Effects of Regulated Deficit Irrigation on Amino Acid Profiles and Their Derived Volatile Compounds in Cabernet Sauvignon (Vitis vinifera L.) Grapes and Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Conditions and Materials
2.2. Samples and Vinifications
2.3. HPLC Determination of Amino Acids
Derivatization of Amino Acids
2.4. Determination of Volatile Compounds by GC-MS
2.4.1. Extraction of Volatile Compounds
2.4.2. GC-MS Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Weather Conditions and Physicochemical Parameters of Grape Berries
3.2. Amino Acid Profiles of Grape Berries and Wines
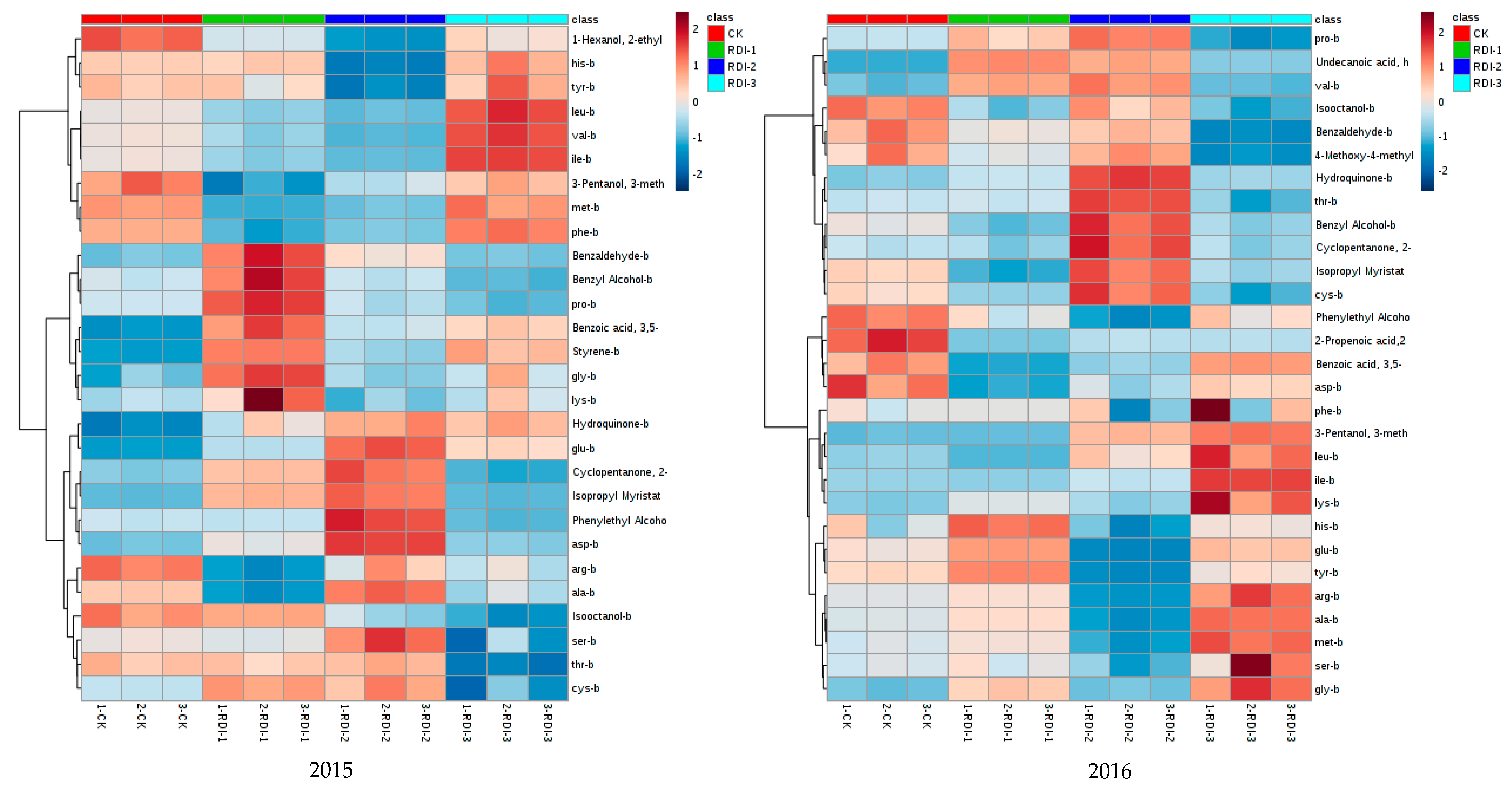
3.3. Volatile Compounds Derived from Amino Acids in Grape Berries and Wines
3.3.1. Volatile Compounds Derived from Amino Acids in Grape Berries
3.3.2. Volatile Compounds Derived from Amino Acids in Wines
3.4. The Correlation Analysis between Volatile Compounds and Amino Acids in Grape Berries and Wines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bell, S.J.; Henschke, P. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145. [Google Scholar] [CrossRef] [PubMed]
- Bisson, L.F.; Karpel, J.E. Genetics of yeast impacting wine quality. Annu. Rev. Food Sci. Technol. 2010, 1, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A. Volatile flavour of wine: Correlation between instrumental analysis and sensory perception. Food 1998, 42, 351–363. [Google Scholar] [CrossRef]
- Ardo, Y. Flavour formation by amino acid catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Procopio, S.; Krause, D.; Hofmann, T.; Becker, T. Significant amino acids in aroma compound profiling during yeast fermentation analyzed by pls regression. LWT. Food Sci. Technol. 2013, 51, 423–432. [Google Scholar]
- Wang, Y.Q.; Ye, D.Q.; Liu, P.T.; Duan, L.L.; Duan, C.Q.; Yan, G.L. Synergistic effects of branched-chain amino acids and phenylalanine addition on major volatile compounds in wine during alcoholic fermentation. S. Afr. J. Enol. Viticult. 2016, 37, 169–175. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Garde-Cerdán, T.; Zalacain, A.; Garcia, R.; Cabrita, M.J.; Salinas, M.R. Vine-shoot waste aqueous extract applied as foliar fertilizer to grapevines: Effect on amino acids and fermentative volatile content. Food Chem. 2016, 197, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Bouzas-Cid, Y.; Díaz-Losada, E.; Trigo-Córdoba, E.; Falqué, E.; Orriols, I.; Garde-Cerdán, T.; Mirás-Avalos, J.M. Effects of irrigation over three years on the amino acid composition of Albariño (Vitis vinifera L.) musts and wines in two different terroirs. Sci. Hortic. 