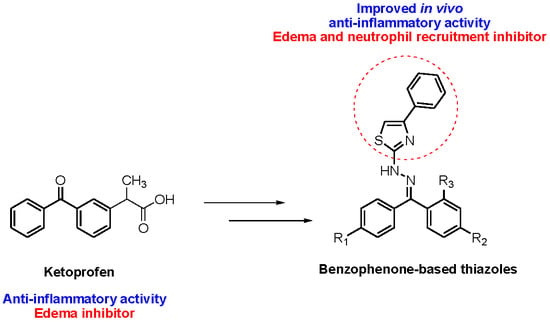
Design and Synthesis of New Benzophenone Derivatives with In Vivo Anti-Inflammatory Activity through Dual Inhibition of Edema and Neutrophil Recruitment
Abstract
1. Introduction
2. Results and Discussion
2.1. Docking Studies
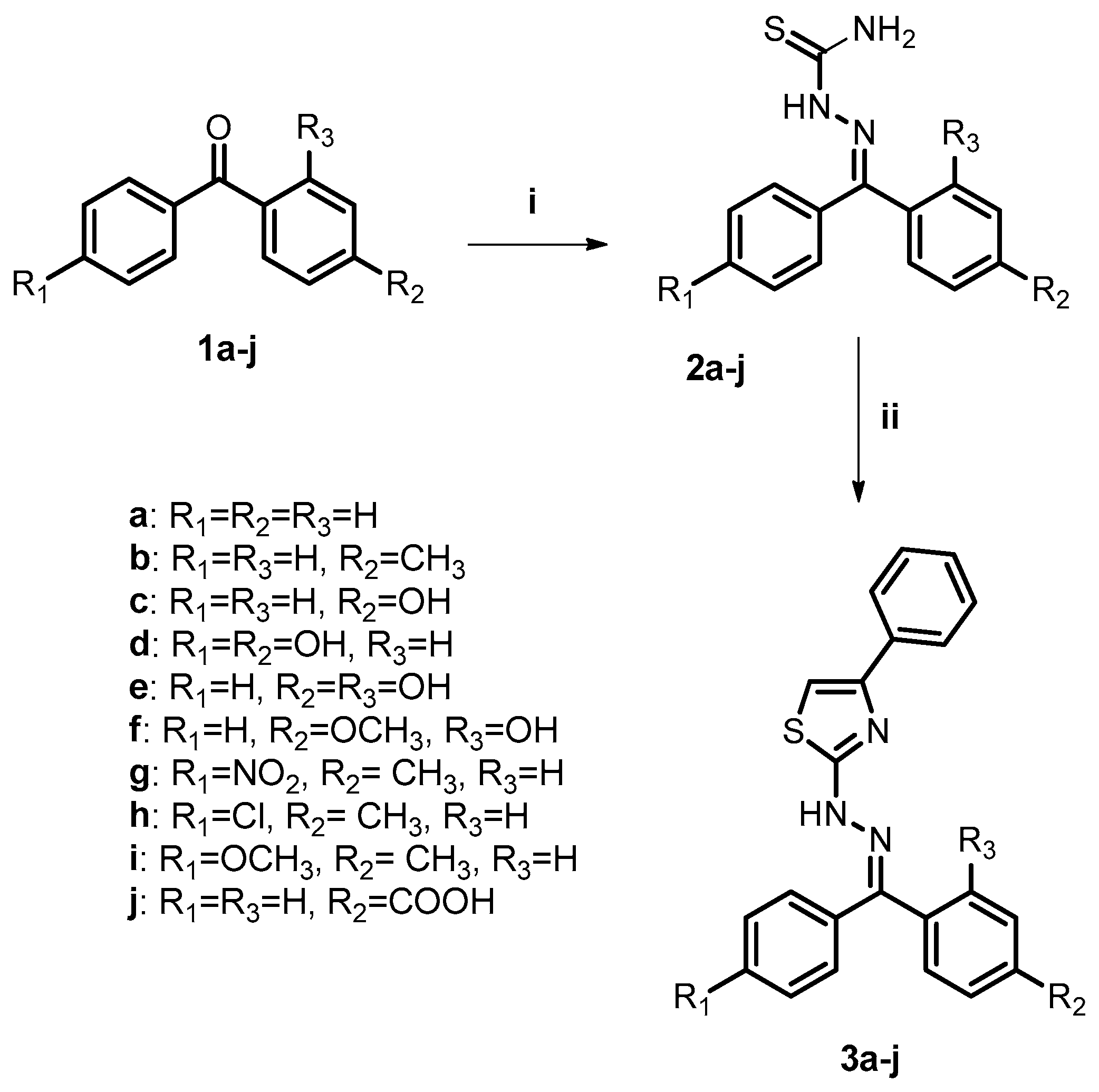
2.2. Chemistry
2.3. Biological Assays
2.3.1. Croton Oil-Induced Ear Edema
2.3.2. Recruitment of Neutrophils Determination
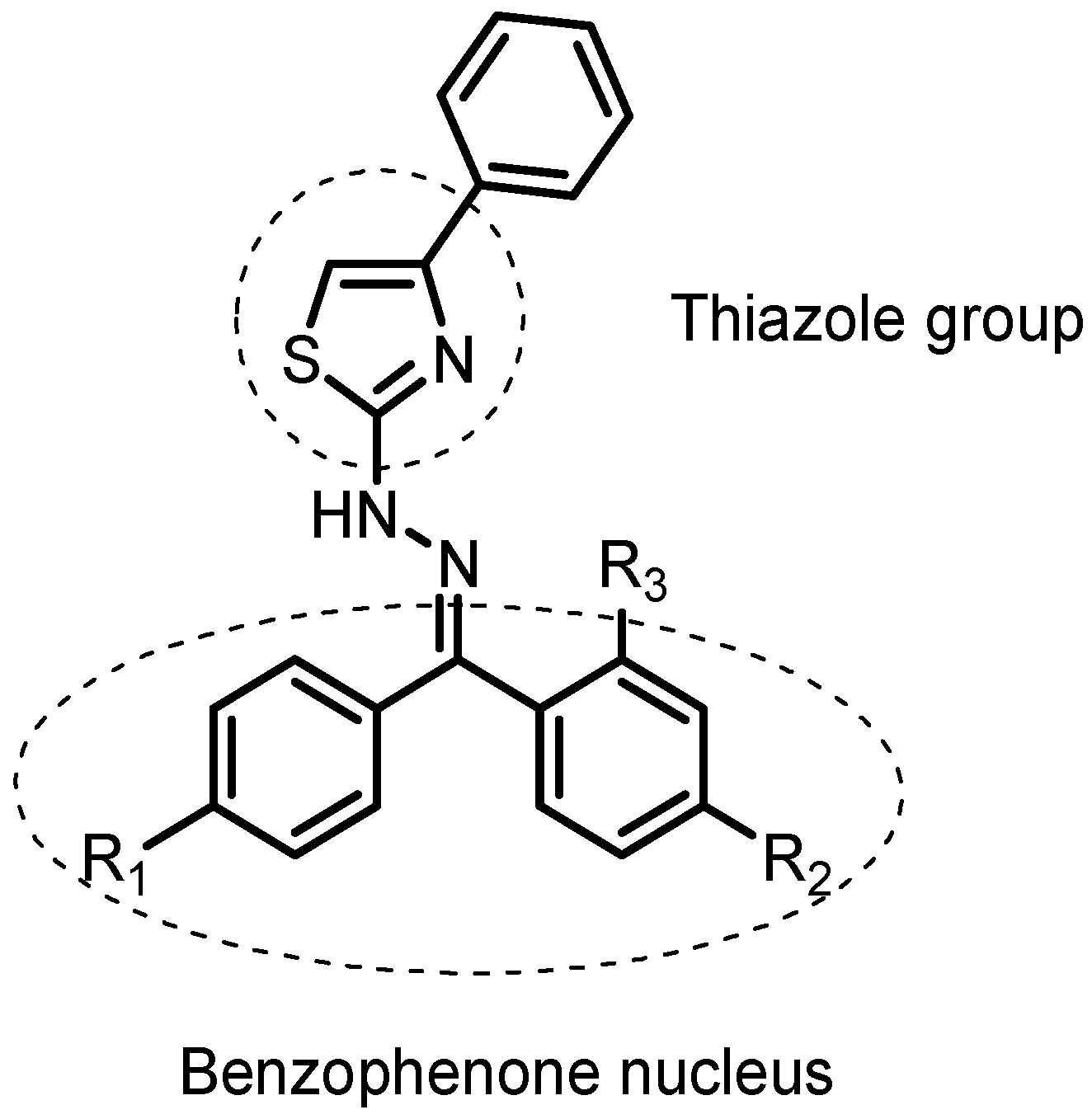
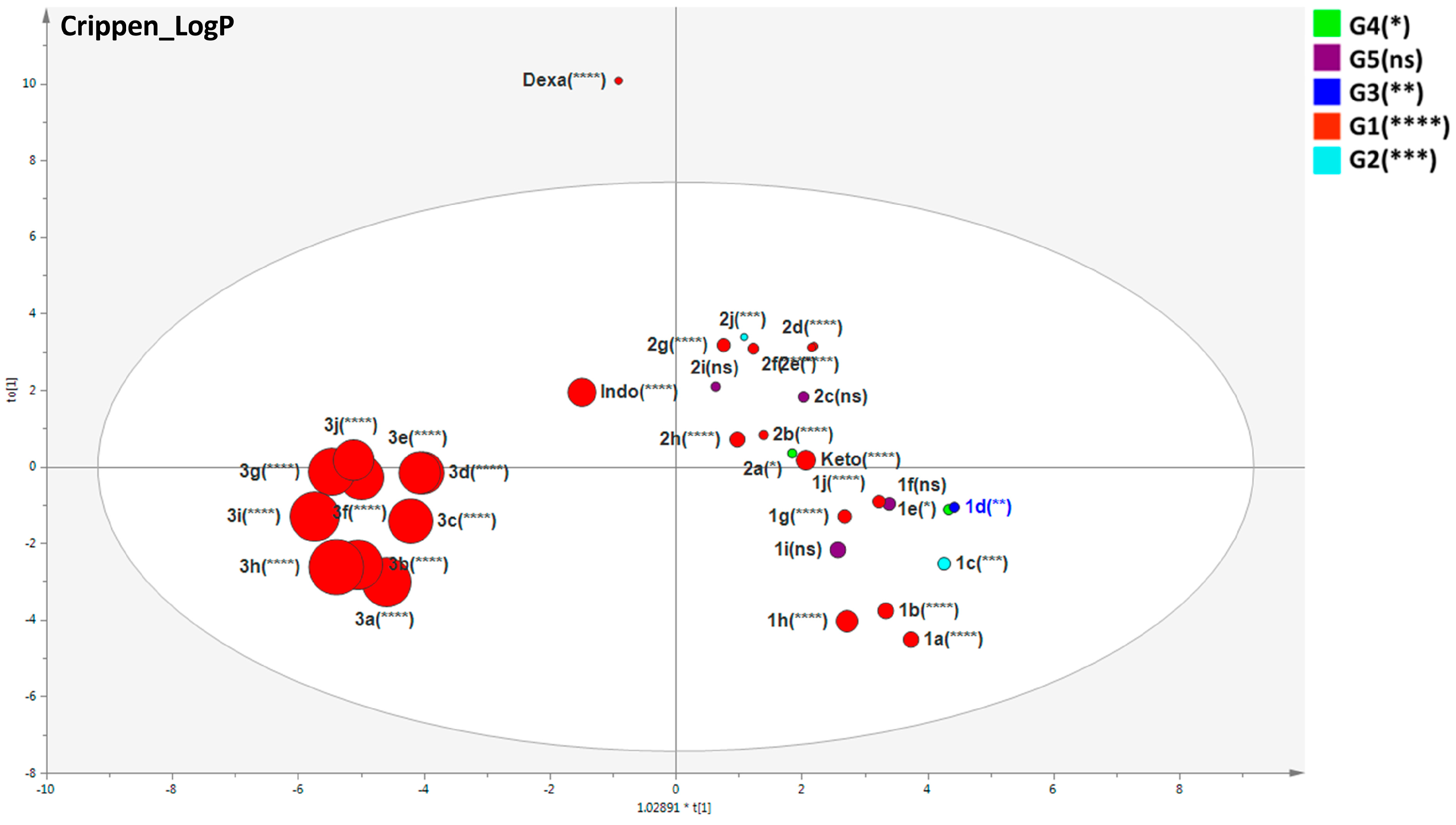
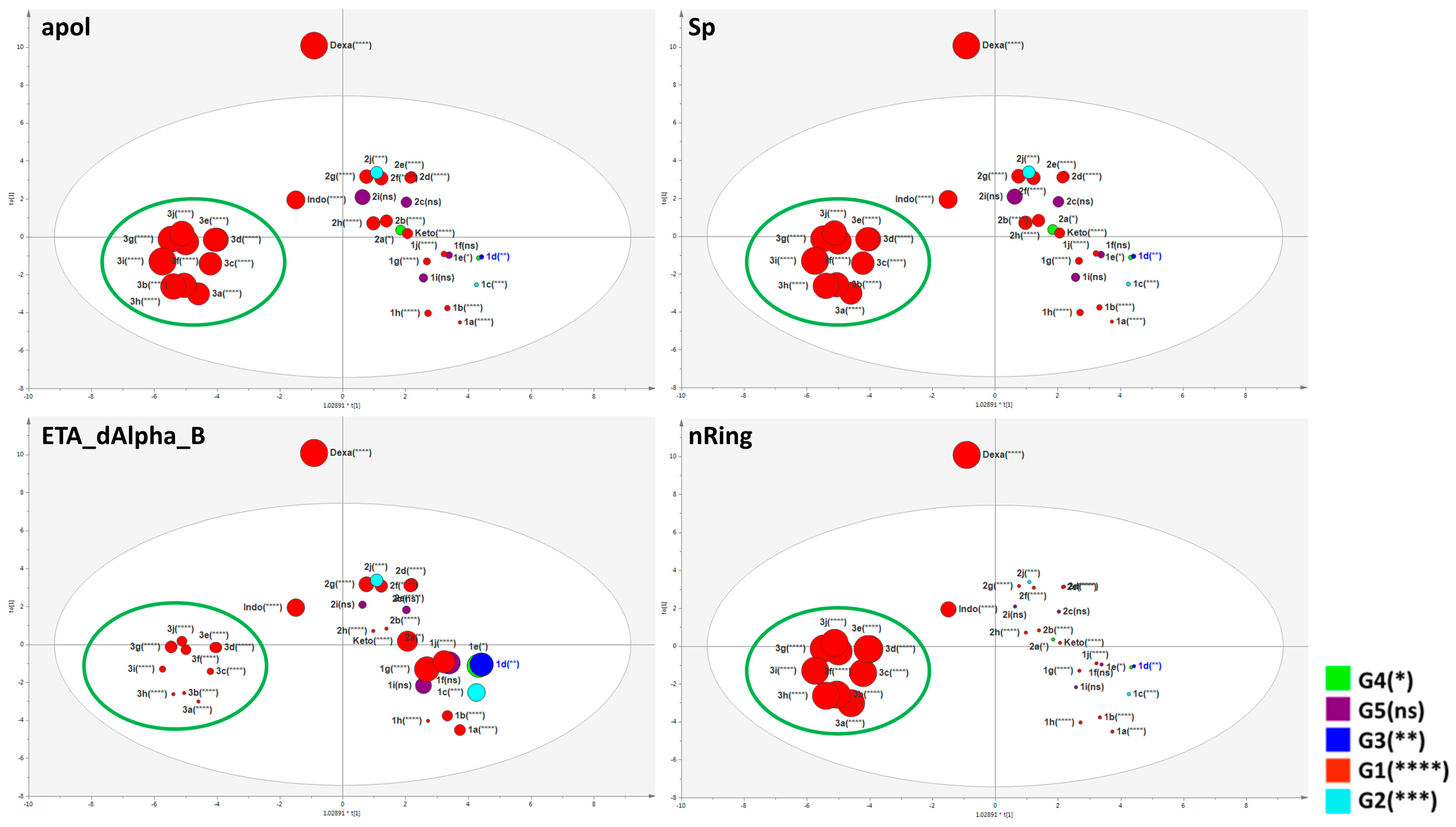
2.4. Important Molecular Features for the Anti-Inflammatory Activity of Designed Derivatives
3. Materials and Methods
3.1. General Information
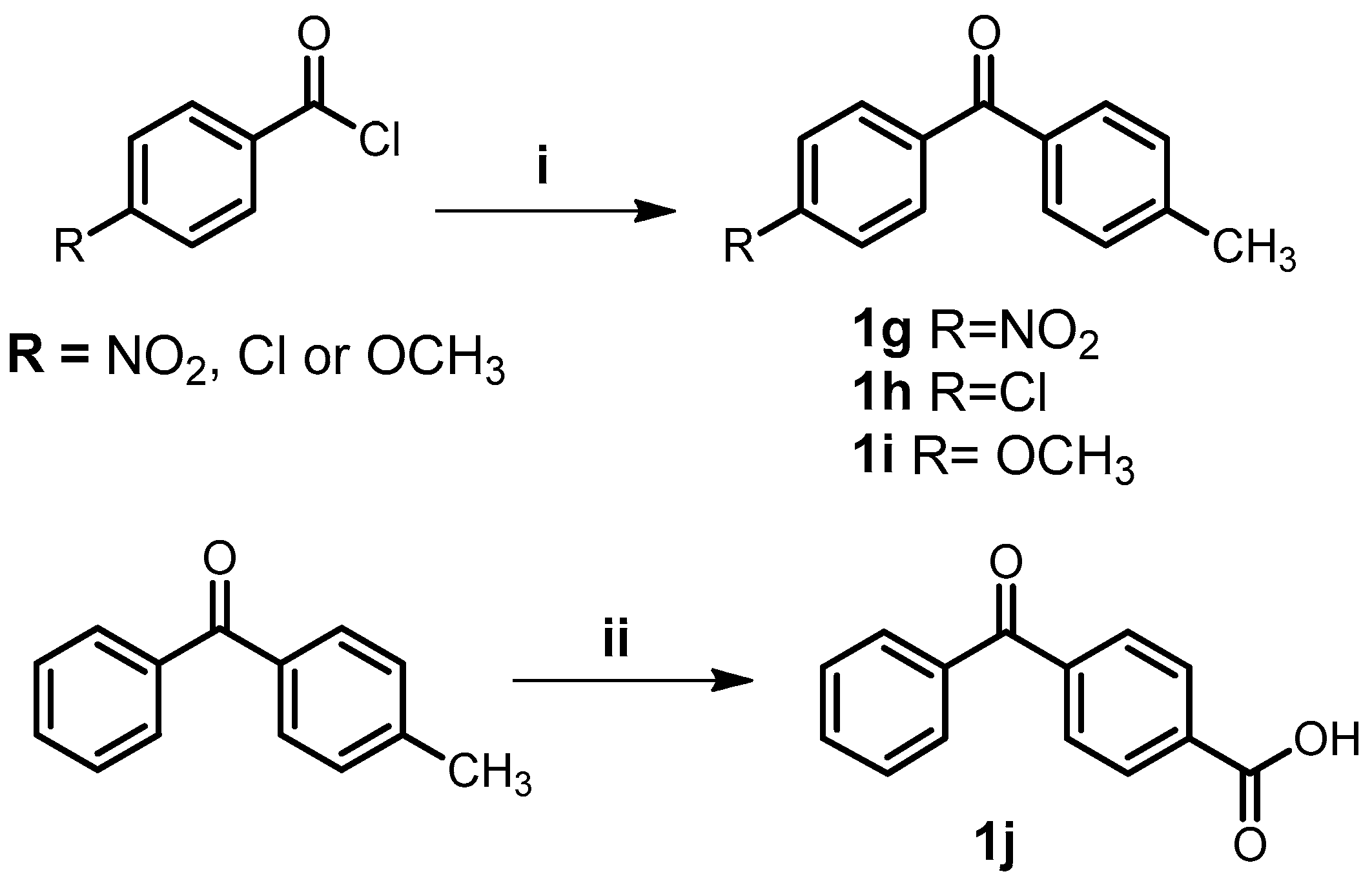
3.2. General Procedure for the Synthesis of Benzophenones (1g–i)
3.3. Synthesis of 4-Benzoylbenzoic Acid (1j)
3.4. General Procedure for the Synthesis of Thiosemicarbazones (2a–j)
3.5. General Procedure for the Synthesis of Thiazole Derivatives (3a–j)
3.6. Biological Assays
3.6.1. Animals
3.6.2. In Vivo Anti-Inflammatory Assay-Croton Oil Ear Edema
3.6.3. Neutrophil Recruitment
3.7. Molecular Docking Studies
3.8. Structure-Activity Relationship
3.8.1. Datasets
3.8.2. Descriptors Calculation on PaDEL
3.8.3. Multivariate Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Amir, M.; Shikha, K. Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of some new 2-[(2,6-dichloroanilino) phenyl]acetic acid derivatives. Eur. J. Med. Chem. 2004, 39, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Parente, L. Pros and cons of selective inhibition of cyclooxygenase-2 versus dual lipoxygenase/cyclooxygenase inhibition: is two better than one? J. Rheumatol. 2001, 28, 2375–2382. [Google Scholar] [PubMed]
