Breakthroughs in Medicinal Chemistry: New Targets and Mechanisms, New Drugs, New Hopes-3
1. Introduction
2. A Hydrogen Peroxide Sensitive Prodrug Strategy for the Targeted Delivery of Methotrexate in Rheumatoid Arthritis
3. A Novel Modulator of Rod Opsin Showing in Vivo Efficacy for Retinal Degeneration
4. Combining Innovative 2D NMR Techniques and Deep Neural Networks to Assist Natural Products Discovery
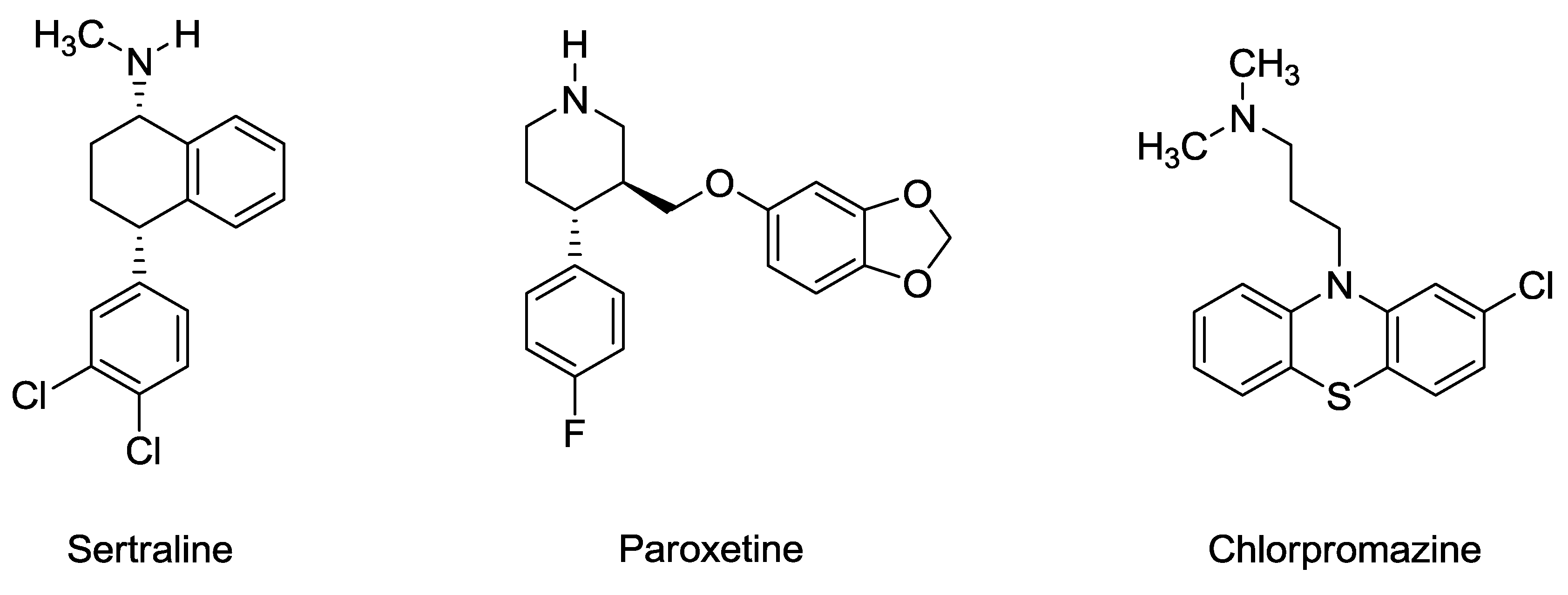
5. Sertraline, Paroxetine, and Chlorpromazine are Rapidly Acting Anthelmintic Drugs Capable of Clinical Repurposing
6. Turning a Gram-Positive-Only into an Effective Gram-Negative Antibiotic Using the Bacterial Machinery
7. Cannabinoid-Induced Cell Death in Endometrial Cancer Cells: Involvement of TRPV1 Receptors in Apoptosis
8. Fenarimols, New Drug Candidates for a Great Social Problem
9. Late-Stage Lead Diversification: Metabolism Giving a Helping Hand at the Nanomole Scale
10. Controlling Insatiable Appetite with a Melanocortin-4 Receptor Agonist in Patients with Leptin Receptor Defect
11. Phosphate Prodrug Strategy Is Applicable in Colon Drug Delivery
12. EphA2 Receptor Is a Key Player in the Metastatic Onset of Ewing Sarcoma
13. Discovery of Sulfonylfluoride Peptidomimetics as Targeted Covalent Inhibitors of Prolyl Oligopeptidase
14. Nanobiotics: A Bioinspired Approach to Fight Antibacterial Resistance
15. Enrichment-triggered Prodrug Activation: A New Concept for Targeted-releasing Prodrug Design
16. Following the Assembly of the Hepatitis B Virus Capsid in Real Time by Mass-Spectrometry
17. Unlocking Promising Avenues to Identify 'Novel' Therapeutic Target Proteins and Candidate Drugs
18. MK-7622: A First-in-Class M1 Positive Allosteric Modulator Development Candidate
19. A Novel Class of Docosahexaenoyl Ethanolamide (DHEA) Epoxides that Exhibit Anti-Inflammatory and Anti-Tumorigenic Properties
20. Click Chemistry-based Discovery of Orally Active Hypoxia Inducing Factor Prolyl Hydroxylase Inhibitors with Favorable Safety Profiles for the Treatment of Anemia
21. Acyclovir as an Ionic Liquid Cation or Anion Can Improve Aqueous Solubility
Author Contributions
Funding
Conflicts of Interest
References
- Peiro Cadahia, J.; Bondebjerg, J.; Hansen, C.A.; Previtali, V.; Hansen, A.E.; Andresen, T.L.; Clausen, M.H. Synthesis and evaluation of hydrogen peroxide sensitive prodrugs of methotrexate and aminopterin for the treatment of rheumatoid arthritis. J. Med. Chem. 2018, 61, 3503–3515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Jastrzebska, B.; Golczak, M.; Gulati, S.; Tang, H.; Seibel, W.; Li, X.; Jin, H.; Han, Y.; et al. A novel small molecule chaperone of rod opsin and its potential therapy for retinal degeneration. Nat. Commun. 2018, 9, 1976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Idelbayev, N.; Roberts, Y.; Tao, Y.; Nannapaneni, B.M.; Duggan, J.; Min, E.C.; Lin, E.C.; Gerwick, G.W.; Cottrell, W.H. Small molecule accurate recognition technology (smart) to enhance natural products research. Sci. Rep. 2017, 7, 14243. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Erez, T.; Reynolds, I.J.; Kumar, D.; Ross, J.; Koytiger, G.; Kusko, R.; Zeskind, B.; Risso, S.; Kagan, E.; et al. Drug repurposing from the perspective of pharmaceutical companies. Br. J. Pharmacol. 