2018, 227, 313–325. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Portu, J.; Santamaría, P.; López, R.; Garde-Cerdán, T. Effects on grape amino acid concentration through foliar application of three different elicitors. Food Res. Int. 2017, 99, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.; Hilbert, G.; Luquin, J.; Goicoechea, N.; Antolín, M.C. Flavonoid and amino acid profiling on Vitis vinifera L. cv Tempranillo subjected to deficit irrigation under elevated temperatures. J. Food Compost. Anal. 2017, 62, 51–62. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Gonzalo-Diago, A.; Moreno-Simunovic, Y.; Martínez-Gil, A.M. Effect of different foliar nitrogen applications on the must amino acids and glutathione composition in Cabernet Sauvignon vineyard. LWT-Food Sci. Technol. 2017, 75, 14–154. [Google Scholar]
- Ji, X.W.; Cheng, Z.Y.; Zhao, X. Effect of regulated deficit drip irrigation on yield and quality of wine grape in desert oasis. J. Arid Land Res. Environ. 2015, 4, 184–188. [Google Scholar]
- Li, Y.S.; Zhao, X.H.; Wang, H. Research advance and prospect of regulated deficit irrigation on grapevines. Agric. Res. Arid Areas 2013, 1, 23–241. [Google Scholar]
- Wang, S.J.; Liu, Q.B.; Yu, H.Y.; Sun, R. The Theory and Technological System of Regulated Deficit Irrigation for Grape. Agric. Mech. Res. 2005, 2, 8–9. [Google Scholar]
- Fang, Y.L.; Sun, W.; Wan, L. Effects of Regulated Deficit Irrigation (RDI) on Wine Grape Growth and Fruit Quality. Sci. Agric. Sin. 2013, 46, 2730–2738. [Google Scholar]
- Ju, Y.L.; Wang, T.M.; Zhao, X.F.; Liu, M.; Min, Z.; Zhang, J.X.; Fang, Y.L. Effects of regulated deficit irrigation on fruit development and seed phenolic compounds of Cabernet Sauvignon. Sino-Overseas Grapevine Wine 2017, 4, 18–24. [Google Scholar]
- Song, J.; Shellie, K.C.; Wang, H.; Qian, M.C. Influence of deficit irrigation and kaolin particle film on grape composition and volatile compounds in Merlot grape (Vitis vinifera L.). Food Chem. 2012, 134, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration Guidelines for Computing Crop Water Requirements (FAO Irrigation and Drainage Paper no. 56); Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- OIV. International Code of Oenological Practices. 2012. Available online: http://www.oiv.int/oiv/info/enpratiquesoenologiques (accessed on 1 January 2012).
