Structural Divergence in O-GlcNAc Glycans Displayed on Epidermal Growth Factor-like Repeats of Mammalian Notch1
Abstract
1. Introduction
2. Results
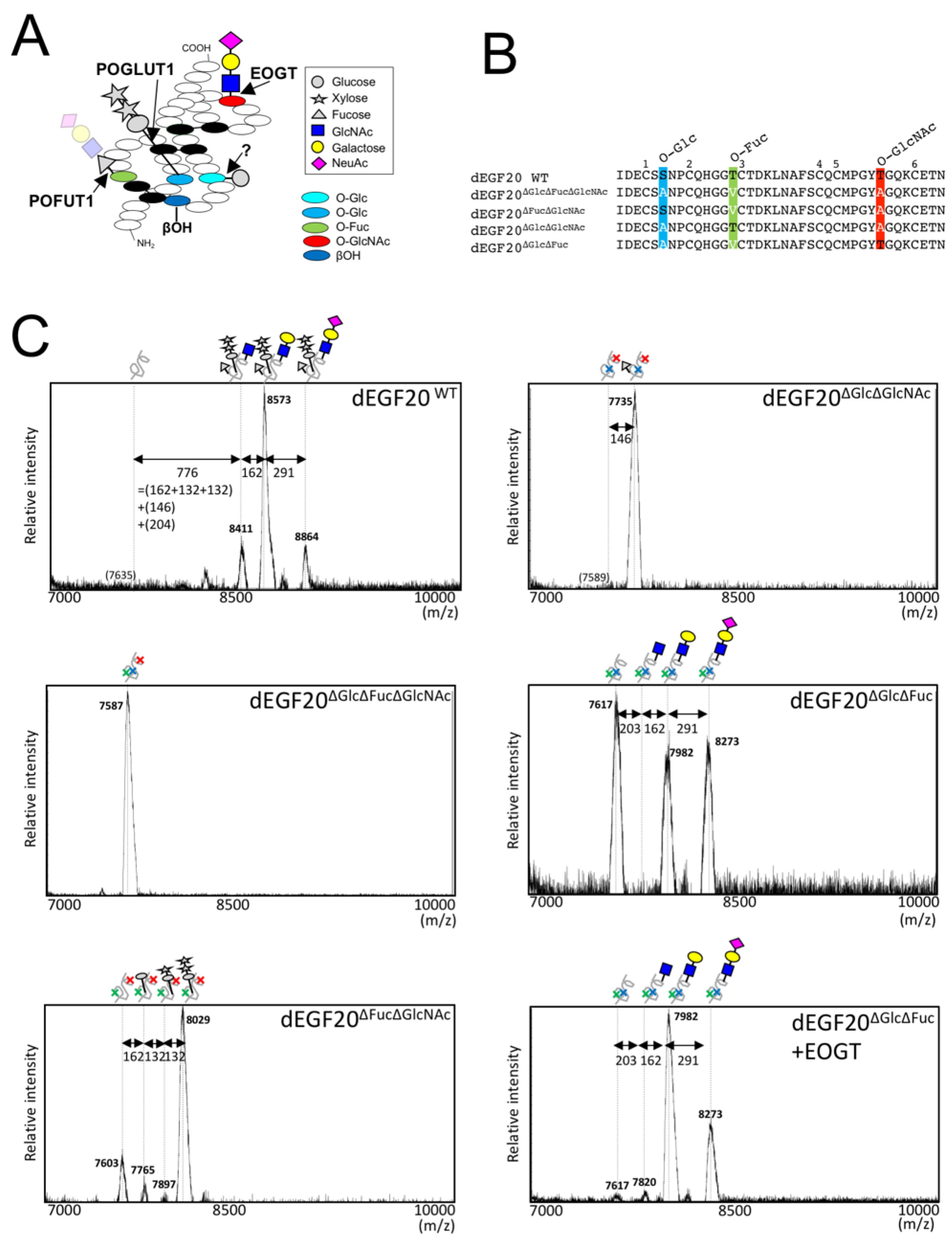
2.1. Drosophila Notch EGF20 Expressed in HEK293T Cells is Modified with O-GlcNAc Glycan
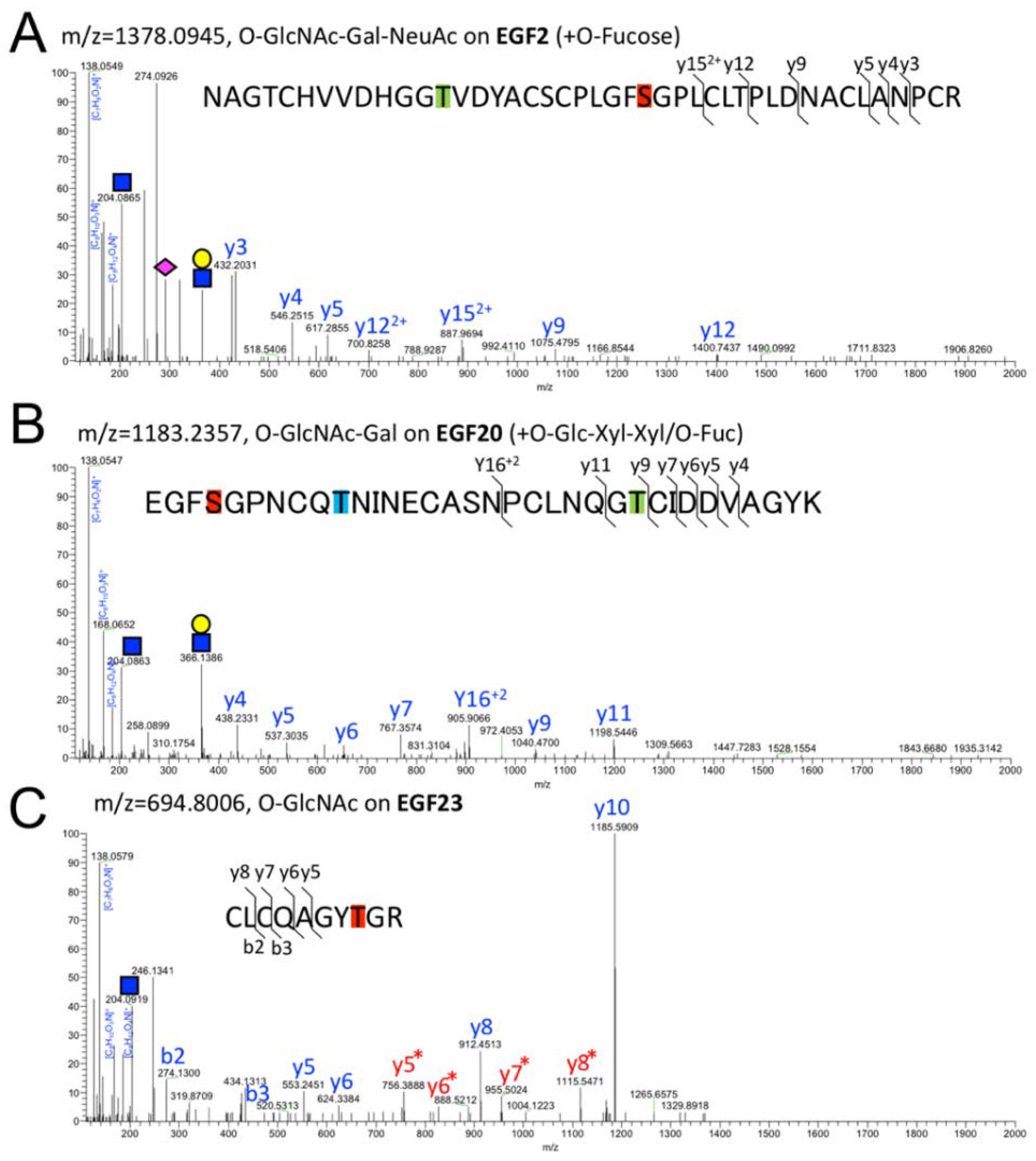
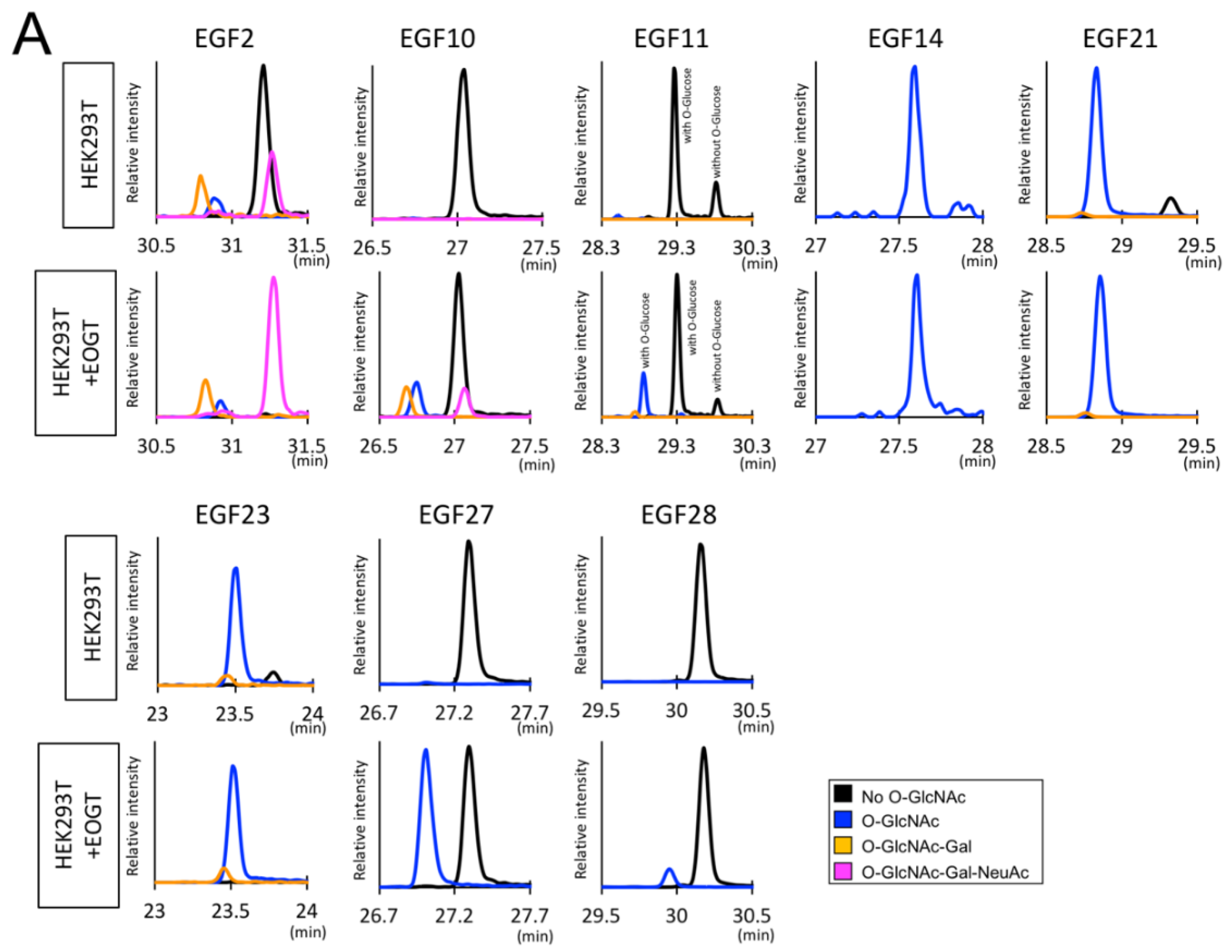
2.2. Detection of O-GlcNAc Oligosaccharide by LC-MS/MS
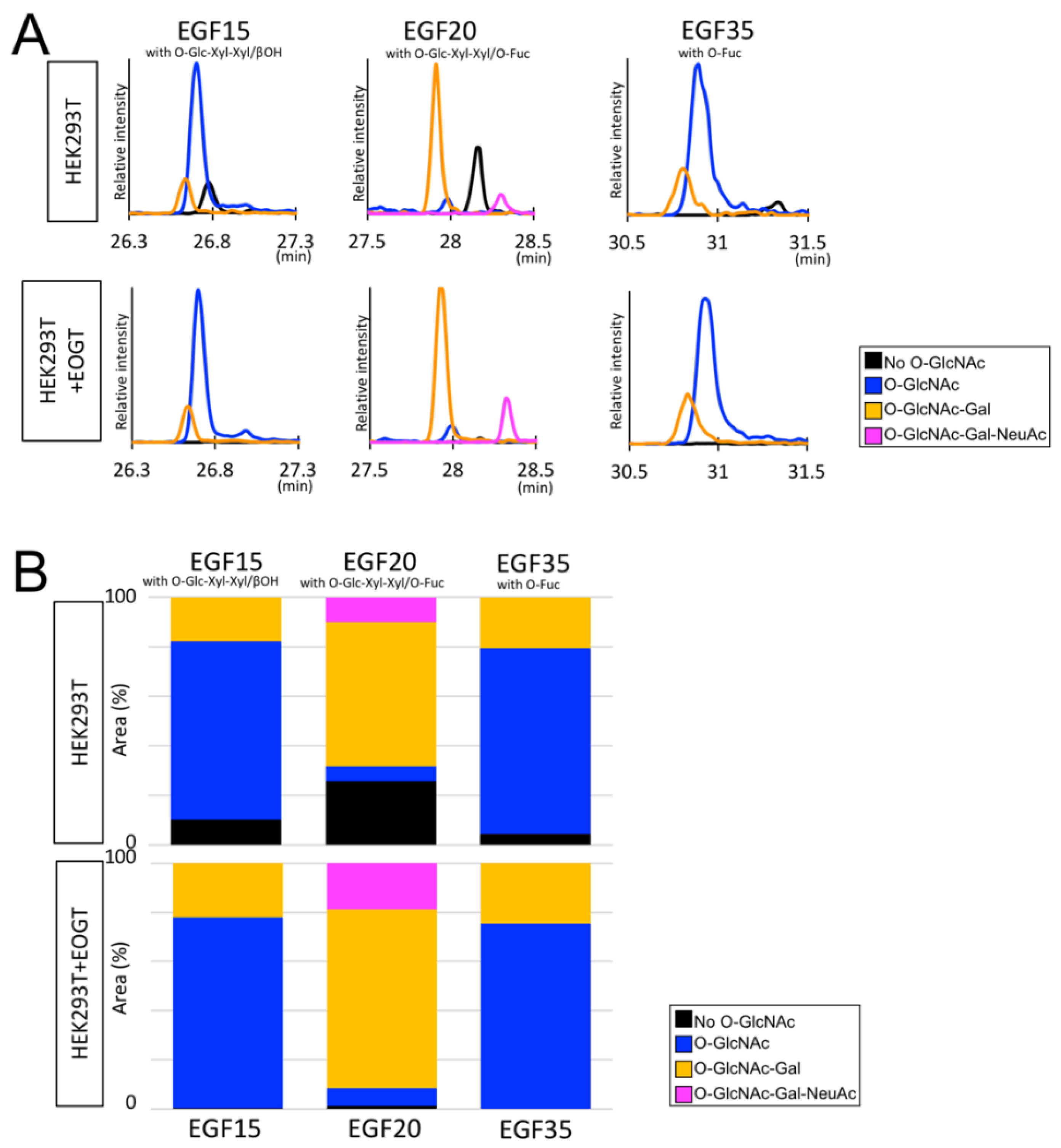
2.3. Structural Differences in O-GlcNAc Dlycan among the EGF Domain of Mouse Notch1 EGF
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plasmid Constructs
4.3. Cell Culture and Transfection
4.4. Sample Preparation
4.5. MALDI-TOF-MS Analysis
4.6. Hybrid Quadrupole FT Linear Ion Trap MS Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsuura, A.; Ito, M.; Sakaidani, Y.; Kondo, T.; Murakami, K.; Furukawa, K.; Nadano, D.; Matsuda, T.; Okajima, T. O-linked N-acetylglucosamine is present on the extracellular domain of notch receptors. J. Biol. Chem. 2008, 283, 35486–35495. [Google Scholar] [CrossRef] [PubMed]
- Hart, G.W. Nutrient Regulation of Cellular Metabolism & Physiology by O-GlcNAcylation. J. Biol. Chem. 2014, 20, 208–213. [Google Scholar]
- Sakaidani, Y.; Nomura, T.; Matsuura, A.; Ito, M.; Suzuki, E.; Murakami, K.; Nadano, D.; Matsuda, T.; Furukawa, K.; Okajima, T. O-linked-N-acetylglucosamine on extracellular protein domains mediates epithelial cell-matrix interactions. Nat. Commun. 2011, 2, 583. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Sawaguchi, S.; Furukawa, K.; Okajima, T. N-acetylglucosamine modification in the lumen of the endoplasmic reticulum. Biochim. Biophys. Acta 2015, 1850, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Varshney, S.; Stanley, P. EOGT and O-GlcNAc on secreted and membrane proteins. Biochem. Soc. Trans. 2017, 45, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Tashima, Y.; Stanley, P. Antibodies that Detect O-GlcNAc on the Extracellular Domain of Cell Surface Glycoproteins. J. Biol. Chem. 2014. [Google Scholar] [CrossRef] [PubMed]
- Sawaguchi, S.; Varshney, S.; Ogawa, M.; Sakaidani, Y.; Yagi, H.; Takeshita, K.; Murohara, T.; Kato, K.; Sundaram, S.; Stanley, P.; et al. O-GlcNAc on NOTCH1 EGF repeats regulates ligand-induced Notch signaling and vascular development in mammals. eLife 2017, 6, e24419. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Sawaguchi, S.; Kawai, T.; Nadano, D.; Matsuda, T.; Yagi, H.; Kato, K.; Furukawa, K.; Okajima, T. Impaired O-linked N-acetylglucosaminylation in the endoplasmic reticulum by mutated EGF domain-specific O-linked N-acetylglucosamine transferase found in Adams-Oliver syndrome. J. Biol. Chem. 2014, 290, 2137–2149. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, R.; Aglan, M.; Keppler-Noreuil, K.; Faqeih, E.; Ansari, S.; Horton, K.; Ashour, A.; Zaki, M.S.; Al-Zahrani, F.; CuetO-González, A.M.; et al. Mutations in EOGT confirm the genetic heterogeneity of autosomal-recessive Adams-Oliver syndrome. Am. J. Hum. Genet. 