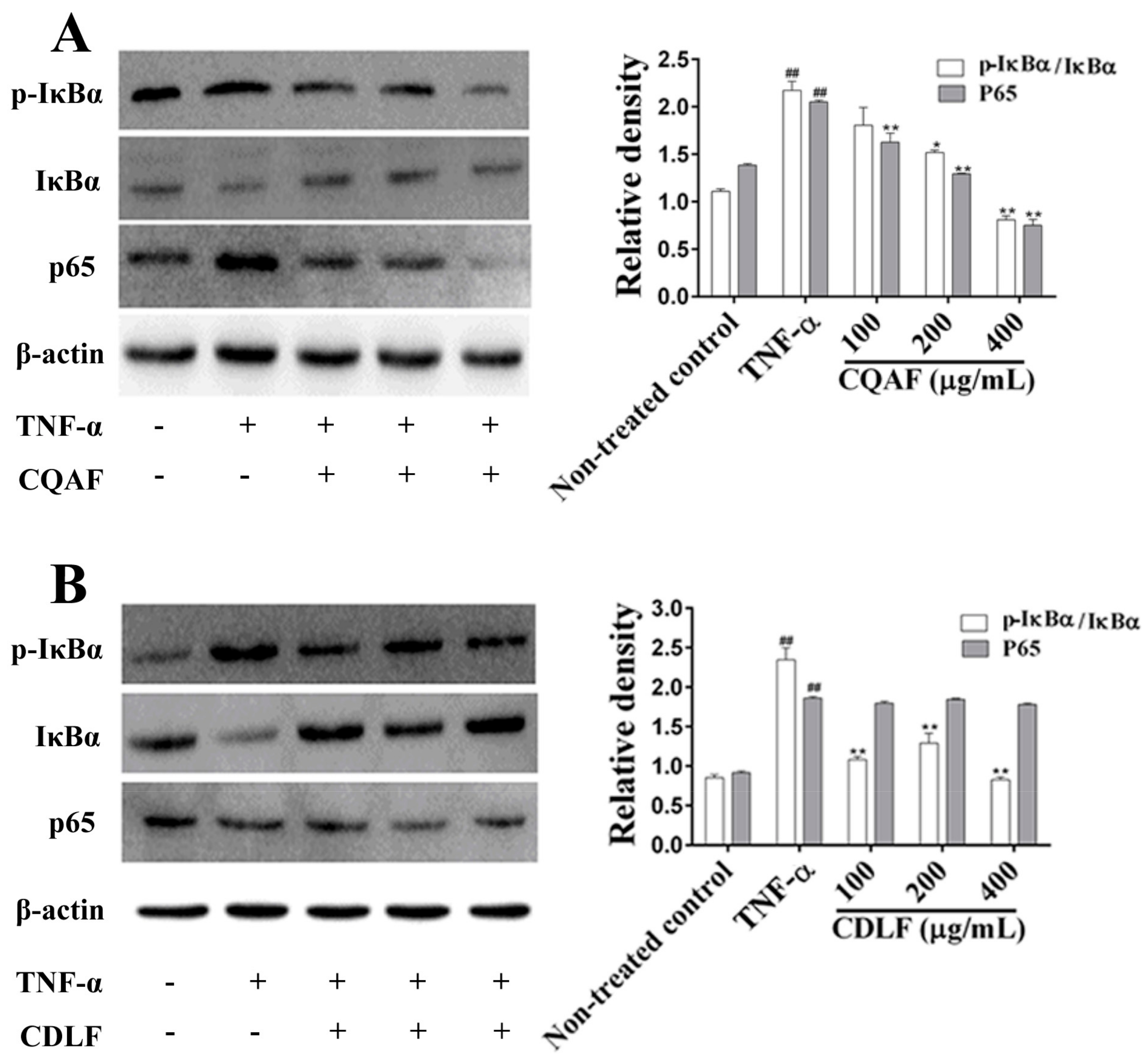
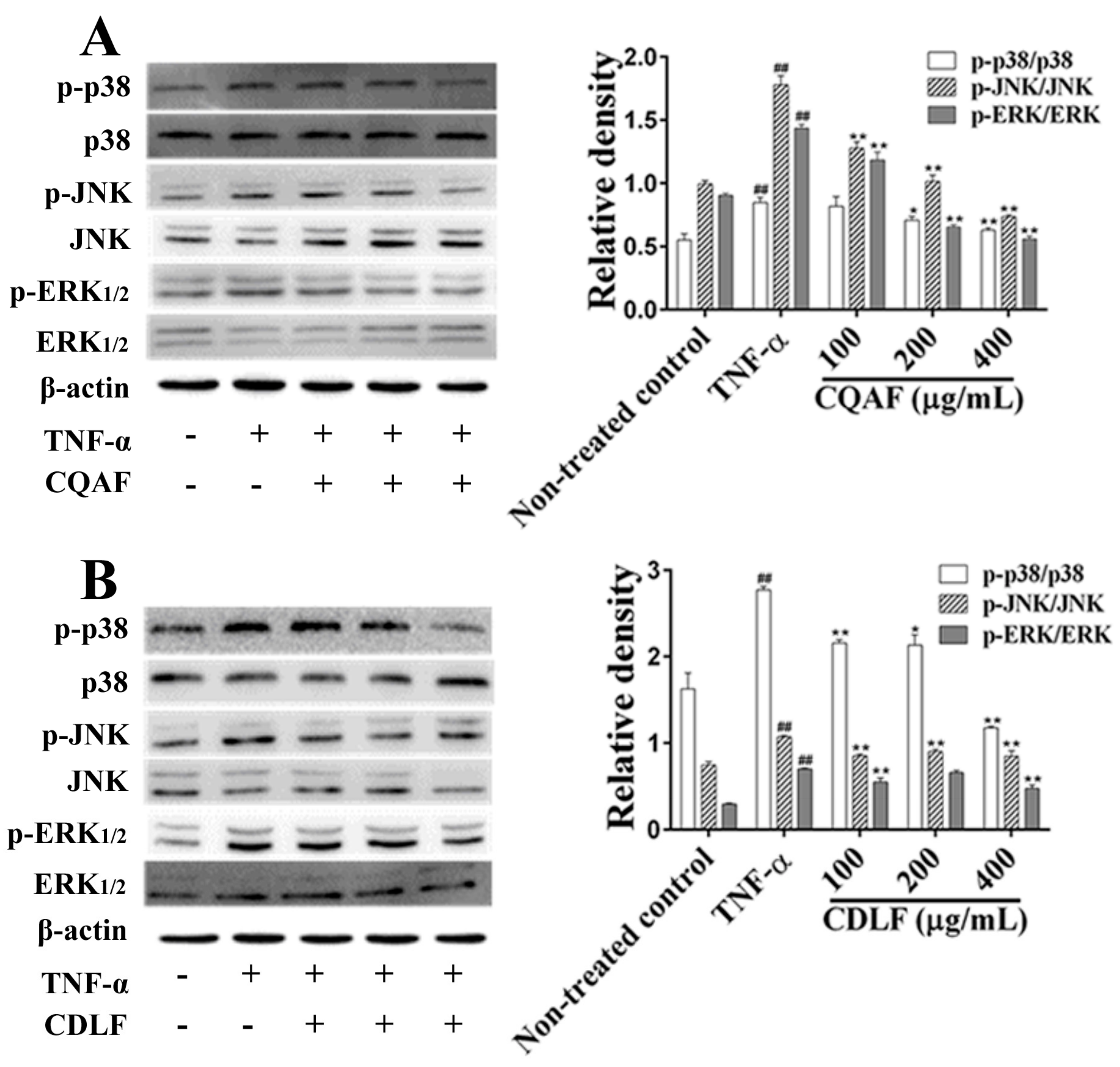
2.5. Effects of CDLF and CQAF on the Activation of NF-κB and MAPK Pathways in MH7A Cells
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Preparation of CDLF and CQAF
4.3. Animals
4.4. Induction of CIA and Treatment
4.5. Histological Examination of Ankle Joints
4.6. Measurement of Serum Levels of Cytokines
4.7. Measurement of Serum NO, SOD, and MDA
4.8. Cell Culture and Treatment
4.9. Quantitative Real-Time PCR Analysis
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RA | rheumatoid arthritis |
| FLS | fibroblast-like synoviocytes |
| LPS | lipopolysaccharides |
| MH7A | human rheumatoid arthritis synovial fibroblast cell line |
| CII | collagen type II from bovine nasal septum |
| TCM | traditional Chinese medicine |
| CFA | Freund’s adjuvant complete |
| CDLF | cardenolide-rich fractions |
| CQAF | caffeoylquinic-acid-rich fractions |
| TGT | tripterygium glycosides tablet |
| NF-κB | nuclear factor κB |
| MAPK | mitogen-activated protein kinase |
References
- Huang, D.; Chen, Y.; Chen, W.; Liu, Y.; Yao, F.; Xue, D.; Sun, L. Anti-inflammatory effects of the extract of gnaphalium affine d. Don in vivo and in vitro. J. Ethnopharmacol. 2015, 176, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Chen, J.; Wang, X.; Pan, Z.; Ke, Y.; Zhou, Z.; Xie, J.; Lv, G.; Luo, X. Kruppel-like factor 4 regulates the expression of inducible nitric oxide synthase induced by tnf-alpha in human fibroblast-like synoviocyte mh7a cells. Mol. Cell Biochem. 2017, 438, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.S.; Lee, E.G.; Jeon, H.S.; Chae, H.J.; Park, S.J.; Lee, Y.C.; Yoo, W.H. Quercetin inhibits il-1beta-induced proliferation and production of mmps, cox-2, and pge2 by rheumatoid synovial fibroblast. Inflammation 2012, 35, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gupta, Y.K.; Singh, S.; Patil, A. Glorisa superba hydroalcoholic extract from tubers attenuates experimental arthritis by downregulating inflammatory mediators, and phosphorylation of erk/jnk/p-38. Immunol. Investig. 2016, 45, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, N.; Wondimu, A.; Ullrich, K.; Schmidt, M.F. Synovial mmp-3 and timp-1 levels and their correlation with cytokine expression in canine rheumatoid arthritis. Vet. Immunol. Immunopathol. 