- Liu, R.; Tian, Y.; Wen, Y.; Gao, Q.; Pan, Q. Effects of rain-shelter cultivation on the contents of amino acids in wine grape berry. Sino-Overseas Grapevine Wine 2012, 4, 15–19. [Google Scholar]
- Garde-Cerdán, T.; López, R.; Portu, J.; González-Arenzana, L.; López-Alfaro, I.; Santamaría, P. Study of the effects of proline, phenylalanine, and urea foliar application to tempranillo vineyards on grape amino acid content. comparison with commercial nitrogen fertilisers. Food Chem. 2014, 163, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.L.; Liu, M.; Zhao, X.F.; Zeng, J.; Min, Z.; Fang, Y.L. Effects of Filed Management Practices and Harvest Time on Fatty Acid Composition of Cabernet Sauvignon and Chardonnay (Vitis vinifera L.) Berries Skins. Food Sci. 2017, 38, 107–113. [Google Scholar]
- Traverso, J.A.; Pulido, A.; Rodríguez-García, M.I.; Alché, J.D. Thiol-based redox regulation in sexual plant reproduction: New insights and perspectives. Front. Plant Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis Current Protocols in Bioinformatics. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar]
- Shellie, K.C. Vine and berry response of Merlot (Vitis vinifera L.) to differential water stress. Am. J. Enol. Vitic. 2006, 57, 514–518. [Google Scholar]
- Koundouras, S.; Marinos, V.; Gkoulioti, A.; Kotseridis, Y.; van Leeuwen, C. Influence of vineyard location and vine water status on fruit maturation of nonirrigated cv. Agiorgitiko (Vitis vinifera L.). Effects on wine phenolic and aroma components. J. Agric. Food Chem. 2006, 54, 5077–5086. [Google Scholar] [CrossRef] [PubMed]
- Valero, E.; Millan, C.; Ortega, J.M.; Mauricio, J.C. Concentration of amino acids in wine after the end of fermentation by Saccharomyces cerevisiae strains. J. Sci. Food Agric. 2003, 83, 830–835. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, A.J.; Carrascosa, A.V.; Martin-Alvarez, P.J.; Moreno-Arribas, V.; Polo, M.C. Influence of the yeast strain on the changes of the amino acids, peptides and proteins during sparkling wine production by the traditional method. J. Ind. Microbiol. Biotechnol. 2002, 29, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Orte, P.; Ibarz, M.; Cacho, J.; Ferreira, V. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005, 89, 163–174. [Google Scholar] [CrossRef]