2018, 175, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Weeks, J.C.; Roberts, W.M.; Leasure, C.; Suzuki, B.M.; Robinson, K.J.; Currey, H.; Wangchuk, P.; Eichenberger, R.M.; Saxton, A.D.; Bird, T.D.; et al. Sertraline, paroxetine, and chlorpromazine are rapidly acting anthelmintic drugs capable of clinical repurposing. Sci. Rep. 2018, 8, 975. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Miller, P.A.; Vakulenko, S.B.; Stewart, N.K.; Boggess, W.C.; Miller, M.J. A synthetic dual drug sideromycin induces Gram-negative bacteria to commit suicide with a Gram-positive antibiotic. J. Med. Chem. 2018, 61, 3845–3854. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. Cannabinoid-induced cell death in endometrial cancer cells: involvement of TRPV1 receptors in apoptosis. J Physiol Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Melse, Y.; Konings, M.; Duong, H.P.; Eadie, K.; Laleu, B.; Perry, B.; Todd, M.H.; Ioset, J.-R.; van de Sande, W.W.J. Addressing the most neglected diseases through an open research model: The discovery of fenarimols as novel drug candidates for eumycetoma. PLOS Negl. Trop. Dis. 2018, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S.; Walker, G.S.; Sharma, R.; Jenkinson, S.; Tran, T.P.; Stepan, A.F. Lead diversification at the nanomole scale using liver microsomes and quantitative nuclear magnetic resonance spectroscopy: Application to phosphodiesterase 2 inhibitors. J. Med. Chem. 2018, 61, 3626–3640. [Google Scholar] [CrossRef] [PubMed]
- Clément, K.; Biebermann, H.; Sadaf Farooqi, I.; Van der Ploeg, L.; Wolters, B.; Poitou, C.; Puder, L.; Fiedorek, F.; Gottesdiener, K.; Kleinau, G; et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 2018, 24, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A.; Krystal, M.R.; Nowicka-Sans, B.; Langley, D.R.; Conlon, D.A.; Eastgate, M.D.; Grasela, D.M.; Timmins, P.; Wang, T.; Kadow, J.F. Inhibitors of HIV-1 attachment: the discovery and development of temsavir and its prodrug fostemsavir. J. Med. Chem. 2018, 61, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Monclús, S.; López-Alemany, R.; Almacellas-Rabaiget, O.; Herrero-Martín, D.; Huertas-Martinez, J.; Lagares-Tena, L.; Rello-Varona, S. EphA2 receptor is a key player in the metastatic onset of Ewing sarcoma. Inter. J. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, S.; Prades, R.; Mendieta, L.; Brouwer, A.J.; Streefkerk, J.; Nevola, L.; Tarragó, T.; Liskamp, R.M.J.; Giralt, E. Targeted covalent inhibition of prolyl oligopeptidase (POP): Discovery of sulfonylfluoride peptidomimetics. Cell Chem. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Hou, J.; Chen, Q.; Yuan, W.; Cheng, B.; Sun, Y.; Jin, Y.; Ge, L.; Ben-Sasson, S.A.; Chen, J.; et al. Self-assembling myristoylated human α-defensin 5 as a next-generation nanobiotics potentiates therapeutic efficacy in bacterial infection. ACS Nano 2018. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ji, X.; Yu, B.; Ji, K.; Gallo, D.; Csizmadia, E.; Zhu, M.; Choudhury, M.R.; De La Cruz, L.K.C.; Chittavong, V.; et al. Enrichment-triggered prodrug activation demonstrated through mitochondria-targeted delivery of doxorubicin and carbon monoxide. Nat. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lutomski, C.A.; Lyktey, N.A.; Pierson, E.E.; Zhao, Z.; Zlotnick, A.; Jarrold, M.F. Multiple pathways in capsid assemble. J. Am. Chem. Soc. 2018, 140, 5784–5790. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Beshore, D.C.; Di Marco, C.N.; Chang, R.K.; Greshock, T.J.; Ma, L.; Wittmann, M.; Seager, M.A.; Koeplinger, K.A.; Thompson, C.D.; Fuerst, J.; et al. MK-7622: A first-in-class M1 positive allosteric modulator development candidate. ACS Med. Chem. Lett. 2018. [Google Scholar] [CrossRef]