- Mukherjee, D. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001, 286, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Palomer, A.; Cabré, F.; Pascual, J.; Campos, J.; Trujillo, M.A.; Entrena, A.; Gallo, M.A.; García, L.; Mauleón, D.; Espinosa, A. Identification of novel cyclooxygenase-2 selective inhibitors using pharmacophore models. J. Med. Chem. 2002, 45, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Parnham, M.J. Antirheumatic agents and leukocyte recruitment. Biochem. Pharmacol. 1999, 58, 209–215. [Google Scholar] [CrossRef]
- Venkatesha, S.H.; Berman, B.M.; Moudgil, K.D. Herbal medicinal products target defined biochemical and molecular mediators of inflammatory autoimmune arthritis. Bioorg. Med. Chem. 2011, 19, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.; Patrignani, P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J. Pain Res. 2015, 8, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Musa, K.A.K.; Palwai, V.R.; Eriksson, L.A. New nonsteroidal anti-inflammatory molecules with reduced photodegradation side effects and enhanced COX-2 selectivity. Int. J. Quantum Chem. 2011, 111, 1184–1195. [Google Scholar] [CrossRef]
- Rajić, Z.; Hadjipavlou-Litina, D.; Pontiki, E.; Balzarini, J.; Zorc, B. The novel amidocarbamate derivatives of ketoprofen: Synthesis and biological activity. Med. Chem. Res. 2011, 20, 210–219. [Google Scholar] [CrossRef]
- Ghate, M.; Kusanur, R.A.; Kulkarni, M. V Synthesis and in vivo analgesic and anti-inflammatory activity of some bi heterocyclic coumarin derivatives. Eur. J. Med. Chem. 2005, 40, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Malini, K.V.; Rao, B.S.; Sarojini, B.K.; Kumari, N.S. Synthesis of some new 2,4-disubstituted thiazoles as possible antibacterial and anti-inflammatory agents. Eur. J. Med. Chem. 2003, 38, 313–318. [Google Scholar] [CrossRef]
- Küçükgüzel, Ş.G.; Küçükgüzel, İ.; Tatar, E.; Rollas, S.; Şahin, F.; Güllüce, M.; De Clercq, E.; Kabasakal, L. Synthesis of some novel heterocyclic compounds derived from diflunisal hydrazide as potential anti-infective and anti-inflammatory agents. Eur. J. Med. Chem. 2007, 42, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Ragab, H.M.A.; Bekhit, A.A.; Rostom, S.A.F.; Bekhit, A.E.