2003, 91, 199–204. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Noack, M.; Miossec, P. Selected cytokine pathways in rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Yue, Y.; Hu, Y.; Cheng, W.; Liu, R.; Pan, X.; Zhang, P. Genistein suppresses tumor necrosis factor alpha-induced inflammation via modulating reactive oxygen species/akt/nuclear factor kappab and adenosine monophosphate-activated protein kinase signal pathways in human synoviocyte mh7a cells. Drug Des. Dev. Ther. 2014, 8, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhang, T.; Yin, C.; Zhou, J.; Huang, R.; Gao, S.; Zheng, L.; Wang, X.; Manyande, A.; Tian, X.; et al. Effects of the stem extracts of schisandra glaucescens diels on collagen-induced arthritis in balb/c mice. J. Ethnopharmacol. 2016, 194, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Huang, M.; Zhang, Y.; Li, H.; Zheng, H.; Yu, L.; Chu, K. Extracts of Bauhinia championii (benth.) benth. Inhibit nf-<kappa>b-signaling in a rat model of collagen-induced arthritis and primary synovial cells. J. Ethnopharmacol. 2016, 185, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.B.; Yu, S.S.; Chen, X.G.; Wu, X.F.; Ma, S.G.; Qu, J.; Hu, Y.C.; Liu, J.; Lv, H.N. Cytotoxic cardenolides from the stems of Periploca forrestii. Steroids 2012, 77, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, R.; Zhou, Y.; Chen, Z.; Tang, W.; Liu, Q.; Zuo, J.P.; Zhao, W. Immunosuppressive pregnane glycosides from periploca sepium and Periploca forrestii. Phytochemistry 2008, 69, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.-H.; Zhou, X.; Zhao, C.; Chen, H.-G. The research progress on the chemical component and the pharmacologyaction of Periploca forrestii schltr. Guangdong Chem. 2012, 39, 20–21. [Google Scholar]
- Liu, Y.; Li, M.; He, Q.; Yang, X.; Ruan, F.; Sun, G. Periploca forrestii saponin ameliorates murine cfa-induced arthritis by suppressing cytokine production. Mediat. Inflamm. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, B.; Xiong, D.-D.; Li, Y.-J.; Zou, H.; Ma, S.; Sun, J.; Lu, Y.; Zheng, L. Chemical constituents from periploca radix. Chin. Tradit. Herbal Drugs 2017, 48, 1513–1518. [Google Scholar]
