A Short and Efficient Total Synthesis of Ficuseptamines A and B
Abstract
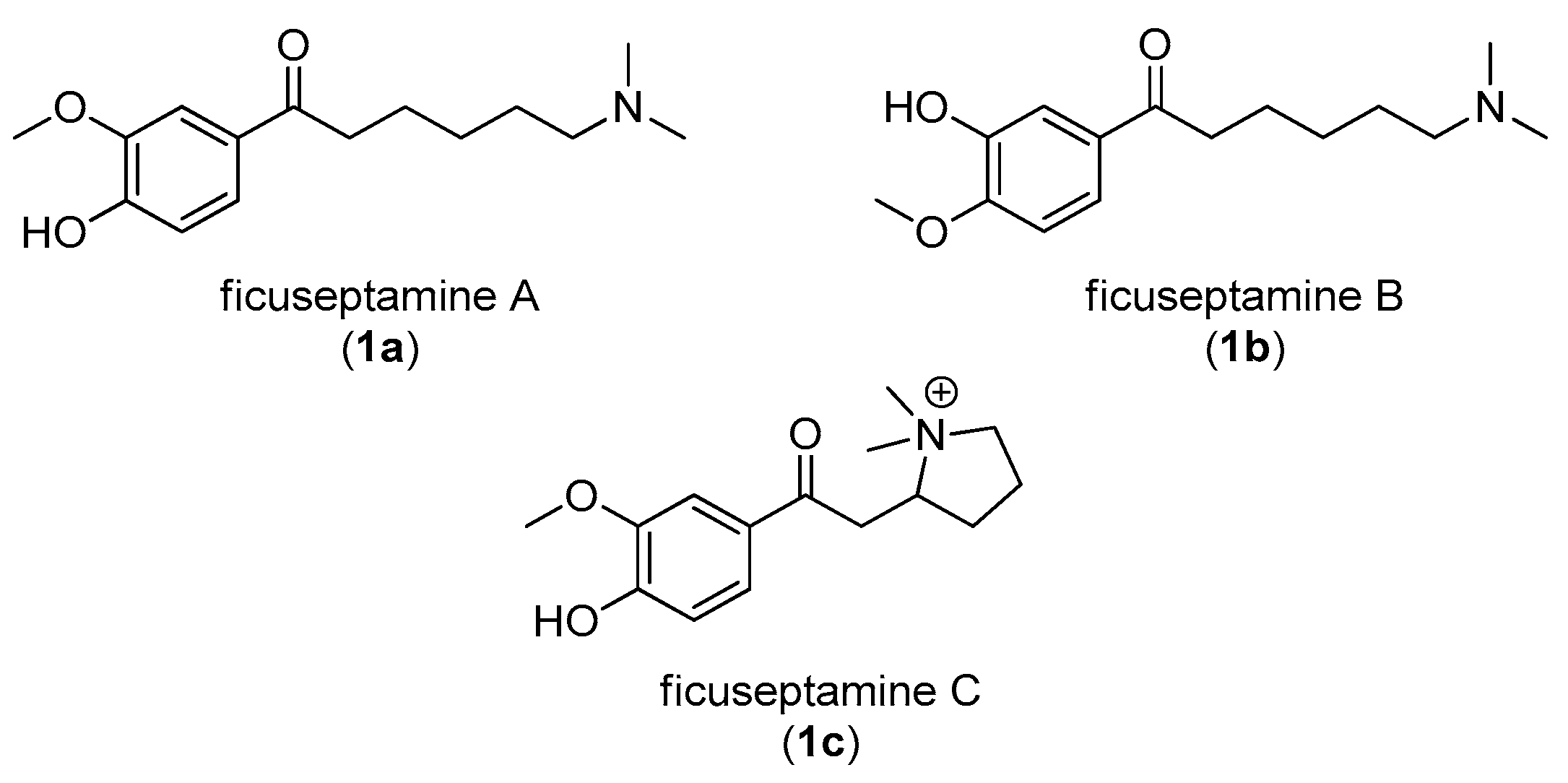
1. Introduction
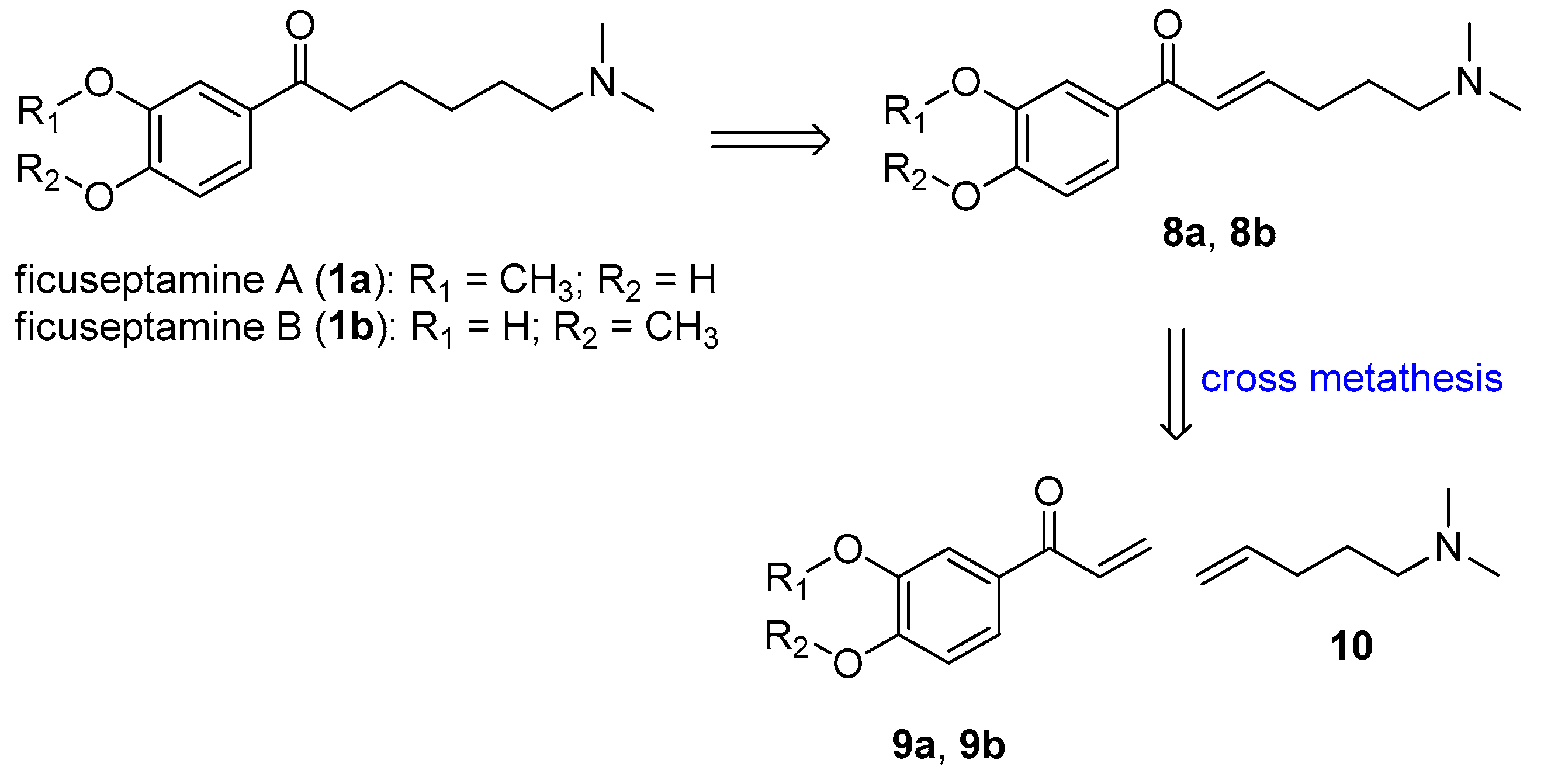
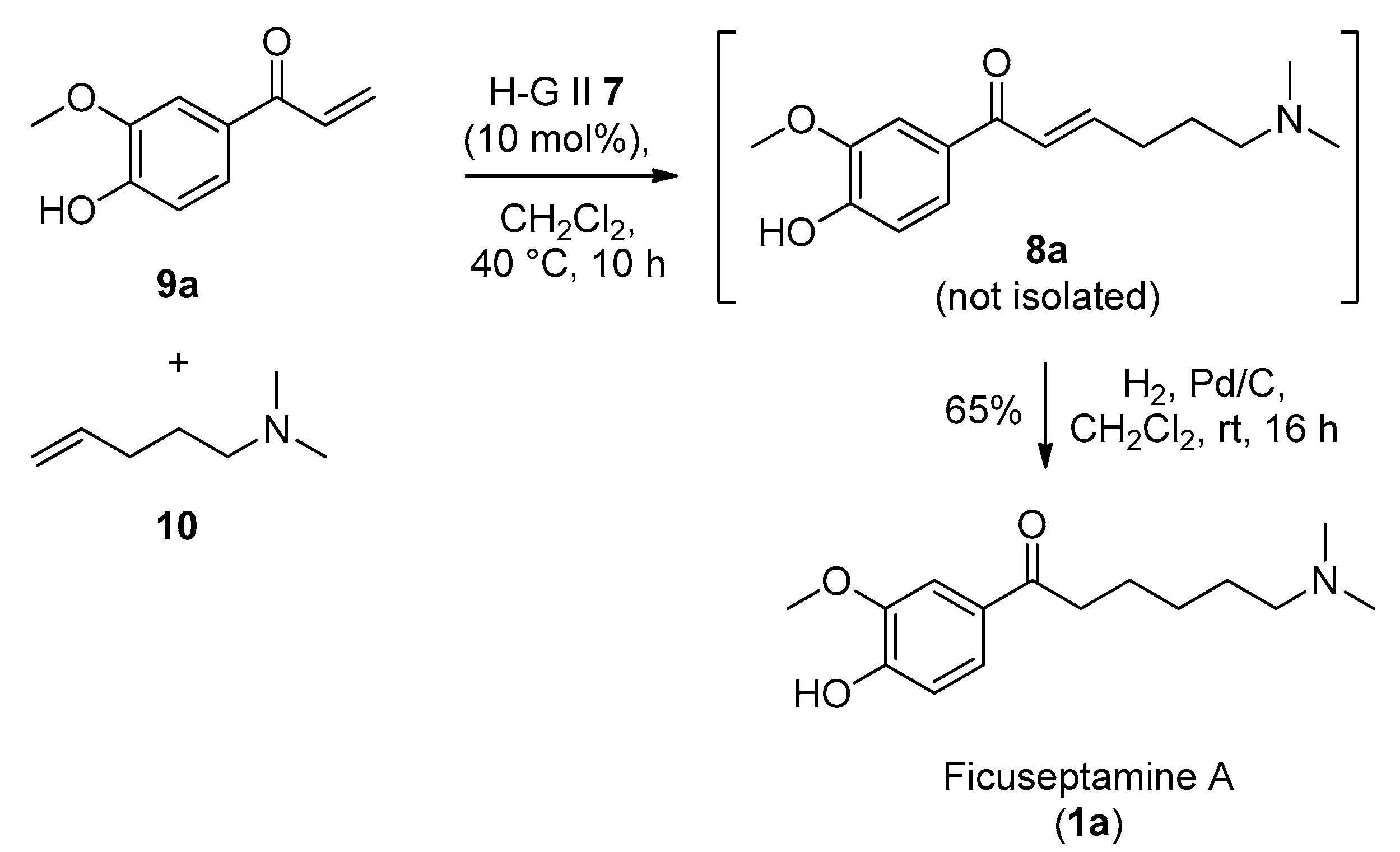
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Chemistry Experimental
4.2. Experimental Procedures for Chemical Synthesis and Characterization Data of Compounds
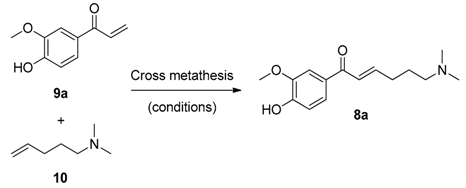
4.2.1. Synthesis of 1-(4-Hydroxy-3-methoxyphenyl)prop-2-en-1-one (9a)
4.2.2. Synthesis of Ficuseptamine A by a One-Pot Cross Metathesis/Hydrogenation Procedure (1a)
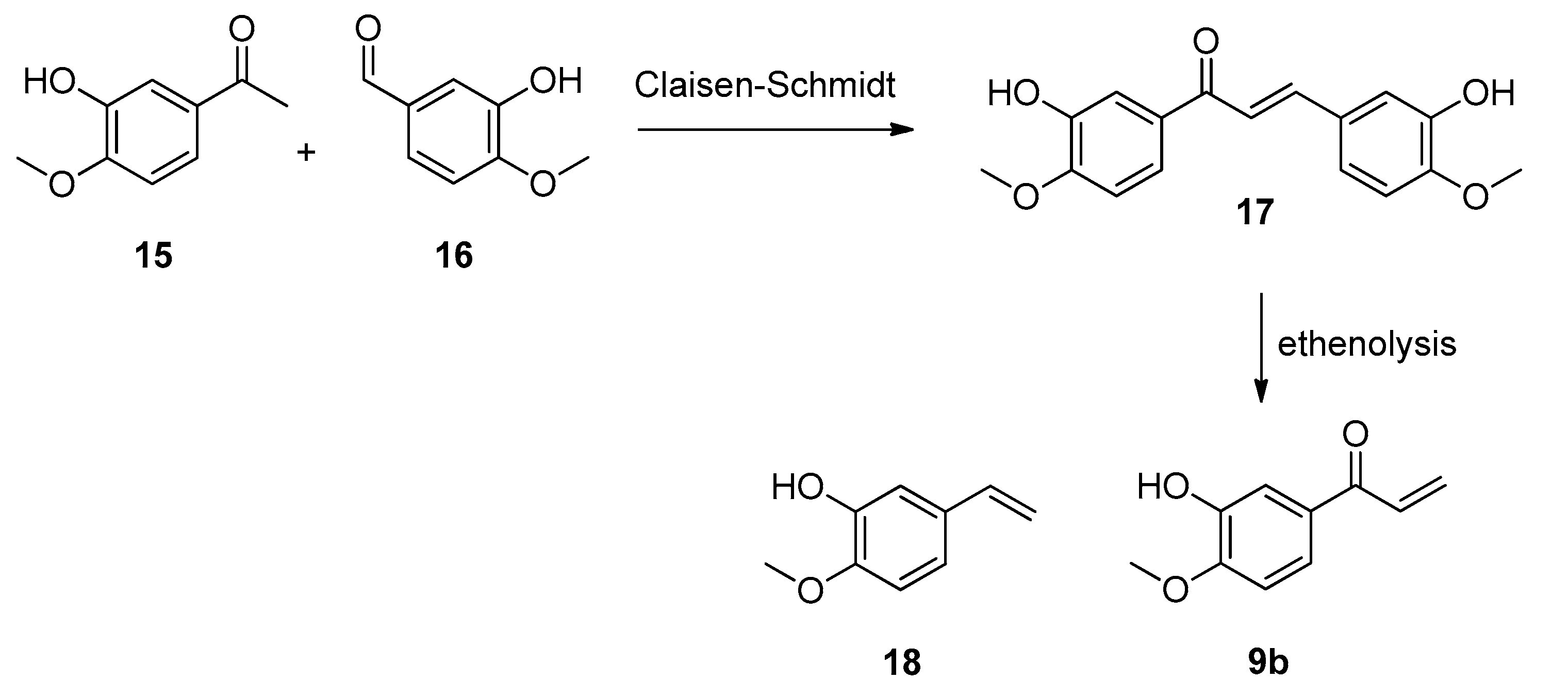
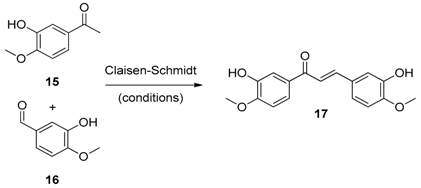
4.2.3. Synthesis of (2E)-1,3-Bis(3-Hydroxy-4-methoxyphenyl)prop-2-en-1-one (17)
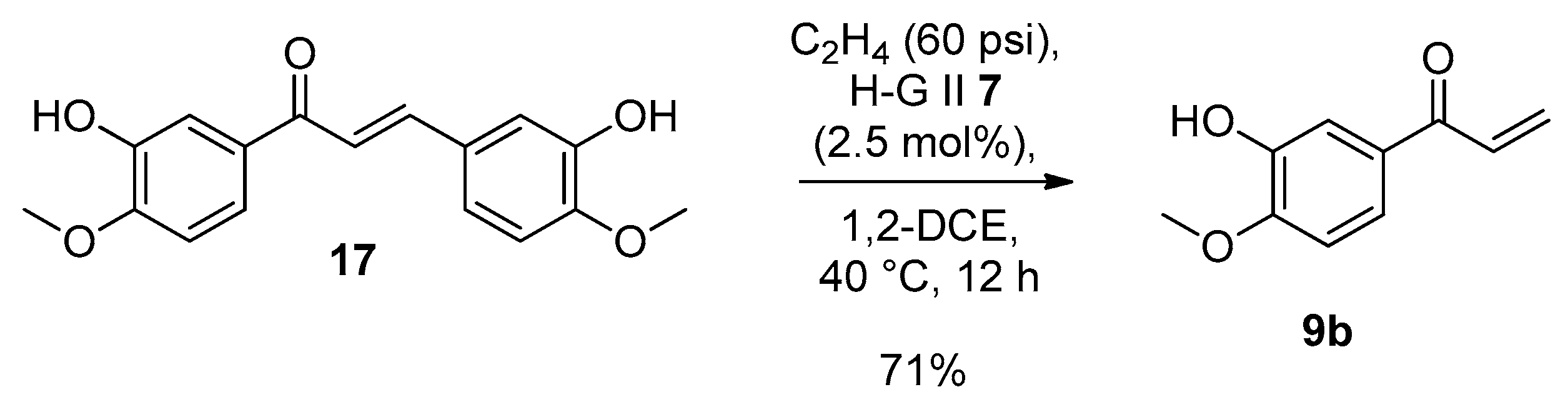
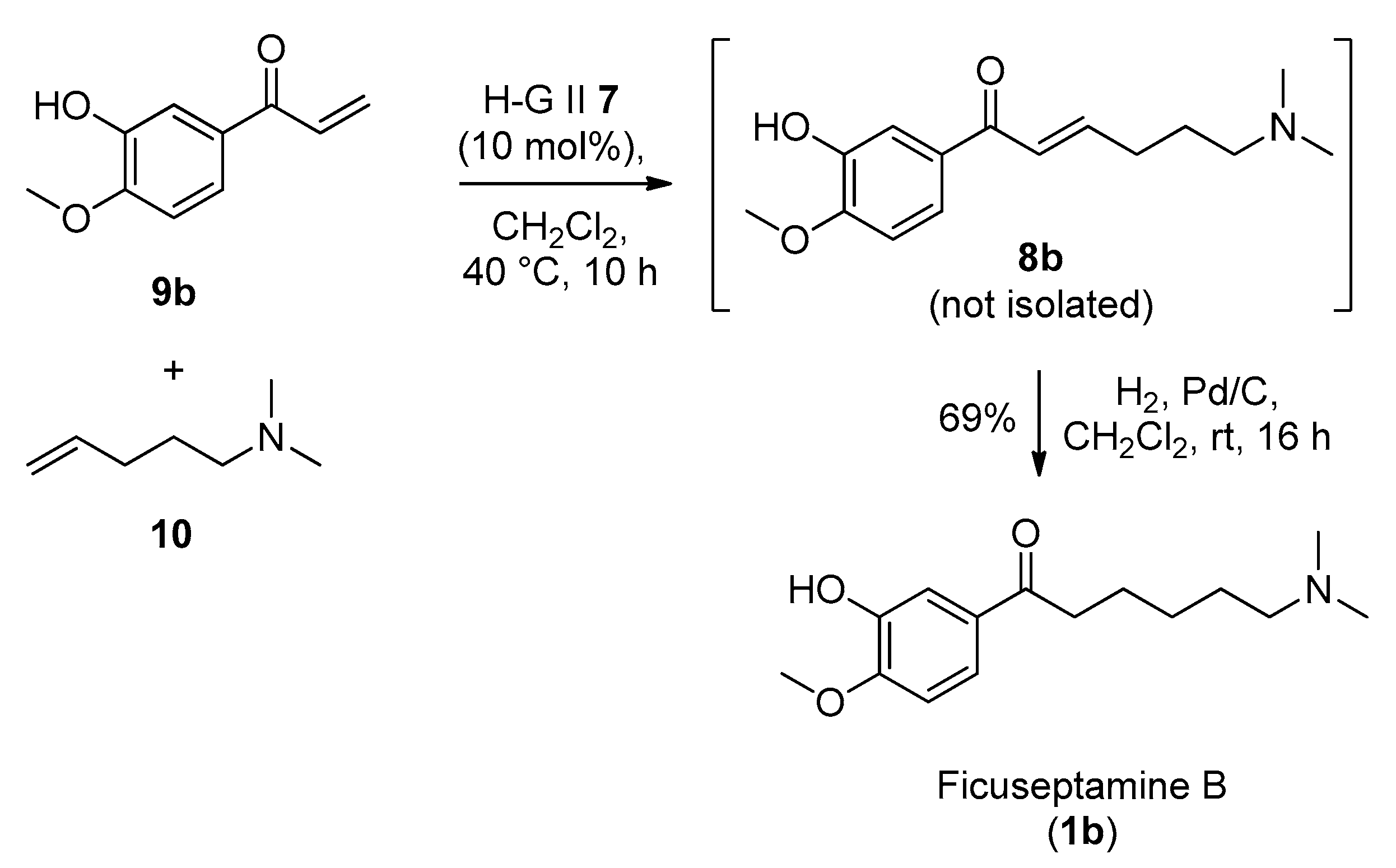
4.2.4. Synthesis of 1-(3-Hydroxy-4-methoxyphenyl)prop-2-en-1-one (9b)
4.2.5. Synthesis of Ficuseptamine B by a One-Pot Cross Metathesis/Hydrogenation Procedure (1b)