- Guan, L.; Wu, B.; Hilbert, G.; Li, S.; Gomès, E.; Delrot, S.; Dai, Z. Cluster shading modifies amino acids in grape (Vitis vinifera L.) berries in a genotype-and tissue-dependent manner. Food Res. Int. 2017, 98, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Lorenzo, C.; Martínez-Gil, A.M.; Lara, J.F.; Pardo, F.; Salinas, R. Evolution of nitrogen compounds during grape ripening from organic and nonorganic Monastrell–nitrogen consumption and volatile formation in alcoholic fermentation. Res. Org. Farming 2009, 8, 123–138. [Google Scholar]
Sample Availability: Not available. |
| Parameter | RDI-1 | RDI-2 | RDI-3 | Control | ||||
|---|---|---|---|---|---|---|---|---|
| R | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | 2015 | 2016 |
| Yield (ton/ha) | 7.12b | 7.70b | 7.77ab | 7.94b | 8.36a | 8.58ab | 9.06a | 9.57a |
| Weight (g/100 berries) | 110.14b | 118.18b | 105.01c | 110.10c | 118.38a | 124.81a | 122.50a | 122.70a |
| Total soluble solids (Brix) | 23.92a | 23.45a | 23.53ab | 23.95a | 23.56ab | 23.31a | 22.30b | 23.26a |
| Titratable acidity (g/L tartaric acid) | 4.42b | 3.25b | 4.81ab | 3.14b | 5.12ab | 3.51a | 5.46a | 3.71a |
| pH | 3.90a | 4.13b | 3.72b | 4.31a | 3.69c | 3.92c | 3.67c | 3.86c |
| Midday stem water potential from fruit set to harvest (MPa) | −0.56 | −0.58 | −0.49 | −0.52 | −0.38 | −0.43 | −0.30 | −0.34 |
| Family | Amino Acid | 2015 | 2016 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RDI-1 | RDI-2 | RDI-3 | CK | RDI-1 | RDI-2 | RDI-3 | CK | ||
| Serine | Cys | 5.96 ± 1.81a | 7.92 ± 1.13a | 10.78 ± 0.05a | 8.55 ± 0.40a | 5.76 ± 0.07a | 6.70 ± 0.69a | 8.43 ± 0.49a | 6.17 ± 0.84a |
| Ser | 35.26 ± 1.21b | 38.56 ± 1.02ab | 41.24 ± 3.01a | 39.25 ± 1.78ab | 33.58 ± 0.24ab | 28.543 ± 2.01b | 42.75 ± 2.06a | 30.52 ± 0.78b | |
| Gly | 3.18 ± 0.45c | 3.40 ± 0.20c | 7.26 ± 0.43a | 7.82 ± 0.10b | 4.92 ± 0.01ab | 2.08 ± 0.29b | 8.96 ± 0.18a | 2.77 ± 0.11b | |
| Aromatic amino acids | Phe | 15.58 ± 0.85c | 16.85 ± 0.45c | 26.41 ± 0.77a | 20.00 ± 0.88b | 17.12 ± 1.39b | 15.26 ± 1.33c | 18.71 ± 0.97a | 16.18 ± 0.90bc |
| Trp | 11.24 ± 0.48ab | 6.95 ± 0.21c | 15.49 ± 0.89a | 9.54 ± 0.32b | 15.88 ± 0.54a | 6.21 ± 0.81c | 14.39 ± 0.31a | 8.82 ± 0.35b | |
| Aspartate | Ile | 8.61 ± 1.04c | 7.62 ± 0.64c | 17.62 ± 1.05a | 12.34 ± 0.62b | 10.65 ± 0.95b | 12.06 ± 0.95b | 19.17 ± 0.92a | 8.14 ± 0.98b |
