In-Depth Aroma and Sensory Profiling of Unfamiliar Table-Grape Cultivars
Abstract
:1. Introduction
2. Results
2.1. Grape Maturity and Harvest
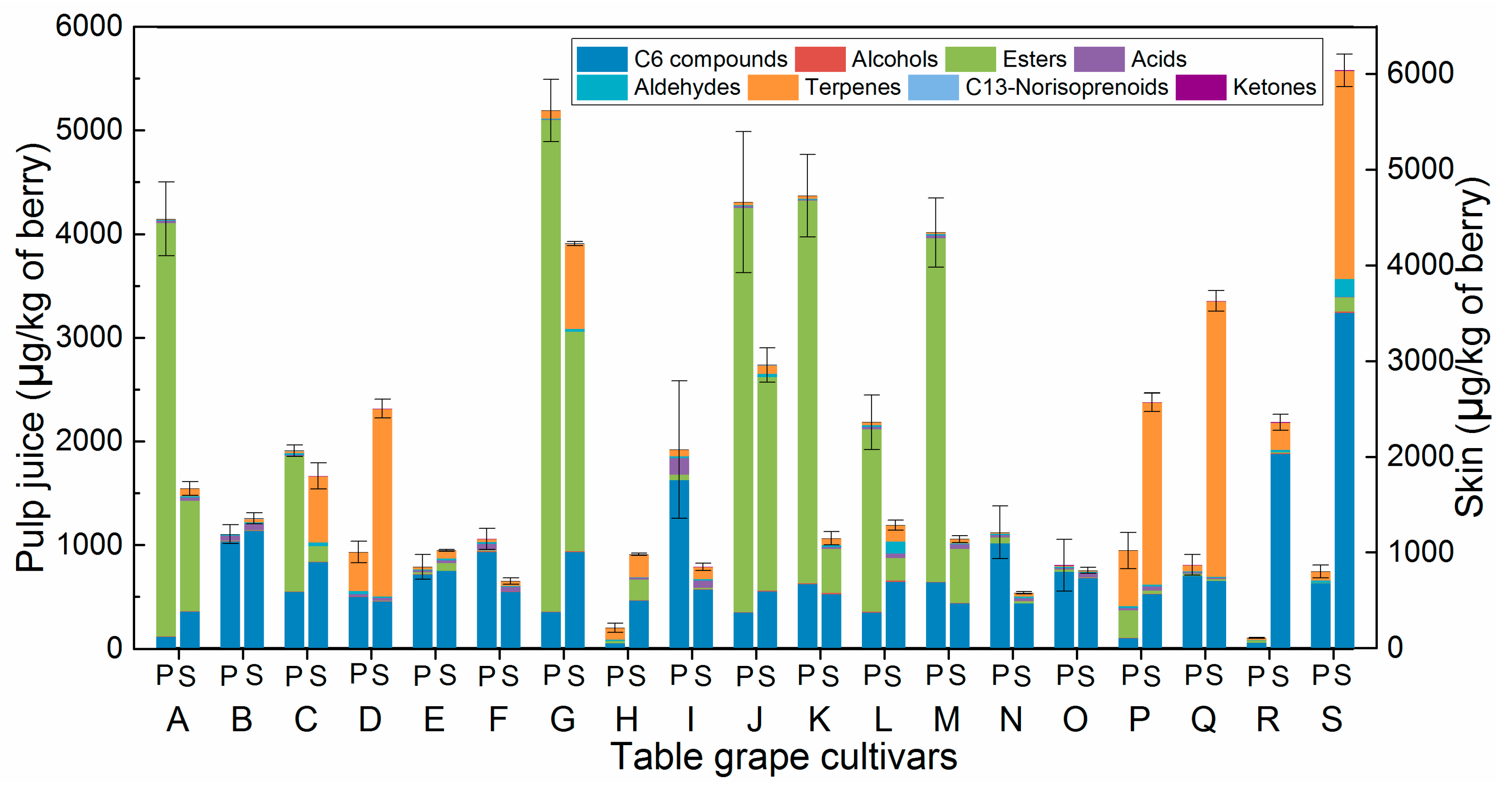
2.2. The Volatile Compounds of Unfamiliar Cultivars
2.3. Odor-Active Compounds of Unfamiliar Table Grapes
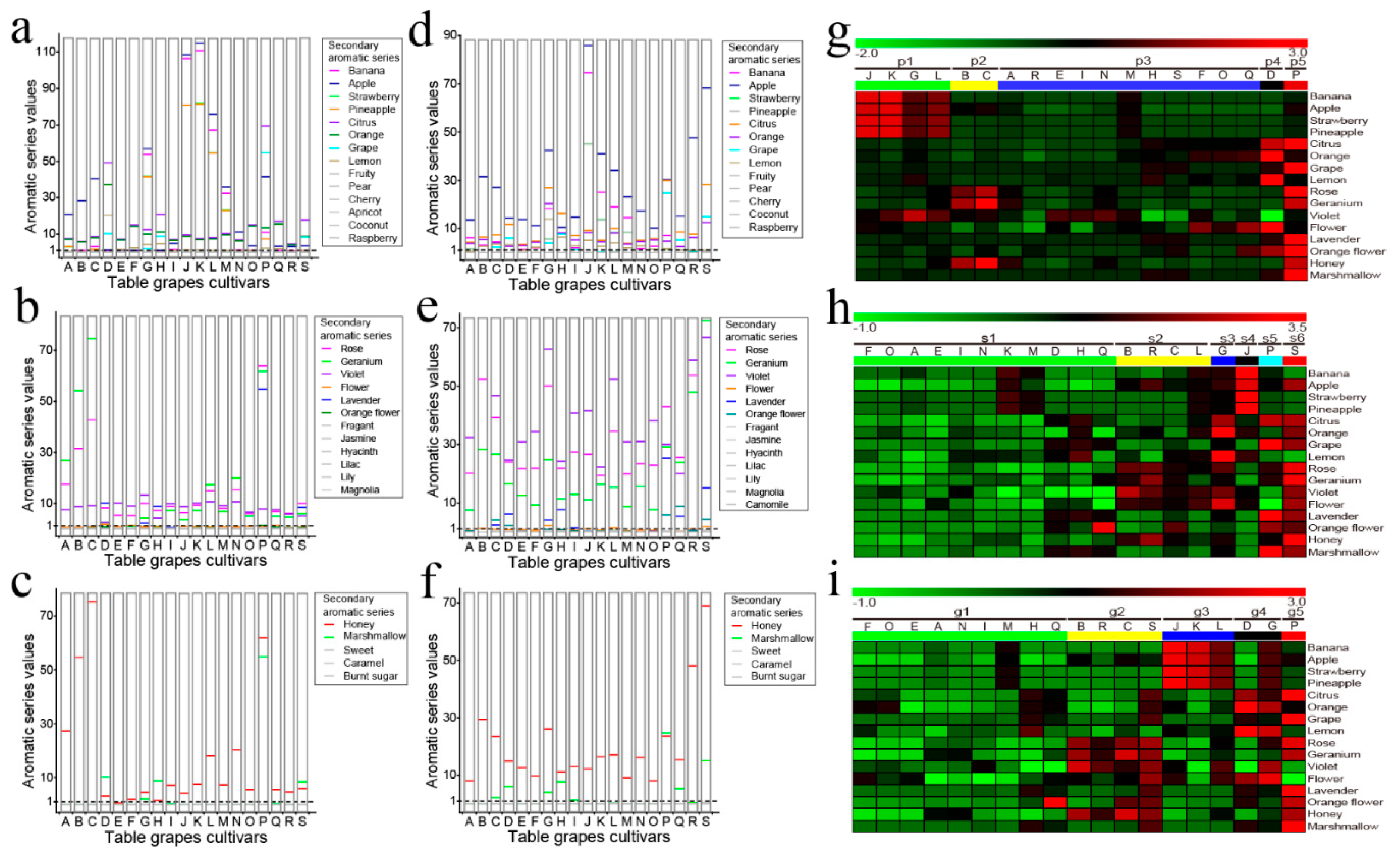
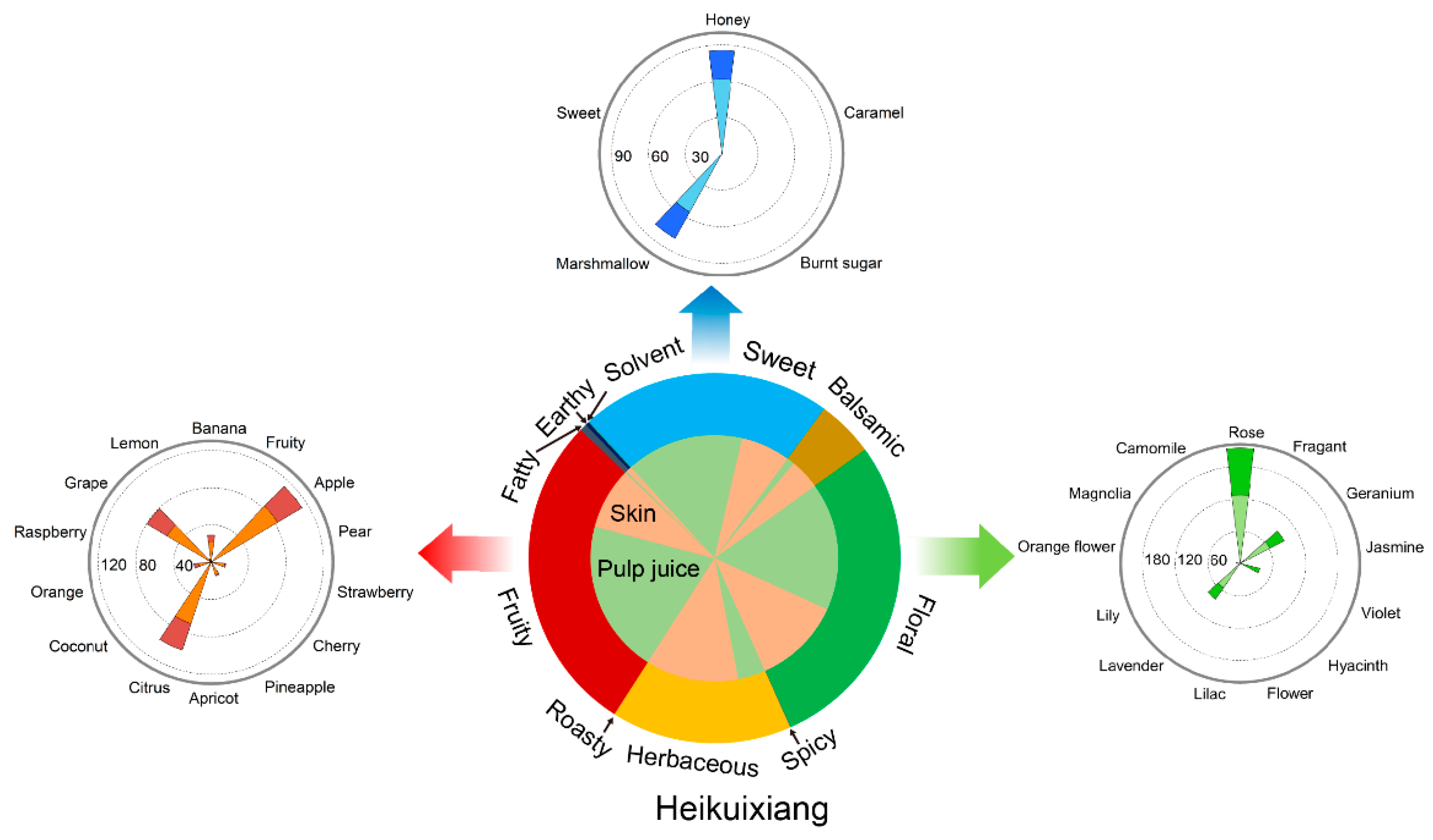
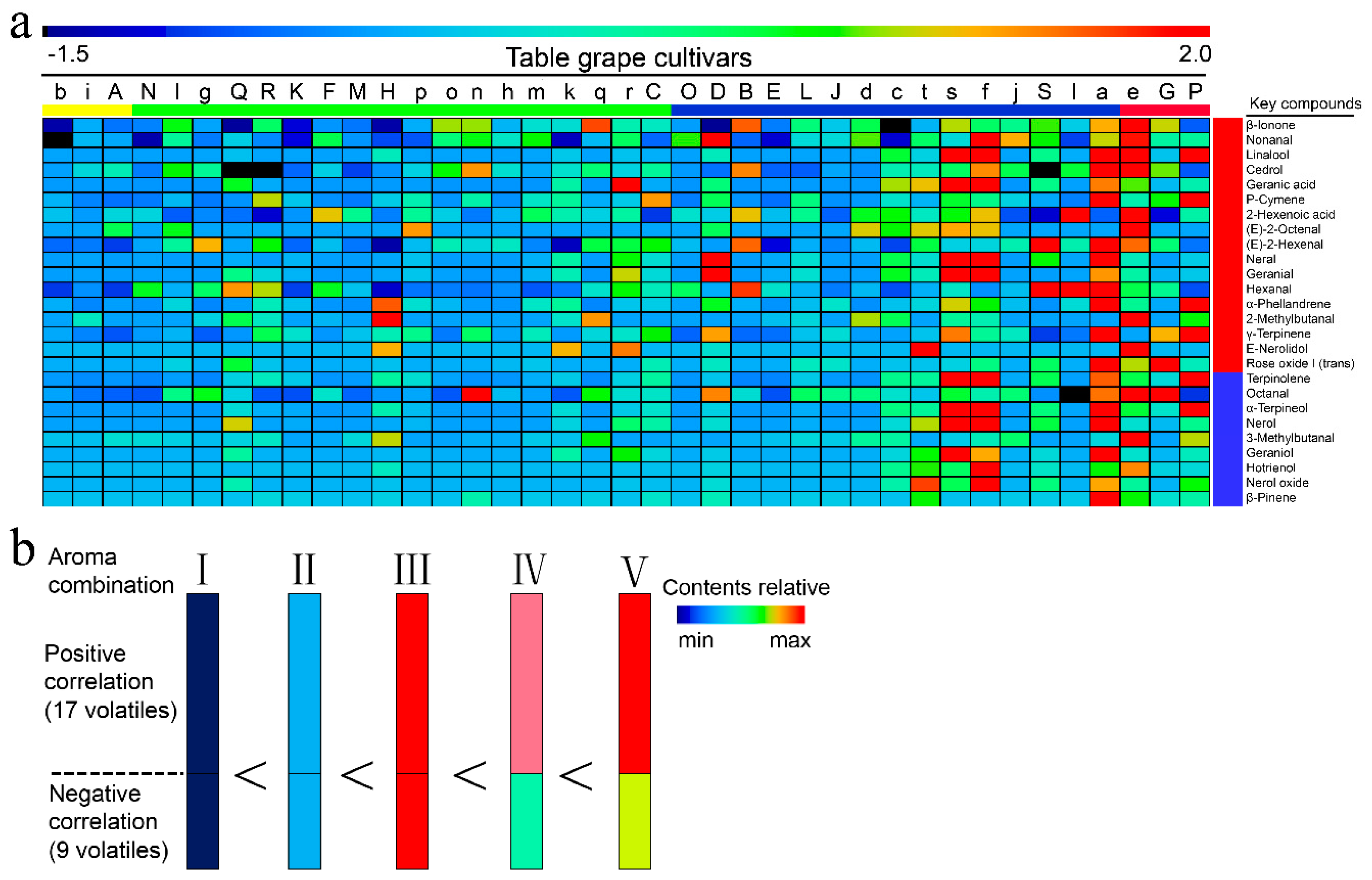
2.4. Primary and Secondary Aromatic Series and Aroma Fingerprints
2.5. Regression Model to Predict Liking Scores
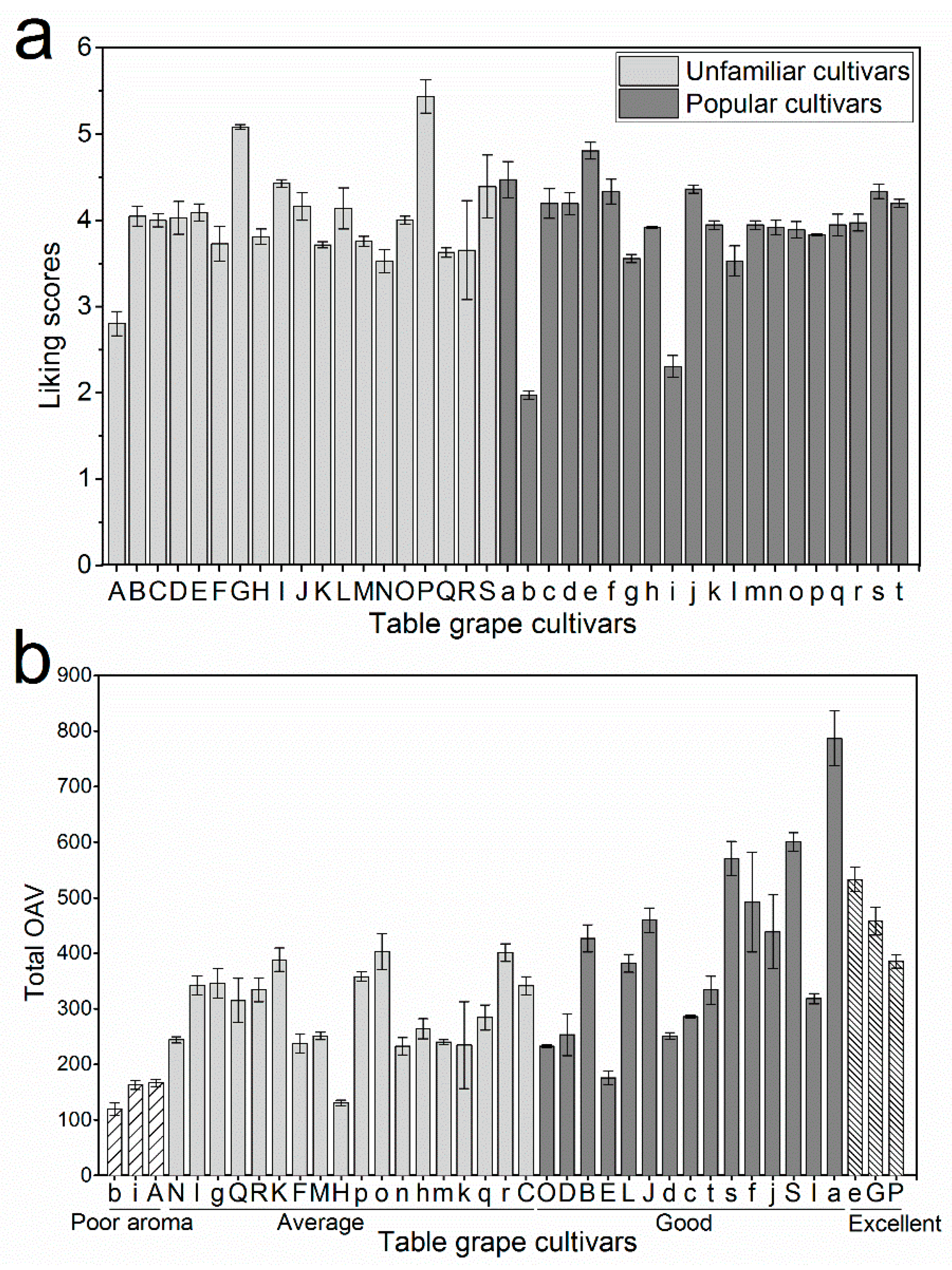
2.6. Key Aroma Compounds Associated with Consumer-Liking Scores
2.7. Relationship between Aromatic Series and Consumer Liking
3. Discussion
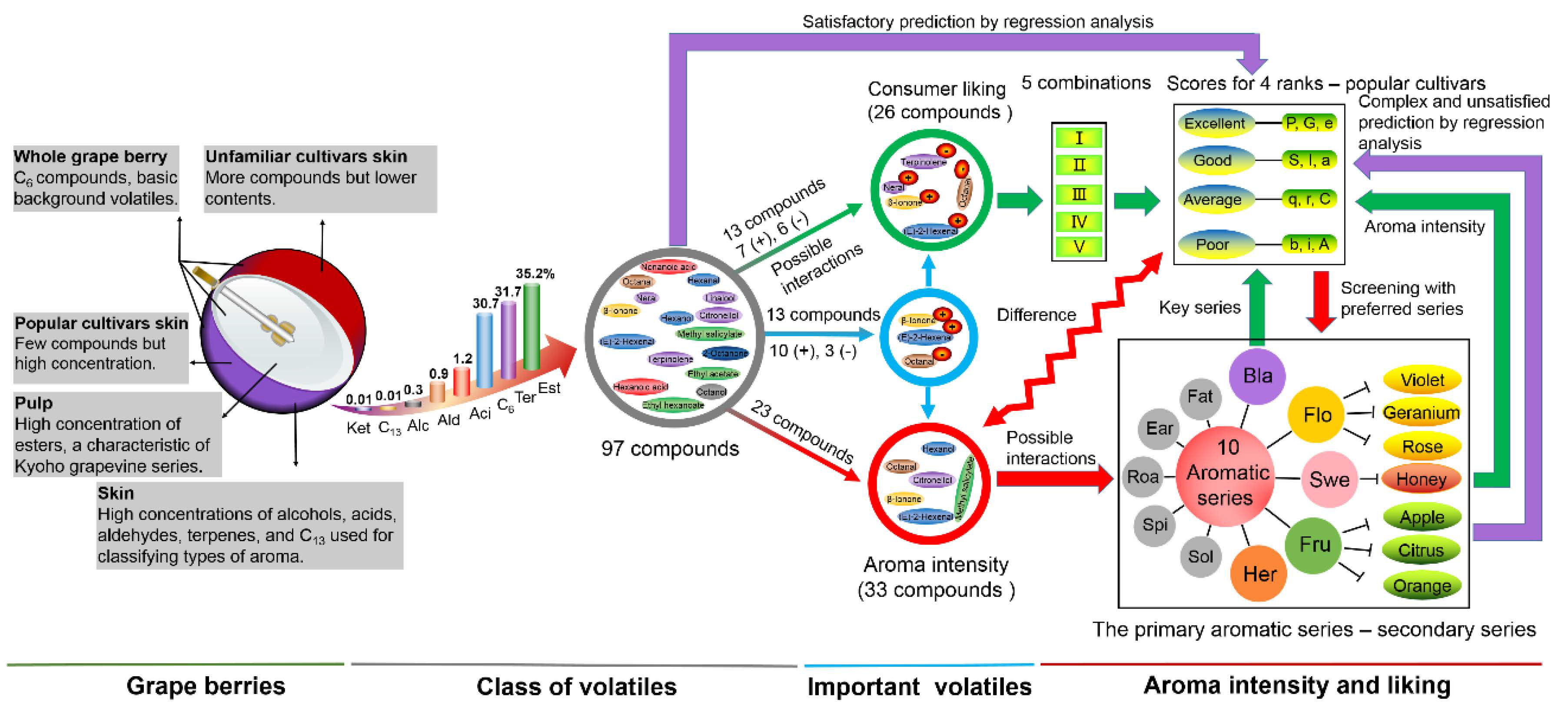
3.1. Relationships among Key Aroma Compounds, Odor-Active Compounds, Aromatic Series, and Consumer-Liking Scores
3.2. The Screening of Unfamiliar Cultivars
3.3. Comprehensive Description of Aroma Characteristics in Table Grapes
4. Materials and Methods
4.1. Materials
4.2. Chemicals
4.3. Sample Preparation
4.4. SPME-GC-MS
4.5. Calculation of the Aromatic Series Values
4.6. Sensory Analysis and Consumer-Liking Scores
4.7. Statistical Analysis
4.8. Chemometric Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SPME | solid-phase microextraction |
| GC-MS | gas chromatography/mass spectrometry |
| DVB/CAR/PDMS | divinylbenzene/carboxen/polydimethylsiloxane |
| TSS | total soluble solids |
| TA | titratable acidity |