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ueda, J.-Y.; Takagi, M.; Shin-ya, K. Aminocaprophenone- and pyrrolidine-type alkaloids from the leaves of Ficus septica. J. Nat. Prod. 2009, 72, 2181–2183. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, U.; Moody, C.J. A short synthesis of the triazolopyrimidine antibiotic essramycin. J. Nat. Prod. 2010, 73, 1938–1939. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zeng, Y.H.; Osman, K.; Shinde, K.; Rahman, M.; Gibbons, S.; Mu, Q. Norlignans, acylphloroglucinols, and a dimeric xanthone from Hypericum chinense. J. Nat. Prod. 2010, 73, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Pais, G.C.G.; Zhang, X.; Marchand, C.; Neamati, N.; Cowansage, K.; Svarovskaia, E.S.; Pathak, V.K.; Tang, Y.; Nicklaus, M.; Pommier, Y.; et al. Structure activity of 3-aryl-1,3-diketo-containing compounds as HIV-1 integrase inhibitors. J. Med. Chem. 2002, 45, 3184–3194. [Google Scholar] [CrossRef] [PubMed]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Salyer, A.E.; Kim, E.H.; Jiang, X.; Jarrad, R.E.; Powers, M.S.; Kirchhoff, A.M.; Salvador, T.K.; Chester, J.A.; Hockerman, G.H.; et al. Evaluation of difluoromethyl ketones as agonists of the γ-aminobutyric acid type B (GABAB) receptor. J. Med. Chem. 2013, 56, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.A. Recent applications of ring-closing metathesis in the synthesis of lactams and macrolactams. Chem. Commun. 2010, 46, 9100–9106. [Google Scholar] [CrossRef] [PubMed]
- Connon, S.J.; Blechert, S. Recent developments in olefin cross-metathesis. Angew. Chem. Int. Ed. 2003, 42, 1900–1923. [Google Scholar] [CrossRef] [PubMed]
- Hoveyda, A.H.; Zhugralin, A.R. The remarkable metal-catalysed olefin metathesis reaction. Nature 2007, 450, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Metathesis reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4490–4527. [Google Scholar] [CrossRef] [PubMed]