- McDougle, D.R.; Watson, J.E.; Abdeen, A.A.; Adili, R.; Caputo, M.P.; Krapf, J.E.; Johnson, R.W.; Kilian, K.A.; Holinstat, M.; Das, A. Anti-inflammatory omega-3 endocannabinoid epoxides. Proc. Natl. Acad. Sci. USA 2017, 114, E6034–E6043. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Watson, J. E.; Hong, I.; Fan, T.M.; Das, A. Anti-tumorigenic properties of omega-3 endocannabinoid epoxides. J. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, Z.; Li, Z.; Gu, J.; You, Q.; Zhang, X. Click chemistry–based discovery of [3-hydroxy-5-(1H-1,2,3-triazol-4-yl)picolinoyl]glycines as orally active hypoxia inducing factor prolyl hydroxylase inhibitors with favorable safety profiles for the treatment of anemia. J. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hough, W.L.; Smiglak, M.; Rodríguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Ferraz, R.; Teixeira, V.; Rodrigues, D.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Petrovski, Z.; Branco, L.C. Antibacterial activity of Ionic Liquids based on ampicillin against resistant bacteria. RSC Adv. 2014, 4, 4301–4307. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Cojocaru, O.A.; Kelley, S.P.; Bica, K.; Wallace, S.P.; Gurau, G.; Rogers, R.D. Acyclovir as an ionic liquid cation or anion can improve aqueous solubility. ACS Omega 2017, 2, 3483–3493. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangoni, A.A.; Tuccinardi, T.; Collina, S.; Vanden Eynde, J.J.; Muñoz-Torrero, D.; Karaman, R.; Siciliano, C.; De Sousa, M.E.; Prokai-Tatrai, K.; Rautio, J.; et al. Breakthroughs in Medicinal Chemistry: New Targets and Mechanisms, New Drugs, New Hopes-3. Molecules 2018, 23, 1596. https://doi.org/10.3390/molecules23071596
Mangoni AA, Tuccinardi T, Collina S, Vanden Eynde JJ, Muñoz-Torrero D, Karaman R, Siciliano C, De Sousa ME, Prokai-Tatrai K, Rautio J, et al. Breakthroughs in Medicinal Chemistry: New Targets and Mechanisms, New Drugs, New Hopes-3. Molecules. 2018; 23(7):1596. https://doi.org/10.3390/molecules23071596
Chicago/Turabian StyleMangoni, Arduino A., Tiziano Tuccinardi, Simona Collina, Jean Jacques Vanden Eynde, Diego Muñoz-Torrero, Rafik Karaman, Carlo Siciliano, Maria Emília De Sousa, Katalin Prokai-Tatrai, Jarkko Rautio, and et al. 2018. "Breakthroughs in Medicinal Chemistry: New Targets and Mechanisms, New Drugs, New Hopes-3" Molecules 23, no. 7: 1596. https://doi.org/10.3390/molecules23071596
APA StyleMangoni, A. A., Tuccinardi, T., Collina, S., Vanden Eynde, J. J., Muñoz-Torrero, D., Karaman, R., Siciliano, C., De Sousa, M. E., Prokai-Tatrai, K., Rautio, J., Guillou, C., Gütschow, M., Galdiero, S., Liu, H., Agrofoglio, L. A., Sabatier, J.-M., Hulme, C., Kokotos, G., You, Q., & Gomes, P. A. C. (2018). Breakthroughs in Medicinal Chemistry: New Targets and Mechanisms, New Drugs, New Hopes-3. Molecules, 23(7), 1596. https://doi.org/10.3390/molecules23071596





