| Asp | 20.16 ± 0.86b | 25.31 ± 0.73a | 25.08 ± 0.76a | 21.55 ± 0.56b | 18.99 ± 1.61bc | 18.28 ± 0.79c | 23.16 ± 0.44a | 21.34 ± 1.08ab | |
| Lys | 2.51 ± 0.81b | 2.45 ± 0.21b | 5.21 ± 0.21a | 3.45 ± 0.12b | 3.01 ± 0.32b | 1.98 ± 0.08b | 4.30 ± 0.05a | 2.68 ± 0.0.31b | |
| Met | 1.64 ± 0.04c | 1.58 ± 0.15c | 4.95 ± 0.75a | 3.21 ± 0.09b | 2.41 ± 0.21b | 1.02 ± 0.01d | 5.01 ± 0.02a | 1.95 ± 0.04c | |
| Thr | 47.62 ± 2.68b | 51.26 ± 1.53b | 57.24 ± 0.92a | 57.32 ± 1.46a | 47.76 ± 1.23ab | 47.85 ± 0.69ab | 49.56 ± 0.60a | 44.55 ± 3.15b | |
| Glutamate | Arg | 330.19 ± 2.61b | 336.47 ± 3.13b | 354.54 ± 1.52a | 346.57 ± 1.26a | 320.54 ± 1.01b | 308.47 ± 0.30c | 334.85 ± 6.09a | 317.55 ± 4.37bc |
| Glu | 69.74 ± 1.53c | 82.42 ± 2.13b | 89.85 ± 0.84a | 70.45 ± 0.17c | 64.45 ± 2.01a | 40.54 ± 0.99c | 66.37 ± 0.66a | 54.25 ± 1.45b | |
| His | 16.32 ± 1.04c | 15.21 ± 0.98c | 27.39 ± 2.30a | 21.52 ± 1.51b | 22.17 ± 1.64ab | 15.05 ± 0.95c | 25.45 ± 2.01a | 18.95 ± 1.01bc | |
| Pro | 195.77 ± 1.61a | 139.45 ± 2.59b | 122.26 ± 10.36b | 105.12 ± 5.526c | 155.49 ± 0.82a | 145.46 ± 2.04b | 119.89 ± 3.80c | 115.64 ± 1.84c | |
| Pyruvate | Leu | 15.95 ± 1.58c | 24.58 ± 1.08b | 31.05 ± 22.85a | 21.95 ± 2.84b | 17.82 ± 1.84b | 15.24 ± 2.01b | 29.54 ± 2.54a | 14.85 ± 0.54b |
| Ala | 35.78 ± 4.18b | 63.83 ± 3.69a | 74.70 ± 59.24a | 67.94 ± 0.67a | 51.65 ± 2.75b | 31.20 ± 0.87d | 71.03 ± 2.74a | 43.57 ± 0.72c | |
| Val | 13.51 ± 1.59c | 12.95 ± 1.52c | 27.62 ± 2.81a | 20.12 ± 0.43b | 22.57 ± 1.24a | 20.14 ± 0.76a | 24.32 ± 2.31a | 15.89 ± 0.81b | |
| Family | Amino Acid | 2015 | 2016 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RDI-1 | RDI-2 | RDI-3 | CK | RDI-1 | RDI-2 | RDI-3 | CK | ||
| Serine | Cys | 3.18 ± 0.13b | 4.12 ± 0.06b | 7.22 ± 0.13a | 6.41 ± 0.26ab | 2.94 ± 0.05b | 3.21 ± 0.67b | 5.99 ± 0.02a | 5.96 ± 0.19a |
| Ser | 1.40 ± 0.03c | 3.75 ± 0.43b | 6.21 ± 0.61a | 2.11 ± 0.15c | 1.05 ± 0.05c | 5.66 ± 0.43b | 8.82 ± 0.42a | 4.30 ± 0.40b | |
| Gly | 2.32 ± 0.77c | 4.62 ± 0.93b | 6.32 ± 0.42a | 3.05 ± 0.38c | 2.74 ± 0.10c | 4.41 ± 0.69c | 5.83 ± 0.06a | 3.75 ± 0.77b | |
| Aromatic amino acids | Trp | 2.06 ± 0.22c | 3.34 ± 0.37b | 4.07 ± 0.75a | 1.73 ± 0.51c | 2.55 ± 0.50b | 4.04 ± 0.37b | 5.51 ± 0.74a | 2.51 ± 0.54c |