| ANOVA | one-way analysis of variance |
| HCA | hierarchical cluster analysis |
| OPLS | orthogonal projection to latent structures |
| PCA | principal component analysis |
| UV | unit variance |
| RMSECV | root-mean-square error of cross-validation |
| RMSEE | root-mean-square error of estimation |
| RMSEP | root-mean-square error of prediction |
| VIP | variable importance in projection |
| OAVs | the odor activity values |
| AC | aroma combinations |
References
- Bartoshuk, L.M.; Klee, H.J. Better fruits and vegetables through sensory analysis. Curr. Biol. 2013, 23, R374–R378. [Google Scholar] [CrossRef] [PubMed]
- Lasers, O. The chemical interactions underlying tomato flavor preferences. Curr. Biol. 2012, 22, 1035–1039. [Google Scholar]
- Spence, C. Multisensory flavor perception. Cell 2015, 161, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, G.M. Smell images and the flavour system in the human brain. Nature 2006, 444, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.A.; Klee, H.J. Plant Volatile Compounds: Sensory Cues for Health and Nutritional Value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Tufariello, M.; Siciliano, P. Analytical characterisation of Negroamaro red wines by “Aroma Wheels”. Food Chem. 2013, 141, 2906–2915. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.; Snitz, K.; Yablonka, A.; Khan, R.M.; Gafsou, D.; Schneidman, E.; Sobel, N. Perceptual convergence of multi-component mixtures in olfaction implies an olfactory white. Proc. Natl. Acad. Sci. USA 2012, 109, 19959–19964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tieman, D.; Zhu, G.; Resende, M.F.