-D.A. Compounds containing azole scaffolds as cyclooxygenase inhibitors: A review. Curr. Top. Med. Chem. 2016, 16, 3569–3581. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Srivastava, V.K.; Kumar, A. Synthesis and antiinflammatory activity of heterocyclic indole derivatives. Eur. J. Med. Chem. 2004, 39, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rajput, C.S.; Bhati, S.K. Synthesis of 3-[4′-(p-chlorophenyl)-thiazol-2′-yl]-2-[(substituted azetidinone/thiazolidinone)-aminomethyl]-6-bromoquinazolin-4-ones as anti-inflammatory agent. Bioorg. Med. Chem. 2007, 15, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.M.; Girodeau, J.; Foster, S.J. Selective chiral inhibitors of 5-lipoxygenase with anti-inflammatory activity. Br. J. Pharmacol. 1990, 101, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Rizk, O.H.; Mahran, M.A.; El-Khawass, S.M.; Shams El-Dine, S.A.; Ibrahim, E.S.A. Synthesis of some new antimicrobial thiadiazolyl and oxadiazolyl quinoline derivatives. Med. Chem. Res. 2005, 14, 260–273. [Google Scholar] [CrossRef]
- Araniciu, C.; Pârvu, A.E.; Palage, M.D.; Oniga, S.D.; Benedec, D.; Oniga, I.; Oniga, O. The effect of some 4,2 and 5,2 bisthiazole derivatives on nitro-oxidative stress and phagocytosis in acute experimental inflammation. Molecules 2014, 19, 9240–9256. [Google Scholar] [CrossRef] [PubMed]
- Tamaian, R.; Moţ, A.; Silaghi-Dumitrescu, R.; Ionuţ, I.; Stana, A.; Oniga, O.; Nastasə, C.; Benedec, D.; Tiperciuc, B.; McPhee, D.J. Study of the relationships between the structure, lipophilicity and biological activity of some thiazolyl-carbonyl-thiosemicarbazides and thiazolyl-azoles. Molecules 2015, 20, 22188–22201. [Google Scholar] [CrossRef] [PubMed]