| Phe | 1.88 ± 0.02c | 2.62 ± 0.09b | 4.14 ± 0.52a | 1.03 ± 0.04c | 2.05 ± 0.22c | 1.51 ± 0.33c | 3.67 ± 0.12a | 2.62 ± 0.11b | |
| Aspartate | Ile | 0.24 ± 0.06c | 2.03 ± 0.26b | 3.64 ± 0.17a | 1.83 ± 0.29c | 0.65 ± 0.16d | 1.63 ± 0.05c | 2.21 ± 0.05a | 1.46 ± 0.15b |
| Lys | 1.59 ± 0.01c | 2.08 ± 0.12b | 3.7 ± 0.07a | 1.86 ± 0.18c | 1.04 ± 0.17b | 2.91 ± 0.34ab | 3.29 ± 0.09a | 1.69 ± 0.15b | |
| Met | 0.4 ± 0.03c | 0.49 ± 0.09c | 1.56 ± 0.06a | 0.69 ± 0.04b | 0.55 ± 1.09c | 0.51 ± 0.08c | 1.82 ± 0.04a | 0.66 ± 0.07b | |
| Asp | 2.79 ± 0.21c | 3.63 ± 0.15b | 4.6 ± 0.29a | 2.07 ± 0.10c | 1.06 ± 0.09c | 3.55 ± 0.18a | 3.19 ± 0.41a | 1.89 ± 0.26b | |
| Thr | 1.12 ± 0.07c | 2.78 ± 0.14b | 2.12 ± 0.07a | 1.05 ± 0.02c | 1.48 ± 0.14c | 1.84 ± 0.02c | 2.4 ± 0.15a | 1.85 ± 0.20b | |
| Glutamate | Pro | 16.24 ± 0.89c | 15.57 ± 2.39b | 14.51 ± 3.48a | 9.47 ± 0.55c | 13.49 ± 1.14c | 12.74 ± 1.51b | 13.47 ± 2.60a | 10.06 ± 1.10c |
| His | 1.43 ± 0.14a | 1.59 ± 0.04a | 1.91 ± 0.07a | 0.55 ± 0.02b | 1.56 ± 0.05b | 1.86 ± 0.01b | 2.51 ± 0.06a | 0.56 ± 0.01c | |
| Arg | 12.59 ± 1.98b | 10.52 ± 1.12c | 15.00 ± 0.16a | 10.75 ± 0.91c | 15.06 ± 1.51b | 13.28 ± 1.00b | 17.87 ± 1.73a | 14.11 ± 1.28b | |
| Glu | 4.65 ± 1.73b | 2.44 ± 0.53c | 7.74 ± 0.17a | 5.74 ± 0.92ab | 3.41 ± 2.13c | 2.44 ± 0.13c | 8.66 ± 1.04a | 6.26 ± 1.07b | |
| Pyruvate | Leu | 5.4 ± 0.79c | 8.67 ± 0.59b | 10.66 ± 1.28a | 6.76 ± 0.51c | 6.09 ± 0.14c | 7.06 ± 0.40b | 9.23 ± 0.26a | 5.88 ± 0.54c |
| Ala | 5.72 ± 0.28c | 6.13 ± 0.48b | 9.49 ± 0.94a | 4.31 ± 0.14c | 6.91 ± 1.36c | 7.11 ± 1.55c | 10.55 ± 0.43a | 8.51 ± 0.96b | |
| Val | 1.27 ± 0.06c | 2.45 ± 0.18b | 4.71 ± 0.45a | 1.1 ± 0.12c | 1.09 ± 0.71b | 1.33 ± 0.05b | 3.78 ± 0.49a | 1.08 ± 0.41b | |
| Compounds | 2015 | 2016 | ||||||
|---|---|---|---|---|---|---|---|---|
| RDI-1 | RDI-2 | RDI-3 | CK | RDI-1 | RDI-2 | RDI-3 | CK | |
| Benzeneacetic acid, methyl ester | 3.74 ± 0.23a | 1.62 ± 0.10c | 4.2 ± 0.26a | 2.56 ± 0.16b | 1.43 ± 0.09c | 1.72 ± 0.10c | 4.05 ± 0.24a | 2.54 ± 0.15b |
| 1-propenyl-benzene | 0.52 ± 0.03c | 1.02 ± 0.06a | 0.77 ± 0.05b | 0.8 ± 0.05b | 1.29 ± 0.08b | 1.89 ± 0.11b | 1.11 ± 0.07a | 1.33 ± 0.08b |
| Benzaldehyde | 2.86 ± 0.18b | 2.98 ± 0.18b | 4.96 ± 0.31a | 1.87 ± 0.12c | 3.17 ± 0.19b | 2.11 ± 0.13c | 5.37 ± 0.32a | 2.97 ± 0.18bc |