2013, 92, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Silberstein, E.; Perez, Y.; Landau, D.; Elbedour, K.; Langer, Y.; Kadir, R.; Volodarsky, M.; Sivan, S.; Narkis, G.; et al. Autosomal recessive Adams-Oliver syndrome caused by homozygous mutation in EOGT, encoding an EGF domain-specific O-GlcNAc transferase. Eur. J. Hum. Genet. 2014, 22, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Jenny, A.; Stanley, P. The EGF repeat-specific O-GlcNAc-transferase Eogt interacts with notch signaling and pyrimidine metabolism pathways in Drosophila. PLoS ONE 2013, 8, e62835. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, S.; Haltiwanger, R.S. Deciphering the Fringe-Mediated Notch Code: Identification of Activating and Inhibiting Sites Allowing Discrimination between Ligands. Dev. Cell 2017, 40, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, L.; Shi, S.; Harris, R.J.; Spellman, M.W.; Stanley, P.; Haltiwanger, R.S. Modification of epidermal growth factor-like repeats with O-fucose. Molecular cloning and expression of a novel GDP-fucose protein O-fucosyltransferase. J. Biol. Chem. 2001, 276, 40338–40345. [Google Scholar] [CrossRef] [PubMed]
- Moloney, D.J.; Panin, V.M.; Johnston, S.H.; Chen, J.; Shao, L.; Wilson, R.; Wang, Y.; Stanley, P.; Irvine, K.D.; Haltiwanger, R.S.; et al. Fringe is a glycosyltransferase that modifies Notch. Nature 2000, 406, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Valdivia, R.; Takeuchi, H.; Samarghandi, A.; Lopez, M.; Leonardi, J.; Haltiwanger, R.S.; Jafar-Nejad, H. Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi. Development 2011, 138, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Rana, N.A.; Nita-Lazar, A.; Takeuchi, H.; Kakuda, S.; Luther, K.B.; Haltiwanger, R.S. O-glucose trisaccharide is present at high but variable stoichiometry at multiple sites on mouse Notch1. J. Biol. Chem. 2011, 286, 31623–31637. [Google Scholar] [CrossRef] [PubMed]
- Sethi, M.K.; Buettner, F.F.; Krylov, V.B.; Takeuchi, H.; Nifantiev, N.E.; Haltiwanger, R.S.; Gerardy-Schahn, R.; Bakker, H. Identification of glycosyltransferase 8 family members as xylosyltransferases acting on O-glucosylated notch epidermal growth factor repeats. J. Biol. Chem. 2010, 285, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Takeuchi, M.; LeBarron, J.; Kantharia, J.; London, E.; Bakker, H.; Haltiwanger, R.S.; Li, H.; Takeuchi, H. Notch-modifying xylosyltransferase structures support an SNi-like retaining mechanism. Nat. Chem. Biol. 2015, 11, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Sethi, M.K.; Buettner, F.F.; Ashikov, A.; Krylov, V.B.; Takeuchi, H.; Nifantiev, N.E.; Haltiwanger, R.S.; Gerardy-Schahn, R.; Bakker, H. Molecular cloning of a xylosyltransferase that transfers the second xylose to O-glucosylated epidermal growth factor repeats of notch. J. Biol. Chem. 2012, 287, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Luca, V.C.; Kim, B.C.; Ge, C.; Kakuda, S.; Wu, D.; Roein-Peikar, M.; Haltiwanger, R.S.; Zhu, C.; Ha, T.; Garcia, K.C. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science 2017, 355, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Andrawes, M.B.; Xu, X.; Liu, H.; Ficarro, S.B.; Marto, J.A.; Aster, J.C.; Blacklow, S.C. Intrinsic selectivity of Notch 1 for Delta-like 4 over Delta-like 1. J. Biol. Chem. 2013, 288, 25477–25489. [Google Scholar] [CrossRef] [PubMed]