- Dong, L.; Zhang, Y.; Wang, X.; Dong, Y.X.; Zheng, L.; Li, Y.J.; Ni, J.M. In vivo and in vitro anti-inflammatory effects of ethanol fraction from Periploca forrestii schltr. Chin. J. Integr. Med. 2017, 23, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, J.; Ke, X.; Qu, W.; Zhang, J.; Feng, F.; Liu, W. The therapeutic effects of Periploca forrestii schltr. Stem extracts on collagen-induced arthritis by inhibiting the activation of src/nf-kappab signaling pathway in rats. J. Ethnopharmacol. 2017, 202, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Baier, A.; Meineckel, I.; Gay, S.; Pap, T. Apoptosis in rheumatoid arthritis. Curr. Opin. Rheumatol. 2003, 15, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Kyei, S.; Koffuor, G.A.; Boampong, J.N. Antiarthritic effect of aqueous and ethanolic leaf extracts of pistia stratiotes in adjuvant-induced arthritis in sprague-dawley rats. J. Exp. Pharmacol. 2012, 4, 41–51. [Google Scholar] [PubMed]
- Tanwar, A.; Chawla, R.; Ansari, M.M.; Neha; Thakur, P.; Chakotiya, A.S.; Goel, R.; Ojha, H.; Asif, M.; Basu, M.; et al. In vivo anti-arthritic efficacy of Camellia sinensis (L.) in collagen induced arthritis model. Biomed. Pharmacother. 2017, 87, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.D.; Latham, K.A.; Rosloniec, E.F. Collagen-induced arthritis. Nat. Protoc. 2007, 2, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Foyet, H.S.; Tsala, D.E.; Zogo Essono Bodo, J.C.; Carine, A.N.; Heroyne, L.T.; Oben, E.K. Anti-inflammatory and anti-arthritic activity of a methanol extract from vitellaria paradoxa stem bark. Pharm. Res. 2014, 7, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Ananth, D.A.; Rameshkumar, A.; Jeyadevi, R.; Aseervatham, G.S.; Sripriya, J.; Bose, P.C.; Sivasudha, T. Amelioratory effect of flavonoids rich pergularia daemia extract against cfa induced arthritic rats. Biomed. Pharmacother. 2016, 80, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, C.G.; Sanz, I.; Cook, M.C. Dysregulation of germinal centres in autoimmune disease. Nat. Rev. Immunol. 2009, 9, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.; Panayi, G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.B.; Yokota, H. Altered mrna level of matrix metalloproteinase-13 in mh7a synovial cells under mechanical loading and unloading. Bone 2001, 28, 399–403. [Google Scholar] [CrossRef]