| 3,4-Dimethyl-benzoic acid | 2.53 ± 0.16c | 4.77 ± 0.3a | 3.37 ± 0.21bc | 3.6 ± 0.22b | 4.99 ± 0.3a | 5.13 ± 0.31a | 4.52 ± 0.27a | 4.68 ± 0.28a |
| Styrene | 2.76 ± 0.17ab | 3 ± 0.19a | 2.65 ± 0.16ab | 2.29 ± 0.14b | 5.34 ± 0.32a | 6.75 ± 0.4a | 6.43 ± 0.38a | 5.6 ± 0.33a |
| Benzyl alcohol | 0.48 ± 0.03b | 0.85 ± 0.05a | 0.89 ± 0.06a | 0.63 ± 0.04b | 0.65 ± 0.04ab | 0.61 ± 0.04b | 0.79 ± 0.05a | 0.57 ± 0.03b |
| Phenylethyl alcohol | 56.43 ± 3.5a | 52.1 ± 3.23a | 55.35 ± 3.43a | 49.57 ± 3.07a | 58.27 ± 3.48a | 57.16 ± 3.41a | 55.83 ± 3.33a | 53.71 ± 3.21a |
| Furfural | 2.46 ± 0.15a | 2.31 ± 0.14a | 2.32 ± 0.14a | 2.41 ± 0.15a | ndb | nd | nd | nd |
| 3-Furaldehyde | 0.27 ± 0.02b | 0.61 ± 0.04a | 0.56 ± 0.03a | 0.16 ± 0.01b | nd | nd | nd | nd |
| 2,3,4,6-Tetramethylphenol | 1.85 ± 0.11a | 0.85 ± 0.05b | 1.55 ± 0.1a | 1.88 ± 0.12a | 2.6 ± 0.16ab | 2.65 ± 0.16ab | 3.21 ± 0.19a | 2.36 ± 0.14b |
| Total amount of aromatic compounds | 73.89 ± 4.58a | 70.11 ± 4.35a | 76.62 ± 4.75a | 65.78 ± 4.08a | 78.19 ± 4.67a | 78.45 ± 4.68a | 81.87 ± 4.89a | 74.26 ± 4.43a |
| Dihydro-3-methyl-2,5-furandione | nd | nd | nd | nd | 0.45 ± 0.03ab | 0.42 ± 0.03b | 0.55 ± 0.03a | 0.49 ± 0.03ab |
| 2-Methyl-1-propanol | 12 ± 0.74a | 11.34 ± 0.7ab | 10.57 ± 0.66ab | 9.25 ± 0.57b | 22.74 ± 1.36a | 16.22 ± 0.97b | 22.2 ± 1.33a | 16.16 ± 0.96b |
| 3-Methyl-1-pentanol | 2.73 ± 0.17c | 5.41 ± 0.34a | 4.41 ± 0.27ab | 3.42 ± 0.21bc | nd | nd | nd | nd |
| 3-Methyl-1-butanol | 192.09 ± 11.91a | 203.06 ± 12.59a | 222.58 ± 13.8a | 202.78 ± 12.57a | 315.86 ± 18.85ab | 305.71 ± 18.25ab | 353.97 ± 21.13a | 275.11 ± 16.42b |
| 3-Methyl-2-pentanol | 0.86 ± 0.05b | 0.59 ± 0.04c | 1.21 ± 0.07a | 0.98 ± 0.06ab | 1.28 ± 0.08bc | 1.08 ± 0.06c | 1.63 ± 0.1a | 1.55 ± 0.09ab |
| 2-Propanol, 1-butoxy- | 0.54 ± 0.03b | 1.31 ± 0.08a | 0.55 ± 0.03b | 0.35 ± 0.02b | nd | nd | nd | nd |
| Total amount of branched alcohols | 208.22 ± 12.91a | 221.72 ± 13.75a | 239.31 ± 14.84a | 216.79 ± 13.44a | 339.87 ± 20.29ab | 323.01 ± 19.28ab | 377.8 ± 22.55a | 292.82 ± 17.48b |
| 2-Butanone, 3-hydroxy- | 0.95 ± 0.06b | 0.42 ± 0.03c | 1.74 ± 0.11a | 0.88 ± 0.05b | 0.88 ± 0.05b | 1.43 ± 0.09a | 1.38 ± 0.08a | 1.25 ± 0.07a |
| Total amount of branched ketones | 0.95 ± 0.06b | 0.42 ± 0.03c | 1.74 ± 0.11a | 0.88 ± 0.05b | 0.88 ± 0.05b | 1.43 ± 0.09a | 1.38 ± 0.08a | 1.25 ± 0.07a |