- Monkovic, D.D.; VanDusen, W.J.; Petroski, C.J.; Garsky, V.M.; Sardana, M.K.; Zavodszky, P.; Stern, A.M.; Friedman, P.A. Invertebrate aspartyl/asparaginyl beta-hydroxylase: Potential modification of endogenous epidermal growth factor-like modules. Biochem. Biophys. Res. Commun. 1992, 189, 233–241. [Google Scholar] [CrossRef]
- Silbermann, E.; Moskal, P.; Bowling, N.; Tong, M.; de la Monte, S.M. Role of aspartyl-(asparaginyl)-β-hydroxylase mediated notch signaling in cerebellar development and function. Behav. Brain Funct. 2010, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Sakaidani, Y.; Ichiyanagi, N.; Saito, C.; Nomura, T.; Ito, M.; Nishio, Y.; Nadano, D.; Matsuda, T.; Furukawa, K.; Okajima, T. O-linked-N-acetylglucosamine modification of mammalian Notch receptors by an atypical O-GlcNAc transferase Eogt1. Biochem. Biophys. Res. Commun. 2012, 419, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.M.; Rana, N.A.; Moss, H.; Leonardi, J.; Jafar-Nejad, H.; Haltiwanger, R.S. Mapping Sites of O-Glycosylation and Fringe Elongation on Drosophila Notch. J. Biol. Chem. 2016, 291, 16348–16360. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cheng, K.; Lo, C.Y.; Li, J.; Qu, J.; Neelamegham, S. A Comprehensive, Open-source Platform for Mass Spectrometry-based Glycoproteomics Data Analysis. Mol. Cell. Proteom. 