- Fürstner, A. Metathesis in total synthesis. Chem. Commun. 2011, 47, 6505–6511. [Google Scholar] [CrossRef] [PubMed]
- Chatare, V.K.; Andrade, R.B. Total synthesis of (−)-albocycline. Angew. Chem. Int. Ed. 2017, 56, 5909–5911. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.B.; Marx, V.M.; Pederson, R.L.; Grubbs, R.H. Concise syntheses of insect pheromones using Z-selective cross metathesis. Angew. Chem. Int. Ed. 2013, 52, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.J.; Speed, A.W.H.; Schrock, R.R.; Amir, H.; Hoveyda, A.H. Catalytic Z-selective cross-metathesis with secondary silyl- and benzyl-protected allylic ethers: Mechanistic aspects and applications to natural product synthesis. Angew. Chem. Int. Ed. 2013, 52, 8395–8400. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.B.; Grubbs, R.H. Z-selective cross metathesis with ruthenium catalysts: Synthetic applications and mechanistic implications. Angew. Chem. Int. Ed. 2015, 54, 5018–5024. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.A.; Brown, F.K. A convenient approach to acyclic unsaturated amino acids via ring-closing metathesis. Chem. Commun. 2010, 46, 3013–3015. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Wenzel, A.G.; Salguero, T.T.; Day, M.W.; Grubbs, R.H. Decomposition of ruthenium olefin metathesis catalysts. J. Am. Chem. Soc. 2007, 129, 7961–7968. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Choi, T.-L.; Sanders, D.P.; Grubbs, R.H. A general model for selectivity in olefin cross metathesis. J. Am. Chem. Soc. 2003, 125, 11360–11370. [Google Scholar] [CrossRef] [PubMed]
- Boulard, L.; BouzBouz, S.; Cossy, J.; Franck, X.; Figadère, B. Two successive one-pot reactions leading to the expeditious synthesis of (−)-centrolobine. Tetrahedron Lett. 2004, 45, 6603–6605. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Bidange, J.; Fischmeister, C.; Bruneau, C. Ethenolysis: A green catalytic tool to cleave carbon—Carbon double bonds. Chem. Eur. J. 2016, 22, 12226–12244. [Google Scholar] [CrossRef] [PubMed]
- Petrov, O.; Ivanova, Y.; Gerova, M. SOCl2/EtOH: Catalytic system for synthesis of chalcones. Catal. Commun. 2008, 9, 315–316. [Google Scholar] [CrossRef]
- Clark, J.R.; French, J.M.; Diver, S.T. Alkene metathesis approach to β-unsubstituted anti-allylic alcohols and their use in ene-yne metathesis. J. Org. Chem. 2012, 77, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Sanders, D.P.; Lee, C.W.; Grubbs, R.H. Prevention of undesirable isomerization during olefin metathesis. J. Am. Chem. Soc. 2005, 127, 17160–17161. [Google Scholar] [CrossRef] [PubMed]
- Perrone, R.; Berardi, F.; Colabufo, N.A.; Lacivita, E.; Leopoldo, M.; Tortorella, V. Synthesis and structure-affinity relationships of 1-[ω-(4-aryl-1-piperazinyl) alkyl]-1-aryl ketones as 5-HT7 receptor ligands. J. Med. Chem. 2003, 46, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Leow, P.