| 2-Methyl-1-butyl acetate | 10.5 ± 0.03b | 10.8 ± 0.05a | 10.7 ± 0.04a | 10.53 ± 0.03b | 10.78 ± 0.05b | 11.07 ± 0.06a | 10.93 ± 0.06ab | 10.94 ± 0.06ab |
| Phenylmethyl acetate | 10.24 ± 0.01a | 10.14 ± 0.01b | 10.24 ± 0.01a | 10.15 ± 0.01b | 10.33 ± 0.02b | 10.24 ± 0.01c | 10.41 ± 0.02a | 10.27 ± 0.02bc |
| Phenethyl acetate | 11.34 ± 0.01b | 12.98 ± 0.03a | 11.47 ± 0.02b | 11.59 ± 0.01b | 11.29 ± 0.05b | 12.85 ± 0.06a | 11.39 ± 0.01b | 11.41 ± 0.02b |
| Isoamyl octanoate | 15.36 ± 0.03b | 19.21 ± 0.01a | 19.38 ± 0.11a | 20.62 ± 0.06a | 14.98 ± 0.07b | 20.35 ± 0.05a | 21.68 ± 0.09a | 21.92 ± 0.03a |
| Isoamyl hexanoate | 12.35 ± 0.06b | 15.34 ± 0.13a | 15.03 ± 0.1a | 14.95 ± 0.11a | 12.68 ± 0.05b | 16.92 ± 0.19a | 16.28 ± 0.14a | 15.62 ± 0.02a |
| Total amount of branched esters | 59.79 ± 0.05b | 68.47 ± 0.06a | 66.82 ± 0.06a | 67.84 ± 0.04a | 60.06 ± 0.07b | 71.43 ± 0.08a | 70.69 ± 0.08a | 70.16 ± 0.07a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, Y.-l.; Xu, G.-q.; Yue, X.-f.; Zhao, X.-f.; Tu, T.-y.; Zhang, J.-x.; Fang, Y.-l. Effects of Regulated Deficit Irrigation on Amino Acid Profiles and Their Derived Volatile Compounds in Cabernet Sauvignon (Vitis vinifera L.) Grapes and Wines. Molecules 2018, 23, 1983. https://doi.org/10.3390/molecules23081983
Ju Y-l, Xu G-q, Yue X-f, Zhao X-f, Tu T-y, Zhang J-x, Fang Y-l. Effects of Regulated Deficit Irrigation on Amino Acid Profiles and Their Derived Volatile Compounds in Cabernet Sauvignon (Vitis vinifera L.) Grapes and Wines. Molecules. 2018; 23(8):1983. https://doi.org/10.3390/molecules23081983
Chicago/Turabian StyleJu, Yan-lun, Guo-qian Xu, Xiao-feng Yue, Xian-fang Zhao, Ting-yao Tu, Jun-xiang Zhang, and Yu-lin Fang. 2018. "Effects of Regulated Deficit Irrigation on Amino Acid Profiles and Their Derived Volatile Compounds in Cabernet Sauvignon (Vitis vinifera L.) Grapes and Wines" Molecules 23, no. 8: 1983. https://doi.org/10.3390/molecules23081983
APA StyleJu, Y.-l., Xu, G.-q., Yue, X.-f., Zhao, X.-f., Tu, T.-y., Zhang, J.-x., & Fang, Y.-l. (2018). Effects of Regulated Deficit Irrigation on Amino Acid Profiles and Their Derived Volatile Compounds in Cabernet Sauvignon (Vitis vinifera L.) Grapes and Wines. Molecules, 23(8), 1983. https://doi.org/10.3390/molecules23081983