- Schenone, S.; Brullo, C.; Bruno, O.; Bondavalli, F.; Ranise, A.; Filippelli, W.; Rinaldi, B.; Capuano, A.; Falcone, G. New 1,3,4-thiadiazole derivatives endowed with analgesic and anti-inflammatory activities. Bioorg. Med. Chem. 2006, 14, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, C.M.; Oniga, O.; Pârvu, A.; Tiperciuc, B.; Verite, P.; Pîrnǎu, A.; Crişan, O.; Bojiţǎ, M.; Pop, R. Synthesis and anti-inflammatory evaluation of some new acyl-hydrazones bearing 2-aryl-thiazole. Eur. J. Med. Chem. 2011, 46, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Kukol, A. Consensus virtual screening approaches to predict protein ligands. Eur. J. Med. Chem. 2011, 46, 4661–4664. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Abdel-Gawad, H.; Awad, G.E.A.; Badria, F.A. Synthesis, antimicrobial, antioxidant, anti-inflammatory, and analgesic activities of some new 3-(2{\textasciiacutex}-thienyl)pyrazole-based heterocycles. Med. Chem. Res. 2012, 21, 1418–1426. [Google Scholar] [CrossRef]
- Alegaon, S.G.; Alagawadi, K.R.; Garg, M.K.; Dushyant, K.; Vinod, D. 1,3,4-Trisubstituted pyrazole analogues as promising anti-inflammatory agents. Bioorg. Chem. 2014, 54, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Blobaum, A.L.; Marnett, L.J. Structural and Functional Basis of Cyclooxygenase Inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.L.; de Lima, R.S.; Moreira, D.R.M.; Cardoso, M.V.O.; Gouveia de Brito, A.C.; Farias dos Santos, L.M.; Hernandes, M.Z.; Kiperstok, A.C.; de Lima, R.S.; Soares, M.B.P. Synthesis, docking, and in vitro activity of thiosemicarbazones, aminoacyl-thiosemicarbazides and acyl-thiazolidones against Trypanosoma cruzi. Bioorg. Med. Chem. 2006, 14, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Dannhardt, G. COX-1/COX-2 inhibitors based on the methanone moiety. Eur. J. Med. Chem. 2002, 37, 147–161. [Google Scholar] [CrossRef]
- Hecker, E. Cocarcinogenic principles from the seed oil of Croton tiglium and from other Euphorbiaceae. Cancer Res. 1968, 28, 2338–2348. [Google Scholar] [PubMed]