-C.; Bahety, P.; Boon, C.P.; Lee, C.Y.; Tan, K.L.; Yang, T.; Ee, P.-L.R. Functionalized curcumin analogs as potent modulators of the Wnt/β-catenin signaling pathway. Eur. J. Med. Chem. 2014, 71, 67–80. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the author. |
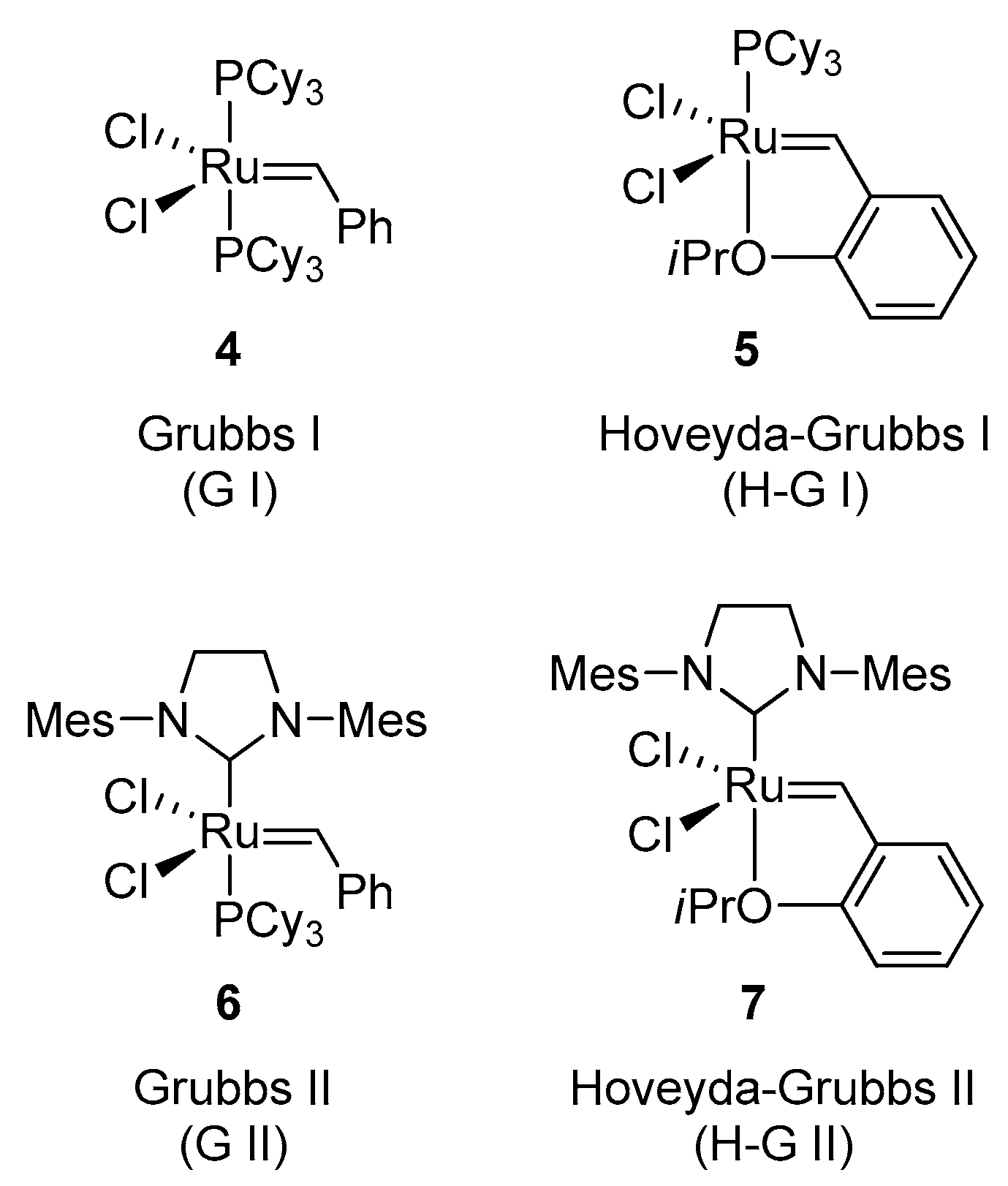

| E | Substrate 9a (equiv.) | Substrate 10 (equiv.) | Catalyst (Loading) | Solvent | T (°C) | Yield % a |
|---|---|---|---|---|---|---|
| 1 | 2 | 1 | G I 4 (5 mol %) | CH2Cl2 | 40 | 22 |
| 2 | 2 | 1 | G I 4 (10 mol %) | CH2Cl2 | 40 | 15 |
| 3 | 2 | 1 | G II 5 (5 mol %) | CH2Cl2 | 40 | 37 |
| 4 | 2 | 1 | G II 5 (10 mol %) | CH2Cl2 | 40 | 41 |
| 5 | 2 | 1 | G II 5 (10 mol %) | Toluene | 80 | 47 |
| 6 | 2 | 1 | H-G II 7 (5 mol %) | CH2Cl2 | 40 | 68 |
| 7 | 2 | 1 | H-G II 7 (10 mol %) | CH2Cl2 | 40 | 76 |