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.; Taylor, M.; Zhang, B. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.T.; Bohlmann, J. The molecular basis for wine grape quality—A volatile subject. Science 2006, 311, 804–805. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6, 31116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguerol-Pato, R.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Santiago, J.L.; Martinez, M.C.; Simal-Gandara, J. Aroma potential of Brancellao grapes from different cluster positions. Food Chem. 2012, 132, 112–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez García-Carpintero, E.; Sánchez-Palomo, E.; Gómez Gallego, M.A.; González-Viñas, M.A. Free and bound volatile compounds as markers of aromatic typicalness of Moravia Dulce, Rojal and Tortosí red wines. Food Chem. 2012, 131, 90–98. [Google Scholar] [CrossRef]
- Panighel, A.; Maoz, I.; De Rosso, M.; De Marchi, F.; Dalla Vedova, A.; Gardiman, M.; Bavaresco, L.; Flamini, R. Identification of saffron aroma compound beta-isophorone (3,5,5-trimethyl-3-cyclohexen-1-one) in some V. vinifera grape varieties. Food Chem. 2014, 145, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Lamorte, S.A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva di Troia grapes by aromatic series. Food Res. Int. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- Nan, L.J.; Liu, L.Y.; Zhao, X.H.; Qiu, S.; Wang, H.; Li, H. Effect of alternative new pruning system and harvesting times on aroma compounds of young wines from Ecolly (Vitis vinifera) in a new grape growing region of the Weibei Plateau in China. Sci. Hortic. 2013, 162, 181–187. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of cabernet sauvignon grapes (Vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Genisheva, Z.; Bescansa, L.; Masa, A.; Oliveira, J.M. Changes in free and bound fractions of aroma compounds of four Vitis vinifera cultivars at the last ripening stages. Phytochemistry 2012, 74, 196–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Martínez, M.; Santiago, J.; Simal-Gándara, J. Floral, spicy and herbaceous active odorants in Gran Negro grapes from shoulders and tips into the cluster, and comparison with Brancellao and Mouratón varieties. Food Chem. 2012, 135, 2771–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asproudi, A.; Petrozziello, M.; Cavalletto, S.; Guidoni, S. Grape aroma precursors in cv. Nebbiolo as affected by vine microclimate. Food Chem. 2016, 211, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Skinkis, P.A.; Qian, M.C. Pinot noir wine volatile and anthocyanin composition under different levels of vine fruit zone leaf removal. Food Chem. 2017, 214, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Verzera, A.; Tripodi, G.; Dima, G.; Condurso, C.; Scacco, A.; Cincotta, F.; Giglio, D.M.L.; Santangelo, T.; Sparacio, A. Leaf removal and wine composition of Vitis vinifera L. cv. Nero d’Avola: The volatile aroma constituents. J. Sci. Food Agric. 2016, 96, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Sparacio, A.; Giglio, D.M.L.; Sparla, S.; Verzera, A. Effects of cluster thinning on wine quality of Syrah cultivar (Vitis vinifera L.). Eur. Food Res. Technol. 2016, 242, 1719–1726. [Google Scholar] [CrossRef]
- Lan, Y.B.; Qian, X.; Yang, Z.J.; Xiang, X.F.; Yang, W.X.; Liu, T.; Zhu, B.Q.; Pan, Q.H.; Duan, C.Q. Striking changes in volatile profiles at sub-zero temperatures during over-ripening of ‘Beibinghong’ grapes in Northeastern China. Food Chem. 2016, 212, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Rolle, L.; Torchio, F.; Giacosa, S.; Rio Segade, S. Berry density and size as factors related to the physicochemical characteristics of Muscat Hamburg table grapes (Vitis vinifera L.). Food Chem. 2015, 173, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Liang, Z.; Fan, P.; Wu, B.; Yang, L.; Wang, Y.; Li, S. Volatiles of grape berries evaluated at the germplasm level by headspace-SPME with GC-MS. Food Chem. 2009, 114, 1106–1114. [Google Scholar] [CrossRef]
- Yang, C.X.; Wang, Y.J.; Wu, B.H.; Fang, J.B.; Li, S.H. Volatile compounds evolution of three table grapes with different flavour during and after maturation. Food Chem. 2011, 128, 823–830. [Google Scholar] [CrossRef]