2017, 16, 2032–2047. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Viner, R.; Teo, C.F.; Boons, G.J.; Horn, D.; Wells, L. Combining high-energy C-trap dissociation and electron transfer dissociation for protein O-GlcNAc modification site assignment. J. Proteome Res. 2011, 10, 4088–4104. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Moloney, D.J.; Stanley, P. Fringe modulation of Jagged1-induced Notch signaling requires the action of beta 4galactosyltransferase-1. Proc. Natl. Acad. Sci. USA 2001, 98, 13716–13721. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, L.; Shi, S.; Stanley, P. Expression of Notch signaling pathway genes in mouse embryos lacking beta4galactosyltransferase-1. Gene Expr. Patterns 2006, 6, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Tashima, Y.; Stanley, P. Galactose differentially modulates lunatic and manic fringe effects on Delta1-induced NOTCH signaling. J. Biol. Chem. 2012, 287, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.; McKay, P.F.; Arokiasamy, S.; Patel, R.K.; Klein, K.; Shattock, R.J. Pulmonary delivery of DNA vaccine constructs using deacylated PEI elicits immune responses and protects against viral challenge infection. J. Control. Release 2013, 170, 452–459. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| EGF Domain | Type of O-GlcNAc Glycan | Other Modification | Sequence | Precursor Ion | Charge State | RT (min) |
|---|---|---|---|---|---|---|
| EGF2 | O-GlcNAc | O-Fuc | NAGTCHVVDHGGTVDYACSCPLGFSGPLCLTPLDNACLANPCR | 1264.8098 | 3 | 30.93 |