| 8 | 2 | 1 | H-G II 7 (10 mol %) | Toluene | 80 | 52 |
| 9 | 1 | 2 | H-G II 7 (10 mol %) | CH2Cl2 | 40 | 42 |
| Entry | Catalyst | Equivalent | Time (h) | Yield (%) a |
|---|---|---|---|---|
| 1 | NaOH b | 2 | 16 | 46 |
| 2 | Cs2CO3 | 2 | 16 | 41 |
| 3 | SOCl2 | 1 | 4 | 83 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, H.M.A. A Short and Efficient Total Synthesis of Ficuseptamines A and B. Molecules 2018, 23, 1865. https://doi.org/10.3390/molecules23081865
Hassan HMA. A Short and Efficient Total Synthesis of Ficuseptamines A and B. Molecules. 2018; 23(8):1865. https://doi.org/10.3390/molecules23081865
Chicago/Turabian StyleHassan, Hani Mutlak A. 2018. "A Short and Efficient Total Synthesis of Ficuseptamines A and B" Molecules 23, no. 8: 1865. https://doi.org/10.3390/molecules23081865
APA StyleHassan, H. M. A. (2018). A Short and Efficient Total Synthesis of Ficuseptamines A and B. Molecules, 23(8), 1865. https://doi.org/10.3390/molecules23081865