- Ruiz-García, L.; Hellín, P.; Flores, P.; Fenoll, J. Prediction of Muscat aroma in table grape by analysis of rose oxide. Food Chem. 2014, 154, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Fenoll, J.; Manso, A.; Hellin, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ikoma, Y. Effect of postharvest temperature on the muscat flavor and aroma volatile content in the berries of ‘Shine Muscat’ (Vitis labruscana Baily × V. vinifera L.). Postharvest Biol. Technol. 2016, 112, 256–265. [Google Scholar] [CrossRef]
- Wang, X.-J.; Tao, Y.-S.; Wu, Y.; An, R.-Y.; Yue, Z.-Y. Aroma compounds and characteristics of noble-rot wines of Chardonnay grapes artificially botrytized in the vineyard. Food Chem. 2017, 226, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Genisheva, Z.; Masa, A.; Oliveira, J.M. Correlation between volatile composition and sensory properties in Spanish Albariño wines. Microchem. J. 2010, 95, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Organisation Internationale de la Vigne et du Vin (OIV). Resolution VITI 1/2008: OIV Standard on Minimum Maturity Requirements for Table Grapes; Organisation Internationale de la Vigne et du Vin: Paris, France, 2008. [Google Scholar]
- Bondada, B.; Harbertson, E.; Shrestha, P.M.; Keller, M. Temporal extension of ripening beyond its physiological limits imposes physical and osmotic challenges perturbing metabolism in grape (Vitis vinifera L.) berries. Sci. Hortic. 