- Rao, T.S.; Currie, J.L.; Shaffer, A.F.; Isakson, P.C. Comparative evaluation of arachidonic acid (AA)- and tetradecanoylphorbol acetate (TPA)-induced dermal inflammation. Inflammation 1993, 17, 723–741. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; Dri, P.; Delbello, G.; Zilli, C.; Loggia, R. Della The Croton oil ear test revisited. Agents Actions 1986, 17, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Chagas-Paula, D.A.; Oliveira, R.B.D.; Da Silva, V.C.; Gobbo-Neto, L.; Gasparoto, T.H.; Campanelli, A.P.; Faccioli, L.H.; Da Costa, F.B. Chlorogenic acids from Tithonia diversifolia demonstrate better anti-inflammatory effect than indomethacin and its sesquiterpene lactones. J. Ethnopharmacol. 2011, 136, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Natural Products as Leads to Potential Drugs: An Old Process or the New Hope for Drug Discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta -Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, R.; Choi, Y.H.; Kim, H.K. Ethnopharmacology and systems biology: A perfect holistic match. J. Ethnopharmacol. 2005, 100, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Michaux, C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur. J. Med. Chem. 2003, 38, 645–659. [Google Scholar] [CrossRef]
- Chagas-Paula, D.A.; Zhang, T.; Da Costa, F.B.; Edrada-Ebel, R. A metabolomic approach to target compounds from the Asteraceae family for dual COX and LOX inhibition. Metabolites 2015, 5, 404–430. [Google Scholar] [CrossRef] [PubMed]
- Yuliana, N.D.; Khatib, A.; Verpoorte, R.; Choi, Y.H. Comprehensive extraction method integrated with NMR metabolomics: A new bioactivity screening method for plants, adenosine A1 receptor binding compounds in Orthosiphon stamineus Benth. Anal. Chem. 2011, 83, 6902–6906. [Google Scholar] [CrossRef] [PubMed]
- Findeis, M.A.; Kaiser, E.T. Nitrobenzophenone oxime based resins for the solid-phase synthesis of protected peptide segments. J. Org. Chem. 1989, 54, 3478–3482. [Google Scholar] [CrossRef]