| EGF2 | O-GlcNAc-Gal | O-Fuc | NAGTCHVVDHGGTVDYACSCPLGFSGPLCLTPLDNACLANPCR | 1305.3207 | 3 | 30.82 |
| EGF2 | O-GlcNAc-Gal-NeuAc | O-Fuc | NAGTCHVVDHGGTVDYACSCPLGFSGPLCLTPLDNACLANPCR | 1378.0945 | 3 | 31.27 |
| EGF10 | O-GlcNAc | none | AICTCPSGYTGPACSQDVDECALGANPCEHAGK | 1253.5182 | 3 | 26.75 |
| EGF10 | O-GlcNAc-Gal | none | AICTCPSGYTGPACSQDVDECALGANPCEHAGK | 980.6537 | 4 | 26.68 |
| EGF10 | O-GlcNAc-Gal-NeuAc | none | AICTCPSGYTGPACSQDVDECALGANPCEHAGK | 1053.4281 | 4 | 27.06 |
| EGF11 | O-GlcNAc | O-Glc βOH | CLNTLGS FECQCLQGYTGPR | 915.3972 | 3 | 28.79 |
| EGF11 | O-GlcNAc-Gal | O-Glc βOH | CLNTLGS FECQCLQGYTGPR | 969.0807 | 3 | 28.67 |
| EGF14 | O-GlcNAc | βOH | CLDGPNTYTCVCTEGYTGTHCEVDIDECDPDPCHYGSCK | 1216.8245 | 4 | 27.61 |
| EGF15 | O-GlcNAc | O-Glc-Xyl βOH | DGVATFTCLCQPGYT GHHCETNINECHSQPCR | 1081.1904 | 4 | 26.71 |
| EGF15 | O-GlcNAc | O-Glc-Xyl-Xyl βOH | DGVATFTCLCQPGYT GHHCETNINECHSQPCR | 1114.2009 | 4 | 26.70 |
| EGF15 | O-GlcNAc-Gal | O-Glc-Xyl-Xyl βOH | DGVATFTCLCQPGYTGHHCETNINECHSQPCR | 1154.9644 | 4 | 26.64 |
| EGF20 | O-GlcNAc | O-Glc-Xyl-Xyl O-Fuc | EGFSGPNCQTNINECAS NPCLNQGT CIDDVAGYK | 1142.7227 | 4 | 27.99 |
| EGF20 | O-GlcNAc-Gal | O-Glc-Xyl-Xyl O-Fuc | EGFSGPNCQTNINECAS NPCLNQGT CIDDVAGYK | 1183.2357 | 4 | 27.92 |
| EGF20 | O-GlcNAc-Gal-NeuAc | O-Glc-Xyl-Xyl O-Fuc | EGFSGPNCQTNINECAS NPCLNQGT CIDDVAGYK | 1256.0098 | 4 | 28.32 |
| EGF21 | O-GlcNAc | none | CNCPLPYTGATCEVVLAPCATSPCK | 1010.7797 | 3 | 28.85 |
| EGF21 | O-GlcNAc-Gal | none | CNCPLPYTGATCEVVLAPCATSPCK | 1064.797 | 3 | 28.77 |