2017, 219, 135–143. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Bescansa, L.; Masa, A.; Oliveira, J.M. Volatile composition of wines from cvs. Blanco lexítimo, Agudelo and Serradelo (Vitis vinifera) grown in Betanzos (NW Spain). J. Inst. Brew. 2009, 115, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Tikunov, Y.; Lommen, A.; de Vos, C.H.; Verhoeven, H.A.; Bino, R.J.; Hall, R.D.; Bovy, A.G. A novel approach for nontargeted data analysis for metabolomics. Large-scale profiling of tomato fruit volatiles. Plant Physiol. 2005, 139, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- González Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Relationships between Godello white wine sensory properties and its aromatic fingerprinting obtained by GC-MS. Food Chem. 2011, 129, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Schüttler, A.; Friedel, M.; Jung, R.; Rauhut, D.; Darriet, P. Characterizing aromatic typicality of Riesling wines: Merging volatile compositional and sensory aspects. Food Res. Int. 2015, 69, 26–37. [Google Scholar] [CrossRef]
- Mimura, N.; Isogai, A.; Iwashita, K.; Bamba, T.; Fukusaki, E. Gas chromatography/mass spectrometry based component profiling and quality prediction for Japanese sake. J. Biosci. Bioeng. 2014, 118, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Cagliani, L.R.; Pellegrino, G.; Giugno, G.; Consonni, R. Quantification of Coffea arabica and Coffea canephora var. robusta in roasted and ground coffee blends. Talanta 2013, 106, 169–173. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Attribute | No. of Latent Variable | R2X | R2Y | Q2Y | R2 | RMSEE | RMSEcv |