- Chen, H.-S.; Kuo, S.-C.; Teng, C.-M.; Lee, F.-Y.; Wang, J.-P.; Lee, Y.-C.; Kuo, C.-W.; Huang, C.-C.; Wu, C.-C.; Huang, L.-J. Synthesis and antiplatelet activity of ethyl 4-(1-benzyl-1H-indazol-3-yl)benzoate (YD-3) derivatives. Bioorg. Med. Chem. 2008, 16, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.D.K.; Chavarria, G.E.; Charlton-Sevcik, A.K.; Yoo, G.K.; Song, J.; Strecker, T.E.; Siim, B.G.; Chaplin, D.J.; Trawick, M.L.; Pinney, K.G. Functionalized benzophenone, thiophene, pyridine, and fluorene thiosemicarbazone derivatives as inhibitors of cathepsin L. Bioorg. Med. Chem. Lett. 2010, 20, 6610–6615. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, N.C.; da Cruz, L.F.; da Silva Villela, F.; do Nascimento Pereira, G.A.; de Siqueira-Neto, J.L.; Kellar, D.; Suzuki, B.M.; Ray, D.; de Souza, T.B.; Alves, R.J.; et al. Synthesis of a sugar-based thiosemicarbazone series and structure-activity relationship versus the parasite cysteine proteases rhodesain, cruzain, and Schistosoma mansoni cathepsin B1. Antimicrob. Agents Chemother. 2015, 59, 2666–2677. [Google Scholar] [CrossRef] [PubMed]
- Dassault Systèmes. BIOVIA Discovery Studio Modeling Environment; Dassault Systèmes Biovia: San Diego, CA, USA, 2016. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.W. PaDEL-descriptor: An open source software to calculate molecular descriptors and fingerprints. J. Comput. Chem. 2011, 32, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Abraham, M.H.; Zissimos, A.M. Fast calculation of van der Waals volume as a sum of atomic and bond contributions and its application to drug compounds. J. Org. Chem. 2003, 68, 7368–7373. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1g–j, 2a, (E,Z)-2b, (E,Z)-2c, 2d, (E,Z)-2e–2j, 3a, (E,Z)-3b, (E,Z)-3c, 3d, (E,Z)-3e–3j are available from the authors. |
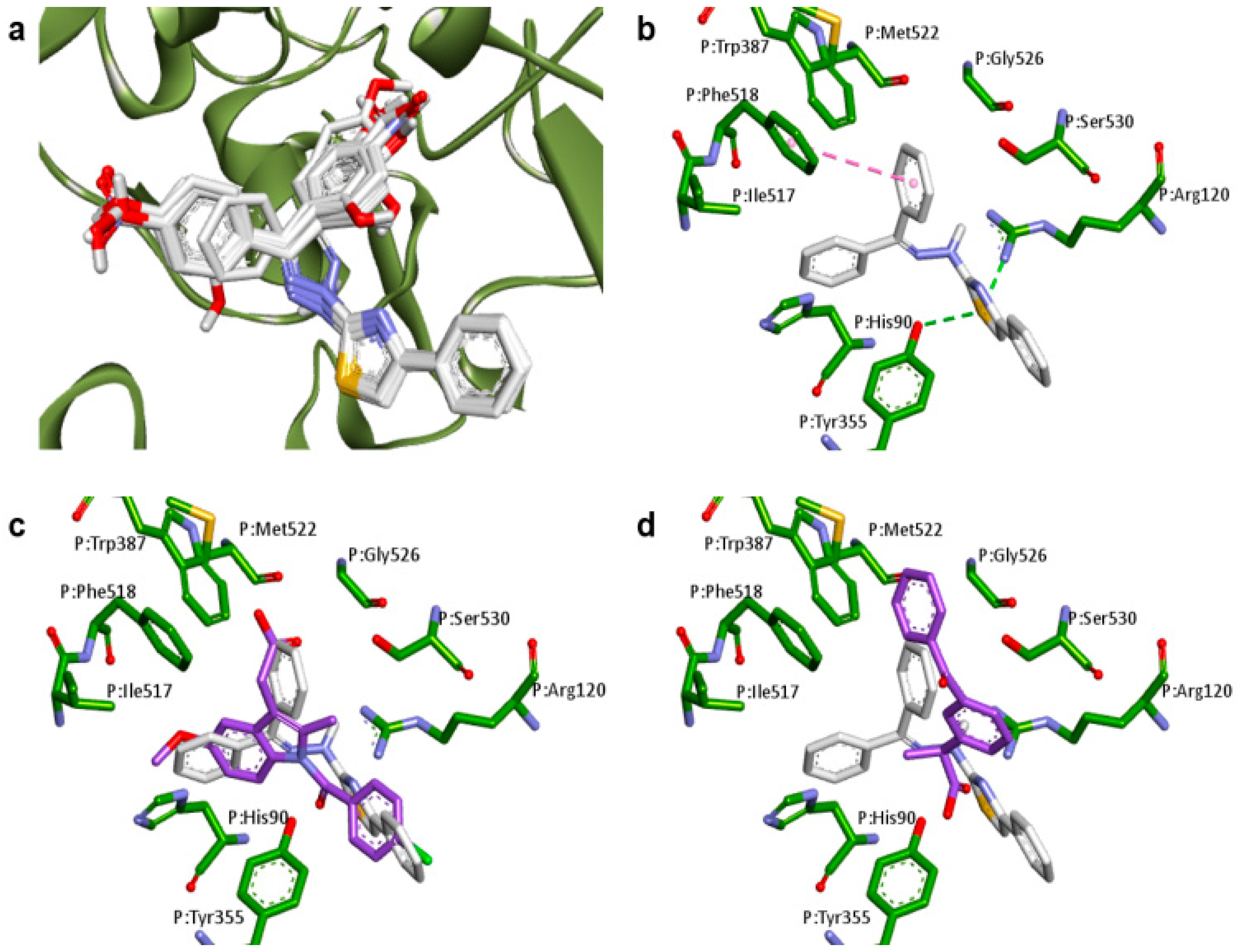
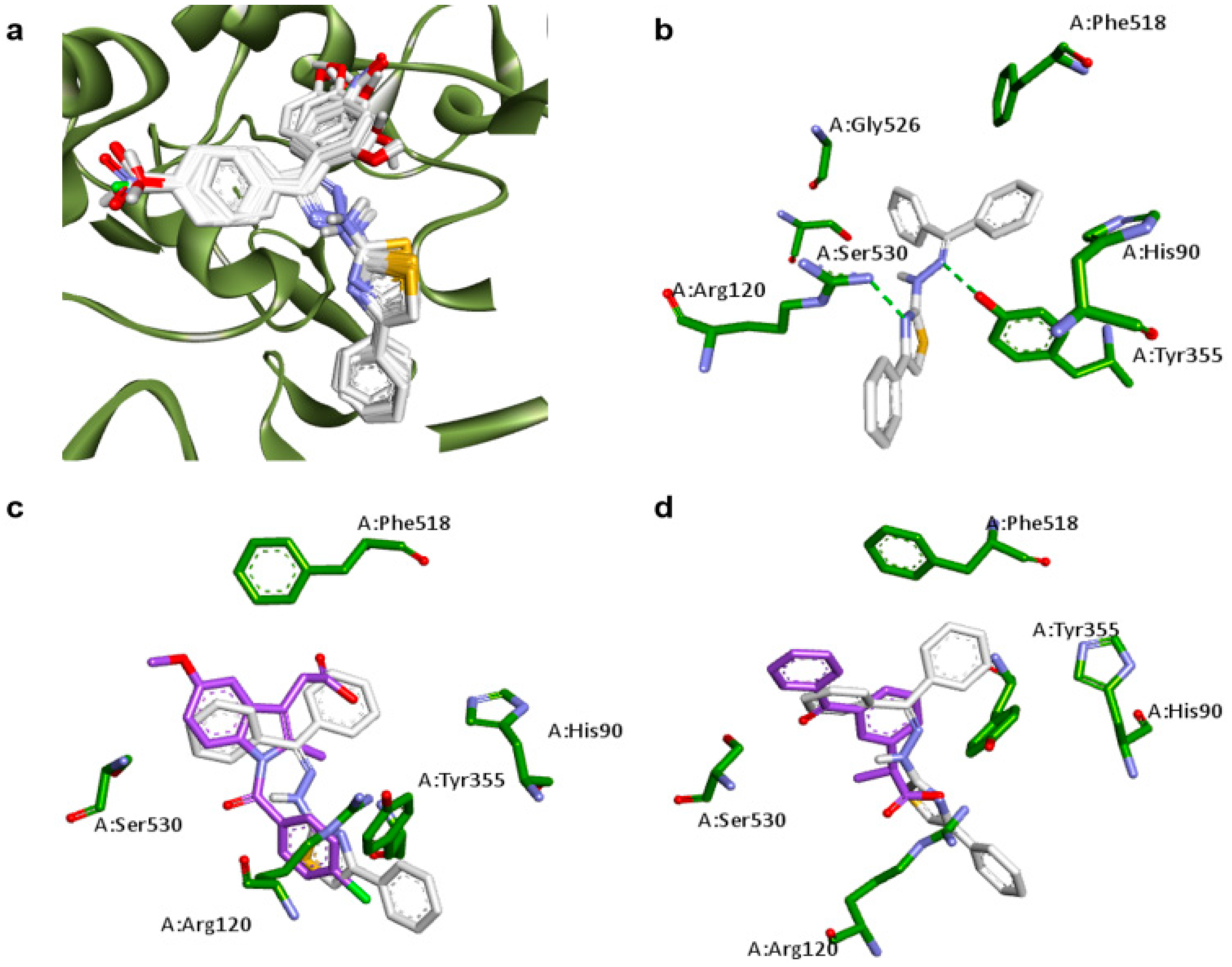
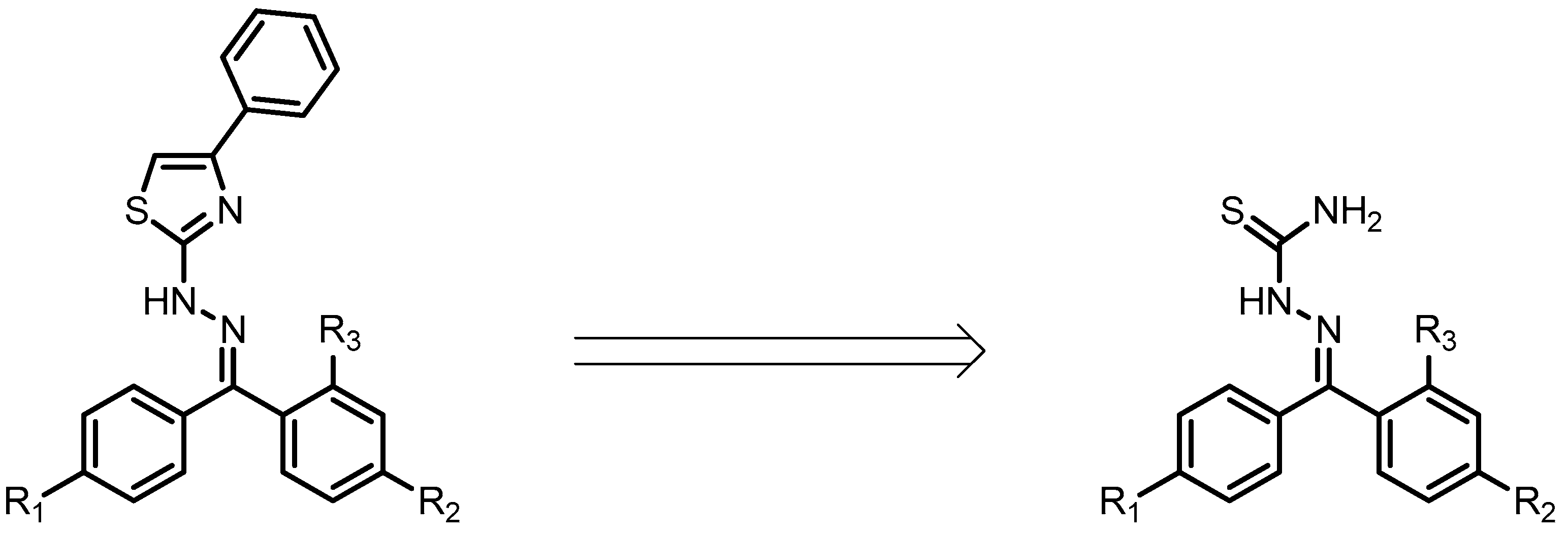
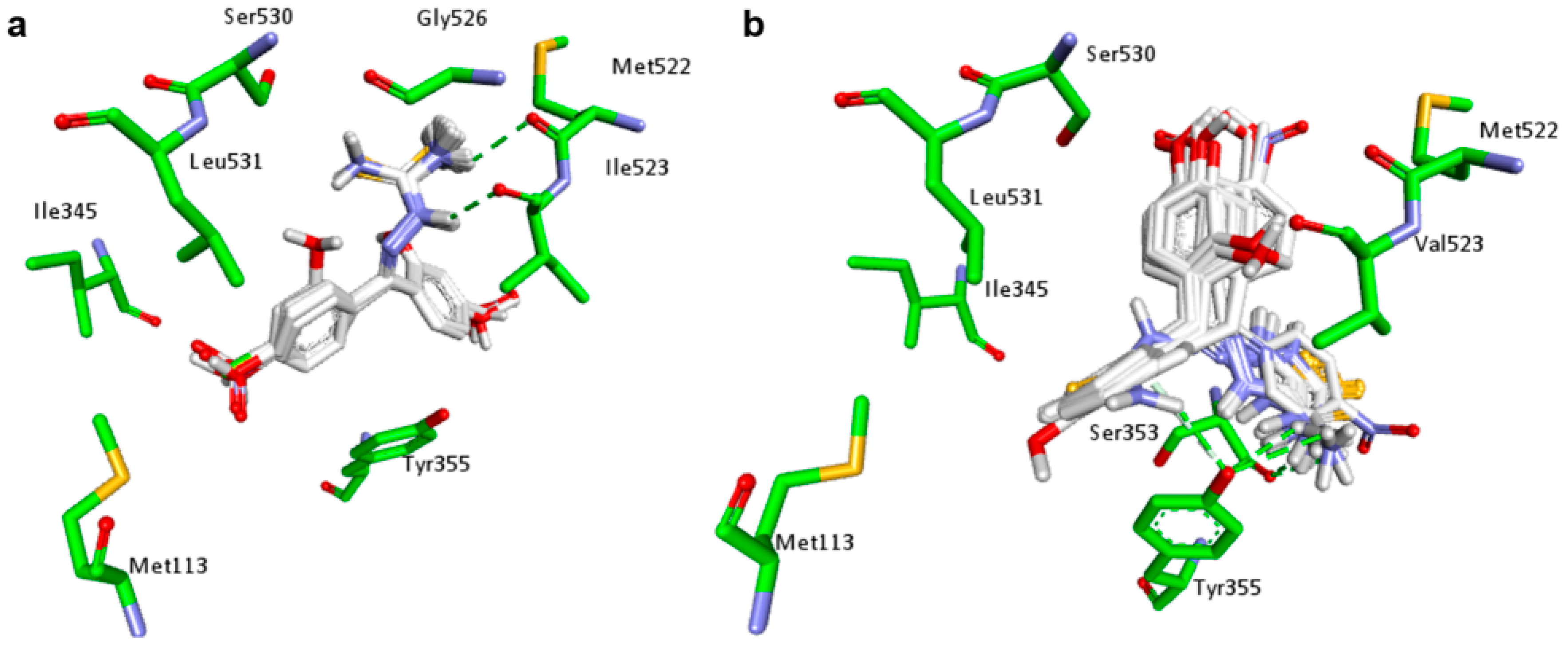
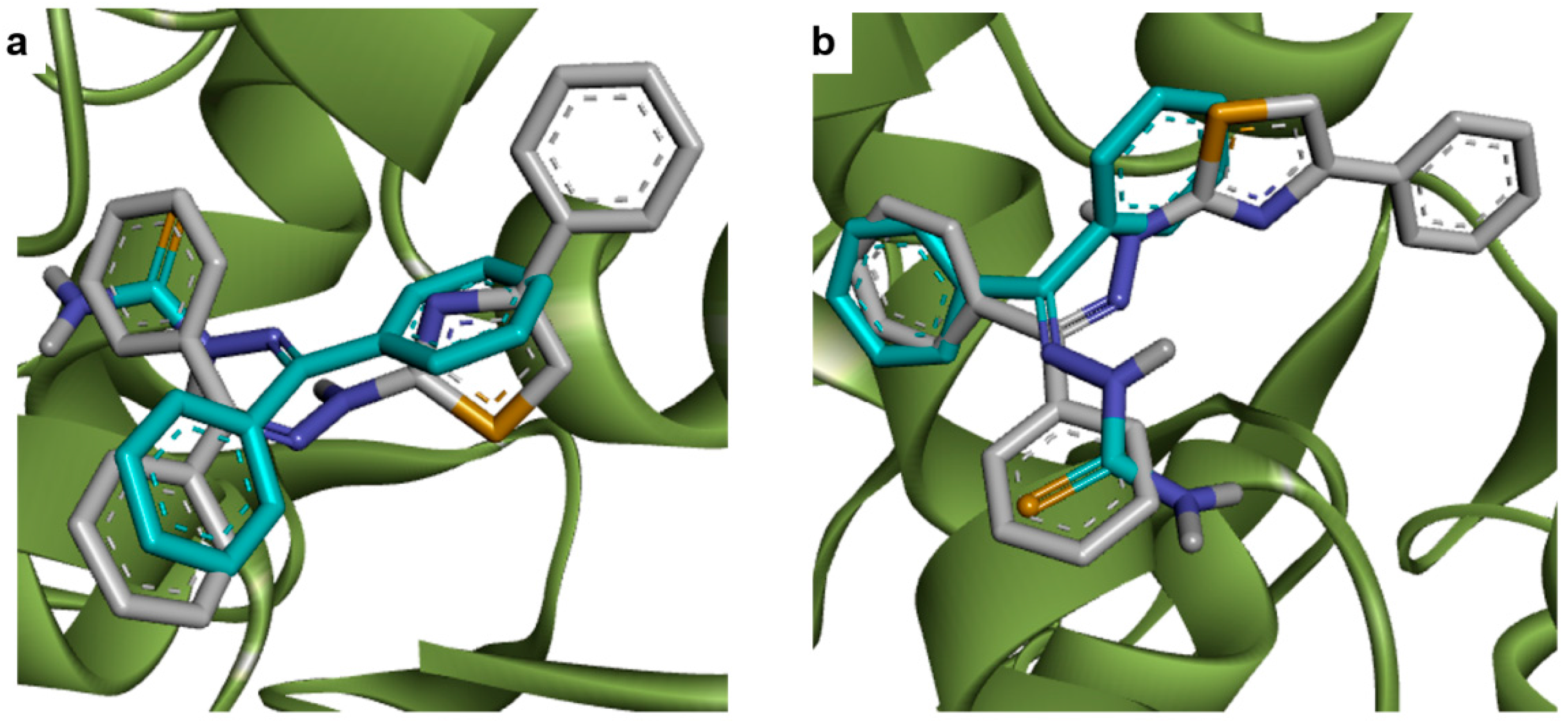
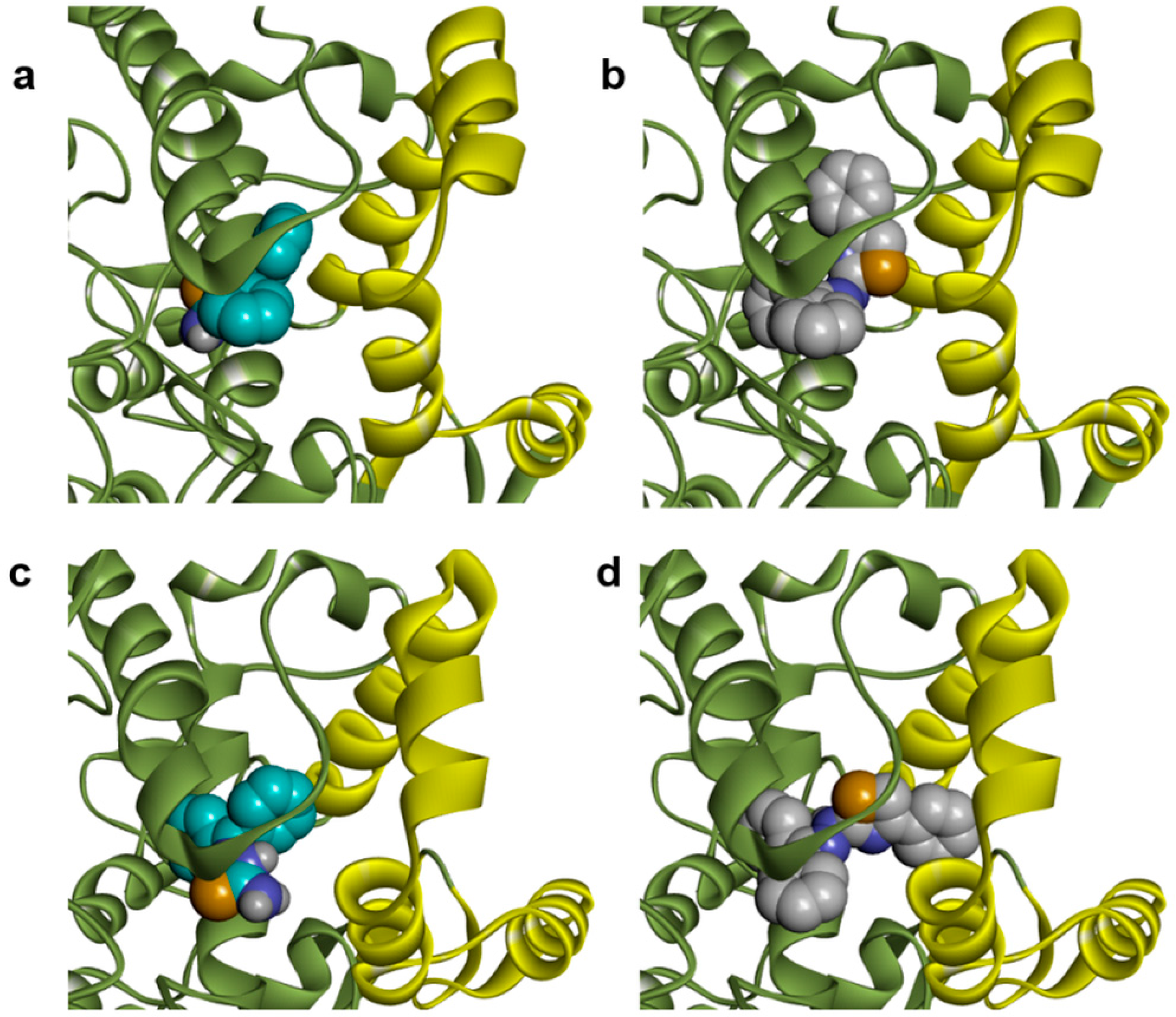
| Compounds | Predicted Binding Energy (kcal/mol) | |
|---|---|---|
| COX-1 | COX-2 | |
| PDB ID: 2OYU | PDB ID: 3NT1 | |
| (Regioisomer) | (Regioisomer) | |