- Xu, J.; Itoh, Y.; Hayashi, H.; Takii, T.; Miyazawa, K.; Onozaki, K. Dihydrotestosterone inhibits interleukin-1alpha or tumor necrosis factor alpha-induced proinflammatory cytokine production via androgen receptor-dependent inhibition of nuclear factor-kappab activation in rheumatoid fibroblast-like synovial cell line. Biol. Pharm. Bull. 2011, 34, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Corral, L.G.; Haslett, P.A.; Muller, G.W.; Chen, R.; Wong, L.M.; Ocampo, C.J.; Patterson, R.T.; Stirling, D.I.; Kaplan, G. Differential cytokine modulation and t cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of tnf-alpha. J. Immunol. 1999, 163, 380–386. [Google Scholar] [PubMed]
- Chen, Y.; Wang, Q.W.; Zuo, J.; Chen, J.W.; Li, X. Anti-arthritic activity of ethanol extract of claoxylon indicum on freund’s complete adjuvant-induced arthritis in mice. BMC Complement. Altern. Med. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lew, J.H.; Kim, T.W.; Kang, H. Effect of an herbal mixture of cinnamon cortex, persicae semen, and natril sulfas on collagen-induced arthritis and lipopolysaccharides-induced nuclear factor-kappa b signaling. Chin. J. Integr. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Huang, M.; Zhang, Y.; Li, H.; Zheng, H.; Yu, L.; Chu, K.; Lin, Y.; Chen, L. Extracts of Bauhinia championii (benth.) benth. Attenuate the in flammatory response in a rat model of collagen-induced arthritis. Mol. Med. Rep. 2016, 13, 4167–4174. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by erk, jnk, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Erickson, E.A.; Long, D.L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Gao, R.F.; Yuan, F.L.; Zhao, M.D. Recombinant human endostatin suppresses mouse osteoclast formation by inhibiting the nf-kappab and mapks signaling pathways. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, J.; Zhang, M.; Nie, L. Protective effect of asarum extract in rats with adjuvant arthritis. Exp. Ther. Med. 2014, 8, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Jeyadevi, R.; Sivasudha, T.; Rameshkumar, A.; Dinesh Kumar, L. Anti-arthritic activity of the Indian leafy vegetable Cardiospermum halicacabum in wistar rats and uplc-qtof-ms/ms identification of the putative active phenolic components. Inflamm. Res. 2013, 62, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y. Chondroprotective and anti-nociceptive effects of caffeoylquinic acid in osteoarthritis by downregulating catabolic activity and oxidative damage in chondrocytes. Biomed. Pharmacother. 2017, 93, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Song, H.P.; Li, X.; Yu, R.; Zeng, G.; Yuan, Z.Y.; Wang, W.; Huang, H.Y.; Cai, X. Phenotypic characterization of type II collagen-induced arthritis in wistar rats. Exp. Ther. Med. 2015, 10, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the CDLF and CQAF are available from the authors. |




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Wang, X.; He, Y.-L.; Wang, Y.; Dong, L.; Ma, X.; Zheng, L.; Liu, C.-H.; Wang, G.-C.; Zheng, J.; et al. In Vivo and In Vitro Anti-Arthritic Effects of Cardenolide-Rich and Caffeoylquinic Acid-Rich Fractions of Periploca forrestii. Molecules 2018, 23, 1988. https://doi.org/10.3390/molecules23081988
Liu T, Wang X, He Y-L, Wang Y, Dong L, Ma X, Zheng L, Liu C-H, Wang G-C, Zheng J, et al. In Vivo and In Vitro Anti-Arthritic Effects of Cardenolide-Rich and Caffeoylquinic Acid-Rich Fractions of Periploca forrestii. Molecules. 2018; 23(8):1988. https://doi.org/10.3390/molecules23081988
Chicago/Turabian StyleLiu, Ting, Xia Wang, Yan-Ling He, Yang Wang, Li Dong, Xue Ma, Lin Zheng, Chun-Hua Liu, Guang-Cheng Wang, Jiang Zheng, and et al. 2018. "In Vivo and In Vitro Anti-Arthritic Effects of Cardenolide-Rich and Caffeoylquinic Acid-Rich Fractions of Periploca forrestii" Molecules 23, no. 8: 1988. https://doi.org/10.3390/molecules23081988
APA StyleLiu, T., Wang, X., He, Y.-L., Wang, Y., Dong, L., Ma, X., Zheng, L., Liu, C.-H., Wang, G.-C., Zheng, J., Lan, Y.-Y., & Li, Y.-J. (2018). In Vivo and In Vitro Anti-Arthritic Effects of Cardenolide-Rich and Caffeoylquinic Acid-Rich Fractions of Periploca forrestii. Molecules, 23(8), 1988. https://doi.org/10.3390/molecules23081988