|---|---|---|---|---|---|---|---|
| Pulp juice | 1 + 6 + 0 | 0.693 | 0.919 | 0.799 | 0.9188 | 0.2016 | 0.2952 |
| Skin | 1 + 5 + 0 | 0.643 | 0.865 | 0.725 | 0.8645 | 0.2601 | 0.3484 |
| Grape berries | 1 + 7 + 0 | 0.753 | 0.942 | 0.834 | 0.9419 | 0.17215 | 0.2681 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhang, W.; Duan, S.; Song, S.; Xu, W.; Zhang, C.; Bondada, B.; Ma, C.; Wang, S. In-Depth Aroma and Sensory Profiling of Unfamiliar Table-Grape Cultivars. Molecules 2018, 23, 1703. https://doi.org/10.3390/molecules23071703
Wu Y, Zhang W, Duan S, Song S, Xu W, Zhang C, Bondada B, Ma C, Wang S. In-Depth Aroma and Sensory Profiling of Unfamiliar Table-Grape Cultivars. Molecules. 2018; 23(7):1703. https://doi.org/10.3390/molecules23071703
Chicago/Turabian StyleWu, Yusen, Wenwen Zhang, Shuyan Duan, Shiren Song, Wenping Xu, Caixi Zhang, Bhaskar Bondada, Chao Ma, and Shiping Wang. 2018. "In-Depth Aroma and Sensory Profiling of Unfamiliar Table-Grape Cultivars" Molecules 23, no. 7: 1703. https://doi.org/10.3390/molecules23071703
APA StyleWu, Y., Zhang, W., Duan, S., Song, S., Xu, W., Zhang, C., Bondada, B., Ma, C., & Wang, S. (2018). In-Depth Aroma and Sensory Profiling of Unfamiliar Table-Grape Cultivars. Molecules, 23(7), 1703. https://doi.org/10.3390/molecules23071703