| 3a | −8.1 | −8.0 |
| 3b | −8.2 (E)/−8.4 (Z) | −8.5 (E)/−8.0 (Z) |
| 3c | −8.1 (E)/−7.9 (Z) | −7.9 (E)/−7.6 (Z) |
| 3d | −8.2 | −7.9 |
| 3e | −8.0 (E)/−7.3 (Z) | −7.4 (E)/−7.6 (Z) |
| 3f | −7.9 (E)/−7.4 (Z) | −7.2 (E)/−7.7 (Z) |
| 3g | −8.2 (E)/−9.2 (Z) | −8.7 (E)/−8.7 (Z) |
| 3h | −8.6 (E)/−8.7 (Z) | −8.2 (E)/−8.5 (Z) |
| 3i | −8.2 (E)/−8.7 (Z) | −8.2 (E)/−8.7 (Z) |
| 3j | −7.8 (E)/−8.7 (Z) | −9.1 (E)/−8.2 (Z) |
| Indomethacin | −7.8 | −5.7 |
| S-Ketoprofen | −8.8 | −8.9 |
| Compound | Predicted Binding Energy (kcal/mol) | |
|---|---|---|
| COX-1 | COX-2 | |
| PDB ID: 2OYU | PDB ID: 3NT1 | |
| (Regioisomer) | (Regioisomer) | |
| 2a | −8.1 | −7.4 |
| 2b | −8.6 (E)/−8.6 (Z) | −8.0 (E)/−8.2 (Z) |
| 2c | −8.1 (E)/−8.1 (Z) | −7.6 (E)/−7.8 (Z) |
| 2d | −8.1 | −6.8 |
| 2e | −7.7 (E)/−7.7 (Z) | −7.4 (E)/−7.1 (Z) |
| 2f | −7.9 (E)/−8.1 (Z) | −7.7 (E)/−7.5 (Z) |
| 2g | −9.0 (E)/−9.3 (Z) | −8.0 (E)/−8.0 (Z) |
| 2h | −9.0 (E)/−9.0 (Z) | −7.3 (E)/−7.8 (Z) |
| 2i | −8.8 (E)/−9.0 (Z) | −7.4 (E)/−7.8 (Z) |
| 2j | −8.6 (E)/−8.7 (Z) | −8.5 (E)/−8.6 (Z) |
| Compounds | Ear Edema | Neutrophil Recruitment | Compounds | Ear Edema | Neutrophil Recruitment |
|---|---|---|---|---|---|
| 2a | 28 ** | ns | 3c | 55 **** | 66 ** |
| 2b | 42 **** | ns | 3d | 64 **** | ns |
| 2c | ns | ns | 3e | 73 **** | ns |
| 2d | 50 **** | ns | 3f | 74 **** | ns |
| 2e | 69 **** | 52 * | 3g | 51 **** | ns |
| 2f | 72 **** | ns | 3h | 72 **** | ns |
| 2g | 74 **** | ns | 3i | 47 **** | ns |
| 2h | 76 **** | ns | 3j | 75 **** | ns |
| 2i | ns | ns | Indomethacin | 71 **** | ns |
| 2j | 38 **** | ns | Ketoprofen | 68 **** | ns |
| 3a | 48 **** | 68 ** | Dexamethasone | 82 **** | 54 * |
| 3b | 69 **** | ns | Vehicle | 0 | 0 |
| Var ID | VIP | Coeff. G1 (****) | Coeff. G5 (ns) |
|---|---|---|---|
| Sv | 1.50867 | 0.041 | −0.022 |
| apol | 1.50866 | 0.041 | −0.022 |
| VABC | 1.50828 | 0.041 | −0.022 |
| Sp | 1.50668 | 0.041 | −0.022 |
| CrippenLogP | 1.45515 | 0.039 | −0.021 |
| Spe | 1.44239 | 0.039 | −0.021 |