| EGF23 | O-GlcNAc | none | CLCQAGYTGR | 694.8006 | 2 | 23.51 |
| EGF23 | O-GlcNAc-Gal | none | CLCQAGYTGR | 775.8269 | 2 | 23.46 |
| EGF27 | O-GlcNAc | none | CTCPQGYTGLNCQNLVR | 749.0013 | 3 | 27.01 |
| EGF28 | O-GlcNAc | none | SGWTGVNCDVLSVSCEVAAQK | 824.0469 | 3 | 29.95 |
| EGF35 | O-GlcNAc | O-Fuc | SPTCLCLGSFTGPECQFPASSPCVGSNPCYNQGT CEPTSENPFYR | 1368.322 | 4 | 30.93 |
| EGF35 | O-GlcNAc-Gal | O-Fuc | SPTCLCLGSFTGPECQFPASSPCVGSNPCYNQGT CEPTSENPFYR | 1408.8363 | 4 | 30.82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, M.; Senoo, Y.; Ikeda, K.; Takeuchi, H.; Okajima, T. Structural Divergence in O-GlcNAc Glycans Displayed on Epidermal Growth Factor-like Repeats of Mammalian Notch1. Molecules 2018, 23, 1745. https://doi.org/10.3390/molecules23071745
Ogawa M, Senoo Y, Ikeda K, Takeuchi H, Okajima T. Structural Divergence in O-GlcNAc Glycans Displayed on Epidermal Growth Factor-like Repeats of Mammalian Notch1. Molecules. 2018; 23(7):1745. https://doi.org/10.3390/molecules23071745
Chicago/Turabian StyleOgawa, Mitsutaka, Yuya Senoo, Kazutaka Ikeda, Hideyuki Takeuchi, and Tetsuya Okajima. 2018. "Structural Divergence in O-GlcNAc Glycans Displayed on Epidermal Growth Factor-like Repeats of Mammalian Notch1" Molecules 23, no. 7: 1745. https://doi.org/10.3390/molecules23071745
APA StyleOgawa, M., Senoo, Y., Ikeda, K., Takeuchi, H., & Okajima, T. (2018). Structural Divergence in O-GlcNAc Glycans Displayed on Epidermal Growth Factor-like Repeats of Mammalian Notch1. Molecules, 23(7), 1745. https://doi.org/10.3390/molecules23071745