| nRing | 1.42547 | 0.038 | −0.021 |
| MLogP | 1.30142 | 0.036 | −0.019 |
| LipoaffinityIndex | 1.26585 | 0.034 | −0.018 |
| XLogP | 1.24645 | 0.034 | −0.018 |
| nRotB | 1.07619 | 0.030 | −0.016 |
| nAromBond | 1.0697 | 0.029 | −0.016 |
| naAromAtom | 1.03784 | 0.029 | −0.015 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Januario, J.P.; De Souza, T.B.; Lavorato, S.N.; Maiolini, T.C.S.; Domingos, O.S.; Baldim, J.L.; Folquitto, L.R.S.; Soares, M.G.; Chagas-Paula, D.A.; Dias, D.F.; et al. Design and Synthesis of New Benzophenone Derivatives with In Vivo Anti-Inflammatory Activity through Dual Inhibition of Edema and Neutrophil Recruitment. Molecules 2018, 23, 1859. https://doi.org/10.3390/molecules23081859
Januario JP, De Souza TB, Lavorato SN, Maiolini TCS, Domingos OS, Baldim JL, Folquitto LRS, Soares MG, Chagas-Paula DA, Dias DF, et al. Design and Synthesis of New Benzophenone Derivatives with In Vivo Anti-Inflammatory Activity through Dual Inhibition of Edema and Neutrophil Recruitment. Molecules. 2018; 23(8):1859. https://doi.org/10.3390/molecules23081859
Chicago/Turabian StyleJanuario, Jaqueline P., Thiago B. De Souza, Stefânia N. Lavorato, Tatiane C. S. Maiolini, Olívia S. Domingos, João L. Baldim, Laís R. S. Folquitto, Marisi G. Soares, Daniela A. Chagas-Paula, Danielle F. Dias, and et al. 2018. "Design and Synthesis of New Benzophenone Derivatives with In Vivo Anti-Inflammatory Activity through Dual Inhibition of Edema and Neutrophil Recruitment" Molecules 23, no. 8: 1859. https://doi.org/10.3390/molecules23081859
APA StyleJanuario, J. P., De Souza, T. B., Lavorato, S. N., Maiolini, T. C. S., Domingos, O. S., Baldim, J. L., Folquitto, L. R. S., Soares, M. G., Chagas-Paula, D. A., Dias, D. F., & Dos Santos, M. H. (2018). Design and Synthesis of New Benzophenone Derivatives with In Vivo Anti-Inflammatory Activity through Dual Inhibition of Edema and Neutrophil Recruitment. Molecules, 23(8), 1859. https://doi.org/10.3390/molecules23081859